Abstract
Monoclonal gammopathies of renal significance (MGRS) encompass a remarkable variety of kidney diseases that result from intrinsic nephrotoxic properties of certain monoclonal Igs or their subunits. Effective disease-modifying treatments rely on the targeting of a malignant B-cell clone that may be demonstrable but often is quite hypothetical. Hence, convincing arguments for the genuine monoclonal character of the causative mono-isotypic Ig tissue deposits is needed for design of appropriate treatment strategies. The purpose of this article was to critically analyze distinct situations of suspected MGRS that occur in the practice of pathologists, nephrologists, hematologists, and immunologists. A particular focus of interest is the group of conditions known as proliferative glomerulonephritis with mono-isotypic immunoglobulin deposits (PGNMIDs), which illustrates the difficulties and ambiguities surrounding a definitive assignment of MGRS status.
Keywords: glomerulopathy, IgG3, immunoglobulin isotypes, immunoglobulin variable regions, monoclonal gammopathy, monotypic deposits, PGNMID
Excessive expansion of a B-cell clone with terminal maturation leads to the production of a monoclonal Ig that is usually detectable in blood and/or urine, a condition termed “monoclonal gammopathy.” Thus, a technical definition of monoclonal gammopathies relies on the demonstration of an abnormally abundant homogeneous Ig (also termed “M-spike”) or its monoclonal light chain and/or heavy-chain subunits on analysis of serum and/or urine proteins by zone electrophoresis and derivative methods including immunoelectrophoresis, immunofixation, and immunoblotting.
In recent years, the monoclonal gammopathy spectrum has gradually expanded to include conditions with mono-isotypic Ig tissue deposition but lacking evidence of a circulating monoclonal Ig component. We direct our discussion to the controversies surrounding this unusual situation.
Asymptomatic monoclonal gammopathies are found in more than 3% of the general population older than 50 years.1 Their discovery is generally fortuitous, and because only a small proportion undergoes a malignant evolution, they are termed monoclonal gammopathy of undetermined significance (MGUS). The overall risk of MGUS progression to myeloma or, more rarely, a lymphoplasmacytic lymphoma is approximately 1% per year. Although this risk remains relatively stable over the time of follow-up, it is augmented by factors such as serum free light chain imbalance, high level of circulating monoclonal Ig, and presence of IgM isotype.2
Apart from “classical MGUS,” certain conditions or physiological situations, including aging, immunosuppressive treatments, or other immunodeficiency states, quite frequently feature very small amounts of serum monoclonal Ig that may be demonstrable only by a sensitive method such as immunoblotting. These “micro-peaks” are often multiple and/or transient, and the frequencies of their isotypes are remarkably different from those observed in malignant lymphoproliferative diseases. Among the IgG subclasses, IgG3 overwhelmingly predominates in these micro-peaks, whereas IgG3 subclass is very rare in myeloma or classical MGUS. In addition, although the monoclonal Ig in both myeloma and classical MGUS is approximately twice more frequently of the kappa than lambda isotype (i.e., with kappa:lambda ratio approximately 2), the monoclonal Ig in micro-peaks has similar frequencies of kappa and lambda isotype (ratio approximately 1).3, 4, 5, 6, 7
We will come back to this distinction between MGUS, a condition that can evolve into hematologic malignancy, and “secondary” micropeaks, as herein may lie a key to understanding the pathogenesis of certain kidney complications of monoclonal Ig.
Pathogenic Monoclonal Ig
Certain monoclonal Ig, or subunits thereof, display intrinsic pathogenic properties that cause deposition in various organs. These may relate to antigen-binding antibody specificity (as occurs in anti-myelin monoclonal IgM related peripheral neuropathies) or to conformational properties of the monoclonal component that promote its deposition in tissues or within cellular compartments. These physicochemical properties govern protein folding, charge, glycosylation, self-aggregation, and resistance to proteolysis, among others. Ig-related pathological conditions thus encompass a wide spectrum of diseases that have been termed “monoclonal gammopathies of clinical significance.”8 In most monoclonal gammopathies of clinical significance in which a pathogenic role of the monoclonal Ig is proven or suspected, efficient disease-modifying therapies are directed to the underlying B-cell proliferation, with a difficult issue often being which stage of B-cell differentiation (lymphocyte or plasma cell) to target. Here we focus on monoclonal gammopathies of clinical significance with kidney involvement (i.e., so-called “monoclonal gammopathies of renal significance” [MGRS]).
