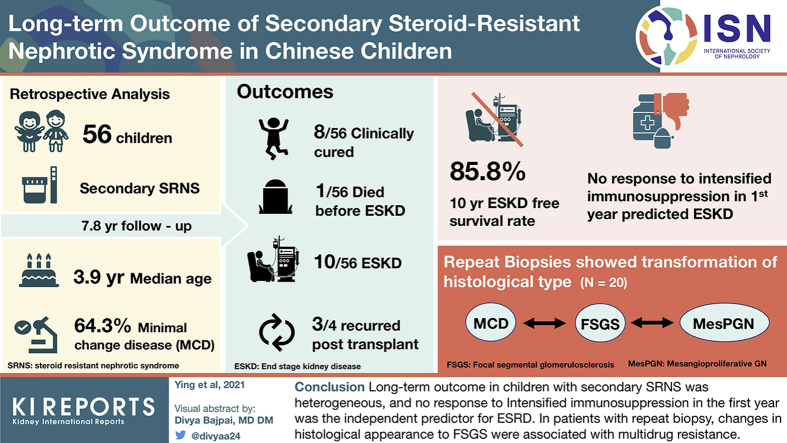
Abstract
Introduction
Secondary steroid-resistant nephrotic syndrome (SRNS) refers to the condition when patients with initial steroid-sensitive nephrotic syndrome develop steroid resistance in subsequent relapses. Long-term outcomes of secondary SRNS in children are uncertain.
Methods
This was a single-center retrospective study of 56 children with secondary SRNS between 2006 and 2016. The survival curve was estimated using the Kaplan-Meier method. Independent risk factors for end-stage renal disease (ESRD) were determined using Cox proportional hazards model.
Results
The median time from nephrotic syndrome onset to secondary SRNS was 7.8 months. Biopsy results at diagnosis secondary SRNS showed that 64.3% of cases were minimal change disease (MCD). No remission was observed in seven (12.5%) patients within the first year. The mean follow-up time was 7.8 ± 3.2 years. Eight patients were clinically cured, one died before ESRD, 10 reached ESRD, and 75.0% (3 of 4) of patients recurred post-transplantation. The 10-year ESRD-free survival rate was 85.8%. No response to intensified immunosuppression (IIS) in the first year was the independent predictor for ESRD. Repeat biopsies were performed in 20 cases, revealing that the reclassification from MCD to mesangial hypercellularity and focal segmental glomerulosclerosis (FSGS) in two when secondary steroid resistance appeared, from MCD and mesangial hypercellularity to FSGS in seven who developed multidrug resistance, and from FSGS to MCD and mesangial hypercellularity in two with favorable outcomes.
Conclusions
The long-term outcome in children with secondary SRNS was heterogeneous, and no response to IIS in the first year was the independent predictor for ESRD. In patients with repeat biopsy, changes in histological appearance to FSGS were associated with multidrug resistance.
Keywords: children, histology, immunosuppression, outcome, secondary steroid-resistant nephrotic syndrome
Graphical abstract
Idiopathic nephrotic syndrome is the most common glomerular disease in childhood, with an incidence of 1.15 to 16.9 per 100,000 children.1 Based on the initial standard steroid treatment response, idiopathic nephrotic syndrome can be classified into steroid-sensitive nephrotic syndrome (SSNS) or SRNS. However, the steroid responsiveness may alter throughout the disease course. Some initial SRNS patients who respond to IIS may develop secondary SSNS, whereas some with initial SSNS may develop secondary SRNS in subsequent relapses.2 Secondary SRNS, which is also called late SRNS or late steroid non-responders,3 accounted for 13.8% to 35.9% of SRNS according to published cohort studies of idiopathic nephrotic syndrome.4, 5, 6 Although it was recognized decades ago, secondary SRNS is still not fully understood. Recent studies have shown that secondary SRNS is associated with a high risk of post-transplantation recurrence which is a major cause of allograft loss.7,8 Therefore, it is crucial to deeply understand the long-term renal outcomes of this SRNS subtype and manage to reduce the risk of kidney failure. Previous studies focused on secondary SRNS have relatively small sample sizes and present conflicting results.9 Hence, this relatively large sample size study aims to investigate the long-term outcome of secondary SRNS.
