Abstract
Introduction
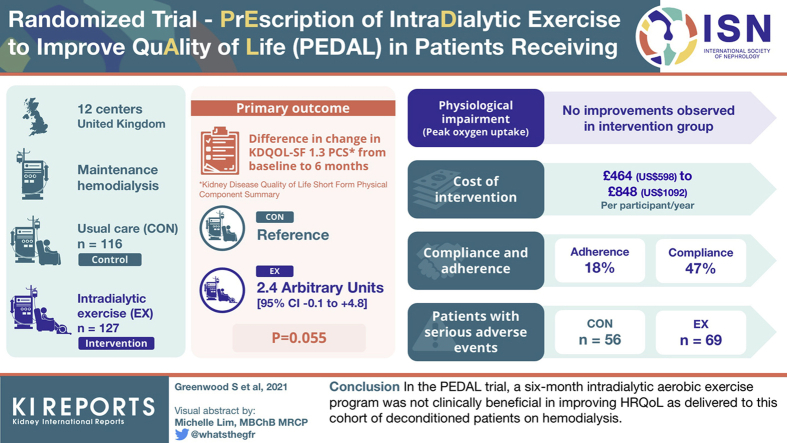
Whether clinically implementable exercise interventions in people receiving hemodialysis (HD) therapy improve health-related quality of life (HRQoL) remains unknown. The PrEscription of intraDialytic exercise to improve quAlity of Life (PEDAL) study evaluated the clinical benefit and cost-effectiveness of a 6-month intradialytic exercise program.
Methods
In a multicenter, single-blinded, randomized, controlled trial, people receiving HD were randomly assigned to (i) intradialytic exercise training (exercise intervention group [EX]) and (ii) usual care (control group [CON]). Primary outcome was change in Kidney Disease Quality of Life Short-Form Physical Component Summary (KDQOL-SF 1.3 PCS) from baseline to 6 months. Cost-effectiveness was determined using health economic analysis; physiological impairment was evaluated by peak oxygen uptake; and harms were recorded.
Results
We randomized 379 participants; 335 and 243 patients (EX n = 127; CON n = 116) completed baseline and 6-month assessments, respectively. Mean difference in change PCS from baseline to 6 months between EX and CON was 2.4 (95% confidence interval [CI]: −0.1 to 4.8) arbitrary units (P = 0.055); no improvements were observed in peak oxygen uptake or secondary outcome measures. Participants in the intervention group had poor compliance (47%) and poor adherence (18%) to the exercise prescription. Cost of delivering intervention ranged from US$598 to US$1092 per participant per year. The number of participants with harms was similar between EX (n = 69) and CON (n = 56). A primary limitation was the lack of an attention CON. Many patients also withdrew from the study or were too unwell to complete all physiological outcome assessments.
Conclusions
A 6-month intradialytic aerobic exercise program was not clinically beneficial in improving HRQoL as delivered to this cohort of deconditioned patients on HD.
Keywords: chronic kidney disease, physical activity, physical function, rehabilitation
Graphical abstract
Introduction
Improved HD techniques and management of coexisting disease has improved the average life expectancy of patients receiving HD therapy globally, but disability and associated symptoms remain highly prevalent accounting for more life years lost to disability.1 In the UK, 48% of the HD population report severe functional dependencies,2 which impact on HRQoL.3 Components of HRQoL, particularly the domain of physical functioning, stand out as the strongest predictor of survival, hospitalizations, and morbidity.4 Knight et al.5 and Lowrie et al.6 report multiple symptoms that affect the physical component of HRQoL.7 Moreover, higher levels of physical activity are associated with better scores in HRQoL measures, physical functioning, depression, and burden of kidney disease symptoms.8
The physical component of HRQoL, therefore, may be targeted with interventions to enhance physical activity. In patients receiving HD therapy, systematic reviews indicate that a range of exercise training interventions improve physical function and alleviate disability symptoms.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 9 Of particular interest are studies investigating intradialytic exercise, as the environment of unit-based HD provides a platform for longer-term sustainable implementation of exercise rehabilitation programs.23 The pre-existing need for patients to attend for standard thrice weekly, 4 hour-long HD sessions provides an opportunity to deliver a structured and supervised rehabilitation program with reduced patient burden regarding time, effort, and travel costs.24,25 Thus, physical activity behaviors could be promoted using an implementation model that integrates physical activity into the main health care system for patients receiving HD therapy.
Nevertheless, very few dialysis units have chosen to implement this physical rehabilitation option in the UK. A barrier to implementation has been a lack of high-quality, adequately powered randomized controlled trials of intradialytic exercise with patient-reported outcomes (HRQoL), health economics (cost-effectiveness), and harms (serious adverse events [SAEs]) as the primary outcomes. Thus, the balance of benefits to costs and harms has been impossible to evaluate. Consequently, the PrEscription of intraDialytic exercise to improve quAlity of Life (PEDAL) trial was commissioned by the National Institute for Health Research to evaluate whether intradialytic exercise was able to improve HRQoL in patients receiving HD therapy. The primary objective was to determine, in stage 5 chronic kidney disease (CKD) patients receiving maintenance HD, whether usual care augmented by intradialytic exercise training for a period of 6 months improved KDQOL-SF 1.3 PCS.
Methods
Trial Design and Oversight
We conducted this pragmatic prospective randomized controlled trial in 5 regions (London, Scotland, Wales, North-West England, and Midlands), across a total of 12 HD units, in the UK. The trial recruited prevalent patients with stage 5 dimensions of CKD receiving HD therapy. Briefly, the intervention consisted of using a modified cycle ergometer to perform aerobic exercise in a semirecumbent position, 3 times per week during the first 2 hours of HD. Twice per week, after the aerobic cycling exercise, participants completed lower extremity muscular conditioning exercises. These included 3 sets of 10 to 15 repetitions of dynamic resistance exercises for all major muscle groups. All exercises were performed against body weight before progression with ankle weights and TheraBands (Akron, OH). The exercise program was delivered and supervised by physiotherapy assistants.
London Fulham Research Ethics Committee approved the protocol (14/LO/1851), and all the participants provided written informed consent. The study was registered prospectively (ISRCTN N83508514). The trial protocol and details on inclusion/exclusion criteria, randomization procedure, and exercise intervention and prescription have been described elsewhere.26 The Consolidated Standards of Reporting Trials Extension for Patient Report Outcomes also suggested reporting all the multi-item scales from the KDQOL-SF instrument.
Primary Outcome
The primary outcome for this study was the change in KDQOL-SF 1.3 PCS from baseline to 6 months.27 The KDQOL-SF 1.3 instrument was chosen because of its validity in patients with CKD and inclusion of a generic core that has been widely used in CKD and other populations. The KDQOL-SF 1.3 is a disease-specific QoL measure that includes 43 kidney disease–targeted items and 36 items providing a generic core and an overall health-rating item. The questionnaire was completed by patients using pen and paper, with queries answered by research officers blinded to treatment allocation. Scoring followed currently recommended methods.28 Thus, the PCS score can be interpreted as follows: a score above or below 50 is above or below the average, respectively, in the US general population, whereas a 1-point difference in the score is one-tenth of a SD. Analysis of within-trial change in the KDQOL-SF 1.3 PCS score from baseline, adjusted for baseline levels and randomization minimization variables, suggested that the study had 80% power to detect a 4-point difference with only 87 participants per group (with complete data at baseline and 6-month follow-up).
