Abstract
Background
Apheresis is the gold standard for idiopathic nephrotic syndrome (INS) relapse after transplantation, but it remains unknown whether such treatment is useful for adults with refractory INS on native kidneys.
Methods
This retrospective study included patients older than 16 years with biopsy-proven refractory (persistent nephrotic syndrome on corticosteroids plus at least 1 immunosuppressive drug) INS treated by apheresis and followed for at least 3 months.
Results
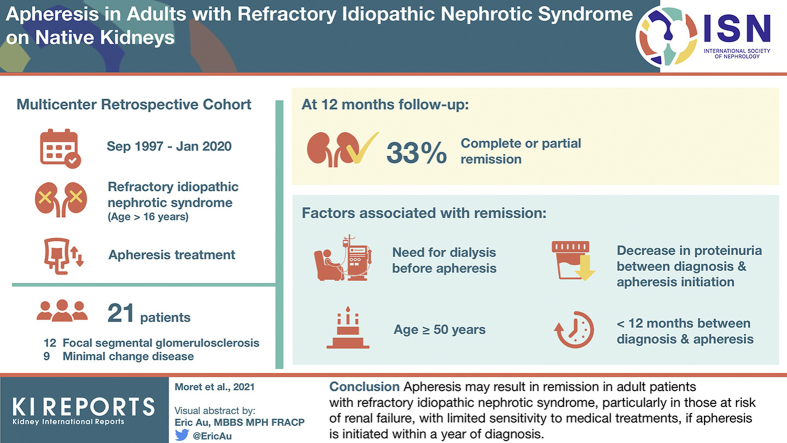
Between September 1997 and January 2020, 21 patients (focal segmental glomerulosclerosis: 12, minimal change nephrotic syndrome: 9, men: 67%, median age: 34 years) were identified. At last follow-up (12 months), 7 of 21 patients were in complete or partial remission. Remission was associated with older age (51 vs. 30 years, P = 0.05), lower proteinuria (3.9 vs. 7.3 g/d, P = 0.03), and lower estimated glomerular filtration rate (eGFR) (28.0 vs. 48.5 ml/min per 1.73 m2, P = 0.05) at apheresis. The need for dialysis before apheresis (odds ratio [OR] 22.0 [1.00–524], P = 0.026), age ≥50 years (OR: 22.6 [1.00–524], P = 0.006), a marked (>4.5 g/d) decrease in proteinuria (OR: 9.17 [1.15–73.2], P = 0.041), and a short (<12 months) time between diagnosis and apheresis (OR: 10.8 [1–117], P = 0.043) were significantly associated with remission. Three of 7 patients in remission who were initially on dialysis became dialysis-free; by contrast, none of the 14 patients without remission was initially on dialysis, but 5 of 14 had become dialysis-dependent (P = 0.01).
Conclusion
Apheresis may result in remission in adult patients with refractory INS, particularly in those at risk of renal failure, with limited sensitivity to medical treatments, if apheresis is initiated within a year of diagnosis.
Keywords: apheresis, focal segmental glomerulosclerosis, minimal change nephrotic syndrome, nephrotic syndrome
Graphical abstract
See Commentary on Page 2019
INS, a primary glomerular disease with 2 underlying histological variants (minimal change nephrotic syndrome [MCNS] and primary focal-segmental glomerulosclerosis [FSGS]), accounts for 90% of the cases of glomerular disease in children and 20% of those in adults.1, 2, 3 Its pathogenesis remains poorly understood, but, given its high sensitivity to corticosteroids and immunosuppressive drugs, INS is currently considered to be an immune-mediated disease.4 The absence of inflammation, immune cell infiltrates, Ig and complement deposits (except IgM and C3 complement deposition in some patients with FSGS) on renal biopsy strongly suggests that INS may be caused by 1 or more putative circulating factors that increase glomerular capillary permeability, leading to disorganization of the podocyte cytoskeleton and subsequent proteinuria.5 In both adults and children, the first-line treatment for INS is corticosteroids.6,7 Such treatment leads to complete remission of nephrotic syndrome in approximately 75% to 95% for adult patients with MCNS and approximately 32% to 47% of adult patients with FSGS.8,9 Patients not achieving remission on corticosteroid treatment are considered to have steroid-resistant nephrotic syndrome (SRNS). Patients with SRNS or with steroid-dependent nephrotic syndrome require second-line treatment with immunosuppressive agents, such as calcineurin inhibitors, cyclophosphamide, mycophenolate mofetil, and B-cell–depleting agents.2,3 However, the persistence of nephrotic syndrome despite the use of at least 2 lines of treatment is associated with an increase in the risk of end-stage kidney disease.5 A recent meta-analysis based on 423 patients with posttransplant INS recurrence showed that plasma exchange resulted in remission in 75% of cases (with complete remission in 46.8%).10 Apheresis is now considered the gold standard treatment for INS recurrence on the graft.11 By extrapolation, apheresis should also probably be considered in adult patients with refractory INS on native kidneys. Unfortunately, published data regarding the potential value of apheresis for the treatment of native kidneys in adult patients are scarce and inconclusive. Most of the available data originate from Japan and relate to patients with low-density lipoprotein (LDL)-apheresis.12, 13, 14, 15, 16
In this French multicenter retrospective study, we investigated the effect of apheresis in 21 adult patients with biopsy-proven refractory INS despite treatment with corticosteroids and at least one immunosuppressive drug.
Methods
Study Design and Participants
This retrospective study, instigated by the network of the French rare disease centers dedicated to INS management, was conducted by sending a questionnaire to all French nephrology departments, asking them to identify patients older than 16 years who had undergone apheresis to treat refractory INS on native kidneys. The patients at each hospital were identified from electronic medical records, including pathological and clinical diagnosis databases. The inclusion criteria were biopsy-proven MCNS or FSGS on the native kidneys in patients requiring at least 3 sessions of apheresis, followed for at least 3 months. The different types of apheresis considered were specified: plasma exchange (PE), immuno-adsorption (IA), LDL-A per dextran column and double-filtration plasmapheresis. The study was performed in accordance with the Helsinki Declaration, with the approval of our local institutional review board (research project no. 2020–042).
Data Collection
Demographic, clinical, and biological data were recorded for each patient at the time of INS diagnosis, at the start of apheresis, and at various time points after the initiation of apheresis (1 month, 3 months, 6 months, and last follow-up visit). Searches for monogenic mutations, targeted sequencing of genes involved in SRNS, and genotyping data for 2 risk alleles (the G1 and G2 variant alleles) of the gene encoding APOL1, were systematically noted, when available.17,18 High-risk APOL1 genotypes were defined as the 2 risk alleles in any combination (homozygous G1/G1, homozygous G2/G2, or compound heterozygous G1/G2).18 All patients tested for genetic mutations gave written informed consent for such tests. The type, number, and duration of apheresis were recorded, together with the number and type of immunosuppressive agents used in addition to corticosteroids for the treatment of INS.
