Abstract
As an important regulator involved in cell activity, microRNAs (miRNAs) are important in the process of exercise influencing bone metabolism. The present study aimed to detect and select differentially expressed miRNAs in the bone tissues of mice trained on a treadmill, predict the target genes of these differentially expressed miRNAs and lay a foundation for exploring the effect of treadmill training on bone metabolism through miRNAs. In this experiment, after the mice were trained on a treadmill for 8 weeks, the mechanical properties of mouse femur bone were assessed, and the alkaline phosphatase (ALP) activity and osteocalcin (OCN) protein levels of the bone were assayed. miRNA microarray and reverse transcription-quantitative (RT-q)PCR were performed to select and validate differentially expressed miRNAs in the bone, and the target genes of these miRNAs were predicted with bioinformatics methods. In addition, the differentially expressed miRNAs in the bone tissues were compared with those in mechanically strained osteocytes in vitro. Treadmill training improved the mechanical properties of the femur bones of mice, and elevated the ALP activity and OCN protein level in the bone. In addition, 122 differentially expressed miRNAs were detected in the bone, of which nine were validated via RT-qPCR. Among the target genes of these differentially expressed miRNAs, certain candidates were involved in bone metabolism. A total of eight miRNAs were differentially expressed in both bone tissue and osteocytes, exhibiting the same expression trends, and various target genes of these eight miRNAs were also involved in bone metabolism. Treadmill training resulted in altered miRNA expression profiles in the bones of mice (mainly in osteocytes) and the differentially expressed miRNAs may serve important roles in regulating bone metabolism and osteogenic differentiation.
Keywords: bone, microRNA, treadmill exercise, bioinformatics, osteocyte
Introduction
Bone tissue is a typical mechanoresponsive tissue. Lack of mechanical stimulation is the major cause of the loss of bone mass and osteoporosis (1,2). Exercise is able to apply mechanical stimulation to bone tissue, and the mechanical stress produced by exercise may increase the bone turnover rate and bone density (3), stimulate osteoblast activity, increase bone mass and promote bone reconstruction and metabolism (4,5). Thus, exercise is one of the primary modifiable factors associated with improved bone health outcomes (6).
Certain types of exercise may result in improved bone strength, even after menopause (7). An increasing number of trials have indicated that treadmill exercise may promote bone health, particularly by suppressing estrogen deficiency-induced osteoporosis, for instance, treadmill training could increase bone mineral density in specific parts of rats, such as cortical bone (8), and treadmill exercise could increase bone density, improve the bone trabecular microstructure of mice, and inhibit osteoporosis and osteoclast activation in bone tissues of ovariectomized mice (9,10). In addition, the mechanical loading of treadmill exercise substantially enhances the osteogenic response (11). However, these studies have not fully revealed the response of bone tissue to running motion stimulation at the molecular level, particularly in terms of regulation by microRNAs (miRNAs/miRs).
miRNAs, a class of small noncoding RNAs, are able to inhibit the expression of negative regulatory genes and protein-coding genes by binding to target mRNAs (12). MiRNAs participate in vital activities, including development, organ formation, tumorigenesis, cell proliferation, differentiation and apoptosis, by modulating gene expression (12,13). MiRNAs have multiple roles in osteoblasts and osteoclasts in the presence of mechanical cyclical stretch (14), fluid shear stress (15), compressive force (16), orthodontic force (17) and microgravity (18). In addition, miRNAs regulate osteogenesis, which may be translated into novel therapeutic approaches for orthodontic conditions and bone fractures, as well as for systemic diseases, such as osteoporosis (19).
Osteocytes account for >90% of the adult bone cell population and are critical sensors of mechanical loading in bone (20). In a previous study by our group, differentially expressed miRNAs [40 miRNAs, 10 of which were confirmed via reverse transcription-quantitative (RT-q)PCR] were identified in MLO-Y4 osteocytes mechanically stimulated in vitro (21). As osteocytes are dominant and mechanosensitive, it was speculated that the same miRNAs may be differentially expressed in mechanically stimulated osteocytes in vitro and in the bone tissue of mice subjected to exercise on a treadmill. These miRNAs are likely to be involved in the response to mechanical strain and bone metabolism.
In the present study, to explore the effect of treadmill exercise on the expression of miRNAs in the bone tissues of mice, the differentially expressed miRNAs in the bone tissues of mice trained on a treadmill were screened using a miRNA microarray and verified by RT-qPCR, and the target genes of these differentially expressed miRNAs were predicted. In addition, the differentially expressed miRNAs in mechanically stimulated osteocytes were compared with the differentially expressed miRNAs in the bone tissues of the mice. Specific differentially expressed miRNAs that exhibited the same expression trends were selected and their target genes were predicted.