Although the concept behind MGRS had been known for several decades before it was promoted (see, for instance, Osserman et al.,9 who actually introduced the concept of “primary” amyloidosis), the term MGRS implies that the pathological consequences of monoclonal Ig should take priority over the hematological status. Thus, defining MGRS as a nosological entity proves especially useful when making treatment decisions for management of those patients with monoclonal Ig occurring in the absence of a clinically overt onco-hematological disease.10 In such patients, however, the monoclonal nature of pathogenic Ig must be sufficiently convincing.
We will not undertake a systematic review of all the pathological conditions that can comprise MGRS, as their distinctive features have been published (e.g., see Sethi et al.11). It is worth noting that several so-called MGRS, including the relatively frequent AL-amyloidosis and non-amyloid light chain deposition disease, actually involve other organs in addition to the kidney. On the other hand, examples of “pure” MGRS include light chain proximal tubulopathy (although some may associate with crystal-storing histiocytosis), PGNMID, and most cases of heavy-chain deposition disease. Certain glomerular diseases, including immunotactoid glomerulonephritis and fibrillary glomerulonephritis (FGN), can exhibit either monoclonal Ig or polyclonal Ig deposits. Of note, it has long been known that genuine cases of MGRS may feature an undetectable circulating monoclonal component, as illustrated for instance by non-amyloid light chain deposition disease with unusual N-glycosylation of variable domains.12
The pathophysiology of most MGRS remains poorly understood. Hypotheses on monoclonal Ig deposition or accumulation have been proposed, but how deposited Ig causes functional injury has been explored in only a few conditions (see, for instance, Herrera et al.13 for a recent review). Consequently, the main therapeutic strategy is based on targeting the B-cell monoclonal proliferation responsible for the production of nephrotoxic Ig or Ig subunits. In clinical practice, however, a precise identification of the underlying hematological disorder often proves unattainable and thus the therapeutic decision-making remains empirical.
How to Validate a Diagnosis of MGRS?
The monoclonal nature of deposited Ig or Ig subunits has been strictly demonstrated in many, but not all, so-called MGRS. Because this issue has important therapeutic implications, different situations should be considered, as proposed in the following.
-
1.
Genuine MGRS: the monoclonal nature of tissue-deposited Ig is proven.
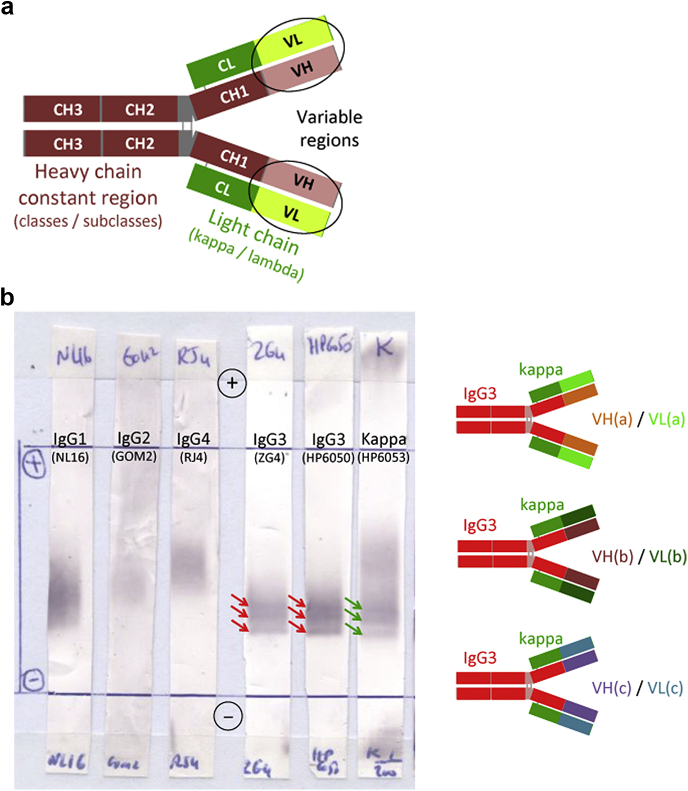
Figure 1a schematically recalls the structural bases of Ig diversity: although isotypes (i.e., Ig classes and subclasses, and light chain kappa/lambda types) define a maximum of 18 distinct Ig species, most of the variability of Ig molecules is borne by the variable (VH and VL) domains that result from somatic rearrangements of gene segments followed by hypermutation events, which may generate close to 1012 different structures. As a consequence, a rigorous definition of monoclonality rests on the demonstration of single variable domains. In biological fluids, an empirical evidence of monoclonal Ig is its narrow migration (i.e., a peak) on zone electrophoresis.