Secondary Outcomes
HRQoL, Cost-Effectiveness, and Harms
From the KDQOL-SF 1.3, the multi-item scale of energy/fatigue and the kidney disease–targeted items (burden of kidney disease) were presented as prespecified. In addition, the remaining 7 multi-item scales were presented. Then, a generic preference-based measure of HRQoL was obtained using the EuroQol 5-dimension descriptive system (EQ-5D-5L).29 The EQ-5D-5L comprises the following 5 dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EQ—visual analog scale was also obtained, whereby participants reported their self-rated evaluation of their health state on a 0 to 100 visual analog scale. Costs of delivering the PEDAL intervention were calculated, including exercise equipment, assumed to cost £1000 with a lifetime of 10 years and maintenance costs of £50 per year. Staff costs were assumed to include one ×0.6 full-time equivalent physiotherapy assistant (mid band 4 Agenda for Change scale, annual employer costs from £25,866 outside London to £34,787 in London) per 12 to 20 participants (to reflect different geographic spacing of kidney units in rural and urban areas) and one ×1.0 full-time equivalent supervisor (mid band 8 Agenda for Change, annual employer costs from £55,078.00 outside London to £71,418.96 in London) per 80 participants.
Physical Function
Upper limits of exercise tolerance were assessed by peak oxygen uptake determined by an incremental cycling protocol.26 Physical function limitations were assessed by the sit-to-stand-6030 and gait speed in 10 m.31 Physical activity behaviors were captured by the International Physical Activity Questionnaire Short-Form32; ability to undertake activities of daily livings was recorded by the Duke Activity Status Index33; and fear of falling was assessed by the Tinetti Falls Efficacy Scale.34
Cardiovascular Risk and Clinical Measures
Arterial stiffness was assessed by the carotid–femoral pulse wave velocity,35 measured using the Vicorder system (Skidmore Industries, UK) and by following the current recommendations.35 Measures of body mass index and waist circumference were also recorded. Clinical data included cause of kidney disease, comorbidities, routine clinical blood tests (hemoglobin, serum phosphate, and parathyroid hormone), and medications (including erythropoiesis-stimulating agents).
Harms
Harms were actively recorded in both groups by the physiotherapy assistants from baseline to the end of the 6-month follow-up period (n = 335). Relationship to the intervention was evaluated by the lead clinician at each center, who was not blinded to treatment allocation. SAEs were reviewed by a data safety monitoring committee; rules for stopping the trial were that the committee identified a marked increase in expected or unexpected SAEs owing to the testing or intervention procedures. Data on hospitalizations and deaths (all-cause mortality and cardiovascular mortality) were collected by reviews of clinical databases and records at each study visit.
Compliance and Adherence (Fidelity) to Exercise Prescription
General compliance was recorded as the percentage of exercise sessions completed out of the total prescribed for the 6-month follow-up period. Adherence (fidelity) was recorded as the percentage of patients who adhered exactly to the prescribed exercise (cycling and muscle conditioning exercises) at the prescribed intensity and cycling time duration for each session across the 6 months. In addition, the percentage of patients who temporarily (>2 weeks) paused exercise was noted. These data were recorded by physiotherapy assistants through completion of sessional exercise diaries.
Statistical Analyses
The primary outcome measure (change from baseline to 6 months in KDQOL-SF 1.3 PCS) was compared between the control and intervention groups using a normal linear model adjusting for baseline KDQOL-SF 1.3 PCS and the randomization minimization variables (age, gender, diabetes status). The findings are presented as the adjusted mean difference (95% CI) between the treatment groups. Significance was set at P ≤ 0.05. The main analysis was carried out on research participants with PCS assessments at baseline and 6 months. A total of 2 sensitivity analyses were also carried out, first imputing a score of 0 for those who died before 6 months and second based on all participants with a baseline PCS using the method of multiple imputation. As results were consistent between methods, only the main analysis is reported herein.
Secondary continuous outcomes were analyzed as for the primary outcome. For health economic data, we estimated the mean between-group difference in costs of the intervention and the mean between-group difference in quality-adjusted life years accrued by participants during the study, estimated as the area under the health utility curve from study entry (i.e., randomized and attended baseline visit) to follow-up (6 months after). Costs in the CON were set to 0. Estimated between-group differences in cost and quality-adjusted life years were obtained by the method of recycled prediction in 5000 bootstrap samples. The distribution of these quantities was summarized and presented graphically in the incremental cost effectiveness plane. Time-to-event outcomes (cardiovascular and all-cause mortality) were calculated as time from randomization and were compared between treatment groups using Cox proportional hazard regression models. The results are reported as the adjusted hazard ratio for intervention versus control (95% CI). Data involving counts of events (hospitalizations) were compared between treatment groups using negative binomial regression models adjusting for length of follow-up. The results are reported as adjusted rate ratio (95% CI). Harms (SAEs) were tabulated by system organ class and body system using the Medical Dictionary for Regulatory Activities Terminology.36 Recurrent events were counted separately. Compliance and adherence data were tabulated and presented visually.
Results
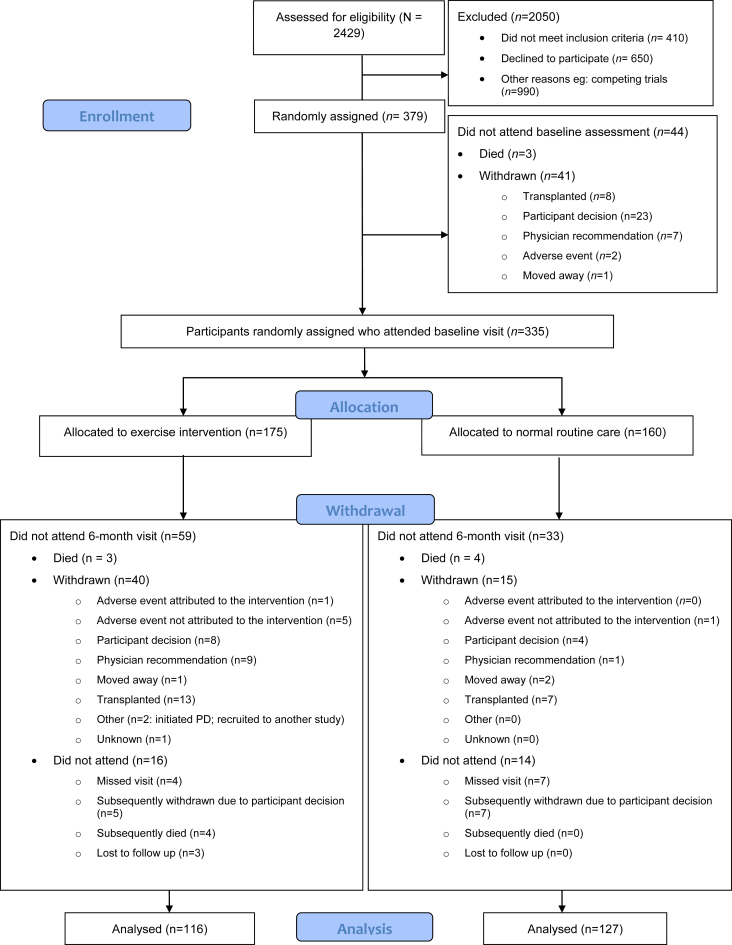
Patient flow, including recruitment to and retention in the trial, is detailed in Figure 1. A total of 2409 patients were screened for eligibility. Nevertheless, 410 were not eligible per inclusion criteria, 660 patients declined to participate, and 990 patients were not eligible to participate owing to competing trials in this same population within the UK. A total of 335 participants attended a baseline study visit, 175 patients who were randomized to EX and 160 participants to CON. The primary outcome was known for 243 participants (73%) who attended a baseline visit, 116 participants (66%) in the exercise group, and 127 participants (79%) in the usual care group. More patients withdrew from EX (40; 34.5%) than CON (15; 11.8%) owing to participant decision, physician recommendation because of medical concerns, and transplantation. Apart from an increased number of smokers in the group of patients who withdrew from EX, no obvious differences in characteristics of the withdrawn and not withdrawn groups were present (Table 1).