Materials and methods
Treadmill running exercise
A total of 22 male BALB/c mice (age, 8 weeks old; weight, 25-30 g; purchased from Hunan Anshengmei Pharmaceutical Research Institute Co., Ltd.) were randomly divided into two groups. The treadmill training group (treadmill speed, 13 m/min; slope, 9˚; training for 40 min per day at the same time each day, 6 days/week) and the control group (no treadmill training). All mice had free access to food and water under a relative humidity of 40-70%, temperature of 22-25˚C and 12-h light/dark cycle. The training lasted for 8 weeks. All of the experimental protocols were performed according to the Guidelines for Animal Research of Guilin Medical University (Guide for the Care and Use of Laboratory Animals) and were approved by the Animal Ethics Committee of Guilin Medical University (approval no. 2019-0013; Guilin, China).
Bone tissue sampling
After 8 weeks of treadmill running exercise, the mice were all euthanized in transparent plastic boxes with CO2 at a flow rate of 20% chamber air exchange/min (1.2 l/min) in December 2019. Death was confirmed by exposing the thorax to observe the lack of heartbeat, as well as observing pupil dilation and unresponsiveness to light. The femurs of the mice were collected and the soft tissue of the bone surface was removed. There were 11 mice in the treadmill training group and 11 rats in the control group, six of them were tested for bone mechanical properties. After testing the mechanical properties of the bone tissues of six mice, five of them were subsequently tested for alkaline phosphatase (ALP) activity and osteocalcin (OCN). The last five of the mice were used for the miRNA microarray and RT-qPCR assays. The femur bone was used for each of the assays.
Application of mechanical stimulation to osteocytes
MLO-Y4 osteocytes (purchased from Guangzhou Jennio Biotech Co., Ltd.) were seeded in α-MEM (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.) and 1% penicillin (Invitrogen; Thermo Fisher Scientific, Inc.) in the loading culture chamber of a four-point bending loading device (Institute of Medical Equipment, Academy of Military Medical Sciences, Tianjin, China). The osteocytes were seeded at a density of 2.5x104 cells/cm2 in mechanical loading plates and cultivated until they reached confluence. Cyclic mechanical tensile strain (2,500 µε, 0.5 Hz, 8 h) was applied to the cells, the cells were cultured in serum-free medium (Invitrogen; Thermo Fisher Scientific, Inc.) during the application of mechanical load. Cells not subjected to mechanical stimulation were used as the control group. These procedures were performed according to a previously outlined method (21).
Mechanical properties of bone tissue
Using a material testing machine (RGM6010; Anhui Regal Electronic Technology Co., Ltd.), the mechanical properties of the mouse femur bones were determined in a classical three-point bending experiment with the following parameters: Preload, 0.5 N; loading rate, 2 mm/min; and span, 10 mm (sufficient to break the bone tissue). The fracture (breaking) load, maximum elastic load, maximum bending stress and bending modulus of the mouse femurs were determined.
ALP and OCN measurements
The bone tissues (femurs) of the mice were weighed and fully ground with 1.5 ml PBS in a tissue grinder, and subsequently, the grinding solution was transferred to a 2-ml Eppendorf tube and centrifuged at 4˚C and 2x103 x g for 5 min. The supernatant was then discarded, and subsequently, 0.2 ml RIPA lysis buffer (Invitrogen; Thermo Fisher Scientific, Inc.) was added to the precipitate at the proportions of 1:10 (g:ml), and then the lysate was sonicated in an ultrasonic cell grinder (200 W, 30 cycles/6 sec). After centrifugation (6x103 x g, 20 min, 4˚C), the protein content of the supernatant was determined by a BCA protein assay kit (Beyotime Institute of Biotechnology). The ALP activity of the supernatant was detected by using an ALP kit (cat. no. A059-2-1; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocol. In addition, the OCN content of the bone tissue protein solution was detected using a mouse OCN ELISA kit (cat. no. H152; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocol.
miRNA microarray and RT-qPCR
After the bone tissues of the mice were completely shredded and ground in liquid nitrogen, the total RNA was purified by using TRIzol® RNA extraction reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol.