Figure 1.
Mono-isotypy may not be considered absolute proof of monoclonal origin. (a) Schematic representation of an Ig molecule showing the distinct locations of isotype determinants (heavy-chain class/subclass and light chain type) and highly variable VH and VL domains. CH1, CH2, and CH3 represent the constant heavy-chain domains; CL is the constant light chain domain. (b) Serum oligoclonal mono-isotypic Ig pattern as demonstrated by single-pressure immunoblot after thin layer zone electrophoresis in a patient with PGNMID: Three distinct micro-peaks are revealed with 2 anti-IgG3 antibodies (clones ZG4 and HP6050, red arrows) and an anti-kappa antibody (clone HP6053, green arrows). The patient’s kidney biopsy had shown mesangial and subendothelial IgG3 kappa mono-isotypic glomerular deposits by immunofluorescence (not illustrated). The right panel shows a schematic representation of 3 distinct serum IgG3 kappa molecules with identical constant regions but different variable regions, conferring the 3 distinct electrophoretic mobilities observed in the immunoblot (left).
For the same reasons, the diagnosis of a monoclonal Ig in tissue deposits requires not only the demonstration of single (restricted) heavy and light chain isotypes, but also evidence of homogeneous variable domains either by empirical approaches or by determination of amino acid sequences. An empirical approach could be, for instance, the immunohistological demonstration of restricted reactivity with antibodies specific for epitopes expressed by small sets of VH or VL regions, such as cross-reactive idiotypes.14 Such antibodies recognize restricted antigenic determinants (once termed “public” idiotopes) that are encoded by nonmutated, germline parts of the Ig variable gene (VH or VL) segments. Reactivity of mono-isotypic Ig deposits with an antibody that has one anti-idiotype specificity would be a strong argument for monoclonal origin. Of note, such antibodies are not in routine diagnostic use by renal pathology laboratories, whose antibody armamentarium typically includes only commercially available antibodies directed to the constant regions of gamma, mu and alpha Ig heavy chains, IgG subclasses (1–4), and kappa and lambda light chain isotypes.
Determination of variable region amino acid sequences has long been performed by Edman degradation on eluted or extracted tissue-deposited material. In recent years, peptide primary structures have been deduced from analyses of mass spectrometry spectra obtained from microdissected tissue biopsies.
N-terminal sequencing studies on 2 cases of AL-amyloidosis by Glenner et al.15 demonstrated the presence, in each case, of a single light chain variable (VL) domain in kidney deposits. These elegant analyses were followed by the evidence of identity between tissue amyloid substance and the circulating monoclonal light chain.16 The first reported case of heavy-chain (AH) amyloidosis also showed a strictly homogeneous structure of the deposited protein and its identity with a circulating monoclonal component, as well as the CH1-CH2 constant domain deletion.17 Thanks to the unique property of amyloid fibrils to resuspend in water (but not in buffered saline), many additional protein analyses could be performed and clearly showed that all Ig subunits that undergo amyloidogenesis are monoclonal. In non-amyloid light chain deposition disease, N-terminal sequencing and N-glycosidase treatment allowed demonstration of the identity of kidney-deposited Ig light chain and its circulating counterpart, as well as the N-glycosylation of both deposited and bone marrow–secreted Ig light chain.18
Molecular analyses of deposited proteins are now becoming available through the development of sensitive targeted proteomic studies using mass spectrometry performed on laser micro-dissected tissue samples.19 This approach proved superior for defining the contents of deposited material, for instance in immunotactoid glomerulopathy20 and heavy-chain deposition disease with unusual delta chain isotype21; however, to date it has not been used much for analysis of the clonality of Ig deposits. The presence of polyclonal Ig circulating in the glomerular capillary lumina at the time of tissue fixation and the relatively small quantity of some glomerular Ig deposits makes this technique less useful in PGNMID than in renal amyloidosis, for example.