Figure 1.
CONSORT diagram of the flow of patients across the various phases of the trial. CONSORT, Consolidated Standards of Reporting Trials; PD, progression disease.
Table 1.
Baseline characteristics of all patients in the trial, stratified by group, and according to withdrawal from the trial
| Baseline characteristic | CON, not withdrawn |
EX, not withdrawn |
CON, withdrawn |
EX, withdrawn |
||||
|---|---|---|---|---|---|---|---|---|
| N | Summary | N | Summary | N | Summary | N | Summary | |
| Age | ||||||||
| Mean (SD) | 145 | 59.8 (14.1) | 135 | 60.5 (15.0) | 15 | 52.8 (19.9) | 40 | 56.8 (13.3) |
| Median (Q1, Q3) | 59.7 (50.5, 71.0) | 62.1 (47.9, 72.9) | 56.1 (34.7, 61.2) | 56.3 (49.6, 64.3) | ||||
| Gender | ||||||||
| n (%) Female | 145 | 55 (38) | 135 | 56 (42) | 15 | 4 (27) | 40 | 11 (28) |
| Ethnicity | ||||||||
| n (%) White | 145 | 67 (46) | 135 | 73 (54) | 15 | 10 (67) | 40 | 19 (48) |
| n (%) Black Caribbean | 26 (18) | 17 (13) | 1 (7) | 3 (8) | ||||
| n (%) Black African | 33 (23) | 24 (18) | 1 (7) | 10 (25) | ||||
| n (%) South Asian | 15 (10) | 16 (129) | 2 (13) | 6 (15) | ||||
| n (%) Chinese | 1 (1) | 1 (1) | 0 (0) | 0 (0) | ||||
| n (%) Othera | 3 (2) | 4 (3) | 1 (7) | 2 (5) | ||||
| Weight (kg) | ||||||||
| Mean (SD) | 143 | 80.8 (20.5) | 135 | 79.2 (18.8) | 15 | 82.5 (13.8) | 40 | 82.8 (24.8) |
| Median (Q1, Q3) | 77.0 (66.1, 92.2) | 76.4 (65.4, 90.8) | 83.0 (67.5, 91.5) | 78.5 (67.4, 90.7) | ||||
| BMI (kg/m2) | ||||||||
| Mean (SD) | 143 | 28.8 (6.5) | 135 | 28.5 (6.5) | 15 | 28.8 (5.5) | 40 | 29.2 (8.8) |
| Median (Q1, Q3) | 28.0 (24.5, 32.0) | 27.0 (23.8, 32.2) | 27.8 (24.2, 32.4) | 27.6 (22.3, 32.6) | ||||
| Smoking | ||||||||
| n (%) Current | 145 | 19 (13.1) | 135 | 18 (13.3) | 15 | 0 (0.0) | 40 | 5 (12.5) |
| n (%) Former | 45 (31.0) | 39 (28.9) | 4 (26.7) | 10 (25.0) | ||||
| n (%) Never | 81 (55.9) | 78 (57.8) | 11 (73.3) | 25 (62.5) | ||||
| SBP (mm Hg) | ||||||||
| Mean (SD) | 142 | 138.6 (23.4) | 135 | 134.4 (21.3) | 15 | 133.9 (22.6) | 40 | 134.1 (17.5) |
| Median (Q1, Q3) | 138.0 (121.8, 153.9) | 133.7 (121.3, 147.5) | 130.0 (115.0, 152.2) | 131.5 (121.0, 142.8) | ||||
| DBP (mm Hg) | ||||||||
| Mean (SD) | 142 | 73.4 (13.7) | 135 | 72.6 (15.4) | 15 | 75.5 (15.4) | 40 | 76.9 (10.0) |
| Median (Q1, Q3) | 73.3 (63.2, 81.7) | 71.3 (61.3, 82.7) | 74.0 (67.0, 80.7) | 76.8 (70.8, 81.5) | ||||
| Peripheral vascular disease | ||||||||
| n (%) Yes | 145 | 6 (4.1) | 135 | 5 (3.7) | 15 | 0 (0.0) | 40 | 0 (0.0) |
| Diabetes | ||||||||
| n (%) Yes | 145 | 59 (40.7) | 135 | 52 (38.5) | 15 | 6 (40.0) | 40 | 15 (37.5) |
| Hypertension | ||||||||
| n (%) Yes | 145 | 116 (80.0) | 135 | 101 (74.8) | 15 | 11 (73.3) | 40 | 33 (82.5) |
| Hyperlipidemia | ||||||||
| n (%) Yes | 145 | 39 (26.9) | 135 | 23 (17.0) | 15 | 4 (26.7) | 40 | 5 (12.5) |
| Previous MI | ||||||||
| n (%) Yes | 145 | 21 (14.5) | 135 | 14 (10.4) | 15 | 0 (0.0) | 40 | 6 (15.0) |
| Heart failure | ||||||||
| n (%) Yes | 145 | 17 (11.7) | 135 | 14 (10.4) | 15 | 0 (0.0) | 40 | 1 (2.5) |
| Cerebrovascular events | ||||||||
| n (%) Yes | 145 | 17 (11.7) | 135 | 8 (5.9) | 15 | 1 (6.7) | 40 | 0 (0.0) |
| Cardiovascular | ||||||||
| n (%) Yes | 145 | 25 (17.2) | 135 | 30 (22.2) | 15 | 2 (13.3) | 40 | 12 (30.0) |
| Musculoskeletal and orthopedic condition | ||||||||
| n (%) Yes | 145 | 19 (13.1) | 135 | 16 (11.9) | 15 | 1 (6.7) | 40 | 7 (17.5) |
| Hb | ||||||||
| Mean (SD) | 141 | 110.2 (12.1) | 127 | 109.8 (14.1) | 15 | 118.1 (14.2) | 37 | 108.9 (15.8) |
| Median (Q1, Q3) | 109.0 (103.0, 119.0) | 110.0 (102.0, 118.5) | 115.0 (109.0, 124.0) | 110.0 (100.0, 120.0) | ||||
| CRP (mg/l) | ||||||||
| Mean (SD) | 139 | 15.3 (21.1) | 125 | 11.9 (15.9) | 15 | 12.5 (16.4) | 36 | 21.1 (26.6) |
| Median (Q1, Q3) | 6.6 (3.1, 18.1) | 6.0 (3.0, 14.1) | 8.0 (4.5, 11.0) | 10.9 (4.3, 28.1) | ||||
| Dialysis efficiency (%) | ||||||||
| Mean (SD) | 141 | 71.2 (8.4) | 125 | 71.9 (7.3) | 15 | 71.0 (11.3) | 37 | 71.6 (7.9) |
| Median (Q1, Q3) | 72.0 (66.0, 77.0) | 73.0 (69.0, 76.5) | 74.0 (68.0, 77.8) | 71.8 (66.0, 77.0) | ||||
BMI, body mass index; CON, control group; CRP, C-reactive protein; DBP, diastolic blood pressure; EX, exercise intervention group; Hb, hemoglobin; MI, myocardial infraction; Q, quartile; SBP, systolic blood pressure.