Part of the total RNA was purified with the mirVana miRNA Isolation kit (Ambion; Thermo Fisher Scientific, Inc.) and labeled with Cyanine 3 using the ULS™ mirVana miRNA ArrayLabeling kit (Ambion; Thermo Fisher Scientific, Inc.). Target labeling, hybridization, imaging and data processing were performed using a RiboArray miDETECT mouse array (Guangzhou RiboBio Co., Ltd.) that included all mouse miRNAs according to the manufacturer's protocol. miRNAs were considered to be differentially expressed if expression was >2-fold higher or lower than that in the control group (P<0.05).
Using the total RNA as a template, the miDETECT A Track™ miRNA qRT-PCR Start kit (Guangzhou RiboBio Co., Ltd.) was used to perform poly(A) tailing (to tail RNA) and Uni-RT (RT based on Uni-RT primers), followed by qPCR, according to the manufacturer's protocols of the 7900HT Fast Real Time PCR machine (Applied Biosystems; Thermo Fisher Scientific, Inc.). The miRNA-specific primers miDETECT A Track™ miRNA Forward Primers and qPCR primers for the miRNA mature chain were synthesized by Guangzhou RiboBio Co., Ltd. Following cDNA synthesis using Megaplex™ RNA RT mix (cat. no. 4444766; Applied Biosystems; Thermo Fisher Scientific, Inc.), qPCR was performed using Power SYBR-Green PCR Master Mix (cat. no. 4367659; Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions used were as follows: 10 min at 95˚C, followed by 45 cycles of 15 sec at 95˚C and 1 min at 60˚C. miRNA microarray and RT-qPCR experiments were performed at Guangzhou RiboBio Co., Ltd.
The miRNA microarray and RT-qPCR analysis of MLO-Y4 mouse osteocytes that were stimulated with a mechanical tensile strain of 2500 µε at 0.5 Hz were performed as described in Zeng et al (21), this mechanical tensile strain has been demonstrated to promote the osteoblastic differentiation of osteoblasts in vitro.
miRNA target gene prediction
TargetScan (www.targetscan.org/), MicroRNA.org (www.microrna.org/) and miRDB (http://www.mirdb.org/2/) were used to predict the target genes of the miRNAs, and target genes related to osteogenic differentiation or bone metabolism were identified.
Statistical analysis
Values are expressed as the mean ± standard deviation from three separate experiments (n=5 or 6 mice/group). Data were tested for normality of distribution using the Shapiro-Wilk test and differences between groups were analyzed using one-way ANOVA. Statistical analysis was performed using SPSS software (version 18; SPSS, Inc.) and P<0.05 was considered to indicate a statistically significant difference.
Results
Evaluation of mechanical properties
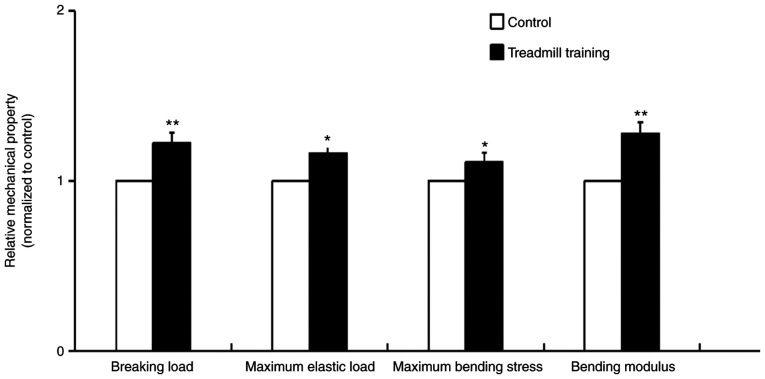
After 8 weeks of treadmill training, the mechanical properties of the femur bone tissues of the mice were significantly improved. These improved properties included the breaking load, maximum elastic load, maximum bending stress and bending modulus, and the breaking load and bending modulus were particularly improved (Fig. 1). This result indicated that treadmill training was able to improve the mechanical strength and elasticity of bone tissue.
Figure 1.
Mechanical properties of the femur bones in mice that were trained on a treadmill for 8 weeks. Training parameters: Speed, 13 m/min; slope, 9˚; training for 40 min/day, 6 days/week. Treadmill training improved the mechanical properties of the femur bone. n=6. *P<0.05, **P<0.01 vs. Control.
ALP activity and OCN expression
After 8 weeks of treadmill training, the ALP activity and OCN content of the femur bone tissues were significantly increased compared with in the control group (Fig. 2), which indicated that treadmill training was able to promote the osteogenic activity of cells in tissue.
Figure 2.
