Abstract
Chronic kidney disease–mineral bone disorder (CKD-MBD) is a common comorbidity in patients with CKD. Characterized by laboratory abnormalities, bone abnormality, and vascular calcification, CKD-MBD encompasses a group of mineral and hormone disturbances that are strongly associated with increased cardiovascular (CV) morbidity and mortality. Abnormal serum phosphate concentrations are an independent risk factor for CV morbidity and mortality, and overall mortality. Phosphate retention plays a central role in initiating and driving many other disturbances in CKD-MBD (e.g., increased parathyroid hormone and fibroblast growth factor 23 concentrations, hypocalcemia, low vitamin D) that are also linked to increased CV risk. Thus, effective phosphate control is a logical therapeutic target for CKD-MBD treatment. Current phosphate management strategies (dietary restrictions, dialysis, phosphate binders) are insufficient to consistently achieve and maintain target phosphate concentrations in patients on dialysis. Phosphate binders reduce available phosphate for intestinal absorption but do not impair the dominant phosphate absorption pathway. Novel therapies that consider new mechanistic understandings of intestinal phosphate absorption are needed. One such therapy is tenapanor, a targeted sodium-hydrogen exchanger isoform 3 inhibitor that has been shown to reduce serum phosphate concentrations in multiple clinical trials. Tenapanor has a novel mechanism of action that reduces intestinal phosphate absorption in the primary paracellular phosphate absorption pathway.
Keywords: chronic kidney disease mineral bone disorder, fibroblast growth factor 23, hyperphosphatemia, parathyroid hormone, phosphate absorption, vascular calcification
Patients with CKD are often affected by CKD-MBD.1 Characterized by hormone and bone abnormalities,2 CKD-MBD is known to be associated with increased CV mortality,3 a significant cause of death in patients on dialysis.4 Phosphate control is a logical and attractive approach for treatment of CKD-MBD due to both the centrality of phosphate homeostasis in the development and progression of CKD-MBD and the independent association between hyperphosphatemia and CV disease (CVD).5,6 Well-managed phosphate would likely decrease the risk of vascular calcification,7 hyperparathyroidism,8 and hypocalcemia9 and decrease fibroblast growth factor 23 (FGF23) production,10 thus attenuating the progression of CKD-MBD and reducing CV mortality risk.
The implementation of effective phosphate control for the treatment of CKD-MBD is stalled by the lack of phosphate management strategies that can achieve and maintain normal phosphate concentrations in patients on dialysis as most patients with CKD on dialysis do not reach phosphate levels <5.5 mg/dl, let alone more normal levels of <4.5 mg.11
Currently, phosphate binders are the pharmacological treatment for hyperphosphatemia.12, 13, 14, 15, 16 However, phosphate binders only bind a portion of dietary phosphate and, in general, require patients to take many pills with meals.12, 13, 14, 15, 16, 17, 18 Moreover, proper adherence to phosphate binders is a challenge.19 The effectiveness of phosphate binders and dietary phosphate restriction are further limited by a maladaptive upregulation of phosphate absorption.20, 21, 22 New phosphate management strategies should incorporate the latest understandings of phosphate absorption—namely, that the paracellular phosphate absorption pathway is the dominant route of intestinal phosphate absorption.23 A novel phosphate absorption inhibitor, tenapanor, has been recently developed. It directly targets the intestinal sodium/hydrogen exchanger isoform 3 (NHE3), leading to reduced sodium absorption.24 In clinical trials, tenapanor has also been shown to reduce serum phosphate concentrations and was generally well tolerated.25, 26, 27 It may offer a novel treatment approach for CKD-MBD.
CKD-MBD Is a Significant Risk Factor for CV Mortality
A major cause of CV mortality in patients with CKD on dialysis is CKD-MBD,3 a common comorbidity in patients with CKD that is characterized by laboratory abnormalities, bone abnormality, and extraskeletal calcification.1,28 As kidney function declines, progressive disruptions in mineral homeostasis (e.g., calcium and phosphate) are associated with abnormalities in circulating hormone concentrations such as increases in parathyroid hormone (PTH) and FGF23 and decreases in calcitriol.29 These mineral and endocrine changes lead to abnormalities in bone turnover and extraskeletal calcification.30, 31, 32, 33 Multiple components of CKD-MBD such as hyperphosphatemia,3 vascular calcification,34 and elevated FGF23 concentrations35 are known to be significantly associated with increased CV morbidity and mortality.36 CVD accounted for ~62% of deaths among patients with CKD on dialysis in 2017.4 Mortality due to CVD in this population is approximately 20 times higher than in a general population.37 Traditional risk factors for CV mortality (e.g., hypertension, diabetes, diet, and lifestyle) alone do not explain the high CV morbidity and mortality in patients with CKD,38 and the established treatment strategies for these risk factors have not seen significant recent advancements. Better control of phosphorus may be necessary to improve clinical outcomes and quality of life in patients on dialysis and reduce unacceptably high mortality risk.6