Patients and Methods
Between 2006 and 2016, 56 consecutive children with secondary SRNS who had been followed up for more than 1 year at the First Affiliated Hospital of Sun Yat-sen University were enrolled in this retrospective study. To avoid selection bias, we reviewed the charts of all patients diagnosing nephrotic syndrome (NS) (age ≤14 years) during the study period. Patients with congenital NS and secondary causes of NS were excluded. The clinical records were reviewed, and relevant data for the course of the disease, laboratory characteristics, biopsy findings, treatment regimen and corresponding response were collected. The final status of the disease was determined via the clinical records and the telephone tracing of patients. The endpoint of follow-up was death, ESRD, or May 31, 2020. The primary endpoint was ESRD.
We categorized IIS responsiveness according to the best antiproteinuric response (complete remission [CR], partial remission [PR], or no remission [NR]) to any IIS protocol used within the first year after secondary SRNS onset to predict long-term outcomes.10 CR was defined as negative or trace first-morning urine dipstick for 3 consecutive days. PR was defined as persistently 1+ to 2+ urine dipstick. NR was defined as persistently 3+ or 4+ urine dipstick. SSNS was defined as patients who achieve CR within 4 weeks of treatment with prednisone at a standard dose (2 mg/kg/d or 60 mg/m2/d, with a maximum of 60 mg/d). Those who do not achieve CR after 4 weeks were considered to have SRNS. Secondary SRNS refers to the condition when initial SSNS patients develop steroid resistance in subsequent relapses.2,3 Calcineurin inhibitor (CNI) resistance and multidrug resistance were defined according to the 2020 International Pediatric Nephrology Association clinical practice recommendations.2 Estimated glomerular filtration rate was calculated using the Schwartz formula.11 ESRD was defined as glomerular filtration rate < 15 ml/min/1.73 m2. Patients who had maintained CR for more than 3 years after the withdrawal of steroids and IIS were considered to be clinically cured cases.
Statistical Analysis
Data were expressed as mean ± standard deviation, median (interquartile range, Q1-Q3), or frequency (percentage). Groups were compared by chi square test or Fisher exact test when appropriate. We used the Kaplan-Meier method to estimate the overall cumulative renal survival rate. Possible predictive variables associated with ESRD were evaluated using the Cox proportional hazards model. The multivariable model incorporated only those variables significant to P < 0.1 in the univariate models. Two-tailed P < 0.05 was considered statistically significant. Statistical analyses were performed with R software 4.0.0 (http://www.R-project.org).
Results
Patient Characteristics
Secondary SRNS accounted for 34.3% of idiopathic SRNS, and the clinical features of the 56 patients with secondary SRNS are presented in Table 1. Of the 56 patients, 40 (71.4%) were male, with a median (interquartile range) age of 3.9 (2.1 to 5.4) years at diagnosis of NS and a median (range) disease duration of 7.8 (1.2 to 116.0) months developing into secondary SRNS, and 41 (73.2%) patients converted within the first year. Twelve (21.4%) cases became secondary SRNS at the first relapse, and 44 (78.6%) patients relapsed more than once. Besides these, 11 (19.6%) patients received IIS before secondary SRNS, 4 of whom received more than one IIS drug sequentially. All 56 cases underwent renal biopsies at diagnosis secondary SRNS. The histology results showed MCD in 36 (64.3%) patients, FSGS in 15 (26.8%) patients, and mesangial hypercellularity in 4 (7.1%) patients. Of the 56 patients, 3 who finally reached ESRD underwent SRNS gene panel tests and the results were all negative.
Table 1.