Definitions
MCNS was diagnosed on the basis of an absence of visible changes on light microscopy examination, and an absence of immunoglobulin and/or complement deposits in immunofluorescence studies.1 FSGS diagnosis was based on the presence of segmentally collapsed glomerular capillaries, with areas of glomerular scarring associated with the focal and segmental granular deposition of IgM and/or C3 within areas of segmental glomerular sclerosis.19 FSGS was considered primary or “idiopathic” when clinicians considered the use of corticosteroid therapy and/or immunosuppressive agents to achieve the remission of nephrotic syndrome. SRNS was defined according to current international treatment guidelines for MCNS and FSGS.20,21 Refractory INS was defined as the persistence of nephrotic syndrome despite the use of corticosteroids associated with at least 1 additional immunosuppressive drug. Patients could be included if apheresis was used as a rescue therapy (i.e., because of a marked deterioration of renal function) or to minimize the adverse effects of prolonged or repeated treatments with immunosuppressive drugs. Acute kidney injury (AKI) was defined according to the Kidney Disease: Improving Global Outcomes criteria.22 Chronic kidney disease (CKD) was defined as an eGFR according to the Chronic Kidney Disease Epidemiology Collaboration equation <60 ml/min per 1.73 m2 for at least 3 months.23 CDK was classified into 5 stages based on the Kidney Disease Outcomes Quality Initiative.24 For patients requiring kidney replacement therapy by intermittent chronic hemodialysis, we considered an eGFR of 0 ml/min per 1.73 m2.
Outcome Measures
All patients were followed for at least 3 months after the initiation of apheresis. Complete remission of nephrotic syndrome was defined as the normalization of urinary protein levels to values within the normal range (proteinuria <0.3 g/d), associated with a serum albumin level >30 g/l. Partial remission was defined as proteinuria between 0.3 and 3 g/d, associated with a serum albumin concentration >30 g/l. An absence of remission was defined as the persistence of nephrotic syndrome (proteinuria greater than 3 g/d, associated with a serum albumin concentration <30 g/l). Successful therapy (remission group) was defined as complete or partial remission, with no requirement for kidney replacement therapy at the time of the last follow-up evaluation.
Statistical Analysis
Continuous variables are expressed as the median and interquartile range, whereas categorical variables are expressed as numbers and percentages. We used Mann-Whitney U tests for comparisons of continuous variables and χ2 or Fisher’s exact tests, as appropriate, for comparisons of categorical variables. Logistic regression was used to assess the likelihood of remission associated with specific baseline parameters; results are expressed as OR and 95% confidence intervals. R software (version 3.6.0) was used for the analyses.
Results
Baseline Characteristics at the Time of INS Diagnosis
Between September 1997 and January 2020, we identified 21 patients (14 [66.7%] men, median age: 34 years [20–49]) with refractory INS who had undergone apheresis with a follow-up of at least 3 months. The demographic, clinical, and biological data and renal biopsy findings for these patients at the time of INS diagnosis are summarized in Table 1. At the time of the first nephrological evaluation, proteinuria was 10 g/d (5.1–10.2) and serum albumin concentration was 19.0 g/l (14.8–23.0). At disease onset, 5 patients (23.8%) had AKI (AKI stage 3 in 1 case of 21 [4.8%]) but none required kidney replacement therapy. Underlying glomerular disease included FSGS in 12 (57.1%) patients and MCNS in 9 (42.9%) patients. Diagnostic tests for a secondary process were negative in all but 2 (9.5%) patients (1 patient with FSGS lesions had a history of parvovirus B19 infection [IgM-positive] and 1 patient had a history of resolved hepatitis B). The first-line treatment was corticosteroids, in all patients. With this therapeutic approach, only 2 (9.5%) patients presented steroid-dependent nephrotic syndrome, whereas 19 (90.5%) had SRNS. Targeted sequencing of an SRNS gene panel, performed in 9 of 19 (42.9%) patients, found no monogenic mutations of SRNS genes, but high-risk apolipoprotein L1 (APOL1) variants were found in 2 (9.5%) patients of African ancestry (1 homozygous G1/G1, 1 compound heterozygous G1/G2). Of note, in 6 of 9 cases, targeted sequencing strategy used to screen for monogenic mutations of SRNS included COL4A genes. A second renal biopsy was performed before the initiation of apheresis in 10 (47.6%) patients (interval between the first and second biopsies: 12 [9–18] months) and revealed FSGS lesions in 7 (70%) cases. Overall, on apheresis, 7 (33.3%) patients achieved remission (4 of 7 [57.1%] patients with complete remission and 3 of 7 [42.9%] patients with partial remission) (Table 1). In the 2 patients with potential secondary forms of FSGS, 1 patient (FSGS occurring after parvovirus B19 infection) did not response to apheresis, whereas the second had partial remission. No differences were observed between these 2 groups (remission vs. no-remission) at baseline, for serum albumin concentration (19.0 g/l [11.2–28.0] vs. 18.1 [14.9–21.2], P = 0.45) and proteinuria level (10.0 g/d [5.0–16.5] vs. 8.4 [5.1–10.1], P = 0.27). The patients achieving remission were older than those without remission (50 years [31.0–52.0] vs. 26.5 [19.3–38.8], P = 0.03).
Table 1.