Continuous variables are revealed as mean (SD) and median (Q1, Q3).
Indian, Pakistani, and Bangladeshi.
Effect of Intradialytic Exercise Training on HRQoL
For the primary outcome, the mean difference in the change in PCS from baseline to 6 months between EX and CON was 2.4 (95% CI: −0.1 to 4.8) arbitrary unit and was not statistically significant (P = 0.055). Similarly, other measures of HRQoL (energy/fatigue, burden of kidney disease, EQ-5D-5L, and EQ-5D visual analog scale: Table 2; the remaining 7 multi-item scales from the KDQOL-SF: Supplementary Table S1) were all unchanged by the intervention.
Table 2.
Response of quality of life to the PEDAL intervention, as assessed by KDQOL-SF 1.3 and EQ-5D-5L questionnaires
| Outcome measure | na | Baseline | Month 6 | Adjusted mean difference in change between EX and CON groupsb | P valuec |
|---|---|---|---|---|---|
| Primary outcome | |||||
| KDQOL-SF 1.3 PCS (AU) | |||||
| CON | 120 | 32.9 (11.3) | 31.8 (11.3) | 2.4 (−0.1 to 4.8) | 0.06 |
| EX | 114 | 33.8 (10.6) | 34.8 (11.6) | ||
| Secondary outcomes | |||||
| KDQOL-SF 1.3 Energy/fatigue (AU) | |||||
| CON | 122 | 39.8 (26.0) | 41.4 (24.9) | 0.1 (−5.6 to 5.8) | 0.97 |
| EX | 114 | 40.3 (27.2) | 41.4 (26.4) | ||
| KDQOL-SF 1.3 burden of kidney disease (AU) | |||||
| CON | 122 | 36.0 (28.6) | 37.3 (29.7) | −1.4 (−7.0 to 4.1) | 0.61 |
| EX | 113 | 37.3 (27.7) | 36.9 (29.0) | ||
| EQ-5D-5L health utility score (AU) | |||||
| CON | 121 | 0.69 (0.25) | 0.68 (0.26) | 0.01 (−0.04 to 0.07) | 0.69 |
| EX | 111 | 0.71 (0.22) | 0.70 (0.25) | ||
| EQ-5D visual analog scale (0–100 scale) | |||||
| CON | 121 | 59.4 (22.7) | 59.3 (20.9) | 3.5 (−1.0 to 8.1) | 0.13 |
| EX | 111 | 60.7 (22.2) | 63.7 (19.3) |
AU, arbitrary unit; CON, control group; EQ-5D, EuroQol 5-dimension descriptive system; EX, exercise intervention group; KDQOL-SF 1.3, Kidney Disease Quality of Life Short-Form; PCS, physical component summary; PEDAL, PrEscription of intraDialytic exercise to improve quAlity of Life.
Data are mean (SD) or mean (95% confidence interval). CON—usual care maintenance hemodialysis. EX—intradialytic exercise training plus usual care maintenance hemodialysis.
Number of participants with baseline and 6-month data available.
Adjusting for baseline data and the randomization minimization variables (age, gender, diabetes status).
Comparison between the control and intervention groups using a normal linear model.
Cost-Effectiveness
The mean (SD) of the area under the EQ-5D-5L curve was 0.665 (0.248) in the CON and 0.653 (0.269) in the intervention group. The mean difference between treatment and intervention groups obtained using the method of recycled predictions was −0.012 (95% CI: −0.069 to 0.043), suggesting no difference in QoL between the intervention and CONs (Figure 2 for an example analysis calculated using a low staff-to-patient ratio, outside London). No significant subgroup effects were found for age, sex, or diabetes at baseline.
Figure 2.
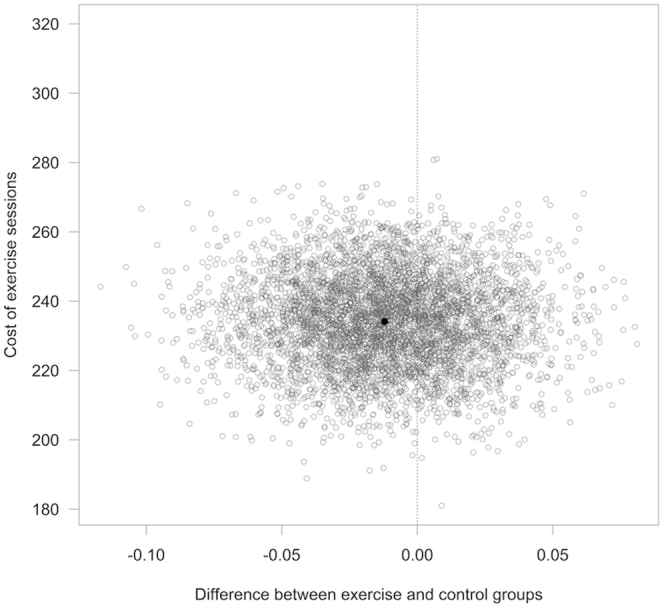
Cost-effectiveness: estimated differences in cost and QALYs on the ICER plane for a low staff-to-patient ratio, outside London (5000 bootstrap samples). ICER, incremental cost effectiveness ratio; QALY, quality-adjusted life year.
Costs from different sources under different scenarios for staff costs are found in Table 3. Average total costs per patient in 6 months range from £232 (US$299) (95% CI: £204–£259) to £424 (US$546) (95% CI: £374–£474), depending on location and staff to patient ratio. The main cost factor was the staff cost for delivering the exercise sessions.
Table 3.
Costs per patient to deliver the PEDAL intervention in the 6-month follow-up period
| Cost source | Outside London |
London |
||
|---|---|---|---|---|
| Low staff-to-patient ratio | High staff-to-patient ratio | Low staff-to-patient ratio | High staff-to-patient ratio | |
| Equipment purchasing and maintenance (£) | 9 (8–10) | 9 (8–10) | 9 (8–10) | 9 (8–10) |
| Staff delivering exercise sessions (£) | 204 (180–228) | 341 (300–381) | 237 (209–265) | 395 (348–441) |
| Training and oversight (£) | 18 (16–20) | 18 (16–20) | 20 (18–23) | 20 (18–23) |
| Total cost per patient in 6 months (£) | 232 (204–259) | 368 (324–412) | 266 (235–298) | 424 (374–474) |
| Estimated difference in cost (recycled predictions) (£) | 234 (209–260) | 372 (331–414) | 269 (240–299) | 428 (380–476) |
PEDAL, PrEscription of intraDialytic exercise to improve quAlity of Life.