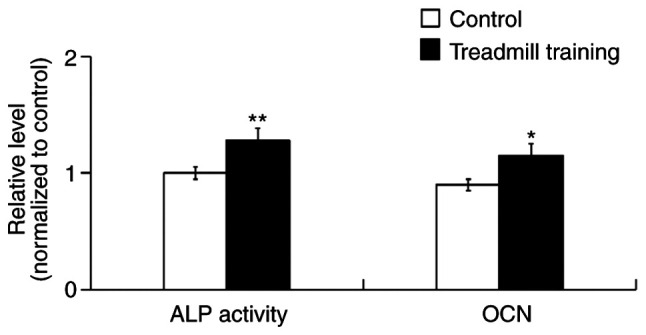
ALP activity and OCN content in the mouse femurs after treadmill training for 8 weeks. Treadmill training increased the ALP activity and OCN content in the femur tissues. n=5. *P<0.05, **P<0.01 vs. Control. ALP, alkaline phosphatase; OCN, osteocalcin.
Differential expression of miRNAs
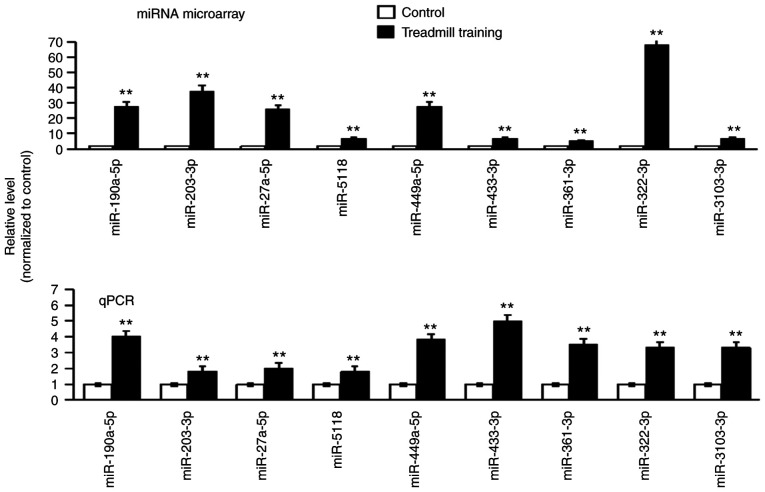
After 8 weeks of treadmill training, the expression of all of the miRNAs in the femur bones was detected via miRNA microarray. A total of 122 miRNAs were indicated to be differentially expressed. From these miRNAs, 19 miRNAs with P<0.01 were selected for further verification (data not shown). The RT-qPCR results suggested that the expression trend of each of the nine miRNAs was the same as that in the miRNA microarray; all of these miRNAs were upregulated (the expression level in the experimental group was higher compared with in the control group; Fig. 3). The nine differentially expressed miRNAs were as follows: miR-190a-5p, miR-203-5p, miR-27a-5p, miR-5118, miR-449a-5p, miR-433-3p, miR-361-3p, miR-322-3p and miR-3103-3p.
Figure 3.
Microarray screening and qPCR verification of differentially expressed miRNAs in bone tissues of mice after treadmill training. The results of the chip screening are provided in the top panel and the results of qPCR are displayed below. n=5. **P<0.01 vs. Control. miR, microRNA; qPCR, quantitative PCR.
Prediction of target genes
Using bioinformatics techniques, certain osteogenic differentiation and bone metabolism-related target genes of the 9 aforementioned differentially expressed miRNAs in bone tissue were predicted and their details are presented in Table I.
Table I.
Predictive target genes of differentially expressed miRNAs in bone tissue.