Demographic, clinical, biological, and pathological findings at the time of idiopathic nephrotic syndrome
| Total population |
Remission |
No remission |
P value | |
|---|---|---|---|---|
| n = 21 | n = 7 | n = 14 | ||
| Clinical data | ||||
| Age (y) [IQR] | 34 [20–49] | 50 [31–52] | 26 [19–38] | 0.03 |
| Min-Max (y) | 16–76 | 16–76 | 16–49 | |
| Men (%) | 14 (66.7) | 6 (85.7) | 8 (57.1) | 0.41 |
| African ancestry (%) | 3 (14.3) | 2 (28.6) | 1 (7.1) | 0.51 |
| Body mass index (kg/m2) [IQR]; n = 19/21, 6/7, 13/14 | 23.8 [21.6–26.9] | 25.1 [23.3–27.5] | 23.4 [21.3–26.5] | 0.32 |
| - < 25 (%) | 11 (57.9) | 3 (50) | 8 (61.5) | |
| - 25–29.9 (%) | 8 (42) | 3 (50) | 5 (38.5) | |
| - ≥ 30 (%) | 0 | 0 | 0 | |
| Comorbid conditions | ||||
| - Hypertension (%) | 2 (9.5) | 1 (14.3) | 1 (7.1) | |
| - History of atopic disease (%) | 2 (9.5) | 1 (14.3) | 1 (7.1) | |
| Renal parameters | ||||
| Systolic blood pressure (mm Hg) [IQR]; n = 18/21, 6/7, 12/14 | 140 [126–155] | 144 [127–161] | 140 [123–153] | 0.67 |
| Diastolic blood pressure (mm Hg) [IQR]; n = 18/21, 6/7, 12/14 | 80 [75–91] | 88 [74–101] | 80 [75–90] | 0.47 |
| Serum albumin level (g/l) [IQR] | 19.0 [14.8–23.0] | 19.0 [11.2–28.0] | 18.1 [14.9–21.2] | 0.45 |
| Proteinuria (g/d) [IQR] | 10.0 [5.1–10.2] | 10.0 [5.0–16.5] | 8.4 [5.1–10.1] | 0.27 |
| Serum creatinine (μmol/l) [IQR] | 93 [77–115] | 110 [80–137] | 86 [74–107] | 0.14 |
| eGFR (mL/min per 1.73 m2) [IQR] | 80 [65–106] | 66 [50–83] | 91 [68–111] | 0.09 |
| Acute kidney injury (AKI)a (%) | 5 (23.8) | 3 (42.9) | 2 (14.3) | 0.37 |
| - AKI stage 1 (%) | 4 (19.1) | 2 (28.6) | 2 (14.3) | |
| - AKI stage 2 (%) | 0 | 0 | 0 | |
| - AKI stage 3 (%) | 1 (4.8) | 1 (14.3) | 0 | |
| Renal biopsy findings | ||||
| Focal segmental glomerulosclerosis (%) | 12 (57.1) | 4 (57.1) | 8 (57.1) | 1 |
| Minimal change nephrotic syndrome (%) | 9 (42.9) | 3 (42.9) | 6 (42.9) | 1 |
| Steroid-resistant nephrotic syndrome (SRNS) (%) | 19 (90.5) | 6 (85.7) | 13 (92.9) | 1 |
| Steroid-dependent nephrotic syndrome (SDNS) (%) | 2 (9.5) | 1 (14.3) | 1 (7.1) | 1 |
| Genetic analysis n = 9/19 | ||||
| Targeted sequencing of genes involved in SRNS (%) | 9 (47.4) | 2 (33.3) | 7 (53.9) | 0.4 |
| Negative for monogenic mutation in SRNS genes (%) | 9 (47.4) | 2 (33.3) | 7 (53.9) | 0.4 |
| Positive for high-risk polymorphism apolipoprotein L1 genotypesb (%) | 2 (10.5) | 1 (16.7) | 1 (7.7) | 0.55 |
| Not performed (%) | 10 (52.6) | 4 (66.7) | 6 (46.1) | 0.4 |
Qualitative data are expressed as n (%), quantitative data as median [interquartile range; IQR], as appropriate.
Glomerular filtration rate (GFR) was estimated (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration equation.
Acute kidney injury was defined according to Kidney Disease Improving Global Outcomes (KDIGO) criteria
High-risk apolipoprotein L1 genotypes were defined as 2 risk alleles in any combination (homozygous G1/G1, homozygous G2/G2, or compound heterozygous G1/G2).
Characteristics of Patients at the Onset of Apheresis
The clinical and biological data of the patients at the start of apheresis are shown in Table 2. For the total population, proteinuria was 5.3 g/day (4.0–10.5) and serum albumin concentration was 17.0 g/l (11.4–22.9). In 18 of the 21 patients (85.7%) nephrotic syndrome persisted despite treatment with corticosteroids and at least 1 immunosuppressive drug. The remaining 3 patients (14.3%) had a proteinuria that was 1.0, 2.6, and 3.0 g/d, and serum albumin concentrations <30 g/l. Between INS diagnosis and the start of apheresis (interval of 10 months [5–25]), eGFR decreased (eGFR change: −29 ml/min per 1.73 m2 [−54.3 to −6.9]) and 3 patients (14.3%) required intermittent hemodialysis. The specific treatments used for INS before apheresis and the type of apheresis are shown in Table 2. All patients received at least 1 immunosuppressive drug in addition to corticosteroids (10 [47.6%] patients had 1 immunosuppressive drug and 11 (52.4%) had 2 or more immunosuppressive drugs). Cyclosporine was the most common immunosuppressive drug used. The type of apheresis was PE in 11 (52.4%) patients, whereas 6 (28.6%) received IA, 2 (9.5%) received double-filtration plasmapheresis, 1 (4.8%) received PE followed by IA, and 1 (4.8%) received LDL-apheresis. The median number of apheresis sessions was 12 (4–39), and the median duration of apheresis was 47 days (17–172). Among the 7 patients who achieved remission under apheresis, 2 displayed nephrotic syndrome relapse when apheresis was stopped. They became “apheresis dependent,” requiring 1 apheresis session per month to maintain remission. In these 2 patients, definitive stopping of apheresis (after 5 and 7 years, respectively) has been associated with residual proteinuria below to 100 mg/mmol. We then compared the main characteristics of patients according to remission status at last follow-up visit (Table 2). The patients who subsequently achieved remission were older (51 years [31–65] vs. 30 [22–40], P = 0.05), had a lower eGFR (28.0 ml/min per 1.73 m2 [0–52.0] vs. 48.5 [39.8–75.0], P = 0.05) and lower proteinuria (3.9 g/d [3.0–5.1] vs. 7.3 [4.9–11.0], P = 0.03) at the initiation of apheresis than those without remission, but serum albumin concentrations did not differ significantly between the 2 groups (20.0 g/l [11.2–28.0] vs. 16.6 [11.4–20.9], P = 0.50). The absolute monthly change in eGFR was larger in patients who achieved remission (−6.8 ml/min per 1.73 m2/month [−12.6 to −3.1] vs. −1.5 [−3.4 to −0.2]; P = 0.04). The number of immunosuppressive drugs before the initiation of apheresis (1 [1–1] vs. 2 [1–3], P = 0.002) and the duration of treatment with corticosteroids before apheresis were lower (5 months [1–7] vs. 15 [6–25.2], P = 0.05) in patients who subsequently achieved remission than in those who did not. PE was performed in 6 of 7 (85.7%) patients in the remission group and in 5 of 14 (35.7%) patients without remission (P = 0.09). IA was used in 0 of 7 patients with remission and in 6 of 14 (42.9%) patients without remission (P = 0.12). The duration of apheresis did not differ significantly between the patients with and without remission. The safety of apheresis was good: venous thrombosis on a catheter was observed in 2 (9.5%) cases and hematoma at the puncture site requiring blood transfusion occurred in 1 (4.8%) patient. Apheresis was stopped due to intolerance in only 1 (4.8%) patient.
Table 2.