Therapeutic approaches for CKD-MBD that may decrease CV mortality include improving dialysis modalities,39 decreasing inflammation,40 and better management of serum phosphorus concentrations.41 Hyperphosphatemia, in particular, is a major remaining modifiable target.42 Among the disturbances in mineral metabolism that fall under CKD-MBD, phosphate retention is by far the greatest risk factor for increased mortality, 2- to 6-fold higher than other top risk factors such as hypercalcemia, hyperparathyroidism, low urea reduction ratio, and anemia (12% vs. 4%, 2%, 5%, and 6%, respectively).43 Abnormal phosphate concentrations have been shown to be an independent risk factor for CV morbidity and mortality in patients with CKD,44 and there is a linear relationship between risk of CVD calcification progression and increasing serum phosphorus concentrations.45 Additionally, the interplay between increasing phosphate retention, increased PTH, and decreased calcium concentrations create a worsening cycle that causes CKD-MBD to progress as CKD progresses.29,46, 47, 48
Phosphate Retention and Elevated Phosphorus Drives Vascular Calcification
Phosphate retention is a known risk factor for decreased vascular health,49 increased vascular calcification,50 and increased CV morbidity and mortality.3 One mechanism by which hyperphosphatemia directly affects vascular health is through increasing the production of reactive oxygen species, thereby causing oxidative damage and endothelial dysfunction.49,51 Even short-term phosphate elevations (2 h) can have a negative effect on endothelium-dependent vasodilation in both healthy individuals and patients with CKD.49
Another primary mechanism by which phosphate retention increases mortality risk is the induction of vascular calcification. High phosphate conditions create a disturbance in the actively regulated vascular calcification process,52 leading to extraskeletal mineralization. Calcium-phosphate deposition in the media of the arterial wall eventually results in increased media thickness and vascular stiffening.34,53 Growing evidence indicates that phosphate retention also induces changes in vascular smooth muscle cells.31,54 Phosphate directly targets the PiT1 receptor on vascular smooth muscle cells, causing them to permanently transform into osteoblast-like cells.52 This transformation is driven by the deposition of hydroxyapatite crystals, which are normally found in the bone.55 Phosphate-induced CV of vascular smooth muscle cells increases the risk of coronary artery events, CV morbidity and mortality, and hypertension.56, 57, 58
Phosphate Retention Drives Derangements in PTH, FGF23, Calcium, and Vitamin D
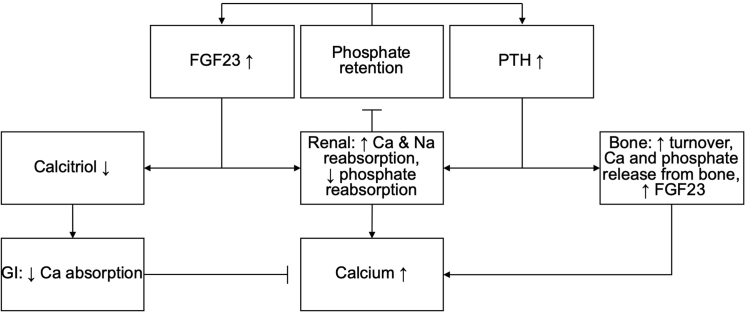
As CKD progresses, reductions in glomerular filtration rate lead to phosphate retention.59 In earlier CKD stages, FGF23 and PTH concentration steadily increase, while phosphate concentrations are stable and even decrease slightly.29 Abnormalities in mineral metabolism (e.g., secondary hyperparathyroidism, decreased vitamin D) are seen in patients before increases in phosphate, indicating that these compensatory mechanisms are at work before hyperphosphatemia develops.29,60 However, increases in PTH and FGF23 have both been associated with increased risk of CVD.61, 62, 63 Hypocalcemia and low vitamin D (both 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D) concentrations have been associated with increased risk of CVD.64,65 Inhibition of calcitriol (1,25-dihydroxyvitamin D) production due to increased FGF2366,67 is associated with increased cardiac contractility,68 coronary artery calcification,69 myocardial fibrosis,70 and proinflammatory effect.71 PTH increases the production of calcitriol (1,25-dihydroxyvitamin D), as well as increases bone resorption, which leads to increasing both serum calcium and phosphate concentrations.72 Thus, by the time hyperphosphatemia develops (>4.5 mg/dl), patients have already experienced the deleterious effects of elevated PTH, elevated FGF23, hypocalcemia, and low vitamin D (Figure 1).
Figure 1.
Physiology of chronic kidney disease (CKD)–mineral bone disorder (MBD). Phosphate retention leads to an increase of fibroblast growth factor 23 (FGF23) and parathyroid hormone (PTH) levels.83,84 FGF23 decreases the levels of calcitriol,91 leading to reduced absorption of calcium in the gastrointestinal tract.128 An association between reduced calcitriol and reduced intestinal phosphate absorption has been shown in animal models.129 Increased PTH leads to higher bone turnover, releasing calcium and phosphate,9,130 and increased FGF23 concentrations.131 Both FGF23 and PTH lead to increased calcium reabsorption as well as decreased phosphate reabsorption in the kidneys.130,132,133 This, in turn, causes calcium concentrations to increase and inhibits phosphate retention.
In CKD stages 4 and 5, phosphate concentrations begin to increase despite elevations in FGF23 and PTH, indicating that compensatory mechanisms are no longer sufficient to maintain phosphate balance and prevent hyperphosphatemia.29 Numerous studies have documented the link between hyperphosphatemia and increased all-cause mortality and CVD in both healthy individuals and patients with CKD.5,6,73,74 It is estimated that patients with poorly managed phosphate have almost 30% greater mortality risk than those who consistently achieve and maintain normal phosphate concentrations.75 Approximately 70% of patients on dialysis have left ventricular hypertrophy, a known risk factor for CVD and mortality that is strongly associated with higher phosphate concentrations.76, 77, 78 Additionally, hyperphosphatemia is directly linked to hypertension, a major CVD risk factor that is seen in up to 90% of patients with CKD.79 Therefore, phosphate control could be a major therapeutic target for the reduction of mortality in patients with CKD.