| miRNA | Target gene | Gene description | (Refs.) |
|---|---|---|---|
| miR-190a-5p | Smad2 | SMAD family member 2 | (39,47) |
| Cacnb2 | Calcium channel voltage-dependent β 2 subunit | (48,49) | |
| miR-203-5p | Mmp13 | Matrix metallopeptidase 13 | (50) |
| Wnt7b | Wingless-related MMTV integration site 7B | (51) | |
| Col3a1 | Collagen type III α 1 | (52,53) | |
| miR-27a-5p | Ngfr | Nerve growth factor receptor | (54) |
| Stk38 | Serine/threonine kinase 38 | (55) | |
| Ube2d2a | Ubiquitin-conjugating enzyme E2D 2A | (56) | |
| miR-5118 | Ngfr | Nerve growth factor receptor | (54) |
| Sh3kbp1 | SH3-domain kinase binding protein 1 | (57) | |
| Ube2d2a | Ubiquitin-conjugating enzyme E2D 2A | (56) | |
| miR-449a-5p | Arhgap26 | Rho GTPase activating protein 26 | (58) |
| Atp2b4 | ATPase Ca++ transporting plasma membrane 4 | (36) | |
| miR-361-3p | Rptor | Regulatory associated protein of MTOR complex 1 | (59) |
| Map3k9 | Mitogen-activated protein kinase kinase kinase 9 | (33,34) | |
| miR-322-3p | Mettl9 | Methyltransferase like 9 | (60) |
| Ptpre | Protein tyrosine phosphatase receptor type E | (37) | |
| miR-3103-3p | Nfrkb | Nuclear factor related to κB binding protein | (35) |
| Arhgap 30 | Rho GTPase-activating protein 30 | (58) | |
| miR-433-3p | Creb1 | cAMP responsive element binding protein 1 | (61) |
| Map2 | Microtubule-associated protein 2 | (62) |
Target genes related to osteogenic differentiation and bone metabolism are listed. miR, microRNA.
Comparison of differentially expressed miRNAs
From the results of the miRNA microarray of the bone tissues and MLO-Y4 osteocytes, 19 miRNAs with the same expression trends in both bones and osteocytes were selected. These miRNAs were analyzed in bone tissues and MLO-Y4 osteocytes using RT-qPCR. The results indicated that each of the eight differentially expressed miRNAs in both the bone tissues and MLO-Y4 osteocytes had the same expression trend; four miRNAs were upregulated and four miRNAs were downregulated (Table II). The target genes of the eight differentially expressed miRNAs were predicted and Table III presents the osteogenic differentiation- and bone metabolism-related target genes, such as dickkopf homolog 2, wingless-related MMTV integration site 2b (Wnt2b), frizzled homolog 5, transforming growth factor β receptor, cAMP responsive element binding protein 1.
Table II.
Differentially expressed miRNAs in both bone tissue and in osteocytes with the same expression level trend, as indicated by miRNA microarray and quantitative PCR.
| A, Upregulated | |
|---|---|
| miRNA | miRbase accession no. |
| miR-5118 | MIMAT0020626 |
| miR-433-3p | MIMAT0001420 |
| miR-190a-5p | MIMAT0000220 |
| miR-470-5p | MIMAT0002111 |
| B, Downregulated | |
| miRNA | miRbase accession no. |
| miR-3082-5P | MIMAT0014872 |
| miR-6348 | MIMAT0025091 |
| miR-669-5P | MIMAT0017346 |
| miR-32-3p | MIMAT0017050 |
The osteocytes were stimulated with mechanical tensile strain (2,500 µε, 0.5 Hz, 8 h). miRNA/miR, microRNA.
Table III.
Predicted target genes of differentially expressed miRNAs both in bone tissue and osteocytes.
| miRNA | Target gene | Gene description | (Refs.) |
|---|---|---|---|
| miR-5118 | DKK2 | Dickkopf homolog 2 | (63) |
| Wnt2b | Wingless related MMTV integration site 2b | (51) | |
| Fzd5 | Frizzled homolog 5 | (64) | |
| Tgfbr1 | Transforming growth factor β receptor | (40,41) | |
| miR-433-3p | Creb1 | cAMP responsive element binding protein 1 | (61) |
| Map2 | Microtubule-associated protein 2 | (62) | |
| miR-190a-5p | Smad2 | SMAD family member 2 | (39,47) |
| miR-470-5p | DKK2 | Dickkopf homolog 2 | (63) |
| ATP2b1 | Plasma membrane calcium ATPase | (59) | |
| Runx2 | Runt related transcription factor 2 | (65) | |
| miR-3082-5p | Gria4 | Glutamate receptor ionotropic AMPA4 | (66,67) |
| Creb1 | cAMP responsive element binding protein 1 | (61) | |
| miR-6348 | Map3k12 | Activated protein kinase kinase kinase 12 | (33,34) |
| Dnm3 | Dynamin 3 | (68) | |
| miR-669-5p | Creb1 | cAMP responsive element binding protein 1 | (61) |
| Cask | Calcium/calmodulin-dependent serine protein kinase | (69) | |
| miR-32-3p | Tgfbr1 | Transforming growth factor β receptor | (40,41) |
| ATP13a3 | ATPase type 13A3 | (70,71) | |
| Dnm3 | Dynamin 3 | (68) |
Target genes related to osteogenic differentiation and bone metabolism are listed. miRNA/miR, microRNA.