Characteristics of patients and treatment at the start of apheresis
| Total population |
Remission |
No remission |
P value | |
|---|---|---|---|---|
| n = 21 | n = 7 | n = 14 | ||
| Clinical and biological characteristics | ||||
| Age (y) [IQR] | 34 [24–50] | 51 [31–65] | 30 [22–40] | 0.05 |
| Min-Max (years) | 16–76 | 16–76 | 16–49 | |
| Hemoglobin level (g/dl) [IQR]; n = 18/21, 6/7, 12/14 | 10.9 [9.2–12.3] | 9.6 [9.1–11.7] | 11.3 [10.1–12.8] | 0.17 |
| Platelet count (109/l) [IQR]; n = 17/21, 6/7, 11/14 | 299 [249–365] | 294 [235–333] | 319 [228–377] | 0.59 |
| Absolute neutrophil count (109/l) [IQR]; n = 16/21, 6/7, 10/14 | 6.1 [4.4–7.7] | 4.6 [2.2–6.7] | 6.4 [5.4–8.5] | 0.14 |
| Renal parameters | ||||
| Systolic blood pressure (mm Hg) [IQR]; n = 18/21, 5/7, 13/14 | 137 [120–146] | 130 [109–141] | 140 [120–146] | 0.27 |
| Diastolic blood pressure (mm Hg) [IQR]; n = 18/21, 5/7, 13/14 | 80 [70–90] | 78 [55–83] | 80 [70–92] | 0.32 |
| Serum albumin concentration (g/l) [IQR] | 17.0 [11.4–22.9] | 20.0 [11.2–28.0] | 16.6 [11.4–20.9] | 0.5 |
| Proteinuria (g/d) [IQR] | 5.3 [4.0–10.5] | 3.9 [3.0–5.1] | 7.3 [4.9–11.0] | 0.03 |
| Absolute change in proteinuria (g/d) [IQR] | −2.9 [−5.4; 2.0] | −5.5 [−14.0; −2.9] | 0.5 [−4.5; 3.6] | 0.03 |
| Absolute monthly change in proteinuria (g/d/mo) [IQR] | 0.0 [−0.6–0.1] | −0.6 [−2.9; −0.3] | 0.0 [−0.2; 0.2] | 0.008 |
| Serum creatinine concentration (μmol/l) [IQR]; n = 18/21, 4/7, 14/14 | 142.5 [110.0–198.5] | 153.5 [112.2–207.5] | 142.5 [103.2–185.5] | 0.87 |
| eGFR (ml/min per 1.73 m2) [IQR]a | 48.0 [25.0–66.5] | 28.0 [0–52.0] | 48.5 [39.8–75.0] | 0.05 |
| CKD stage | ||||
| - I (%) | 2 (9.5) | 0 | 2 (14.3) | 0.79 |
| - II (%) | 3 (14.3) | 0 | 3 (21.4) | 0.51 |
| - III (%) | 10 (47.6) | 3 (42.9) | 7 (50.0) | 1 |
| - IV (%) | 2 (9.5) | 1 (14.3) | 1 (7.1) | 1 |
| - V (%) | 4 (19.1) | 3 (42.9) | 1 (7.1) | 0.17 |
| Time from diagnosis to apheresis (mo) [IQR] | 10 [5–25] | 8 [1–11] | 19 [7–26] | 0.17 |
| Absolute change in eGFR (ml/min per 1.73 m2) [IQR] | −29.0 [−54.3; −6.9] | −28.0 [−83.0; −7.0] | −29.0 [−51.2; −4.7] | 0.68 |
| Absolute monthly change in eGFR (ml/min per 1.73 m2/mo) [IQR] | −2.9 [−6.2; −0.3] | −6.8 [−12.6; −3.1] | −1.5 [−3.4; −0.2] | 0.04 |
| Dialysis before apheresis /at the last follow-up | ||||
| - no/no (%) | 13 (61.9) | 4 (57.1) | 9 (64.3) | } 0.01 |
| - yes/no (%) | 3 (14.3) | 3 (42.9) | 0 | |
| - no/yes (%) | 5 (23.8) | 0 | 5 (35.7) | |
| Specific treatments for INS before apheresis | ||||
| Number of immunosuppressive drugs with corticosteroids | ||||
| - 1 immunosuppressive drug (%) | 10 (47.6) | 7 (100.0) | 3 (20.0) | 0.003 |
| - ≥ 2 immunosuppressive drugs (%) | 11 (52.4) | 0 | 11 (78.6) | 0.003 |
| Number of treatments without corticosteroids [IQR] | 2 [1–3] | 1 | 2 [1–3] | 0.002 |
| Min - Max | 1; 6 | 1 | 1; 6 | |
| Duration of corticosteroid use before apheresis - median [IQR] (month) | 7.0 [5.0–24.0] | 5.0 [1.0–7.0] | 15.0 [6.0–25.2] | 0.05 |
| Immunosuppressive therapy used before apheresis | ||||
| - Calcineurin inhibitor (%) | 13 (61.9) | 4 (57.1) | 9 (64.3) | 1 |
| Cyclosporine (%) | 13 (61.9) | 4 (57.1) | 9 (64.3) | |
| Tacrolimus (%) | 8 (38.1) | 0 | 8 (57.1) | |
| - Cyclophosphamide (%) | 4 (19.1) | 2 (28.6) | 2 (14.3) | 0.84 |
| - Rituximab (%) | 8 (38.1) | 1 (14.3) | 7 (50.0) | 0.27 |
| - Mycophenolate mofetil (%) | 7 (33.3) | 0 | 7 (50.0) | 0.07 |
| - Intravenous immunoglobulin (%) | 1 (4.8) | 0 | 1 (7.1) | 1 |
| Mode of apheresis | ||||
| Type of apheresis | ||||
| - Plasma exchange (%) | 11 (52.4) | 6 (85.7) | 5 (35.7) | 0.09 |
| - Immunoadsorption (%) | 6 (28.6) | 0 | 6 (42.9) | 0.12 |
| - Double-filtration plasmapheresis (%) | 2 (9.5) | 1 (14.3) | 1 (7.1) | 1 |
| - Plasma exchange followed by immunoadsorption (%) | 1 (4.8) | 0 | 1 (7.1) | 1 |
| - Low-density lipoprotein apheresis (%) | 1 (4.8) | 0 | 1 (7.1) | 1 |
| Number of apheresis sessions [IQR] | 12 [4–39] | 11 [4–74] | 12 [7–32] | 0.97 |
| - ≤ 3 (%) | 3 (14.3) | 1 (14.3) | 2 (14.3) | |
| - [4–15](%) | 10 (47.6) | 3 (42.9) | 7 (50) | |
| - ≥ 15 (%) | 8 (38.1) | 3 (42.9) | 5 (35.7) | |
| Duration of apheresis (days) [IQR] | 47 [17–172] | 56 [13–1954] | 40 [21–114] | 0.56 |
| Min-Max | 6; 2296 | 7; 2296 | 6; 338 | |
| Specific treatments for INS after apheresis | ||||
| Dose of corticosteroids at the start of apheresis (mg/d) [IQR] | 15 [1–60] | 30 [7–65] | 10 [0–60] | 0.36 |
| Immunosuppressive therapy used after and during apheresis | ||||
| - Calcineurin inhibitor (%) | 12 (57.1) | 5 (71.5) | 7 (50.0) | 0.64 |
| Cyclosporine (%) | 7 (33.3) | 5 (71.5) | 2 (14.3) | |
| Tacrolimus (%) | 7 (33.3) | 0 | 7 (50.0) | |
| - Cyclophosphamide (%) | 2 (9.5) | 2 (28.6) | 0 | 0.19 |
| - Rituximab (%) | 3 (14.3) | 2 (28.6) | 1 (7.1) | 0.51 |
| - Mycophenolate mofetil (%) | 4 (19.1) | 0 | 4 (28.6) | 0.36 |
| - Intravenous immunoglobulin (%) | 2 (9.5) | 0 | 2 (14.3) | 0.79 |
| - Azathioprine (%) | 2 (9.5) | 1 (14.3) | 1 (7.1) | 1 |
| - Ofatumumab (%) | 1 (4.8) | 0 | 1 (7.1) | 1 |
Qualitative data are expressed as n (%), quantitative data as medians [interquartile range; IQR], as appropriate.