The importance of phosphate as a therapeutic target is compounded by its role as a driver of multiple CKD-MBD components linked to increased risk of CVD. FGF23 and PTH concentrations, both of which have been suggested to have independent pathogenic CV effects,29,80, 81, 82 are key regulators of phosphate that increase in response to phosphate retention.9,83,84 Elevated FGF23 concentrations are the earliest mineral metabolism abnormality observed in CKD.29 Phosphate retention stimulates progressive increases in FGF23 concentrations,67 which directly target the heart to promote left ventricular hypertrophy and congestive heart failure.63,81 Higher FGF23 concentrations are independently associated with a greater risk of CV events, particularly congestive heart failure.63 PTH is a known uremic toxin85,86 and elevated PTH concentrations are associated with various negative effects including pro-inflammatory effect,80 increased bone resorption possibly via increased interleukin 6,87 decrease in cardiac index and mean arterial pressure via impaired myocardial energy production,88 and genesis of cardiac fibrosis.89
Not only are abnormal phosphate, calcium, FGF23, and PTH concentrations independent risk factors for mortality, they are linked in a progressively worsening cycle. Increases in FGF23, triggered by phosphate retention, inhibit calcitriol, and lead to decreased serum calcium.90,91 Hypocalcemia develops, and PTH responds by increasing bone turnover, causing calcium and phosphate release from bones,9,92 which further increases serum phosphate concentrations that continue to stimulate further hypocalcemia and further release of PTH in an unending cycle.9,93 Secondary hyperparathyroidism develops in later CKD stages despite high FGF23 concentrations,94 suggesting resistance of the parathyroid glands to FGF23 regulation.95 A maladaptive upregulation of phosphate absorption observed may further exacerbate hyperphosphatemia and CKD-MBD.20,96,97
The negative effects of phosphate retention are not exclusive to hyperphosphatemia or patients with CKD; elevated phosphate concentrations within the normal range (<4.5 mg/dl) are known to increase the risk of death and CV events even in individuals without CKD.6,73 In individuals with normal renal function, phosphate concentrations >3.9 mg/dl were associated with increased CVD.44 In those with normal renal function, the association between serum phosphate and CV risk is linear.44 A long-term study of individuals without kidney disease found that serum phosphate concentrations >3.5 mg/dl were associated with an approximate 55% increased risk of CVD.73 Although evidence of the association between phosphate and CV risk is observational, the volume of data that link elevated phosphate concentrations and increased CV morbidity and mortality in individuals with and without CKD is significant.41,44,73,98 This connection has been accepted by clinicians and researchers for decades.
Phosphate Control Is Associated With Reduced Risk of CV Morbidity and Mortality and May Halt the Progression of CKD-MBD
Phosphate retention is an independent and impactful modifiable risk factor for theoretically lowering CV morbidity and mortality in patients with CKD.42,44,45,99 Block et al. first reported that increased serum phosphorus concentrations of even 0.5 to 1.0 mg/dl over the reference range (4.0–5.0 mg/dl) resulted in a significant increase in the relative risk of death, with relative risk increasing as serum phosphate increased.5 An association between increased serum phosphate concentrations and increased risk of CVD hospitalizations and mortality5 indicates that a greater degree of phosphate control may correspond to a greater reduction in morbidity and mortality risk. Slinin et al. reported that patients with the worst phosphate control (>7.5 mg/dl) had a 25% greater risk of CV events than those who had serum phosphate <4.5 mg/dl.98 A separate analysis by Block et al. found that patients on dialysis with serum phosphate >6.5 mg/dl had ~27% greater risk of mortality than those with concentrations between 2.4 and 6.5 mg/dl.75 In addition to the degree of phosphate increase, time spent with elevated phosphate concentrations also increases the risk of CV mortality. A longitudinal analysis showed that patients who had phosphate over the KDOQI recommended range (3.5–5.5 mg/dl) for 1 or fewer quarters (3 months) had a 62% higher mortality risk than those who were within the target range for all 4 quarters in a year.100 A large multinational study of patients on dialysis found that the more time patients spent with serum phosphate >4.5 mg/dl over a 6-month period, the greater their risk of CV mortality.41 Elevated phosphate within the normal range was predictive of CV morbidity and mortality even in patients with early-stage CKD,6 thus improved phosphate control and regular monitoring of serum phosphate concentrations would be beneficial for all patients with CKD (Table 1). Reflecting the accepted importance of phosphate control in clinical practice, reduction of phosphate toward normal concentrations is recommended by multiple guidelines.2,101
Table 1.
The degree of phosphate control is associated with reduced risk of cardiovascular (CV) morbidity and mortality
| Author | Year of publication | No. of patients | Study type | Mortality | Morbidity |
|---|---|---|---|---|---|
| Block et al.5 | 2004 | 40,538 | Observational | X |
|
| Slinin et al.98 | 2005 | 14,829 | Observational | X | CV events |
| Block et al.75 | 1998 | 6407 | Observational | X | |
| Danese et al.100 | 2008 | 22,937 | Observational | X | |
| Lopes et al.41 | 2020 | 17,414 | Prospective cohort | X |
Phosphate control may also halt increased production of FGF23, another factor in CKD-MBD. Achieving serum phosphate <4.5 mg/dl has been shown to reduce FGF23 concentrations and associated inflammatory parameters.10 A study from Rodelo-Haad et al. showed that FGF23 concentrations in patients on dialysis who achieved a serum phosphate <4.5 mg/dl decreased to less than half of baseline levels during a 40-week treatment period, whereas FGF23 concentrations in patients with serum phosphate >4.5 mg/dl increased 2- to 4-fold from baseline.10 Early phosphate control or prevention of phosphate retention could lead to moderating the increases in FGF23 and potentially decrease CV toxicity of FGF23.10,63
Effective phosphate control could also potentially break the cycle of worsening hyperparathyroidism and hypocalcemia by removing the stimulus causing PTH concentrations to increase.93,102 Without the “trigger” of phosphate retention or worsening hyperphosphatemia, PTH concentrations would not increase as a compensatory mechanism.84 High serum phosphate concentrations have also been shown to increase PTH synthesis directly.103,104 Because low calcium and vitamin D concentrations are associated with hyperphosphatemia and hyperparathyroidism,9,29 effective phosphate control may minimize the risk of hypocalcemia and low vitamin D.
The intricate regulatory and feedback loops between the various mineral and endocrine disruptions that comprise CKD-MBD make this a challenging condition to manage. Phosphate retention drives many of the disruptions comprising CKD-MBD,105 and thus effective phosphate control would likely attenuate or prevent the development and worsening progression of the PTH–calcium–vitamin D cycle in CKD-MBD, making phosphate control a logical and attractive foundational treatment strategy.