Discussion
Bone is an important structure that bears mechanical loading and has a vital role in maintaining mineral homeostasis (22). Mechanical loading, particularly dynamic loading, is a major determinant in the regulation of the morphology and architecture of bone (13,23). Suitable mechanical loading prevents bone loss or promotes bone formation, and the absence of suitable mechanical loading results in a decline in bone mass (24). Exercise produces mechanical loading, which reduces bone loss, increases bone strength and prevents osteoporosis in aging individuals (25,26).
The effects of exercise on the structure and metabolism of bone tissue, such as cytokines and signaling pathways, have been studied at the cellular and molecular levels. However, only a limited number of studies have investigated the involvement of miRNAs induced by exercise in regulating bone metabolism and osteoblastic differentiation, such as mechanically-induced overexpression of miR-214 not only inhibited the expression of these osteogenic factors, but also attenuated mechanical strain-enhanced osteogenesis in osteoblasts (14).
Running is a normal form of exercise; it applies a dynamic mechanical loading to the bone tissues, particularly the femur and the tibia tissues, which is beneficial for bone health (27). In the present study, mice were forced to run on a treadmill. After 8 weeks of running exercise, it was determined that running training increased the mechanical properties of the femur, and increased the activity of ALP and level of OCN in the bone tissues. ALP activity and OCN levels are important markers of osteogenic differentiation (28,29), and these markers are related to the deposition and mineralization of bone matrix (30). The present results indicated that treadmill training promoted the maturation and differentiation of bone tissue and was beneficial for bone health, which was consistent with the results of dynamic load countering ovariectomy-induced osteoporosis in rats (31) and mechanical stretch increasing osteogenesis-related protein expression in the bones of ovariectomized rats (32).
Subsequently, nine of the numerous differentially expressed miRNAs identified in the screening by miRNA microarray were selected for verification via RT-qPCR analysis, and the target genes of the nine miRNAs related to osteogenic differentiation were predicted. As an example, one target gene of miR-316-3p, Map3k9, is a MAPK signaling molecule (33). MAPKs function to regulate the key transcriptional mediators of osteoblast differentiation, with ERK and p38 MAPKs phosphorylating runt-related transcription factor 2, the master regulator of osteoblast differentiation. ERK also activates ribosomal S6 kinase 2, which in turn phosphorylates activating transcription factor 4, a transcriptional regulator of late-stage osteoblast synthetic functions (34). One target gene of miR-3103-3p, nuclear factor related to kB binding protein, is related to the NF-κB signaling pathway (35). The transcription factor NF-κB is a member of a family of proteins involved in signaling pathways that are essential for normal cellular functions and development (35). Deletion of various components of this pathway results in abnormal skeletal development, research in the last decade has indicated that NF-κB signaling mediates RANK ligand-induced osteoclastogenesis (35). Plasma membrane Ca2+-transporting ATPase 4, a target gene of miR-449a-5p, has been proven to be overexpressed during the maturation or senescence of bone tissue cells (36). In addition, protein tyrosine phosphatase receptor type E, a target gene of miR-322-3p, belongs to the protein tyrosine kinase family and overexpression of this kinase may promote osteogenic differentiation (37). These target genes and their signaling pathways have been confirmed to be related to osteogenic differentiation or bone metabolism.
In addition, the differentially expressed miRNAs in the bones of the exercised mice were compared with the differentially expressed miRNAs in mechanically strained osteocytes in vitro. A total of eight differentially expressed miRNAs in both bone tissues and osteocytes were identified, and each of these miRNAs had the same expression trend in bone tissues and osteocytes. Of these, four miRNAs (miR-5118, miR-433-3p, miR-190a-5p and miR-470-5p) were upregulated and four miRNAs (miR-3082-5p, miR-6438, miR-669-5p and miR-32-3p) were downregulated, and these results were confirmed via miRNA microarray and RT-qPCR. Furthermore, the target genes of the eight differentially expressed miRNAs were predicted and certain target genes were confirmed to be related to osteogenic differentiation or bone metabolism. For instance, Wnt2b, one of the target genes of miR-5118, is a molecule of the Wnt/β-catenin signaling pathway and activation of Wnt/β-catenin signaling may promote osteogenic differentiation and increase mechanically induced bone formation (38). Smad2, a target gene of miR-190a-5p, is a Smad signaling molecule and mechanical stress rapidly inhibits Smad3 phosphorylation, thereby inhibiting the activity of the key transforming growth factor-β (TGF-β) effectors Smad2 and Smad3 in osteocytes, and maintaining bone homeostasis via TGF-β signaling (39). In the present study, downregulation of miR-669m-5p expression was associated with one of its target genes, TGF-β receptor 1, and the reduction in TGF-β signaling, through its effector SMAD3, enhanced the mechanical properties and mineral concentration of the bone matrix as well as the bone mass (40,41).