Data are mean (95% confidence interval). Estimated differences in cost obtained by the method of recycled prediction in 5000 bootstrap samples, setting cost in the control group to 0, adjusted for age, sex, and diabetes at baseline.
Effect of Intradialytic Exercise Training on Secondary Outcomes
Consistent with the lack of change in HRQoL, there were no statistically significant or absolute changes in physical function outcomes (Table 4), cardiovascular risk (arterial stiffness: Table 4), or clinical measures (routine clinical blood tests and medications: data not found). Although mortality was not influenced by the intervention, the number of hospitalizations tended to be higher in the EX group (Table 5). This trend was driven by 11 patients in the EX group who were each hospitalized more than 4 times during the trial for reasons deemed unlikely to be related to the intervention (e.g., fistula issues); in contrast, only 2 patients in the CON group were hospitalized more than 4 times.
Table 4.
Response of secondary outcome measures to the PEDAL intervention
| Outcome measure | na | Baseline | Month 6 | Adjusted mean difference in change between EX and CON groupsb | P valuec |
|---|---|---|---|---|---|
| Peak aerobic capacity (VO2 peak, l/min) | |||||
| CON | 68 | 0.97 (0.38) | 0.96 (0.37) | 0.05 (−0.03 to 0.12) | 0.22 |
| EX | 75 | 0.95 (0.42) | 0.98 (0.43) | ||
| Peak aerobic capacity (VO2 peak, ml/min/kg) | |||||
| CON | 68 | 11.9 (4.5) | 11.8 (4.2) | 0.75 (−0.20 to 1.71) | 0.12 |
| EX | 74 | 11.8 (5.3) | 12.4 (5.7) | ||
| Arterial stiffness by pulse wave velocity (ms)22 | |||||
| CON | 78 | 8.10 (6.78, 9.29) | 7.78 (6.97, 9.13) | 1.01 (0.97–1.06) | 0.54 |
| EX | 78 | 7.92 (6.62, 9.09) | 7.88 (6.98, 9.27) | ||
| DASI (AU) | |||||
| CON | 121 | 23.1 (13.1) | 22.7 (13.4) | 0.35 (−2.23 to 2.93) | 0.79 |
| EX | 112 | 24.9 (13.3) | 24.1 (14.3) | ||
| IPAQ total physical activity (MET, min/wk) [ln(x + 10)] | |||||
| CON | 118 | 423.8 (39.0, 1465.4) | 353.2 (46.1, 1033.1) | 1.36 (0.84–2.21) | 0.21 |
| EX | 106 | 709.5 (153.8, 2515.1) | 591.0 (111.8, 1793.2) | ||
| Gait speed in 10 m (m/s) | |||||
| CON | 84 | 0.86 (0.30) | 0.87 (0.29) | 0.01 (−0.04 to 0.06) | 0.73 |
| EX | 79 | 0.94 (0.29) | 0.94 (0.30) | ||
| Sit-to-stand 60 s (no. of repetitions) | |||||
| CON | 87 | 13.8 (6.6) | 14.4 (7.0) | 1.02 (−0.42 to 2.47) | 0.16 |
| EX | 82 | 15.8 (7.1) | 17.1 (8.1) | ||
| Tinetti Falls Efficacy Scale (AU) [ln(x)] | |||||
| CON | 122 | 22.5 (10.2, 46.8) | 24.5 (11.0, 50.0) | 0.94 (0.80–1.12) | 0.49 |
| EX | 112 | 23.0 (11.8, 49.2) | 24.5 (11.0, 46.2) |
AU, arbitrary units; CON, control group; DASI, Duke Activity Status Index; EX, exercise intervention group; IPAQ, International Physical Activity Questionnaire; MET, metabolic equivalent task; PEDAL, PrEscription of intraDialytic exercise to improve quAlity of Life; VO2, maximum rate of oxygen consumption.
Data are mean (SD), median (IQR), or mean (95% confidence interval); some variables were transformed to enhance model fit: transformations are given in [brackets]. CON—usual care maintenance hemodialysis; EX—intradialytic exercise training plus usual care maintenance hemodialysis.
Number of participants with baseline and 6-month data available.
Adjusting for baseline data and the randomization minimization variables (age, gender, diabetes status): note variables analyzed as log-transformed values are given as ratios.
Comparison between the control and intervention groups using a normal linear model.
Table 5.
Number of hospitalizations and mortality during the PEDAL trial
| Variable | na | No. of hospitalizations (hospitalization rate per person year) |
bIncident rate ratio (95% confidence interval) |
P valuec |
|---|---|---|---|---|
| No. of hospitalizations | ||||
| CON | 160 | 84 (0.54) | 1.39 (0.93–2.08) | 0.109 |
| EX | 175 | 132 (0.85) |
| No. of events (event rate per 100 person years) |
dHazard ratio (95% confidence interval) |
P value | ||
|---|---|---|---|---|
| All-cause mortality | ||||
| CON | 160 | 9 (5.8) | 1.19 (0.48–2.94) | 0.71 |
| EX | 174 | 10 (6.5) | ||
| Cardiovascular mortality | ||||
| CON | 160 | 3 (1.9) | N/A | N/A |
| EX | 174 | 2 (1.3) |
CON, control group; EX, exercise intervention group; N/A, not applicable; PEDAL, PrEscription of intraDialytic exercise to improve quAlity of Life.
CON—usual care maintenance hemodialysis. EX—intradialytic exercise training plus usual care maintenance hemodialysis. N/A—as numbers too small to analyze.
Number of participants with baseline and 6-month data available.
Incident rate ratios have been calculated in negative binomial regression predicting number of hospitalizations from treatment, adjusting for age, sex, and diabetes at baseline.
For all-cause mortality, survival was adjusted for age, sex, and diabetes at baseline; for cardiovascular mortality, survival was adjusted for age and diabetes at baseline.
Hazard ratios have been calculated in Cox proportional hazard regression models predicting survival from treatment.
Harms
There was no noticeable increase in SAEs in the exercise group (Table 6). Nevertheless, there was 1 noticeable SAE: an individual with type 1 diabetes and autonomic neuropathy experienced severe episodes of symptomatic hypotension that were possibly exacerbated by the intervention. The participant was withdrawn.
Table 6.