Clinical and histopathologic characteristics of patients
| Characteristic | N=56 |
|---|---|
| Male | 40 (71.4) |
| Age at diagnosis NS, median (IQR), yrs | 3.9 (2.1-5.4) |
| Time to initial remission, median (IQR), days | 14.0 (7.0-20.8) |
| Time to first relapse, median (IQR), moa | 1.4 (0.5-3.0) |
| Time to secondary SRNS, median (IQR), mo | 7.8 (3.4-18.9) |
| Frequency of relapses before secondary SRNS | |
| Once | 12 (21.4) |
| Infrequently relapsing | 12 (21.4) |
| Frequently relapsing | 23 (41.1) |
| Steroid dependent | 7 (12.5) |
| Uncertain | 2 (3.6) |
| IIS before secondary SRNS, n | |
| Cyclosporine A | 6 |
| Tacrolimus | 6 |
| Cyclophosphamide | 3 |
| Age at diagnosis secondary SRNS, median (IQR), yrs | 4.9 (3.3-7.0) |
| Hypertension | 23 (41.1) |
| eGFR < 90 ml/m/1.73 m2 | 5 (8.9%) |
| Histopathology at diagnosis secondary SRNS | |
| MCD | 36 (64.3) |
| FSGS | 15 (26.8) |
| Mesangial hypercellularity | 4 (7.1) |
| MN | 1 (1.8) |
eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; IIS, intensified immunosuppression; IQR, interquartile range; MCD, minimal change disease; MN, membranous nephropathy; NS, nephrotic syndrome; SRNS, steroid-resistant nephrotic syndrome.
Values are n (%) unless otherwise stated.
Missing data, n = 6.
IIS Treatment and Response
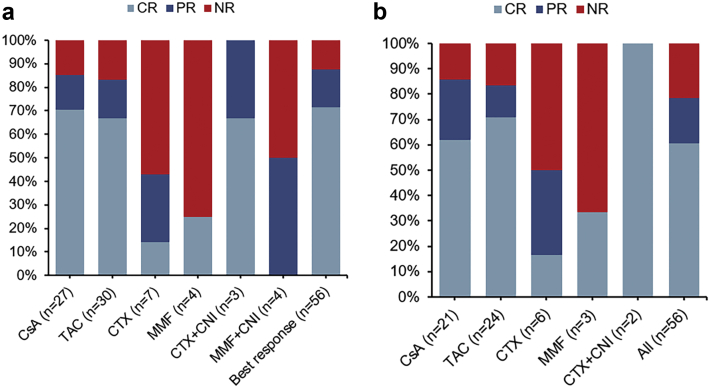
Fifty-six cases received 81 IIS treatment protocols during the first year from diagnosis of secondary SRNS. Thirty-eight patients were treated with one IIS drug, 12 patients were treated with two different IIS drugs, and six patients were treated with three or more IIS drugs. All IIS protocols were combined with oral steroids initially. CR and PR were observed in 40 (71.4%) and 9 (16.1%) patients in the first year, respectively, and 38.8% (19 of 49) of the patients relapsed within the first year. Seven (12.5%) patients showed no response to the IIS in the first year. According to the biopsy results at diagnosis, the combined CR and PR rates of patients with MCD, mesangial hypercellularity, and FSGS in the first year were significantly different (94.4% vs. 100.0% vs. 66.7%, P = 0.03). Response data from 75 (92.6%) IIS treatment protocols are shown in Figure 1a, and the other 6 were excluded for being stopped in advance (exposure time less than 6 months) before CR or PR was observed. The combined CR and PR rates were 85.2% and 83.3% in patients with cyclosporine A and tacrolimus, respectively, whereas 42.9% and 25.0% in patients with cyclophosphamide and mycophenolate mofetil, respectively. Besides, the median time to achieve CR with CNI was 1.0 (range: 0.6 to 3.8) months. Response data of the first IIS protocols are presented in Figure 1b. Twelve, 10, and 34 patients showed NR, PR, and CR when using the first IIS protocols, respectively.
Figure 1.
Response to IIS treatment protocols in 56 children with secondary SRNS. (a) Response to 75 IIS protocols in the first year after secondary SRNS onset, 18 (32.1%) patients were treated with two or more protocols. (b) Response to the first IIS protocol after secondary SRNS onset. CNI, calcineurin inhibitors; CR, complete remission; CsA, cyclosporine A; CTX, cyclophosphamide; IIS, intensified immunosuppression; MMF, mycophenolate mofetil; NR, no remission; PR, partial remission; SRNS, steroid-resistant nephrotic syndrome; TAC, tacrolimus.