Glomerular filtration rate (GFR) was estimated (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
CDK stage was defined according to National Kidney Foundation practice guidelines for CKD.
eGFR = 0 ml/min per 1.73 m2 for the 3 patients on dialysis.
Outcome of Renal Parameters During Follow-Up
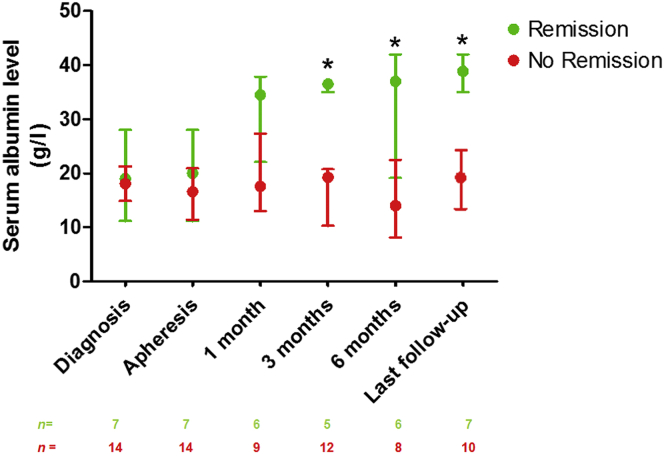
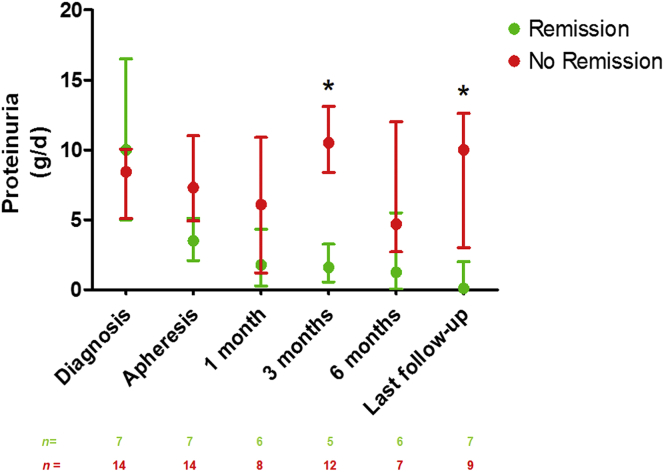
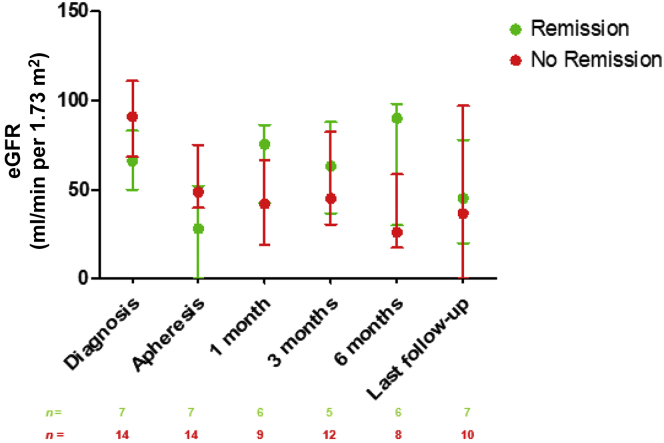
In the total population, 3 months after the initiation of apheresis, serum albumin concentration was 20.0 g/l (13.4–36.0) and proteinuria was 8.5 g/d (0.8–8.9). After a median follow-up of 12 (6.5–43.0) months, serum albumin concentration was at 28.0 g/l (17.7–37.0) and proteinuria was 2.3 g/d (0.4–10.0) (median follow-up was 68.0 months [37.0–15.0] in the remission group and 10.0 months [4.8–15.0] in the nonremission group, P = 0.004) (Figure 1 and Figure 2). As expected, patients from the remission group had a higher serum albumin concentration 3 months after the initiation of apheresis (36.5 g/l [35.0–20.8] vs. 19.3 [10.3–20.8], P = 0.01) and at the last follow-up evaluation than those in the nonremission group (38.9 g/l [35.0–25.2] vs. 19.7 g/l [11.9–25.2], P = 0.001) (Figure 1). Similarly, proteinuria was significantly lower in the remission group than in the nonremission group (1.6 g/d [0.5–13.1] and 10.5 g/d [8.4–13.1], P = 0.004) at 3 months after the initiation of apheresis. The absolute change in proteinuria between INS diagnosis and the start of apheresis was larger in the remission group than in the nonremission group (−5.5 g/d [−14.0 to −2.9] vs. 0.5 g/d [−4.5 to 3.6]; P = 0.03) (Table 2). At the last follow-up evaluation, proteinuria was 0.1 g/d [0.0–12.6] for patients in remission and 10.0 g/d [3.0–12.6] for patients without remission (P = 0.008) (Figure 2). Figure 3 shows the changes in eGFR at the time of INS diagnosis, at apheresis initiation, at 3 and 6 months, and at the last follow-up evaluation. At the last follow-up visit, CKD stage I was observed in 1 of the 7 patients who had achieved remission (14%) and in 4 of the 14 (29%) patients without remission (P = 0.46). CKD stage II–III was observed in 4 of 7 (57%) patients with remission, versus 3 of 14 (21%) patients without remission (P = 0.10), and CKD stage IV–V was observed in 2 of 7 (29%) patients with remission, versus 7 of 14 (50%) patients without remission (P = 0.35). Three (42.9%) patients from the remission group had to start dialysis because of a rapid deterioration of renal function at the time of apheresis. The duration of the disease before the initiation of apheresis was 1, 8, and 11 months, in these 3 patients. All were off dialysis at the last follow-up visit (eGFR: 45 ml/min per 1.73 m2 [20–97]). By contrast, none of the patients who did not achieve remission were on dialysis before apheresis, whereas 5 of 14 (35.7%) of these patients were on dialysis at last follow-up (P = 0.01) (Table 2).