Number of patients with at least 1 SAE by MedDRA system organ class during the PEDAL trial
| Variable | All, n (%) | CON, n (%) | EX, n (%) |
|---|---|---|---|
| Number of randomized patients who attended baseline visit | 335 | 160 | 175 |
| Number of patients with any event | 125 | 56 (35.0) | 69 (39.4) |
| Blood and lymphatic system disorders | 2 (0.6) | 0 (0.0) | 2 (1.1) |
| Cardiac disorders | 15 (4.5) | 6 (3.8) | 9 (5.1) |
| Congenital, familial, and genetic disorders | 1 (0.3) | 1 (0.6) | 0 (0.0) |
| Gastrointestinal disorders | 14 (4.2) | 4 (2.5) | 10 (5.7) |
| General disorders and administration site conditions | 17 (5.1) | 12 (7.5) | 5 (2.9) |
| Hepatobiliary disorders | 3 (0.9) | 1 (0.6) | 2 (1.1) |
| Infections and infestations | 47 (14.0) | 18 (11.2) | 29 (16.6) |
| Injury, poisoning, and procedural complications | 28 (8.4) | 12 (7.5) | 16 (9.1) |
| Investigations | 5 (1.5) | 4 (2.5) | 1 (0.6) |
| Metabolism and nutrition disorders | 17 (5.1) | 4 (2.5) | 13 (7.4) |
| Musculoskeletal and connective tissue disorders | 4 (1.2) | 1 (0.6) | 3 (1.7) |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 (0.3) | 0 (0.0) | 1 (0.6) |
| Nervous system disorders | 8 (2.4) | 3 (1.9) | 5 (2.9) |
| Psychiatric disorders | 4 (1.2) | 1 (0.6) | 3 (1.7) |
| Renal and urinary disorders | 1 (0.3) | 1 (0.6) | 0 (0.0) |
| Reproductive system and breast disorders | 2 (0.6) | 1 (0.6) | 1 (0.6) |
| Respiratory, thoracic and mediastinal disorders | 13 (3.9) | 3 (1.9) | 10 (5.7) |
| Skin and subcutaneous tissue disorders | 1 (0.3) | 0 (0.0) | 1 (0.6) |
| Social circumstances | 1 (0.3) | 1 (0.6) | 0 (0.0) |
| Surgical and medical procedures | 37 (11.0) | 13 (8.1) | 24 (13.7) |
| Vascular disorders | 10 (3.0) | 6 (3.8) | 4 (2.3) |
CON, control group; EX, exercise intervention group; MedDRA, Medical Dictionary for Regulatory Activities; PEDAL, PrEscription of intraDialytic exercise to improve quAlity of Life; SAE, serious adverse event.
CON—usual care maintenance hemodialysis. EX—intradialytic exercise training plus usual care maintenance hemodialysis.
Compliance and Adherence (Fidelity) to the Exercise Prescription
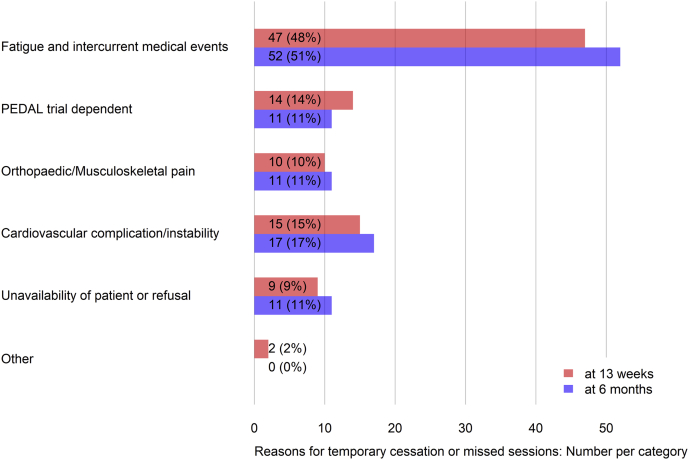
A median (interquartile range) of 47 (28–77)% of exercise training sessions prescribed was completed by participants in EX. Nevertheless, only 18% of patients adhered exactly to the prescribed exercise type, intensity, and duration. Moreover, during the 6-month observation period, only 42% of participants avoided temporary cessation of the exercise intervention (Table 7). Reasons reported were fatigue and intercurrent medical events (Figure 3).
Table 7.
Summary of exercise compliance and adherence to the PEDAL trial intervention during the 6-month follow-up period
| Compliance (percentage of expected sessions completed) | |
|---|---|
| Sample size (n) | 175 |
| Median (IQR) | 47 (28–77) |
| Temporary (>2 wk) cessation of exercise | |
| Sample size (n) | 119 |
| n (%) | 69 (58) |
| Adhered (fidelity to type/intensity/duration) to the exercise prescription | |
| Sample size (n) | 119 |
| n (%) | 21 (18) |
IQR, interquartile range; PEDAL, PrEscription of intraDialytic exercise to improve quAlity of Life.
Figure 3.
Number (%) of recorded incidents of temporary cessation (>2 weeks) or missed exercise sessions with reasons.
Discussion
The aim of the PEDAL trial was to evaluate the clinical value of a 6-month intradialytic exercise program on QoL, compared with usual care, for patients receiving HD therapy. The PEDAL trial was novel in that it was the first to evaluate intradialytic exercise as would most likely be implemented, should health service commissioners include exercise training as part of the service specification for in-center HD. Unfortunately, as delivered, the PEDAL program did not statistically improve HRQoL, as assessed by the KDQOL-SF 1.3 PCS (P = 0.055), nor did it statistically improve QoL as assessed by the prespecified secondary outcomes of EQ-5D-5L, EQ-5D visual analog scale, or the KDQOL-SF 1.3 multi-item scales of energy/fatigue and burden of kidney disease (Table 2).
The lack of statistical improvement in the PCS can be explained in part by the PEDAL participants having poor compliance (only 47% of prescribed exercise sessions were completed) and very poor adherence (only 18% of patients adhered to the prescribed progression of overload regarding type, intensity, and duration of exercise) to the exercise intervention. By design, the PEDAL trial aimed to have inclusive inclusion criteria. Consequently, baseline peak aerobic capacity values of 12 ml/min/kg were considerably lower than typically reported in previous studies (approximately 18 ml/min/kg).10,12,18 This observation, combined with extremely low scores in physical performance (sit-to-stand and gait speed tests), confirms that the PEDAL cohort consisted of participants with severely low functional capacity. Arguably, this makes the PEDAL cohort more representative and its findings generalizable to the current HD population. Nevertheless, perhaps including such participants prevented benefits of the exercise intervention being realized in the relatively short 6-month intervention, and it is possible that some of these highly compromised participants may require a slower rate of overload progression and adaptation/adjustment periods to an aerobic intradialytic exercise intervention. Poor compliance and adherence to implemented renal exercise programs in clinical practice is well documented, with more than 50% of the patients starting exercise reportedly dropping out by 6 months, often owing to fatigue and being unwell.18,37,38
That the PEDAL program was not effective to increase PCS warrants comparison with previous studies. A Cochrane review completed in 2011 concluded that exercise was beneficial for HRQoL in patients with CKD, but unfortunately no meta-analysis or risk of bias assessment was performed, and many of the included studies were not representative of the HD population.9 Other reviews have concluded positive effects of exercise but not on PCS16,21,38 or have relied on studies at high risk of bias and with considerable heterogeneity.17,20 Previous meta-analyses have also included extradialytic exercise programs19,39,40 and intradialytic exercise programs that were intensively supervised (e.g., Ouzouni et al.41) or studies that have delivered progressive resistance training as opposed to aerobic cycling alone.10,15,42 In this regard, 1 meta-analysis22 usefully compared aerobic versus progressive resistance training versus combined exercise; only progressive resistance training increased PCS. Detailed analysis of the very few empirical studies included in reviews that do reveal positive effects of aerobic intradialytic exercise on QoL reveals that they have often used interventions that would be difficult to implement in routine care.41 A recent study by Jeong et al.43 found no significant improvements in physical function or QoL with a combined oral protein supplement and intradialytic cycling program. The authors suggested that a more comprehensive lifestyle management approach would be required to elicit improvements in these parameters. Taken together with the results reported herein, it is highly unlikely that clinically implementable intradialytic aerobic exercise training alone can improve QoL at a whole population level.