Definite proof of monoclonality requires the demonstration of homogeneous variable sequences, which is not frequently feasible. A recent study on Ig light chain-PGNMID nicely demonstrated the presence of single kappa VL domains in mass spectrometry performed on laser micro-dissected tissue–analyzed samples.22 These studies, as well as the large majority of cases with a circulating monoclonal Ig, clearly distinguish light chain-PGNMID from IgG-PGNMID that are discussed as follows.
-
2.
Probable MGRS: Tissue-deposited Ig displays unquestionable mono-isotypy, in the context of a monoclonal proliferation and/or circulating monoclonal Ig with corresponding isotype.
Arguments for identity are based on demonstration of mono-isotypy of the kidney-deposited material that displays the same heavy-chain class and light chain type as those of a monoclonal Ig detected in body fluids. Undoubtedly, immunohistological analyses of IgG subclasses in addition to classical Ig class and light chain type provide more reliable indication of monoclonal origin, as recently shown for instance in immunotactoid glomerulopathy, where polyclonal as well as monoclonal entities were defined.23
In addition to mono-isotypy, there are many examples of biochemical similarity between peripheral and kidney-deposited material that further argue for a monoclonal origin. First, it is tempting to anticipate that ultrastructurally organized deposits (i.e., crystals or 1-dimensional forms such as fibrils or microtubules) would arise from homogeneous precursors. However, this is actually not the case in rare conditions such as FGN and immunotactoid glomerulopathy, among which polyclonal Ig cases are not exceptional or may even be frequent, especially in FGN.24
A convincing example is that of immunotactoid glomerulopathy in which identical microtubular structures with the same Ig isotypes in both malignant B-cells and kidney glomeruli may be considered quasi-proof of identity.25 Another example is light chain proximal tubulopathy, where identical rhombohedric structures with a precise 60-Å periodic striation on electron microscopy were demonstrated in both patient’s intracellular crystals and in those obtained in vitro from the urine monoclonal light chain.26
Finally, situations featuring unusual structures or properties of a circulating monoclonal component constitute strong arguments for their identity with tissue-deposited material. For instance, the deletion of 2 heavy-chain constant domains (CH1 and CH2) in heavy-chain deposition disease, when evidenced in the serum monoclonal Ig, as well as the plasma-cell mRNA precursor and the glomerular deposits themselves can be considered sufficient proof of identity.27
-
3.
Possible MGRS: Tissue-deposited Ig displays mono-isotypy, but there is no detectable monoclonal proliferation or circulating monoclonal Ig of corresponding isotype.
The term “monoclonal Ig deposits” is used routinely in the literature when referring to mono-isotypic Ig deposits, regardless of the immuno-hematological context. Yet, it is conceivable that peculiar pathological mechanisms are linked to restricted isotypes, as proposed for the IgG4 restricted Ig deposits of FGN, for example.28 Strict isotype restrictions of polyclonal Ig have been described in FGN (including some IgG4 with kappa light chain only)28 and IgA nephropathy (with IgA1 lambda).29,30 Almost 10% of IgA nephropathy cases appear to display mono-isotypic mesangial deposits, without evidence of a circulating monoclonal Ig or abnormal serum kappa:lambda ratio and without significant clinical or pathological differences from other polyclonal IgA cases.31 Recent studies on FGN revealed that most so-called “monoclonal FGN” based on light chain restriction actually prove to be polyclonal on analysis of IgG subclasses or examination by paraffin immunofluorescence.24,32 Thus, whether certain mono-isotypic deposits might be considered sufficient for MGRS diagnosis and consequent treatments is a crucial issue in patient management. The relatively common entity of IgG-PGNMID certainly deserves special discussion in this regard.
PGNMID
Immune complex-like nonorganized granular electron dense deposits of mono-isotypic Ig in the mesangium and subendothelial regions with more variable subepithelial involvement define the entity of PGNMID, which was first described in a small series33 and subsequently expanded to 37 cases.34 A prominent feature is the strong predominance of the rare IgG3 subclass, mostly of kappa isotype, which was confirmed in further studies.35 Another important observation is the scarcity of a detectable blood (or urine) monoclonal Ig and the typical absence of any evidence of a monoclonal B-lymphocyte or plasma-cell proliferation on bone marrow biopsy. Of note, in the “Columbia” series,34 only 2 of the 9 patients with a detectable M-spike had IgG3 kidney deposits, as compared with 19 of 23 (83%) patients without a detectable M-spike, in whom the kappa isotype predominated.