The Final Follow-Up Status
The mean follow-up time was 7.8 ± 3.2 years since NS onset. At the last visit, one patient died of severe infection before reaching ESRD, and 10 reached ESRD. Four patients received a transplant, and three (75.0%) recurred post-transplantation. Three patients were in unremitting status (NR in the last year of follow-up), two with chronic kidney disease (CKD) stage 3, and one with CKD stage 2; 29 patients were in intermittently relapsing status, 2 with CKD stage 2 and 27 with CKD stage 1; of these 32 patients, 15.6% (5 of 32) were older than 18 years old. The other 13 cases (23.2%) had withdrawn steroids and IIS, and 8 (61.5%) of them did not relapse beyond 3 years.
Predictors of ESRD
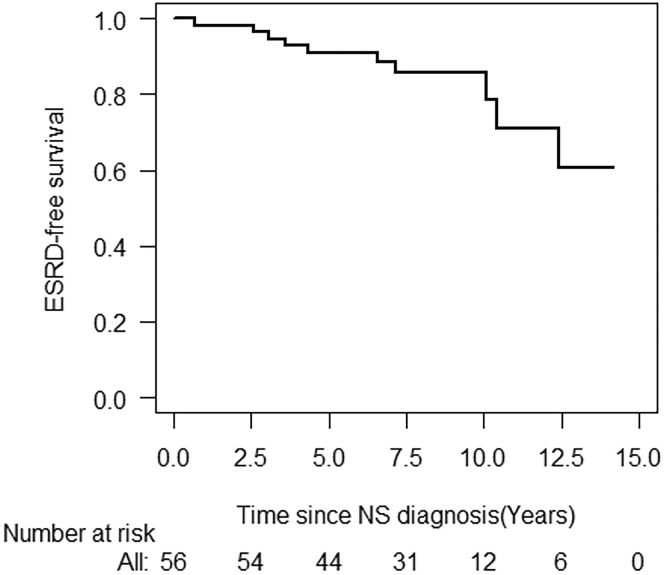
According to the Kaplan-Meier analysis, the overall 5-year and 10-year ESRD-free survival rates were 90.9% (95% confidence interval [CI]: 83.3% to 98.5%) and 85.8% (95% CI: 79.5% to 95.7%), respectively (Figure 2). The predictors of ESRD in univariate Cox regression models were FSGS at diagnosis (hazard ratio: 6.96; 95% CI 1.78 to 27.19, P = 0.005) and no response to IIS in the first year (hazard ratio: 12.41; 95% CI 2.93 to 52.52, P < 0.001), respectively (Table 2). By multivariable Cox regression model, only no response to IIS in the first year (hazard ratio: 6.02; 95% CI: 1.14 to 31.73, P = 0.03) was independently associated with ESRD development (Table 2).
Figure 2.
Overall end-stage renal disease (ESRD)–free survival in 56 Chinese children with secondary steroid-resistant nephrotic syndrome after idiopathic nephrotic syndrome onset. NS, nephrotic syndrome.
Table 2.
Predictors for ESRD in secondary SRNS according to Cox regression analysis
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age at NS onset (reference: ≥ 5 yr) | 1.48 (0.30-7.21) | 0.63 | ||
| Sex (reference: female) | 1.57 (0.33-7.40) | 0.60 | ||
| IIS using before secondary SRNS onset (reference: no) | 2.75 (0.77-9.79) | 0.12 | ||
| Time to secondary SRNS (reference: ≥ 1 yr) | 1.08 (0.28-4.22) | 0.91 | ||
| Hypertension (reference: no) | 2.32 (0.65-8.24) | 0.20 | ||
| FSGS at diagnosis (reference: non-FSGSa) | 6.96 (1.78-27.19) | 0.005 | 3.40 (0.70-17.03) | 0.14 |
| Response to IIS in the first year (reference: CR) | ||||
| NR | 12.41 (2.93-52.52) | <0.001 | 6.02 (1.14-31.73) | 0.03 |
| PR | 2.44 (0.40-14.67) | 0.33 | 2.42 (0.40-14.56) | 0.33 |
CI, confidence interval; CR, complete remission; ESRD, end-stage renal disease; FSGS, focal segmented glomerulosclerosis; HR, hazard ratio; IIS, intensified immunosuppression; NR, no remission; NS, nephrotic syndrome; PR, partial remission; SRNS, steroid-resistant nephrotic syndrome.