Figure 1.
Serum albumin concentration (g/l) at the time of INS diagnosis; at the time of apheresis; and at 1, 3, and 6 months and at last follow-up in patients with and without remission. Treatment efficacy (remission vs. no remission) was evaluated on based on serum albumin concentration and proteinuria at last follow-up. Median follow-up was 12 [6.5–43] months. Data are expressed as medians and interquartile range. ∗P < 0.05 for the comparison of the remission and no-remission groups at each time point.
Figure 2.
Proteinuria (g/d) at the time of INS diagnosis; at the time of apheresis; and at 1, 3, and 6 months and at last follow-up in patients with and without remission. Treatment efficacy (remission vs. no remission) was evaluated based on the results for serum albumin concentration and proteinuria at last follow-up. Median follow-up was 12 [6.5–43] months. Data are expressed as medians and interquartile range. ∗P < 0.05 for the comparison of the remission and no-remission groups at each time point.
Figure 3.
Glomerular filtration rate (ml/min per 1.73 m2) estimated (eGFR) according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation at the time of INS diagnosis; at the time of apheresis; and at 1, 3, and 6 months, and at last follow-up, in patients with and without remission. Treatment efficacy (remission vs. no remission) was evaluated based on the results for serum albumin concentration and urinary protein concentration at last follow-up. Median follow-up was 12 [6.5–43] months. Data are expressed as medians and interquartile range. Patients on dialysis (5 patients in the no-remission group) were considered to have an eGFR of 0 ml/min per 1.73 m2). Remission versus no remission at each time point: P ≥ 0.05.
Identification of Parameters Associated With Remission on Apheresis
In univariate analysis, AKI requiring dialysis at the time of apheresis (OR: 22.0 [1.00–525] P = 0.026), a marked decrease in proteinuria from initial diagnosis to the initiation of apheresis (absolute decrease >4.5 g/d: OR: 9.17 [1.15–73.2], P = 0.041); and age ≥50 years (OR: 22.6 [1.00–524], P = 0.006) were associated with a higher likelihood of remission. A time from diagnosis to apheresis <12 months was also associated with remission (OR: 10.8 [1–117], P = 0.043) (Table 3).
Table 3.
Parameters associated with remission (univariate analysis)
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| All patients (n = 21) | |||
| Age ≥50 y | 22.6 | 1.00–525 | 0.006 |
| Time from diagnosis to apheresis < 12 mo | 10.8 | 1–117 | 0.043 |
| Change in proteinuria before apheresis > 4.5 g/d | 9.17 | 1.15–73.2 | 0.041 |
| Dialysis for acute kidney injury at the time of apheresis | 22.0 | 1.00–524 | 0.026 |
| Dialysis or change in proteinuria > 4.5 g/d before apheresis | 22.0 | 1.86–107 | 0.001 |
CI, confidence interval.
Discussion
In this retrospective study, we assessed the effect of apheresis in adult patients with refractory INS. This treatment was found to be a promising therapeutic approach in refractory INS, because 33% of the patients were in remission at the last follow-up evaluation. Strikingly, apheresis seemed to be more effective in patients with (i) an imminent risk of renal failure requiring kidney replacement therapy, (ii) with limited sensitivity to treatment with corticosteroids and at least 1 additional immunosuppressive drug (defined as a marked decrease in proteinuria of more than 4.5 g/d but without complete or partial remission before apheresis), (iii) with a time from diagnosis to apheresis <12 months, and (iv) an age older than 50 years.
The treatment of refractory INS on native kidneys remains a matter of debate, and the potential value of apheresis for treating this condition in adult patients remains to be determined. Muso25 reported that LDL-apheresis resulted in 50% remission in adults with steroid-resistant NS, but this study included patients with diverse primary glomerular diseases (FSGS, MCNS, IgA nephropathy, and membranous nephropathy), making it difficult to draw definitive conclusions. In a prospective multicenter study from the same group, including 44 adult patients (including 29 with INS) with persistent SRNS after at least 4 weeks of steroid treatment, LDL-apheresis led to remission (defined as proteinuria <1 g/d) in 47.7% of cases after 2 years of follow-up.15 A recent retrospective study by Nattes et al.16 evaluated the efficacy of plasma protein (IA) in 14 children with refractory INS. This study reported a complete and partial remission rate of 64%, but all patients displayed relapse when IA was stopped. In our study, 2 patients with remission became dependent to apheresis, suggesting that this therapeutic approach, in some patients, may be only suspensive. Naciri Bennani et al.26 recently described 3 cases that suggested that double-filtration plasmapheresis or semi-specific IA could successfully treat INS in patients in whom conventional therapies had failed. With the exception of studies on LDL-A, all the available data relating to the potential value of apheresis for treating refractory INS in adulthood originate exclusively from case reports.13,26, 27, 28 Our study focusing exclusively on adult patients with refractory INS suggests that apheresis may be a relevant treatment option in some cases.
The immune mechanisms underlying the pathophysiology of INS are believed to involve a systemic T- and/or B-cell dysfunction.29 It is currently thought that INS is caused by a putative circulating factor, and we can hypothesize that apheresis acts directly, by removing this permeability factor.11,30 Thus, our understanding of INS pathogenesis has dramatically improved during the past decade based on the identification of some potential glomerular permeability factors.31 In this setting, the putative benefits of apheresis observed in some patients might involve the removing of some potential relevant circulating factors including anti-nephrin antibodies,32 circulating anti-CD40 antibodies that may act synergistically with soluble urokinase plasminogen activator receptor to drive glomerular injury,33 or autoantibodies against carboxy-terminal hydrolase L1 ubiquitin (UCHL1), a cytosolic enzyme expressed by podocytes.34 Nevertheless, in our study, accurate mechanisms supporting the improvement with apheresis remain to be determined. Saleem5 recently proposed a new classification of INS based on underlying molecular mechanisms: monogenic disease versus immune-mediated INS. In our study, the sequencing of target genes involved in SRNS yielded negative results in all cases tested (47.4% of patients with SRNS were tested). In addition, a broad spectrum of etiologic agents or associated conditions has been reported in association with secondary forms of FSGS,35 but it is not always easy for clinicians to distinguish between primary and secondary FSGS.36 In our study, we cannot rule out the possibility that some of the patients diagnosed with FSGS had a secondary form of FSGS. As also reported by Sethi et al.,36 all our patients with FSGS diagnosis displayed nephrotic syndrome at disease onset, suggesting a primary, rather than a secondary form of FSGS. Moreover, all patients underwent extensive screening for potential confounding causal agents. One patient tested positive for antibodies against parvovirus 19, which has been identified as a secondary cause of FSGS.37 Two additional patients of African ancestry tested positive for 2 APOL1 risk alleles known to constitute a major genetic risk factor for developing FSGS, regardless of etiology.38 It has been suggested that high-risk APOL1 genotypes do not modify the response to immunosuppression.39 Finally, extensive screening for secondary causes was performed in all patients displaying a deep nephrotic syndrome, and one-third of patients went into remission on apheresis. These findings suggest that INS was due to an immune-mediated mechanism rather than a secondary process.