Effective Treatment of CKD-MBD Is Impeded by Challenges in Current Phosphate Management
Given an understanding that phosphate retention triggers and drives the progressive deterioration in mineral homeostasis, it is logical to target phosphate control as a treatment strategy for CKD-MBD. However, phosphate control is an ongoing clinical challenge; when evaluating the most recent monthly phosphate lab value, 42% of patients on dialysis treated with phosphate binders had phosphate >5.5 mg/dl in the most recent month, whereas a normal serum phosphate concentration of <4.6 mg/dl was achieved in only 23% of patients on dialysis.106
More than 40% of dialysis patients have phosphate concentrations >5.5 mg/dl, and more than 79% have phosphate >5.5 mg/dl,107 indicating that current phosphate management strategies are insufficient to reduce and maintain serum phosphate to normal concentrations. Dietary restriction of phosphate, although foundational, is complicated by “hidden” phosphate in food additives, and many medications that make it difficult for patients to accurately assess their phosphate intake.108,109 Dialysis can only remove ~300 to 1200 mg of phosphate per day (1800–2520 mg/week), far below the average amount of phosphate consumed and absorbed.110, 111, 112 Phosphate binders are the only pharmacological treatment currently indicated for hyperphosphatemia.12, 13, 14, 15, 16 Approximately 88% of patients on dialysis are prescribed phosphate binders,113 which work by binding to dietary phosphate to create insoluble complexes that are then excreted in the gastrointestinal tract.12, 13, 14, 15, 16 Binders do not target or directly act on phosphate absorption pathways (neither the primary paracellular pathway nor the secondary transcellular pathway),12, 13, 14, 15, 16 and each pill can only bind a limited amount of phosphate.18,114,115 The nonspecific binding mechanism may also lead to drug–drug interactions, and the limited binding capacity requires large and/or many pills that are still insufficient to keep up with daily dietary phosphate intake.12, 13, 14, 15, 16,116 Labeled dosing instructions require patients to take 1 to 4 binders with each meal or snack (~3–5 times/day),12, 13, 14, 15, 16 accounting for approximately half of the daily pill burden in patients on dialysis.19 Poor adherence to phosphate binders is also a challenge, with reported nonadherence rates of up to 62%.19 All of these factors likely contribute to our inability to achieve and maintain target serum phosphate concentrations, indicating a need for therapeutic innovations.
The effectiveness of phosphate-restricted diets and phosphate binder use might be tempered by a maladaptive upregulation of phosphate absorption, at least in rodents.20, 21, 22
Consistent with findings from previous animal studies on the effects of dietary phosphate restriction,20 Giral et al. demonstrated that a chronic low-phosphate diet results in adaptive upregulation of the sodium-dependent phosphate transport protein NaPi-2b activity in the jejunum.21 Increased NaPi-2b expression has also been observed in rats treated with phosphate binders.22 These findings are important for the treatment of patients with hyperphosphatemia because they point to a natural limitation of dietary phosphate restriction and phosphate binders as phosphate management strategies.
New Phosphate Control Therapies Are Needed to Treat CKD-MBD
A significant development in the understanding of intestinal phosphate absorption is the newfound importance of the paracellular phosphate absorption pathway. Dietary phosphate is absorbed passively via the nonsaturable paracellular pathway and actively via the saturable transcellular pathway.54,117, 118, 119 Although it was previously believed that the active transcellular phosphate transport pathway was responsible for the majority of phosphate absorption, there is a growing body of evidence that the paracellular pathway is the dominant site of gastrointestinal phosphate absorption when luminal phosphate concentrations are high,23,120,121 consistent with the consumption of a Western diet.110 The paracellular route, in which phosphate passes through the tight junctions between the cell membranes, is the major phosphate absorption pathway under normal dietary conditions that contain high amounts of phosphate.120,121,122,123 Given this new knowledge and the understanding that maladaptive hyperabsorption of phosphate limits the efficacy of low phosphate diets and phosphate binders,21,22 there is a call for new therapies that leverage these data to effectively control phosphate and avoid the negative outcomes associated with hyperphosphatemia and CKD-MBD.
A targeted sodium-dependent phosphate co-transporter type 2b (NaPi2b) inhibitor ASP3325 has been tested as a potential treatment for hyperphosphatemia by lowering transcellular permeability for phosphate, but the study concluded that inhibition of NPT2b with ASP3325 did not reduce serum phosphate concentrations in dialysis patients.122 This is consistent with the decreased importance of this transcellular phosphate absorption pathway in humans on a Western diet. Nicotinamide is another promising NaPi2b inhibitor that efficiently reduces active phosphate absorption via the sodium-dependent transcellular pathway.124,125 Nicotinamide treatment with or without lanthanum carbonate for 12 months did not decrease serum phosphate or FGF23 concentrations in patients with CKD stages 3 or 4.126
The lack of effect of NaPi2b inhibitors may be because most phosphate is absorbed passively through the paracellular pathway, especially in patients with CKD on standard Western diets,23 which potentially makes nicotinamide less efficacious in the treatment of hyperphosphatemia.
Tenapanor is an investigational novel phosphate absorption inhibitor that acts on the primary absorption pathway, providing a new approach to treating hyperphosphatemia.24
Tenapanor has a unique mechanism of action that reduces paracellular absorption of phosphate in the gastrointestinal tract by local inhibition of the NHE3, leading to reduced sodium absorption and proposed conformational changes in claudin proteins present in tight junctions that directly decrease the permeability of the paracellular pathway to phosphate.24 Tenapanor reduced phosphate concentrations in multiple clinical trials and was generally well tolerated. A long-term study in patients with CKD on dialysis showed that 77% of tenapanor-treated patients had a reduction in serum phosphorus (mean 2 mg/dl).27 Tenapanor has also been investigated with phosphate-binders as part of a dual mechanism approach to phosphorus-lowering in a 4-week human study.127 A significantly larger proportion of subjects treated with tenapanor and binders achieved serum phosphorus concentrations of <5.5 mg/dl at week 1 (49% vs. 21%; P < 0.001), week 2 (41% vs. 24%; P = 0.003), week 3 (47% vs. 18%; P < 0.001), and week 4 (37% vs. 22%; P = 0.01) compared with those who only received binders.127
CKD-MBD treatment strategies should reflect the centrality of phosphate within the greater set of mineral and endocrine disruptions. Given the evidence that links phosphate control to reduced CV morbidity and mortality presented here, it is logical that novel phosphate management therapies that more effectively achieve and maintain normal phosphate concentrations would reduce morbidity and mortality. This can be achieved via two pathways: (i) decreasing the risk of all-cause and CV mortality independently associated with phosphate retention and (ii) removing the stimulus for the cascade of laboratory and bone abnormalities that lead to increased risk of CV morbidity and mortality. Clinicians should consider implementing new hyperphosphatemia treatment paradigms to better achieve serum phosphate concentrations <5.5 mg/dl (or ideally, normal values). One emerging option would be to use targeted paracellular phosphate absorption inhibitors as an initial, foundational treatment. If needed, adjunctive binders may be added as part of a dual-mechanism approach for patients with difficult-to-control phosphate.