Non-FSGS indicates patients with MCD or mesangial hypercellularity or membranous nephropathy at diagnosis.
Repeat Histological Findings
Twenty patients underwent repeated renal biopsies. Three were at the time of diagnosis of secondary SRNS, two of them were reclassified from MCD to mesangial hypercellularity and FSGS, respectively, and one remained mesangial hypercellularity. The time interval from the first biopsy was 8.6, 3.3, and 1.4 years, respectively. The other 17 cases underwent repeated biopsies in the later disease course of secondary SRNS, either to guide therapeutic decisions or to assess CNI nephrotoxicity (Table 3). Of these, seven cases were reclassified from MCD and mesangial hypercellularity to FSGS, and all of them developed multidrug resistance, and five of them reached CKD stage 3 or ESRD. Six cases with MCD did not convert, one of them developed CNI resistance, and the other five maintained CR at the last visit. Two patients who achieved CR and PR in the first year were reclassified from FSGS to MCD and mesangial hypercellularity, respectively, one with mesangial hypercellularity was clinically cured, and the other maintained PR with cyclosporine A. CNI toxicity was confirmed in one patient (patient no. 7) after using CNI for 5.3 years. In all patients with repeated biopsy, the rate of multidrug resistance was significantly higher in those who transformed to FSGS than in those who remained MCD or mesangial hypercellularity (87.5% [7 of 8 patients] vs. 12.5% [1 of 8 patients], P = 0.01).
Table 3.
Clinical and histological characteristics of 17 patients performed repeat biopsies in the later disease course of secondary SRNS
| Patient no. | Age at first biopsy, yrs | First histological diagnosis | Treatment before repeat biopsy | Repeat histological diagnosis | Time from the first biopsy, yrs | Treatment after repeat biopsy | Follow-up, yrs | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 6.5 | MCD | CNI, MMF | MCD | 2.6 | CNI | 10.4 | CKD2 |
| 2 | 3.8 | MCD | CNI, CNI+MMF, RTX | FSGS (CELL) | 2.5 | Prednisone only | 4.3 | ESRD |
| 3 | 4.3 | FSGS (NOS) | MMF, CNI, MMF+CNI | FSGS (NOS) | 4.8 | CNI | 6.6 | ESRD |
| 4 | 8.2 | MCD | CTX, CNI | MCD | 3.3 | MMF+RTX, CNI | 6.3 | CKD1 |
| 5 | 5.1 | MCD | CNI, CTX, MMF+CNI, MZR+CNI | FSGS (CELL) | 7.7 | RTX | 10.1 | ESRD |
| 6 | 2.1 | MCD | CTX, CNI | MCD | 2.6 | MMF | 3.6 | CKD1 |
| 7 | 6.0 | FSGS (NOS) | CNI | FSGS (NOS) | 5.4 | MMF | 10.4 | ESRD |
| 8 | 7.3 | MCD | CTX+CNI, CNI, MMF | FSGS (NOS) | 4.6 | CNI | 5.8 | CKD1 |
| 9 | 5.3 | FSGS (NOS) | CNI | MH | 2.8 | CNI | 8.4 | Clinical cure |
| 10 | 2.5 | MCD | CNI | MCD | 2.8 | CNI | 6.7 | CKD1 |
| 11 | 11.0 | MCD | CNI | FSGS (NOS) | 0.9 | CNI+MMF | 2.6 | ESRD |
| 12 | 5.2 | FSGS (CELL) | CTX, CNI, CNI+MMF | MCD | 7.3 | CNI+MMF, CNI | 12.0 | CKD1 |
| 13 | 2.2 | MCD | CNI, CNI+MMF | FSGS (NOS) | 5.3 | RTX, CNI | 8.4 | CKD1 |
| 14 | 2.0 | MH | MMF, CNI | FSGS (NOS) | 2.3 | CTX+CNI, CNI | 13.3 | CKD3 |
| 15 | 2.3 | MCD | CNI, CTX, MMF | FSGS (NOS) | 3.5 | MMF+RTX, CNI, ACTH | 6.0 | CKD3 |
| 16 | 4.4 | MCD | CNI | MCD | 5.8 | MMF+RTX | 9.5 | CKD1 |
| 17 | 6.0 | MCD | CTX, CNI | MCD | 2.6 | CNI | 4.5 | CKD1 |
ACTH, adrenocorticotropic hormone; CELL, cellular; CKD, chronic kidney disease; CNI, calcineurin inhibitors; CTX, cyclophosphamide; ESRD, end-stage renal disease; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MH, mesangial hypercellularity; MMF, mycophenolate mofetil; NOS, not otherwise specified; MZR, mizoribine; RTX, rituximab; SRNS, steroid-resistant nephrotic syndrome.