We found that 3 of the 7 patients (42.9%) for whom it was necessary to initiate dialysis before apheresis achieved remission. Dialysis at the time of apheresis was, therefore, associated with a greater likelihood of remission. An Italian study suggested that significantly altered renal function at the time of initial management has no effect on the response to steroids.40 Previous studies found that AKI during MCNS is a frequent, but reversible process,41 whereas CKD is common in FSGS (30%–45% of cases at presentation).9 Feld et al.27 suggested that the use of plasmapheresis to treat FSGS recurrence on the graft may stabilize renal function. We found that the success of apheresis was associated with better renal outcomes (CKD stage IV–V in 29% of patients in the remission group vs. 50% for patients without remission). This finding is consistent with the report by Terada et al.42 of efficacy for early additional LDL-apheresis in patients with nephrotic syndrome due to MCNS developing AKI requiring dialysis.
We found that a marked decrease in proteinuria without complete remission on treatment with corticosteroids and at least 1 immunosuppressive drug was associated with a higher likelihood of remission. This parameter may be important for selecting the patients most likely to benefit from apheresis. By contrast, the patients who did not achieve remission in our study had a high risk of end-stage kidney disease in the short term. These findings are consistent with previous data showing that patients displaying partial or complete remission from nephrotic syndrome have better renal outcomes concerning the risk of CKD progression.43 It is possible that the relative decrease in proteinuria on corticosteroids and/or immunosuppressive agents reflects the immunological activity of the underlying glomerular disease, and the possibility of improving proteinuria by removing potential circulating factors.
In our study, older age was associated with a greater likelihood of remission. Colliou et al.44 showed that 85% of patients developing INS after the age of 60 years have a complete or partial renal response on corticosteroids and/or immunosuppressive regimens. In the POLARIS (Prospective Observational Survey on the Long-Term Effects of LDL Apheresis on Drug-Resistant Nephrotic Syndrome) study, Muso et al.15 found no effect of age on outcome, whereas previously published case studies reported remission from INS after apheresis in patients younger than 50 years.12, 13, 14 Our data suggest that, regardless of the patient’s age, intensive rescue therapy by apheresis should be considered in patients with INS.
Our study has several limitations. First, this study was retrospective, with a long inclusion period, and suffers from the limitations inherent to this type of approach. It may not, therefore, be representative of all refractory INS cases diagnosed in France during the period study. Second, the limited size of the total study population results in a low power for the statistical analyses comparing the remission and no-remission groups. Third, several types of apheresis with different protocols were used, and there was no control group. Fourth, genetic testing for SRNS was performed in 9 patients, and we cannot exclude the possibility that underlying genetic causes were missed. Of note, all 19 adult patients included in our study with SRNS had an apparent sporadic form of INS. However, until recently, genetic screening was not routinely performed in adult patients in the absence of known family history of steroid-resistant INS. Fifth, electron microscopy analyses are not routinely performed at French nephrology centers. In patients with MCNS, light and immunofluorescence microscopy findings were highly suggestive of this diagnosis, but differential diagnoses could not be definitively ruled out. In addition, we were unable to investigate the characteristics of podocyte foot process to potentially discriminate primary versus maladaptive FSGS. Finally, we observed a significant difference in median follow-up between patients in remission and patients without remission potentially due to the follow-up of patients without remission being stopped when chronic renal replacement therapy was started.
In conclusion, our study shows, for the first time, that apheresis (at least for PE and IA, as only 1 patient was treated with lipid apheresis) can be an effective therapeutic option in adult patients with refractory INS. Apheresis was effective mostly in patients with a high risk of renal failure in the short term, and in those with limited sensitivity to treatment (i.e., a marked but insufficient decrease in proteinuria level). A failure to achieve remission on apheresis was associated with a poor renal outcome. Further prospective studies are warranted to optimize the use of apheresis in adult patients with refractory INS.
Disclosure Statement
VA received consulting fees from Addmedica outside of the submitted work. All the other authors declared no competing interests.
Acknowledgments
We would like to thank all the nephrologists involved in the medical care of the patients included in this study.