Disclosure
Dr. Sprague reports grants and personal fees from Ardelyx, grants from Amgen, grants and personal fees from Opko Pharm, grants and personal fees from Vifor, grants, and personal fees from Reata, outside of submitted work. Dr. Coyne reports personal fees from FBC-Renal Therapies Group, personal fees from Ardelyx, outside the submitted work. Dr. Martin reports grants from Ardelyx, Inc., other fees from Ardelyx, Inc, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
As a review article, there was no prospective study with patients or research study staff and therefore no funding needed. The authors were responsible for the review and screening of literature, conceptualization, writing and revising of this review article. Writing support was provided by Xelay Acumen Group and funded by Ardelyx, Inc.
References
- 1.Chuang S.H., Wong H.C., Vathsala A. Prevalence of chronic kidney disease-mineral and bone disorder in incident peritoneal dialysis patients and its association with short-term outcomes. Singapore Med J. 2016;57:603–609. doi: 10.11622/smedj.2015195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block G.A., Kilpatrick R.D., Lowe K.A. CKD–mineral and bone disorder and risk of death and cardiovascular hospitalization in patients on hemodialysis. Clin J. Am. Soc. Nephrol. 2013;8:2132–2140. doi: 10.2215/CJN.04260413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System . National Institute of Diabetes and Digestive and Kidney Diseases; 2019. 2019 Annual Data Report: Epidemiology of kidney disease in the United States. [Google Scholar]
- 5.Block G.A., Klassen P.S., Lazarus J.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 6.Kestenbaum B., Sampson J.N., Rudser K.D. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 7.Raggi P., Boulay A., Chasan-Taber S. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 8.de Francisco A.L.M., Cobo M.A., Setien M.A. Effect of serum phosphate on parathyroid hormone secretion during hemodialysis. Kidney Int. 1998;54:2140–2145. doi: 10.1046/j.1523-1755.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 9.Goyal R., Jialal I. StatPearls. StatPearls Publishing; 2020. Hyperphosphatemia. [Google Scholar]
- 10.Rodelo-Haad C., Rodríguez-Ortiz M.E., Martin-Malo A. Phosphate control in reducing FGF23 levels in hemodialysis patients. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serum phosphorus (most recent), categories. DOPPS Practice Monitor. 2020 [Google Scholar]
- 12.PhosLo gelcaps (calcium acetate): 667 mg [prescribing information]. Fresenius Medical Care North America, 2011.
- 13.VELPHORO (sucroferric oxyhydroxide) [prescribing information]. Fresenius Medical Care North America, 2013.
- 14.FOSRENAL (lanthanum carbonate) [prescribing information]. Shire US Inc., 2016.
- 15.AURYXIA (ferric citrate) tablets [prescribing information]. Keryx Biopharmaceuticals Inc., 2017.
- 16.RENVELA (sevelamer carbonate) [prescribing information]. Genzyme Corp., 2020.
- 17.Daugirdas J.T., Finn W.F., Emmett M. The phosphate binder equivalent dose. Semin Dial. 2011;24:41–49. doi: 10.1111/j.1525-139X.2011.00849.x. [DOI] [PubMed] [Google Scholar]
- 18.Martin P., Wang P., Robinson A. Comparison of dietary phosphate absorption after single doses of lanthanum carbonate and sevelamer carbonate in healthy volunteers: a balance study. Am J Kidney Dis. 2011;57:700–706. doi: 10.1053/j.ajkd.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 19.Chiu Y.-W., Teitelbaum I., Misra M. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4:1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hattenhauer O., Traebert M., Murer H. Regulation of small intestinal Na-P(i) type IIb cotransporter by dietary phosphate intake. Am J Physiol. 1999;277:G756–G762. doi: 10.1152/ajpgi.1999.277.4.G756. [DOI] [PubMed] [Google Scholar]
- 21.Giral H., Caldas Y., Sutherland E. Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am J Physiol Renal Physiol. 2009;297:F1466–F1475. doi: 10.1152/ajprenal.00279.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiavi S.C., Tang W., Bracken C. Npt2b deletion attenuates hyperphosphatemia associated with CKD. J Am Soc Nephrol. 2012;23:1691–1700. doi: 10.1681/ASN.2011121213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis G.R., Zerwekh J.E., Parker T.F. Absorption of phosphate in the jejunum of patients with chronic renal failure before and after correction of vitamin D deficiency. Gastroenterology. 1983;85:908–916. [PubMed] [Google Scholar]
- 24.King A.J., Siegel M., He Y. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aam6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Block G.A., Rosenbaum D.P., Yan A. Efficacy and safety of tenapanor in patients with hyperphosphatemia receiving maintenance hemodialysis: a randomized phase 3 trial. J Am Soc Nephrol. 2019;30:641–652. doi: 10.1681/ASN.2018080832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenbaum DP, Yang Y. Efficacy of tenapanor for the control of serum phosphorus in patients with chronic kidney disease on dialysis: novel mechanism of action allows for both monotherapy and dual mechanism approaches. Paper presented at the American Society of Nephrology (ASN) Kidney Week [Virtual meetihng], October 20–25, 2020.
- 27.Chertow GM, Yang Y, Rosenbaum DP. Paper presented at the Long-term safety and efficacy of tenapanor for the control of serum phosphorus in patients with chronic kidney disease on dialysis. Presented at the American Society of Nephrology (ASN) Kidney Week 2020 [Virtual meeting], October 20–25, 2020.