Discussion
In this largest-sample-ever study of children with secondary SRNS, we demonstrated that the remission rate in the first year after secondary SRNS onset and overall ESRD-free survival rate were favorable, and no response to IIS in the first year was the independent predictor for ESRD development. Our data also revealed the prognosis' heterogeneity of secondary SRNS, and changes in histological appearance to FSGS were associated with multidrug resistance in patients with repeat biopsy.
The 5-year and 10-year overall ESRD-free survival rates of children with secondary SRNS in this study were 90.9% and 85.8%, respectively. Previous studies focused on secondary SRNS used a relatively small sample size and did not provide the long-term renal survival rate data by survival analysis. We can see the apparent heterogeneity of renal outcome in the small sample size studies. Traninin et al.12 observed that 90% (9 of 10) of patients with secondary SRNS maintained normal renal function after a median follow-up time of 53 months, and Schwaderer et al.13 reported that all 14 patients held stable kidney function during the median observation period of 7.8 years. And Straatmann et al.14 described that 3 of 29 patients developed ESRD after a mean follow-up period of 85 ± 47 months. In contrast, Siegel et al.15 observed that all six patients developed renal insufficiency without reporting the specific follow-up period, and Srivastava et al.16 described that 4 of 12 patients developed renal insufficiency during the follow-up period of 1 to 16 years after secondary SRNS. Bierzynska et al.5 reported that 7 of 25 patients reached ESRD after a mean time of 4.71 years. We could not draw a conclusion from these studies. For previous cohort studies on the overall idiopathic SRNS mainly including initial SRNS, Niaudet et al.17 reports 65%, Trautmann et al.10 reports 74%, and Mekhalli et al.18 reports 75% overall renal survival rate at 5 years; these three studies report 50%, 58%, and 58% overall renal survival at 10 years, respectively. By contrast, secondary SRNS might be the group of SRNS who had a favorable renal prognosis.
Based on previous studies and our findings, we can infer the heterogeneity in the prognosis of secondary SRNS. However, studies focused on the prognostic factors of long outcomes of secondary SRNS are lacking. Straatmann et al.14 observed that the longer time to secondary steroid resistance tended to result in poor renal outcomes (P = 0.07), yet we did not see the trend in our patients (P = 0.91). In this study, no response to IIS in the first year was the only independent predictor for ESRD. In an international multicenter study about idiopathic SRNS, no response to IIS in the first year is also identified as one of the independent risk factors of ESRD.10
The CR and PR rates in the first year were 71.4% and 16.1%, respectively. In the early single-center studies, the remission rate was 88% to 100% with cyclophosphamide or/and CNI.12,13,16 Recently, the results of two multicenter studies involving secondary SRNS are not that much better, reporting the remission rate of 65% to 69%.8,14 In comparison, our data seem favorable. The differences in results among studies might be due to race, sample size, or choice of response criteria. CNI was the most effective IIS, and mycophenolate mofetil was the least effective. Likewise, Sinha et al.19 observed that mycophenolate mofetil was less effective in secondary SRNS than initial SRNS. Aside from this, the time to achieve CR with CNI was favorable in this study, indicating CNI for first-line treatment for secondary SRNS.
MCD was the predominant histology type in our study; this was in agreement with previous studies.4,14,16,20,21 On the other hand, for patients with initial SRNS, FSGS is the primary histology type.5,10,22 Our study showed that MCD at diagnosis was associated with significantly better treatment response than FSGS, which was consistent with the previous two studies in secondary SRNS,12,16 but not consistent with studies in initial SRNS or overall idiopathic SRNS.8,23,24 We could infer that secondary SRNS is a unique part of idiopathic SRNS.