In addition to evaluating potential benefits, the PEDAL study uniquely assessed the cost of delivery of its intervention by recording harms and using health economic methods. The number of hospitalizations, all-cause mortality, and cardiovascular mortality was not noticeably different between the groups. Although these results should be interpreted cautiously owing to the low number of events, there was no increase in SAE in the exercise group either. The economic cost of delivering the PEDAL intervention ranged from £464 (US$598) to £848 (US$1092) per participant per year (depending on pay band of the physiotherapy assistant, whether London weighting was applied, and staff-to-patient ratio). Note this calculation assumed that physiotherapy assistants supervised between 6 and 10 participants per dialysis session without incurring any travel costs and that exercise would be offered as part of a general physiotherapy service (with enough capacity to provide absence cover at no additional cost). It also assumes that patients will only exercise for between 1 and 2 sessions per week (the calculation is based on compliance to the PEDAL intervention, which was only 47%). For comparison purposes, the cost of delivering cardiac rehabilitation is £477 (US$614) per person per year,44 equating to costs of £550 (US$709) to £12,558 (US$16,178) per quality-adjusted life year gained.45 In contrast, PEDAL had no apparent QoL gain, albeit in a relatively short period of observation of 6 months (cost-effectiveness of rehabilitation programs increases with time44,45). The cost of delivery of HD in the UK is approximately £35,000 (US$45,088) per patient per year.46 As the PEDAL trial was not clinically effective at a whole sample level, whether the cost of delivery of intradialytic exercise is justified to enhance patient choice remains a matter for debate.
Limitations
PEDAL was designed to assess a pragmatic, clinically implementable intradialytic exercise intervention. By design, the study relied on a patient-reported outcome measure for its primary outcome; it is recognized that the primary limitation of this study was the lack of an attention CON. In this regard, it is possible that an experimenter effect explains the 2.4 arbitrary unit increase (albeit nonsignificant) in PCS.47 This interpretation is supported by the lack of absolute or statistical changes in objective measures of physical function, cardiovascular risk, and clinical measures (Table 2), consistent with a conclusion that intradialytic aerobic exercise per se had no clinical benefit. In addition, the study was not powered to detect differences in some secondary outcomes, including mortality. Nevertheless, we reported these data to allow a balance of benefits and harms to be evaluated. Future studies should address these concerns by including attention control arms and being adequately powered for all outcome measures. Perhaps the most important finding of the PEDAL study was the observation of poor compliance and adherence when intradialytic exercise was implemented as part of routine care. It is acknowledged that the lack of absolute or significant change in objective measures may in part be due to limitations in the effective implementation of delivering an adequate dose of exercise stimulus as indicated by the very low compliance and adherence data. PEDAL was designed to be a pragmatic intervention, and no additional strategies to address low compliance or adherence were introduced. Thus, future studies need to evaluate whether there are subgroups of patients who may benefit from this type of intervention and whether there is scope to optimize strategies to improve compliance and adherence with intradialytic cycling interventions, implementation settings, and resources to deliver exercise-based interventions to improve effectiveness.
Conclusions
The PEDAL study was a rehabilitation program that could realistically be commissioned as part of routine care. Compliance and adherence with the exercise intervention, as per the study design, were extremely low. In this inclusive sample of people on HD, many of whom were severely deconditioned; the findings therefore suggest that 6 months of intradialytic aerobic exercise did not improve HRQoL.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We are grateful to the research assistants, physiotherapy assistants, and research nurses who facilitated completion of this study. This study is funded by a grant from the National Institute for Health Research (grant number: NIHR-HTA 12/23/09). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health. The funders had no role in the design, collection, analysis, and interpretation of the data or writing of this protocol. SM is supported by NIHR infrasructure in D4D MIC Sheffield, UK.
Author Contributions
SAG, PK, JHM, ICM, IF, AS, and TM conceived and designed the study; IF and CMM analyzed the data; SAG, JHM, and PK interpreted and contextualized the data and drafted the paper; SAG, JHM, PK, DW, SB, KF, MT, PAK, MK, JB, ICM, IF, CMM, SK, CR, IDG, CR, MY, PT, SM, TM, and AS revised the paper; all authors approved the final manuscript.
Footnotes
Table S1. Response of quality of life to the PEDAL intervention, as assessed by the Kidney Disease Quality of Life Short-Form (KDQOL-SF 1.3) multi-item scales.
Consort checklist.
STROBE Statement.
Supplementary Material
Table S1. Response of quality of life to the PEDAL intervention, as assessed by the Kidney Disease Quality of Life Short-Form (KDQOL-SF 1.3) multi-item scales.
Consort checklist.
STROBE Statement.
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jassal S.V., Karaboyas A., Comment L.A. Functional dependence and mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2016;67:283–292. doi: 10.1053/j.ajkd.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stack A.G., Molony D.A., Rives T., Tyson J., Murthy B.V. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis. 2005;45:690–701. doi: 10.1053/j.ajkd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 4.van Loon I.N., Bots M.L., Boereboom F.T.J. Quality of life as indicator of poor outcome in hemodialysis: relation with mortality in different age groups. BMC Nephrol. 2017;18:217. doi: 10.1186/s12882-017-0621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knight E.L., Ofsthun N., Teng M., Lazarus J.M., Curhan G.C. The association between mental health, physical function, and hemodialysis mortality. Kidney Int. 2003;63:1843–1851. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 6.Lowrie E.G., Curtin R.B., LePain N., Schatell D. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41:1286–1292. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 7.Brown S.A., Tyrer F.C., Clarke A.L. Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J. 2017;10:788–796. doi: 10.1093/ckj/sfx057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y.N., Shapiro B., Kim J.C. Association between quality of life and anxiety, depression, physical activity and physical performance in maintenance hemodialysis patients. Chronic Dis Transl Med. 2016;2:110–119. doi: 10.1016/j.cdtm.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heiwe S., Jacobson S.H. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev. 2011;(10):CD003236. doi: 10.1002/14651858.CD003236.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiwe S., Jacobson S.H. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Cheema B.S., Chan D., Fahey P., Atlantis E. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med. 2014;44:1125–1138. doi: 10.1007/s40279-014-0176-8. [DOI] [PubMed] [Google Scholar]
- 12.Segura-Ortí E. Ejercicio en pacientes en hemodiálisis: revisión sistemática de la literature [Exercise in hemodialysis patients: a literature systematic review] Nefrologia. 2010;30:236–246. doi: 10.3265/Nefrologia.pre2010.Jan.10229. [DOI] [PubMed] [Google Scholar]