The histologic features of PGNMID include membranoproliferative, diffuse endocapillary proliferative and variable membranous patterns. The Ig deposits display the anatomic (mostly subendothelial and mesangial) locations and ultrastructural appearance observed in immune complex-mediated glomerulonephritides. Because cryoglobulins represent an archetype of circulating immune complexes, it is appropriate to mention here 2 reported cases of type-1 cryoglobulin with membranoproliferative glomerulonephritis in which the IgG subclasses were analyzed36; both cases displayed IgG3 kappa restricted deposits. There was no demonstrable B-cell proliferation. Thus, although no cryoglobulinemia was detected in reported cases of PGNMID, the pathologic features of type-1 cryoglobulinemic glomerulonephritis and PGNMID are similar, with the exception of focal capillary occlusion by intraluminal deposits in some cryoglobulinemic cases. Of note, Nasr et al.34 described focal fibrillar organization of the deposits in nearly one-third of their PGNMID cases, another feature shared with cryoglobulin-related glomerulopathies. Thus, a continuum might exist between IgG-PGNMID and type-1 cryoglobulinemic glomerulonephritis.
Because monoclonal IgG3 is quite uncommon in plasma-cell dyscrasia, a possible explanation for its frequency in PGNMID is the propensity of this subclass to self-aggregate and precipitate in the glomerulus, as proposed by many authors.33, 34, 35 This hypothesis is quite plausible, yet it provides insufficient proof of monoclonality to justify clone-directed therapy. In addition, as previously mentioned, IgG3 is the most frequent subclass among micro-peaks that are secondary to overt or latent immunodeficiency states, including aging, HIV infection, immunosuppressive treatments, and immune deficiency due to chronic lymphocytic leukemia in which the micro-peaks are not secretion products of the leukemic clones.6 In such conditions monoclonal Igs may be multiple (i.e., oligoclonal) and are often transient, at variance with true MGUS.
Figure 1b illustrates this point with an IgG3-PGNMID case showing 3 distinct serum micro-peaks of the same IgG3 kappa isotype.
A possible hypothesis is that IgG-PGNMID actually encompasses several entities with distinct origins. A recent study using antibodies directed to conformational epitopes at the junctions of the CH1 heavy-chain and CL light chain constant domains found that as many as 20% of cases of apparent IgG3-PGNMID diagnosed by routine immunofluorescence may contain polyclonal deposits as revealed by both IgG3-kappa and IgG3-lambda specific CH1/CL antibodies.37 Indeed, certain patients with PGNMID appear to recover spontaneously whereas others clearly require chemotherapy directed to either a hypothetical B-lymphocyte or plasma-cell clone. Certain cases seem to progress from an isotype-restricted polyclonal to a monoclonal status,38 and progression from granular deposits to fibrillar substructure with the appearance of a serum monoclonal IgG also has been reported.34 Yet other cases of PGNMID progress to end-stage kidney disease and have a latency period of many years before hematologic malignancy is detected at the time of recurrence in the allograft.39
Interestingly, a recent report describes 9 pediatric cases of IgG-PGNMID who showed IgG3 subclass restriction of kidney-deposited material, often with lambda light chain isotype.40 No serum monoclonal Ig was detected by conventional methods in all tested patients. Of note, previous or concurrent infections by group A streptococcus, influenza B virus, or other undetermined pathogen, were observed in 5 cases, suggesting a mechanism of adaptive immune response to infectious pathogens in disease pathogenesis. Recurrences in the allograft were frequent, in one case occurring in 3 consecutive allografts over 20 years, without a detectable monoclonal Ig in the serum or evidence of onco-hematologic malignancy. A possible role of parvovirus B19 infection in 2 cases of IgG3 kappa PGNMID also has been reported.41
In a large series, a high rate (89%) of early recurrence of PGNMID in the allograft was recently reported.42 Only 5 of the 25 patients had a detectable M-spike, of which only 1 had an abnormal serum free light chain ratio; all 5 patients with a serum monoclonal Ig had normal bone marrow biopsy. Recurrent PGNMID in the allograft also was described in a small cohort of patients with underlying autoimmune diseases, including rheumatoid arthritis, Graves disease, and ankylosing spondylitis, suggesting potential mono-isotypic responses as part of an autoimmune antibody repertoire.43 Thus, recurrence in the kidney allograft per se does not seem to be a reliable indicator of the monoclonal origin of PGNMID.