- 28.Moe S., Drüeke T., Cunningham J. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 29.Isakova T., Wahl P., Vargas G.S. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torres A., Lorenzo V., Hernández D. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int. 1995;47:1434–1442. doi: 10.1038/ki.1995.201. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D., Bi X., Liu Y. High phosphate-induced calcification of vascular smooth muscle cells is associated with the TLR4/NF-κb signaling pathway. Kidney Blood Pressure Res. 2017;42:1205–1215. doi: 10.1159/000485874. [DOI] [PubMed] [Google Scholar]
- 32.Barreto F.C., Barreto D.V., Moysés R.M. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73:771–777. doi: 10.1038/sj.ki.5002769. [DOI] [PubMed] [Google Scholar]
- 33.Wang H., Yoshiko Y., Yamamoto R. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23:939–948. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- 34.London G.M., Guérin A.P., Marchais S.J. Arterial media calcification in end-stage renal disease:impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 35.Kendrick J., Cheung A.K., Kaufman J.S. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 37.Foley R.N., Parfrey P.S., Sarnak M.J. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9:S16–S23. [PubMed] [Google Scholar]
- 38.Cheung A.K., Sarnak M.J., Yan G. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 39.Maduell F., Moreso F., Pons M. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–497. doi: 10.1681/ASN.2012080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panichi V., Rizza G.M., Paoletti S. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. results from the RISCAVID study. Nephrol Dial Transplant. 2008;23:2337–2343. doi: 10.1093/ndt/gfm951. [DOI] [PubMed] [Google Scholar]
- 41.Lopes M.B., Karaboyas A., Bieber B. Impact of longer term phosphorus control on cardiovascular mortality in hemodialysis patients using an area under the curve approach: results from the DOPPS. Nephrol Dial Transplant. 2020;35:1794–1801. doi: 10.1093/ndt/gfaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Major R.W., Cheng M.R.I., Grant R.A. Cardiovascular disease risk factors in chronic kidney disease: a systematic review and meta-analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moe S.M., Chertow G.M. The case against calcium-based phosphate binders. Clin J Am Soc Nephrol. 2006;1:697–703. doi: 10.2215/CJN.00560206. [DOI] [PubMed] [Google Scholar]
- 44.McGovern A.P., de Lusignan S., van Vlymen J. Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: a large community based cohort study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shang D., Xie Q., Ge X. Hyperphosphatemia as an independent risk factor for coronary artery calcification progression in peritoneal dialysis patients. BMC Nephrol. 2015;16:107. doi: 10.1186/s12882-015-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slatopolsky E., Caglar S., Gradowska L. On the prevention of secondary hyperparathyroidism in experimental chronic renal disease using “proportional reduction” of dietary phosphorus intake. Kidney Int. 1972;2:147–151. doi: 10.1038/ki.1972.84. [DOI] [PubMed] [Google Scholar]
- 47.Khan M., Jose A., Sharma S. StatPearls. StatPearls Publishing; 2021. Physiology, parathyroid hormone. Available at https://www.ncbi.nlm.nih.gov/books/NBK499940/. Accessed July 26, 2021. [PubMed] [Google Scholar]
- 48.Massry S.G., Coburn J.W., Lee D.B. Skeletal resistance to parathyroid hormone in renal failure. Studies in 105 human subjects. Ann Intern Med. 1973;78:357–364. doi: 10.7326/0003-4819-78-3-357. [DOI] [PubMed] [Google Scholar]
- 49.Shuto E., Taketani Y., Tanaka R. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adeney K.L., Siscovick D.S., Ix J.H. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Marco G.S., Hausberg M., Hillebrand U. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol. 2008;294:F1381–F1387. doi: 10.1152/ajprenal.00003.2008. [DOI] [PubMed] [Google Scholar]
- 52.Jono S., McKee M.D., Murry C.E. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 53.Schwarz U., Buzello M., Ritz E. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant. 2000;15:218–223. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 54.Walton J., Gray T.K. Absorption of inorganic phosphate in the human small intestine. Clin Sci (London) 1979;56:407–412. doi: 10.1042/cs0560407. [DOI] [PubMed] [Google Scholar]
- 55.Lei Y., Sinha A., Nosoudi N. Hydroxyapatite and calcified elastin induce osteoblast-like differentiation in rat aortic smooth muscle cells. Exp Cell Res. 2014;323:198–208. doi: 10.1016/j.yexcr.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Detrano R., Guerci A.D., Carr J.J. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 57.Blacher J., Guerin A.P., Pannier B. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 58.Grossman C., Shemesh J., Dovrish Z. Coronary artery calcification is associated with the development of hypertension. Am J Hyperten. 2012;26:13–19. doi: 10.1093/ajh/hps028. [DOI] [PubMed] [Google Scholar]
- 59.Slatopolsky E., Robson A.M., Elkan I. Control of phosphate excretion in uremic man. J Clin Invest. 1968;47:1865–1874. doi: 10.1172/JCI105877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levin A., Bakris G.L., Molitch M. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 61.De Boer I.H., Gorodetskaya I., Young B. The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol. 2002;13:2762–2769. doi: 10.1097/01.asn.0000034202.91413.eb. [DOI] [PubMed] [Google Scholar]
- 62.Hagström E., Hellman P., Larsson T.E. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 63.Scialla J.J., Xie H., Rahman M. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25:349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaguchi S., Hamano T., Doi Y. Hidden hypocalcemia as a risk factor for cardiovascular events and all-cause mortality among patients undergoing incident hemodialysis. Sci Rep. 2020;10:4418. doi: 10.1038/s41598-020-61459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kendrick J., Targher G., Smits G. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–260. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 66.Portale A.A., Wolf M., Jüppner H. Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol. 2014;9:344–353. doi: 10.2215/CJN.05840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gutierrez O., Isakova T., Rhee E. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 68.Weishaar R.E., Simpson R.U. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79:1706–1712. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watson K.E., Abrolat M.L., Malone L.L. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 70.Artaza J.N., Norris K.C. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol. 2009;200:207–221. doi: 10.1677/JOE-08-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olszowiec-Chlebna M., Koniarek-Maniecka A., Brzozowska A. Vitamin D inhibits pro-inflammatory cytokines in the airways of cystic fibrosis patients infected by Pseudomonas aeruginosa—pilot study. Ital J Pediatr. 2019;45:41. doi: 10.1186/s13052-019-0634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nussey S., Whitehead S. Endocrinology: An Integrated Approach. BIOS Scientific Publishers; 2001. The parathyroid glands and vitamin D. [PubMed] [Google Scholar]