- 13.Smart N., Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrol (Carlton) 2011;16:626–632. doi: 10.1111/j.1440-1797.2011.01471.x. [DOI] [PubMed] [Google Scholar]
- 14.Salhab N., Karavetian M., Kooman J., Fiaccadori E., El Khoury C.F. Effects of intradialytic aerobic exercise on hemodialysis patients: a systematic review and meta-analysis. J Nephrol. 2019;32:549–566. doi: 10.1007/s40620-018-00565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng K., Zhang P., Chen L., Cheng J., Wu C., Chen J. Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol. 2014;40:478–490. doi: 10.1159/000368722. [DOI] [PubMed] [Google Scholar]
- 16.Chung Y.C., Yeh M.L., Liu Y.M. Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Nurs. 2017;26:1801–1813. doi: 10.1111/jocn.13514. [DOI] [PubMed] [Google Scholar]
- 17.Pu J., Jiang Z., Wu W. Efficacy and safety of intradialytic exercise in haemodialysis patients: a systematic review and meta-analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2017-020633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young H.M.L., March D.S., Graham-Brown M.P.M. Effects of intradialytic cycling exercise on exercise capacity, quality of life, physical function and cardiovascular measures in adult haemodialysis patients: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33:1436–1445. doi: 10.1093/ndt/gfy045. [DOI] [PubMed] [Google Scholar]
- 19.Huang M., Lv A., Wang J. Exercise training and outcomes in hemodialysis patients: systematic review and meta-analysis. Am J Nephrol. 2019;50:240–254. doi: 10.1159/000502447. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Q.G., Zhang H.R., Wen X. Exercise interventions on patients with end-stage renal disease: a systematic review. Clin Rehabil. 2019;33:147–156. doi: 10.1177/0269215518817083. [DOI] [PubMed] [Google Scholar]
- 21.Clarkson M.J., Bennett P.N., Fraser S.F., Warmington S.A. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: a systematic review and meta-analysis. Am J Physiol Ren Physiol. 2019;316:F856–F872. doi: 10.1152/ajprenal.00317.2018. [DOI] [PubMed] [Google Scholar]
- 22.Gomes Neto M., de Lacerda F.F.R., Lopes A.A., Martinez B.P., Saquetto M.B. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: systematic review and meta-analysis. Clin Rehabil. 2018;32:1189–1202. doi: 10.1177/0269215518760380. [DOI] [PubMed] [Google Scholar]
- 23.Global Advocacy for Physical Activity (GAPA) the Advocacy Council of the International Society for Physical Activity and Health (ISPAH) NCD prevention: investments [corrected] that work for physical activity [published correction appears in Br J Sports Med. 2013;47:246] Br J Sports Med. 2012;46:709–712. doi: 10.1136/bjsm.2012.091485. [DOI] [PubMed] [Google Scholar]
- 24.Koufaki P., Greenwood S., Painter P., Mercer T. The BASES expert statement on exercise therapy for people with chronic kidney disease. J Sports Sci. 2015;33:1902–1907. doi: 10.1080/02640414.2015.1017733. [DOI] [PubMed] [Google Scholar]
- 25.Sallis R. Exercise is medicine: a call to action for physicians to assess and prescribe exercise. Phys Sportsmed. 2015;43:22–26. doi: 10.1080/00913847.2015.1001938. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood S.A., Koufaki P., Macdonald J. The PrEscription of intradialytic exercise to improve quAlity of Life in patients with chronic kidney disease trial: study design and baseline data for a multicentre randomized controlled trial. Clin Kidney J. 2020;14:1345–1355. doi: 10.1093/ckj/sfaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hays R.D., Kallich J.D., Mapes D.L., Coons S.J., Carter W.B. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 28.Hays K. RAND; Santa Monica, CA: 1997. SF User’s Manual. [Google Scholar]
- 29.Herdman M., Gudex C., Lloyd A. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segura-Orti E., Martinez-Olmos F.J. Test–retest reliability and minimal detectable change scores for sit-to-stand-to-sit tests, the six-minute walk test, the one-leg heel-rise test, and handgrip strength in people undergoing hemodialysis. Phys Ther. 2011;91:1244–1252. doi: 10.2522/ptj.20100141. [DOI] [PubMed] [Google Scholar]
- 31.Abe Y., Matsunaga A., Matsuzawa R. Determinants of slow walking speed in ambulatory patients undergoing maintenance hemodialysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee P.H., Macfarlane D.J., Lam T.H., Stewart S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coutinho-Myrrha M.A., Dias R.C., Fernandes A.A. Duke Activity Status Index for cardiovascular diseases: validation of the Portuguese translation. Arq Bras cardiol. 2014;102:383–390. doi: 10.5935/abc.20140031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tinetti M.E., Richman D., Powell L. Falls efficacy as a measure of fear of falling. J Gerontol. 1990;45:P239–P243. doi: 10.1093/geronj/45.6.p239. [DOI] [PubMed] [Google Scholar]
- 35.Laurent S., Cockcroft J., Van Bortel L. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 36.MedDRA Maintenance and Support Services Organisation . 2010. Introductory Guide to MedDRA Version 13.1. Chantilly. [Google Scholar]
- 37.Greenwood S.A., Castle E., Lindup H. Mortality and morbidity following exercise-based renal rehabilitation in patients with chronic kidney disease: the effect of programme completion and change in exercise capacity. Nephrol Dial Transplant. 2019;34:618–625. doi: 10.1093/ndt/gfy351. [published correction appears in Nephrol Dial Transplant. 2020;35:1452] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannan M., Bronas U.G. Barriers to exercise for patients with renal disease: an integrative review. J Nephrol. 2017;30:729–741. doi: 10.1007/s40620-017-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pei G., Tang Y., Tan L., Tan J., Ge L., Qin W. Aerobic exercise in adults with chronic kidney disease (CKD): a meta-analysis. Int Urol Nephrol. 2019;51:1787–1795. doi: 10.1007/s11255-019-02234-x. [DOI] [PubMed] [Google Scholar]
- 40.Barcellos F.C., Santos I.S., Umpierre D., Bohlke M., Hallal P.C. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J. 2015;8:753–765. doi: 10.1093/ckj/sfv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouzouni S., Kouidi E., Sioulis A., Grekas D., Deligiannis A. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil. 2009;23:53–63. doi: 10.1177/0269215508096760. [DOI] [PubMed] [Google Scholar]
- 42.Matsuzawa R., Hoshi K., Yoneki K. Exercise training in elderly people undergoing hemodialysis: a systematic review and meta-analysis. Kidney Int Rep. 2017;2:1096–1110. doi: 10.1016/j.ekir.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong J.H., Biruete A., Tomayko E.J. Results from the randomized controlled IHOPE trial suggest no effects of oral protein supplementation and exercise training on physical function in hemodialysis patients. Kidney Int. 2019;96:777–786. doi: 10.1016/j.kint.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shields G.E., Wells A., Doherty P., Heagerty A., Buck D., Davies L.M. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart. 2018;104:1403–1410. doi: 10.1136/heartjnl-2017-312809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards K., Jones N., Newton J. The cost-effectiveness of exercise-based cardiac rehabilitation: a systematic review of the characteristics and methodological quality of published literature. Health Econ Rev. 2017;7:37. doi: 10.1186/s13561-017-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baboolal K., McEwan P., Sondhi S., Spiewanowski P., Wechowski J., Wilson K. The cost of renal dialysis in a UK setting--a multicentre study. Nephrol Dial Transplant. 2008;23:1982–1989. doi: 10.1093/ndt/gfm870. [DOI] [PubMed] [Google Scholar]
- 47.Fokkema M., Smits N., Kelderman H., Cuijpers P. Response shifts in mental health interventions: an illustration of longitudinal measurement invariance. Psychol Assess. 2013;25:520–531. doi: 10.1037/a0031669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.