The change of pathology from MCD or mesangial hypercellularity to FSGS when secondary SRNS onset was observed in this study and previous studies.9 To our knowledge, this is the first report of a patient with MCD who developed mesangial hypercellularity at the onset of secondary SRNS. This case supports the theory that increased mesangial cellularity is associated with reduced steroid responsiveness and that mesangial hypercellularity and MCD constitute the same spectrum of glomerular injury.25 In addition, repeated biopsies have shown for the first time the transitions from MCD and mesangial hypercellularity to FSGS before or after the appearance of multidrug resistance. These results reinforce the hypothesis that idiopathic FSGS represents an advanced disease progression stage that is less likely to respond to treatment than the earlier disease stage defined as MCD.26 Investigators suggest that a common etiology of MCD and FSGS exists.26 Moreover, the reclassification from FSGS to MCD and mesangial hypercellularity was also observed in two cases with favorable prognoses, which hint that the process of scarring may be controlled with treatment. Similarly, Watanabe et al.27 found the reassignment from FSGS to MCD in one-third of the children (3 of 9 patients) with initial SRNS which they suspected of having an immune-mediated etiology. In short, we observed the transformation among MCD, mesangial hypercellularity, and FSGS in the disease course in some patients, which had been seldom reported. Indeed, a common etiology might exist that deserves exploration. Importantly, changes in histological appearance to FSGS were associated with multidrug resistance, and 62.5% (5 of 8 patients) reached ESRD or CKD stage 3, which suggested that the severity of FSGS that evolved from MCD or mesangial hypercellularity after treatment may be not less than the original FSGS found at initial SRNS onset.10
This study has several limitations. First, it is a single-center study; therefore, the representation of the broader population may be limited. Second, because our study is retrospective, it is inevitable that data may be missing and there is possibility for information bias. Third, some cases of FSGS might be missed due to renal biopsy sampling error.
In conclusion, secondary SRNS was found to be a unique part of idiopathic SRNS in children; it had relatively favorable remission rates and long-term ESRD-free survival rates. The prognosis was heterogeneous, and no response to IIS in the first year was found to be the independent predictor for ESRD. MCD was the primary histology type at diagnosis and was associated with a higher remission rate. Repeat biopsies revealed the transformation among MCD, mesangial hypercellularity, and FSGS, especially regarding changes in histological appearance to FSGS that were associated with multidrug resistance.
Disclosure
The authors have nothing to disclose.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81970611) and the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2019A1515010694). Mrs. Ying, Mr. Liu, and Mrs. Chen contributed to the work equally.
References
- 1.Noone D.G., Iijima K., Parekh R. Idiopathic nephrotic syndrome in children. Lancet. 2018;392:61–74. doi: 10.1016/S0140-6736(18)30536-1. [DOI] [PubMed] [Google Scholar]
- 2.Trautmann A., Vivarelli M., Samuel S. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35:1529–1561. doi: 10.1007/s00467-020-04519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int. Suppl. 2012;2:139–274. [Google Scholar]
- 4.Kim J.S., Bellew C.A., Silverstein D.M. High incidence of initial and late steroid resistance in childhood nephrotic syndrome. Kidney Int. 2005;68:1275–1281. doi: 10.1111/j.1523-1755.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 5.Bierzynska A., McCarthy H.J., Soderquest K. Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int. 2017;91:937–947. doi: 10.1016/j.kint.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Sato M., Ishikura K., Ando T. Prognosis and acute complications at the first onset of idiopathic nephrotic syndrome in children: a nationwide survey in Japan (JP-SHINE study) Nephrol Dial Transplant. 2019:1–7. doi: 10.1093/ndt/gfz185. [DOI] [PubMed] [Google Scholar]