- 73.Dhingra R., Sullivan L.M., Fox C.S. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 74.Tonelli M., Sacks F., Pfeffer M. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 75.Block G.A., Hulbert-Shearon T.E., Levin N.W. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 76.Foley R.N., Parfrey P.S., Harnett J.D. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 77.Gallieni M., Caputo F., Filippini A. Prevalence and progression of cardiovascular calcifications in peritoneal dialysis patients: a prospective study. Bone. 2012;51:332–337. doi: 10.1016/j.bone.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Zou J., Yu Y., Wu P. Serum phosphorus is related to left ventricular remodeling independent of renal function in hospitalized patients with chronic kidney disease. Int J Cardiol. 2016;221:134–140. doi: 10.1016/j.ijcard.2016.06.181. [DOI] [PubMed] [Google Scholar]
- 79.Cozzolino M., Mangano M., Stucchi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33:iii28–iii34. doi: 10.1093/ndt/gfy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng S.P., Liu C.L., Liu T.P. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm. 2014;2014:709024. doi: 10.1155/2014/709024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faul C., Amaral A.P., Oskouei B. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schlüter K.D., Piper H.M. Trophic effects of catecholamines and parathyroid hormone on adult ventricular cardiomyocytes. Am J Physiol. 1992;263:H1739–H1746. doi: 10.1152/ajpheart.1992.263.6.H1739. [DOI] [PubMed] [Google Scholar]
- 83.Hasegawa H., Nagano N., Urakawa I. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78:975–980. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 84.Centeno P.P., Herberger A., Mun H.-C. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat Commun. 2019;10:4693. doi: 10.1038/s41467-019-12399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bogin E., Massry S.G., Levi J. Effect of parathyroid hormone on osmotic fragility of human erythrocytes. J Clin Invest. 1982;69:1017–1025. doi: 10.1172/JCI110505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avram M.M., Feinfeld D.A., Huatuco A.H. Search for the uremic toxin. Decreased motor-nerve conduction velocity and elevated parathyroid hormone in uremia. N Engl J Med. 1978;298:1000–1003. doi: 10.1056/NEJM197805042981805. [DOI] [PubMed] [Google Scholar]
- 87.Grey A., Mitnick M.A., Masiukiewicz U. A role for interleukin-6 in parathyroid hormone-induced bone resorption in vivo. Endocrinology. 1999;140:4683–4690. doi: 10.1210/endo.140.10.7036. [DOI] [PubMed] [Google Scholar]
- 88.Baczynski R., Massry S.G., Kohan R. Effect of parathyroid hormone on myocardial energy metabolism in the rat. Kidney Int. 1985;27:718–725. doi: 10.1038/ki.1985.71. [DOI] [PubMed] [Google Scholar]
- 89.Amann K., Ritz E., Wiest G. A role of parathyroid hormone for the activation of cardiac fibroblasts in uremia. J Am Soc Nephrol. 1994;4:1814–1819. doi: 10.1681/ASN.V4101814. [DOI] [PubMed] [Google Scholar]
- 90.Heaney R.P., Dowell M.S., Hale C.A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 91.Shimada T., Yamazaki Y., Takahashi M. Vitamin D receptor–independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–F1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 92.Yu E., Sharma S. StatPearls. StatPearls Publishing; 2020. Physiology, Calcium.https://www.ncbi.nlm.nih.gov/books/NBK482128/ Available at: [Google Scholar]
- 93.Almaden Y., Hernandez A., Torregrosa V. High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol. 1998;9:1845–1852. doi: 10.1681/ASN.V9101845. [DOI] [PubMed] [Google Scholar]
- 94.Westerberg P.-A., Linde T., Wikström B. Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol Dial Transplant. 2007;22:3202–3207. doi: 10.1093/ndt/gfm347. [DOI] [PubMed] [Google Scholar]
- 95.Krajisnik T., Olauson H., Mirza M.A. Parathyroid Klotho and FGF-receptor 1 expression decline with renal function in hyperparathyroid patients with chronic kidney disease and kidney transplant recipients. Kidney Int. 2010;78:1024–1032. doi: 10.1038/ki.2010.260. [DOI] [PubMed] [Google Scholar]
- 96.Huber K., Walter C., Schröder B. Phosphate transport in the duodenum and jejunum of goats and its adaptation by dietary phosphate and calcium. Am J Physiol Regul Integr Comp Physiol. 2002;283:R296–R302. doi: 10.1152/ajpregu.00760.2001. [DOI] [PubMed] [Google Scholar]
- 97.Saddoris K.L., Fleet J.C., Radcliffe J.S. Sodium-dependent phosphate uptake in the jejunum is post-transcriptionally regulated in pigs fed a low-phosphorus diet and is independent of dietary calcium concentration. J Nutr. 2010;140:731–736. doi: 10.3945/jn.109.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Slinin Y., Foley R.N., Collins A.J. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16:1788–1793. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 99.Qunibi W.Y. Consequences of hyperphosphatemia in patients with end-stage renal disease (ESRD) Kidney Int Suppl. 2004:S8–S12. doi: 10.1111/j.1523-1755.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- 100.Danese M.D., Belozeroff V., Smirnakis K. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1423–1429. doi: 10.2215/CJN.01060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 102.Craver L., Marco M.P., Martínez I. Mineral metabolism parameters throughout chronic kidney disease stages 1–5—achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22:1171–1176. doi: 10.1093/ndt/gfl718. [DOI] [PubMed] [Google Scholar]
- 103.Hernández A., Concepción M.T., Rodríguez M. High phosphorus diet increases preproPTH mRNA independent of calcium and calcitriol in normal rats. Kidney Int. 1996;50:1872–1878. doi: 10.1038/ki.1996.508. [DOI] [PubMed] [Google Scholar]
- 104.Estepa J.C., Aguilera-Tejero E., Lopez I. Effect of phosphate on parathyroid hormone secretion in vivo. J Bone Miner Res. 1999;14:1848–1854. doi: 10.1359/jbmr.1999.14.11.1848. [DOI] [PubMed] [Google Scholar]
- 105.Bansal V.K. Serum inorganic phosphorus. In: Walker H.K., Hall W.D., Hurst J.W., editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Butterworths; 1990. [PubMed] [Google Scholar]
- 106.RealWorld Dynamix: Dialysis US. Vol. 2020. Spherix Global Insights; 2019.