References
- 1.Vivarelli M., Massella L., Ruggiero B., Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12:332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogan J., Radhakrishnan J. The treatment of minimal change disease in adults. J Am Soc Nephrol. 2013;24:702–711. doi: 10.1681/ASN.2012070734. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahali D., Sendeyo K., Mangier M. Immunopathogenesis of idiopathic nephrotic syndrome with relapse. Semin Immunopathol. 2014;36:421–429. doi: 10.1007/s00281-013-0415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleem M.A. Molecular stratification of idiopathic nephrotic syndrome. Nat Rev Nephrol. 2019;15:750–765. doi: 10.1038/s41581-019-0217-5. [DOI] [PubMed] [Google Scholar]
- 6.Webb N.J.A., Woolley R.L., Lambe T. Long term tapering versus standard prednisolone treatment for first episode of childhood nephrotic syndrome: phase III randomised controlled trial and economic evaluation. BMJ. 2019;365:1800. doi: 10.1136/bmj.l1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rémy P., Audard V., Natella P.A. An open-label randomized controlled trial of low-dose corticosteroid plus enteric-coated mycophenolate sodium versus standard corticosteroid treatment for minimal change nephrotic syndrome in adults (MSN Study) Kidney Int. 2018;94:1217–1226. doi: 10.1016/j.kint.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Korbet S.M., Whittier W.L. Management of adult minimal change disease. Clin J Am Soc Nephrol. 2019;14:911–913. doi: 10.2215/CJN.01920219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korbet S.M. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23:1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 10.Kashgary A., Sontrop J.M., Li L. The role of plasma exchange in treating post-transplant focal segmental glomerulosclerosis: a systematic review and meta-analysis of 77 case-reports and case-series. BMC Nephrol. 2016;17:104. doi: 10.1186/s12882-016-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canaud G., Delville M., Legendre C. Recurrence of focal and segmental glomerulosclerosis after transplantation. Transplantation. 2016;100:284–287. doi: 10.1097/TP.0000000000000902. [DOI] [PubMed] [Google Scholar]
- 12.Araki H., Ono S., Nishizawa Y. Focal segmental glomerular sclerosis ameliorated by long-term hemodialysis therapy with low-density lipoprotein apheresis. Intern Med. 2015;54:2213–2217. doi: 10.2169/internalmedicine.54.4631. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama K., Sakai S., Yamaguchi Y. Complete remission of the nephrotic syndrome due to focal glomerular sclerosis achieved with low density lipoprotein adsorption alone. Nephron. 1996;72:318–320. doi: 10.1159/000188863. [DOI] [PubMed] [Google Scholar]
- 14.Yorioka N., Taniguchi Y., Nishida Y. Low-density lipoprotein apheresis for focal glomerular sclerosis. Ther Apher. 1997;1:370–371. doi: 10.1111/j.1744-9987.1997.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 15.Muso E., Mune M., Hirano T. A prospective observational survey on the long-term effect of LDL apheresis on drug-resistant nephrotic syndrome. Nephron Extra. 2015;5:58–66. doi: 10.1159/000437338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nattes E., Karaa D., Dehoux L. Remission of proteinuria in multidrug-resistant idiopathic nephrotic syndrome following immunoglobulin immunoadsorption. Acta Paediatr. 2019;108:757–762. doi: 10.1111/apa.14582. [DOI] [PubMed] [Google Scholar]
- 17.Gribouval O., Boyer O., Hummel A. Identification of genetic causes for sporadic steroid-resistant nephrotic syndrome in adults. Kidney Int. 2018;94:1013–1022. doi: 10.1016/j.kint.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Freedman B.I., Skorecki K. Gene–gene and gene–environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol. 2014;9:2006–2013. doi: 10.2215/CJN.01330214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Agati V.D., Fogo A.B., Bruijn J.A., Jennette J.C. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Floege J., Barbour S.J., Cattran D.C. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:268–280. doi: 10.1016/j.kint.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Rovin B.H., Caster D.J., Cattran D.C. Management and treatment of glomerular diseases (part 2): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:281–295. doi: 10.1016/j.kint.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;12:1–138. [Google Scholar]
- 23.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 25.Muso E. Beneficial effect of LDL-apheresis in refractory nephrotic syndrome. Clin Exp Nephrol. 2014;18:286–290. doi: 10.1007/s10157-013-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naciri Bennani H., Jouve T., Noble J. Apheresis therapy for steroid-resistant idiopathic nephrotic syndrome: report on a case series. Case Rep Nephrol. 2019;2019:7304786. doi: 10.1155/2019/7304786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feld S.M., Figueroa P., Savin V. Plasmapheresis in the treatment of steroid-resistant focal segmental glomerulosclerosis in native kidneys. Am J Kidney Dis. 1998;32:230–237. doi: 10.1053/ajkd.1998.v32.pm9708606. [DOI] [PubMed] [Google Scholar]
- 28.Haas M., Godfrin Y., Oberbauer R. Plasma immunadsorption treatment in patients with primary focal and segmental glomerulosclerosis. Nephrol Dial Transplant. 1998;13:2013–2016. doi: 10.1093/ndt/13.8.2013. [DOI] [PubMed] [Google Scholar]
- 29.Colucci M., Corpetti G., Emma F., Vivarelli M. Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol. 2018;33:573–584. doi: 10.1007/s00467-017-3677-5. [DOI] [PubMed] [Google Scholar]
- 30.Savin V.J., Sharma R., Sharma M. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 31.Maas R.J., Deegens J.K., Wetzels J.F. Permeability factors in idiopathic nephrotic syndrome: historical perspectives and lessons for the future. Nephrol Dial Transplant. 2014;29:2207–2216. doi: 10.1093/ndt/gfu355. [DOI] [PubMed] [Google Scholar]
- 32.Patrakka J., Ruotsalainen V., Reponen P. Recurrence of nephrotic syndrome in kidney grafts of patients with congenital nephrotic syndrome of the Finnish type: role of nephrin. Transplantation. 2002;73:394–403. doi: 10.1097/00007890-200202150-00013. [DOI] [PubMed] [Google Scholar]
- 33.Delville M., Sigdel T.K., Wei C. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6:256ra136. doi: 10.1126/scitranslmed.3008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jamin A., Berthelot L., Couderc A. Autoantibodies against podocytic UCHL1 are associated with idiopathic nephrotic syndrome relapses and induce proteinuria in mice. J Autoimmun. 2018;89:149–161. doi: 10.1016/j.jaut.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 35.D’Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 36.Sethi S., Glassock R.J., Fervenza F.C. Focal segmental glomerulosclerosis: towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant. 2015;30:375–384. doi: 10.1093/ndt/gfu035. [DOI] [PubMed] [Google Scholar]
- 37.Tanawattanacharoen S., Falk R.J., Jennette J.C., Kopp J.B. Parvovirus B19 DNA in kidney tissue of patients with focal segmental glomerulosclerosis. Am J Kidney Dis. 2000;35:1166–1174. doi: 10.1016/s0272-6386(00)70055-2. [DOI] [PubMed] [Google Scholar]
- 38.Kopp J.B., Nelson G.W., Sampath K. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopp J.B., Winkler C.A., Zhao X. Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol. 2015;26:1443–1448. doi: 10.1681/ASN.2013111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stellato T., Cappelleri A., Farina M. Severe reversible acute renal failure in idiopathic nephrotic syndrome. J Nephrol. 2010;23:717–724. [PubMed] [Google Scholar]
- 41.Waldman M., Crew R.J., Valeri A. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2:445–453. doi: 10.2215/CJN.03531006. [DOI] [PubMed] [Google Scholar]
- 42.Terada K., Mugishima K., Kawasaki S. Low-density lipoprotein apheresis in patients with acute kidney injury due to minimal change disease requiring acute renal replacement therapy. Int J Nephrol Renovasc Dis. 2020;13:157–162. doi: 10.2147/IJNRD.S248610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chun M.J., Korbet S.M., Schwartz M.M., Lewis E.J. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol. 2004;15:2169–2177. doi: 10.1097/01.ASN.0000135051.62500.97. [DOI] [PubMed] [Google Scholar]
- 44.Colliou E., Karras A., Boffa J.-J. Outcomes of older patients (≥60 years) with new-onset idiopathic nephrotic syndrome receiving immunosuppressive regimen: a multicentre study of 116 patients. J Clin Med. 2019;8:298. doi: 10.3390/jcm8030298. [DOI] [PMC free article] [PubMed] [Google Scholar]