In most MGRS, the precise pathophysiological mechanisms of monoclonal Ig-related kidney injury remain poorly understood. Hence, the only available disease-modifying treatments have to target a monoclonal Ig-producing proliferation whose existence is frequently speculative. Of note, effective clinical response to B-cell (either B-lymphocyte or plasma cell) directed chemotherapies may not be considered in itself proof of a monoclonal disease. To this point, anti-CD20 antibodies or proteasome inhibitors have shown efficacy in treating a variety of autoimmune conditions in which the disease-causing antibodies are polyclonal, such as membranous nephropathy, lupus nephritis, and refractory immune-mediated thrombocytopenic purpura.44,45 Optimal evidence of the indisputable monoclonal nature of Ig deposits is definitely a prerequisite to guide therapy in MGRS, at least in the absence of a corresponding circulating monoclonal Ig. A crucial issue remains whether the pathogenic clone is truly malignant or emerges secondarily as part of an initially adaptive immune response to virus or other pathogens, immunodeficiency state, autoimmunity, or other immunologic challenge. Identifying the origin and predicting the behavior of the pathogenic clone remains the key to designing optimal treatment strategies.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors are grateful to Dr. Samih Nasr for kind and fruitful discussions. Supported by Sorbonne Université, Paris.
References
- 1.Kyle R.A., Therneau T.M., Rajkumar S.V. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 2.Kyle R.A., Larson D.R., Therneau T.M. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378:241–249. doi: 10.1056/NEJMoa1709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briault S., Courtois-Capella M., Duarte F. Isotypy of serum monoclonal immunoglobulins in human immunodeficiency virus-infected adults. Clin Exp Immunol. 1988;74:182–184. [PMC free article] [PubMed] [Google Scholar]
- 4.Radl J., Wels J., Hoogeveen C.M. Immunoblotting with (sub)class-specific antibodies reveals a high frequency of monoclonal gammopathies in persons thought to be immunodeficient. Clin Chem. 1988;34:1839–1842. [PubMed] [Google Scholar]
- 5.Connault L., Preud'Homme J.L., Gombert J. Serum monoclonal immunoglobulins in healthy aged people : a study of incidence and isotypy using a sensitive immunoblotting method. Aging: Immunology and Infectious Diseases. 1991;3:37–42. [Google Scholar]
- 6.Beaume A., Brizard A., Dreyfus B. High incidence of serum monoclonal Igs detected by a sensitive immunoblotting technique in B-cell chronic lymphocytic leukemia. Blood. 1994;84:1216–1219. [PubMed] [Google Scholar]
- 7.Touchard G., Pasdeloup T., Parpeix J. High prevalence and usual persistence of serum monoclonal immunoglobulins evidenced by sensitive methods in renal transplant recipients. Nephrol Dial Transplant. 1997;12:1199–1203. doi: 10.1093/ndt/12.6.1199. [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A. Monoclonal gammopathies of clinical significance. Hematology Am Soc Hematol Educ Program. 2020;2020:380–388. doi: 10.1182/hematology.2020000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osserman E.F., Takatsuki K., Talal N. Multiple myeloma I. The pathogenesis of "amyloidosis.". Semin Hematol. 1964;1:3–85. [PubMed] [Google Scholar]
- 10.Leung N., Bridoux F., Hutchison C.A. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120:4292–4295. doi: 10.1182/blood-2012-07-445304. [DOI] [PubMed] [Google Scholar]