- 107.Serum phosphorus (3 month average), categories. In (vol 2021), DOPPS Practice Monitor, 2021
- 108.León J.B., Sullivan C.M., Sehgal A.R. The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J Ren Nutr. 2013;23:265–270.e262. doi: 10.1053/j.jrn.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nelson S.M.L., Sarabia S.R.S., Christilaw E. Phosphate-containing prescription medications contribute to the daily phosphate intake in a third of hemodialysis patients. J Renal Nutr. 2017;27:91–96. doi: 10.1053/j.jrn.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 110.McClure S.T., Chang A.R., Selvin E. Dietary sources of phosphorus among adults in the United States: results from NHANES 2001–2014. Nutrients. 2017;9 doi: 10.3390/nu9020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hou S.H., Zhao J., Ellman C.F. Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis. 1991;18:217–224. doi: 10.1016/s0272-6386(12)80882-1. [DOI] [PubMed] [Google Scholar]
- 112.Bell R.R., Draper H.H., Tzeng D.Y. Physiological responses of human adults to foods containing phosphate additives. J Nutr. 1977;107:42–50. doi: 10.1093/jn/107.1.42. [DOI] [PubMed] [Google Scholar]
- 113.Lopes A.A., Tong L., Thumma J. Phosphate binder use and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS): evaluation of possible confounding by nutritional status. Am J Kidney Dis. 2012;60:90–101. doi: 10.1053/j.ajkd.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mai M.L., Emmett M., Sheikh M.S. Calcium acetate, an effective phosphorus binder in patients with renal failure. Kidney Int. 1989;36:690–695. doi: 10.1038/ki.1989.247. [DOI] [PubMed] [Google Scholar]
- 115.Ramirez J.A., Emmett M., White M.G. The absorption of dietary phosphorus and calcium in hemodialysis patients. Kidney Int. 1986;30:753–759. doi: 10.1038/ki.1986.252. [DOI] [PubMed] [Google Scholar]
- 116.Bover Sanjuán J., Navarro-González J.F., Arenas M.D. Pharmacological interactions of phosphate binders. Nefrologia. 2018;38:573–578. doi: 10.1016/j.nefro.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 117.Danisi G., Straub R.W. Unidirectional influx of phosphate across the mucosal membrane of rabbit small intestine. Pflügers Arch. 1980;385:117–122. doi: 10.1007/BF00588690. [DOI] [PubMed] [Google Scholar]
- 118.McHardy G.J.R., Parsons D.S. The absorption of inorganic phosphate from the small intestine of the rat. Q J Exp Physiol. 1956;41:398–409. [Google Scholar]
- 119.Saurette M., Alexander R.T. Intestinal phosphate absorption: the paracellular pathway predominates? Exp Biol Med (Maywood) 2019;244:646–654. doi: 10.1177/1535370219831220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marks J., Lee G.J., Nadaraja S.P. Experimental and regional variations in Na+-dependent and Na+-independent phosphate transport along the rat small intestine and colon. Physiol Rep. 2015;3 doi: 10.14814/phy2.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vorland C.J., Biruete A., Lachcik P.J. Kidney disease progression does not decrease intestinal phosphorus absorption in a rat model of chronic kidney disease–mineral bone disorder. J Bone Miner Res. 2020;35:333–342. doi: 10.1002/jbmr.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Larsson T.E., Kameoka C., Nakajo I. NPT-IIb inhibition does not improve hyperphosphatemia in CKD. Kidney Int Rep. 2018;3:73–80. doi: 10.1016/j.ekir.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knöpfel T., Himmerkus N., Günzel D. Paracellular transport of phosphate along the intestine. Am J Physiol Gastroint Liver Physiol. 2019;317:G233–G241. doi: 10.1152/ajpgi.00032.2019. [DOI] [PubMed] [Google Scholar]
- 124.Takahashi Y., Tanaka A., Nakamura T. Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65:1099–1104. doi: 10.1111/j.1523-1755.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 125.Katai K., Tanaka H., Tatsumi S. Nicotinamide inhibits sodium-dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant. 1999;14:1195–1201. doi: 10.1093/ndt/14.5.1195. [DOI] [PubMed] [Google Scholar]
- 126.Ix J.H., Isakova T., Larive B. Effects of nicotinamide and lanthanum carbonate on serum phosphate and fibroblast growth factor-23 in CKD: the COMBINE Trial. J Am Soc Nephrol. 2019;30:1096–1108. doi: 10.1681/ASN.2018101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pergola P.E., Rosenbaum D.P., Yang Y. A randomized trial of tenapanor and phosphate binders as a dual-mechanism treatment for hyperphosphatemia in patients on maintenance dialysis (AMPLIFY) J Am Soc Nephrol. 2021;32:1465–1473. doi: 10.1681/ASN.2020101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van Cromphaut S.J., Dewerchin M., Hoenderop J.G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ichida Y., Ohtomo S., Yamamoto T. Evidence of an intestinal phosphate transporter alternative to type IIb sodium-dependent phosphate transporter in rats with chronic kidney disease. Nephrol Dial Transplant. 2020;36:68–75. doi: 10.1093/ndt/gfaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lofrese J.J., Basit H., Lappin S.L. StatPearls. StatPearls Publishing; 2020. Physiology, parathyroid.https://www.ncbi.nlm.nih.gov/books/NBK482510/ Available at: [Google Scholar]
- 131.Lavi-Moshayoff V., Wasserman G., Meir T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889. doi: 10.1152/ajprenal.00360.2010. [DOI] [PubMed] [Google Scholar]
- 132.Andrukhova O., Smorodchenko A., Egerbacher M. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. Embo J. 2014;33:229–246. doi: 10.1002/embj.201284188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shimada T., Hasegawa H., Yamazaki Y. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]