The present results indicated that exercise-induced differentially expressed miRNAs in the bone most likely regulate bone metabolism and osteoblastic differentiation, and the same miRNAs that were differentially expressed in both mechanically stimulated osteocytes in vitro and in the bone tissues of mice subjected to exercise on a treadmill, also probably regulate bone metabolism and osteoblastic differentiation.
As terminally differentiated cells, osteocytes that are mechanically stimulated produce factors such as proteins, peptides and signaling molecules that regulate osteogenic differentiation or bone metabolism (42,43). Mechanical stimuli, such as fluid shear stress, increase the number of exosomes released by osteocytes, these exosomes contain factors such as sclerotinins, NF-κB receptor activators and osteoprotegerin, which regulate osteogenic differentiation (44,45). Certain osteocyte-derived miRNAs, such as miR-218, may influence osteoblastic differentiation (46). Therefore, in the present study, it was hypothesized that these differentially expressed miRNAs in mechanically stimulated osteocytes in vitro and in bone tissue of treadmill trained mice likely regulate osteogenic differentiation or bone metabolism. It is hypothesized that this regulatory mechanism may be via exosomes, thus in the future, how these miRNAs regulate osteogenic differentiation or bone metabolism through exosomes will be studied.
In conclusion, treadmill training resulted in the differential expression of miRNAs in the bone tissues of mice. Certain differentially expressed miRNAs were also observed in osteocytes and these miRNAs probably have an important role in the regulation of bone metabolism and osteogenic differentiation.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the National Nature Science Foundation of China (grant nos. 31660261 and 32071309) and the Natural Science Foundation of Guangxi (grant nos. 2018JIA140050 and 2018GXNSFAA281357).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The datasets generated and/or analyzed during the current study are available in the Gene Expression Omnibus repository, [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE179199 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE179201].
Authors' contributions
YG and JG designed the study, analyzed the data and participated in the bioinformatics analysis. HY and ZC performed all the RT-qPCR analyses at Guangzhou RiboBio Co., Ltd., (experimental equipment and reagents were provided by Guangzhou RiboBio Co., Ltd.)., and assays for ALP activity and OCN levels, and participated in the animal experiments. YW and JW performed the miRNA microarray at Guangzhou RiboBio Co., Ltd., (experimental equipment and reagents were provided by Guangzhou RiboBio Co., Ltd.), and performed the animal experiments. BH, FY and YQ performed the bioinformatics analysis and detected the mechanical properties of the mouse femur bones. YG, HY and ZC wrote and revised the manuscript. All authors have read and approved the final manuscript. YG, JG, HY, ZC, YW, JW, BH, FY and YQ confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study involved animals and was approved by the Animal Ethics Committee of Guilin Medical University (Guilin, China; approval no. 2019-0013). All of the authors declare that the experiments complied with the current laws of China (Guangxi) where they were performed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Belavý DL, Baecker N, Armbrecht G, Beller G, Buehlmeier J, Frings-Meuthen P, Rittweger J, Roth HJ, Heer M, Felsenberg D. Serum sclerostin and DKK 1 in relation to exercise against bone loss in experimental bed rest. Bone Miner Metab. 2016;34:354–365. doi: 10.1007/s00774-015-0681-3. [DOI] [PubMed] [Google Scholar]
- 2.Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: A systematic review and meta analysis. JAMA. 2017;318:2466–2482. doi: 10.1001/jama.2017.19344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamazaki S, Ichimura S, Iwamoto J, Takeda T, Toyama Y. Effect of walking exercise on bone metabolism in postmenopausal women with osteopenia/osteoporosis. Bone Miner Metab. 2004;22:500–508. doi: 10.1007/s00774-004-0514-2. [DOI] [PubMed] [Google Scholar]
- 4.Wen HJ, Huang TH, Li TL, Chong PN, Ang BS. Effects of short-term step aerobics exercise on bone metabolism and functional fitness in postmenopausal women with low bone mass. Osteoporos Int. 2017;28:539–547. doi: 10.1007/s00198-016-3759-4. [DOI] [PubMed] [Google Scholar]