- 11.Sethi S., Rajkumar S.V., D'Agati V.D. The complexity and heterogeneity of monoclonal immunoglobulin-associated renal diseases. J Am Soc Nephrol. 2018;29:1810–1823. doi: 10.1681/ASN.2017121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denoroy L., Déret S., Aucouturier P. Overrepresentation of the V kappa IV subgroup in light chain deposition disease. Immunol Lett. 1994;42:63–66. doi: 10.1016/0165-2478(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 13.Herrera G.A., Teng J., Turbat-Herrera E.A. Understanding mesangial pathobiology in AL-amyloidosis and monoclonal ig light chain deposition disease. Kidney Int Rep. 2020;5:1870–1893. doi: 10.1016/j.ekir.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson K.M., Randen I., Natvig J.B. Human monoclonal rheumatoid factors derived from the polyclonal repertoire of rheumatoid synovial tissue: incidence of cross-reactive idiotopes and expression of VH and V kappa subgroups. Eur J Immunol. 1990;20:863–868. doi: 10.1002/eji.1830200422. [DOI] [PubMed] [Google Scholar]
- 15.Glenner G.G., Terry W., Harada M. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analyses. Science. 1971;172:1150–1151. doi: 10.1126/science.172.3988.1150. [DOI] [PubMed] [Google Scholar]
- 16.Terry W.D., Page D.L., Kimura S. Structural identity of Bence Jones and amyloid fibril proteins in a patient with plasma cell dyscrasia and amyloidosis. J Clin Invest. 1973;52:1276–1281. doi: 10.1172/JCI107295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eulitz M., Weiss D.T., Solomon A. Immunoglobulin heavy-chain-associated amyloidosis. Proc Natl Acad Sci U S A. 1990;87:6542–6546. doi: 10.1073/pnas.87.17.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cogné M., Preud'homme J.L., Bauwens M. Structure of a monoclonal kappa chain of the V kappa IV subgroup in the kidney and plasma cells in light chain deposition disease. J Clin Invest. 1991;87:2186–2190. doi: 10.1172/JCI115252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung A.W.S., Sugumar V., Ren A.H. Emerging role of clinical mass spectrometry in pathology. J Clin Pathol. 2020;73:61–69. doi: 10.1136/jclinpath-2019-206269. [DOI] [PubMed] [Google Scholar]
- 20.Nasr S.H., Fidler M.E., Cornell L.D. Immunotactoid glomerulopathy: clinicopathologic and proteomic study. Nephrol Dial Transplant. 2012;27:4137–4146. doi: 10.1093/ndt/gfs348. [DOI] [PubMed] [Google Scholar]
- 21.Royal V., Quint P., Leblanc M. IgD heavy-chain deposition disease: detection by laser microdissection and mass spectrometry. J Am Soc Nephrol. 2015;26:784–790. doi: 10.1681/ASN.2014050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasr S.H., Larsen C.P., Sirac C. Light chain only variant of proliferative glomerulonephritis with monoclonal immunoglobulin deposits is associated with a high detection rate of the pathogenic plasma cell clone. Kidney Int. 2020;97:589–601. doi: 10.1016/j.kint.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Nasr S.H., Kudose S.S., Said S.M. Immunotactoid glomerulopathy is a rare entity with monoclonal and polyclonal variants. Kidney Int. 2021;99:410–420. doi: 10.1016/j.kint.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 24.Kudose S., Canetta P., Andeen N.K. Diagnostic approach to glomerulonephritis with fibrillar IgG deposits and light chain restriction. Kidney Int Rep. 2021;6:936–945. doi: 10.1016/j.ekir.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Touchard G., Preud'homme J.L., Aucouturier P. Nephrotic syndrome associated with chronic lymphocytic leukemia: an immunological and pathological study. Clin Nephrol. 1989;31:107–116. [PubMed] [Google Scholar]
- 26.Aucouturier P., Bauwens M., Khamlichi A.A. Monoclonal Ig L chain and L chain V domain fragment crystallization in myeloma-associated Fanconi's syndrome. J Immunol. 1993;150:3561–3568. [PubMed] [Google Scholar]
- 27.Aucouturier P., Khamlichi A.A., Touchard G. Brief report: heavy-chain deposition disease. N Engl J Med. 1993;329:1389–1393. doi: 10.1056/NEJM199311043291905. [DOI] [PubMed] [Google Scholar]
- 28.Iskandar S.S., Falk R.J., Jennette J.C. Clinical and pathologic features of fibrillary glomerulonephritis. Kidney Int. 1992;42:1401–1407. doi: 10.1038/ki.1992.433. [DOI] [PubMed] [Google Scholar]