- 7.Ding W.Y., Koziell A., McCarthy H.J. Initial steroid sensitivity in children with steroid-resistant nephrotic syndrome predicts post-transplant recurrence. J Am Soc Nephrol. 2014;25:1342–1348. doi: 10.1681/ASN.2013080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason A.E., Sen E.S., Bierzynska A. Response to first course of intensified immunosuppression in genetically-stratified steroid resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2020;15:983–994. doi: 10.2215/CJN.13371019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akchurin O.M., Kaskel F.J. Late steroid resistance in childhood nephrotic syndrome: do we now know more than 40 years ago? Pediatr Nephrol. 2013;28:1157–1160. doi: 10.1007/s00467-013-2509-5. [DOI] [PubMed] [Google Scholar]
- 10.Trautmann A., Schnaidt S., Lipska-Ziętkiewicz B.S. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28:3055–3065. doi: 10.1681/ASN.2016101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz G.J., Brion L.P., Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 12.Trainin E.B., Boichis H., Spitzer A. Late nonresponsiveness to steroids in children with the nephrotic syndrome. J Pediatr. 1975;87:519–523. doi: 10.1016/s0022-3476(75)80812-2. [DOI] [PubMed] [Google Scholar]
- 13.Schwaderer P., Knüppel T., Konrad M. Clinical course and NPHS2 analysis in patients with late steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2008;23:251–256. doi: 10.1007/s00467-007-0653-5. [DOI] [PubMed] [Google Scholar]
- 14.Straatmann C., Ayoob R., Gbadegesin R. Treatment outcome of late steroid-resistant nephrotic syndrome: a study by the Midwest Pediatric Nephrology Consortium. Pediatr Nephrol. 2013;28:1235–1241. doi: 10.1007/s00467-013-2483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel N.J., Goldberg B., Krassner L.S. Long-term follow-up of children with steroid-responsive nephrotic syndrome. J Pediatr. 1972;81:251–258. doi: 10.1016/s0022-3476(72)80291-9. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava R.N., Agarwal R.K., Moudgil A. Late resistance to corticosteroids nephrotic syndrome. J Pediatr. 1985;108:66–70. doi: 10.1016/s0022-3476(86)80770-3. [DOI] [PubMed] [Google Scholar]
- 17.Niaudet P., Boyer O. Idiopathic Nephrotic Syndrome in Children: Clinical Aspects. In: Avner E.D., Harmon W.E., editors. Vol 1. Springer-Verlag Company; Berlin/Heidelberg, Germany: 2016. pp. 839–882. (Pediatric Nephrology). [Google Scholar]
- 18.Mekahli D., Liutkus A., Ranchin B. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: a multicenter study. Pediatr Nephrol. 2009;24:1525–1532. doi: 10.1007/s00467-009-1138-5. [DOI] [PubMed] [Google Scholar]
- 19.Sinha A., Gupta A., Kalaivani M. Mycophenolate mofetil is inferior to tacrolimus in sustaining remission in children with idiopathic steroid-resistant nephrotic syndrome. Kidney Int. 2017;92:248–257. doi: 10.1016/j.kint.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Dai Y., Yang H., Gao P. NPHS2 variation in Chinese southern infants with late steroid-resistant nephrotic syndrome. Ren Fail. 2014;36:1395–1398. doi: 10.3109/0886022X.2014.947515. [DOI] [PubMed] [Google Scholar]
- 21.Bagga A., Sinha A. Individualizing treatment of steroid-resistant nephrotic syndrome: registries to the fore. Clin J Am Soc Nephrol. 2020;15:920–922. doi: 10.2215/CJN.08080520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tullus K., Webb H., Bagga A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child Adolescent Health. 2018;2:880–890. doi: 10.1016/S2352-4642(18)30283-9. [DOI] [PubMed] [Google Scholar]
- 23.Liu I.D., Willis N.S., Craig J.C. Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database Syst Rev. 2019;2019:CD003594. doi: 10.1002/14651858.CD003594.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trautmann A., Bodria M., Ozaltin F. Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol. 2015;10:592–600. doi: 10.2215/CJN.06260614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20:765–771. doi: 10.1038/ki.1981.209. [DOI] [PubMed] [Google Scholar]
- 26.Maas R.J., Deegens J.K., Smeets B. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12:768–776. doi: 10.1038/nrneph.2016.147. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe Y., Fujinaga S., Endo A. Baseline characteristics and long-term outcomes of steroid-resistant nephrotic syndrome in children: impact of initial kidney histology. Pediatr Nephrol. 2020;35:2377–2381. doi: 10.1007/s00467-020-04760-8. [DOI] [PubMed] [Google Scholar]