- 29.Lai K.N., Chui S.H., Lai F.M. Predominant synthesis of IgA with lambda light chain in IgA nephropathy. Kidney Int. 1988;33:584–589. doi: 10.1038/ki.1988.37. [DOI] [PubMed] [Google Scholar]
- 30.Tomana M., Novak J., Julian B.A. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagae H., Tsuchimoto A., Tsuruya K. Clinicopathological significance of monoclonal IgA deposition in patients with IgA nephropathy. Clin Exp Nephrol. 2017;21:266–274. doi: 10.1007/s10157-016-1275-7. [DOI] [PubMed] [Google Scholar]
- 32.Said S.M., Leung N., Alexander M.P. DNAJB9-positive monotypic fibrillary glomerulonephritis is not associated with monoclonal gammopathy in the vast majority of patients. Kidney Int. 2020;98:498–504. doi: 10.1016/j.kint.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 33.Nasr S.H., Markowitz G.S., Stokes M.B. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int. 2004;65:85–96. doi: 10.1111/j.1523-1755.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 34.Nasr S.H., Satoskar A., Markowitz G.S. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055–2064. doi: 10.1681/ASN.2009010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guiard E., Karras A., Plaisier E. Patterns of noncryoglobulinemic glomerulonephritis with monoclonal Ig deposits: correlation with IgG subclass and response to rituximab. Clin J Am Soc Nephrol. 2011;6:1609–1616. doi: 10.2215/CJN.10611110. [DOI] [PubMed] [Google Scholar]
- 36.Karras A., Noël L.H., Droz D. Renal involvement in monoclonal (type I) cryoglobulinemia: two cases associated with IgG3 kappa cryoglobulin. Am J Kidney Dis. 2002;40:1091–1096. doi: 10.1053/ajkd.2002.36350. [DOI] [PubMed] [Google Scholar]
- 37.Nasr SH, Fidler ME, Said SM, et al. Immunofluorescence staining for immunoglobulin heavy chain/light chain on kidney biopsies is a valuable ancillary technique for the diagnosis of monoclonal gammopathy-associated kidney diseases [e-pub ahead of print]. Kidney Int. https://doi.org/10.1016/j.kint.2021.02.038. Accessed March 25, 2021. [DOI] [PubMed]
- 38.Yu X.J., Hu N., Wang S.X. Membranoproliferative glomerulonephritis with deposition of monoclonal IgG evolved from polyclonal IgG: a case report with two consecutive renal biopsies. BMC Nephrol. 2019;20:275. doi: 10.1186/s12882-019-1453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batal I., Markowitz G.S., Wong W. Filgrastim-induced crescentic transformation of recurrent IgG2λ GN. J Am Soc Nephrol. 2016;27:1911–1915. doi: 10.1681/ASN.2016010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller P., Xiao A.Y., Kung V.L. Progression of proliferative glomerulonephritis with monoclonal IgG deposits in pediatric patients. Pediatr Nephrol. 2021;36:927–937. doi: 10.1007/s00467-020-04763-5. [DOI] [PubMed] [Google Scholar]
- 41.Fujita E., Shimizu A., Kaneko T. Proliferative glomerulonephritis with monoclonal immunoglobulin G3κ deposits in association with parvovirus B19 infection. Hum Pathol. 2012;43:2326–2333. doi: 10.1016/j.humpath.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Said S.M., Cosio F.G., Valeri A.M. Proliferative glomerulonephritis with monoclonal immunoglobulin G deposits is associated with high rate of early recurrence in the allograft. Kidney Int. 2018;94:159–169. doi: 10.1016/j.kint.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 43.Nasr S.H., Sethi S., Cornell L.D. Proliferative glomerulonephritis with monoclonal IgG deposits recurs in the allograft. Clin J Am Soc Nephrol. 2011;6:122–132. doi: 10.2215/CJN.05750710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ronco P., Plaisier E., Debiec H. Advances in membranous nephropathy. J Clin Med. 2021;10:607. doi: 10.3390/jcm10040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckman J.D., Rollins-Raval M.A., Raval J.S. Bortezomib for refractory immune-mediated thrombocytopenia purpura. Am J Ther. 2018;25:e270–e272. doi: 10.1097/MJT.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]