- 5.Vainionpää A, Korpelainen R, Väänänen HK, Haapalahti J, Jämsä T, Leppäluoto J. Effect of impact exercise on bone metabolism. Osteoporos Int. 2009;20:1725–1733. doi: 10.1007/s00198-009-0881-6. [DOI] [PubMed] [Google Scholar]
- 6.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC, Zemel BS. The national osteoporosis foundation's position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos Int. 2016;27:1281–1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polidoulis I, Beyene J, Cheung AM. The effect of exercise on pQCT parameters of bone structure and strength in postmenopausal women-a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2012;23:39–51. doi: 10.1007/s00198-011-1734-7. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto J, Yeh JK, Aloia JF. Differential effect of treadmill exercise on three cancellous bone sites in the young growing rat. Bone. 1999;24:163–169. doi: 10.1016/s8756-3282(98)00189-6. [DOI] [PubMed] [Google Scholar]
- 9.Ma T, Li SC, Liang XX, Luo J, Li ZX. Effects of uphill or downhill running on bone mineral density and the indexes of bone histomorphometry in ovariectomized mice. Sports Sci. 2011;31:48–55. (In Chinese) [Google Scholar]
- 10.Ma T, Li SC. Effect of uphill or downhill running on the osteoclast differentiation of ovariectomized mice. Chin J Sports Med. 2015;34:468–474. (In Chinese) [Google Scholar]
- 11.Borer KT, Zheng Q, Jafari A, Javadi S, Kernozek T. Nutrient intake prior to exercise is necessary for increased osteogenic marker response in diabetic postmenopausal women. Nutrients. 2019;11(1494) doi: 10.3390/nu11071494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai Y, Tyagi SC, Tyagi N. Cross-talk of microRNA and hydrogen sulfide: A novel therapeutic approach for bone diseases. Biomed Pharmacother. 2017;92:1073–1084. doi: 10.1016/j.biopha.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanyon LE. Control of bone architecture by functional load bearing. Bone Miner Res. 1992;7 (Suppl 2):S369–S375. doi: 10.1002/jbmr.5650071403. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Y, Guo J, Zhang L, Tong X, Zhang S, Zhou X, Zhang M, Chen X, Lei L, Li H, et al. miR-214 attenuates the osteogenic effects of mechanical loading on osteoblasts. Int J Sports Med. 2019;40:931–940. doi: 10.1055/a-1015-0285. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Sun Z, Wang Y, Hu Z, Zhou H, Zhang L, Hong B, Zhang S, Cao X. MiR-33-5p, a novel mechano-sensitive microRNA promotes osteoblast differentiation by targeting Hmga2. Sci Rep. 2016;6(23170) doi: 10.1038/srep23170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwawaki Y, Mizusawa N, Iwata T, Higaki N, Goto T, Watanabe M, Tomotake Y, Ichikawa T, Yoshimoto K. miR-494-3p induced by compressive force inhibits cell proliferation in MC3T3-E1 cells. J Biosci Bioeng. 2015;120:456–462. doi: 10.1016/j.jbiosc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Chen N, Sui BD, Hu CH, Cao J, Zheng CX, Hou R, Yang ZK, Zhao P, Chen Q, Yang QJ, et al. MicroRNA-21 contributes to orthodontic tooth movement. J Dent Res. 2016;95:1425–1433. doi: 10.1177/0022034516657043. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Jia L, Zheng Y, Li W. Bone remodeling induced by mechanical forces is regulated by miRNAs. Biosci Rep. 2018;38(BSR20180448) doi: 10.1042/BSR20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Wang K, Hu Z, Zhou H, Zhang L, Wang H, Li G, Zhang S, Cao X, Shi F. MicroRNA-139-3p regulates osteoblast differentiation and apoptosis by targeting ELK1 and interacting with long noncoding RNA ODSM. Cell Death Dis. 2018;9(1107) doi: 10.1038/s41419-018-1153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: Master orchestrators of bone. Calcif Tissue Int. 2013;94:5–24. doi: 10.1007/s00223-013-9790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng QC, Wang Y, Gao J, Yan ZX, Li ZH, Zou XQ, Li YN, Wang JH, Guo Y. miR-29b-3p regulated osteoblast differentiation via regulating IGF-1 secretion of mechanically stimulated osteocytes. BioMed Central. 2019;24(11) doi: 10.1186/s11658-019-0136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosley JR. Osteoporosis and bone functional adaptation: Mechanobiological regulation of bone architecture in growing and adult bone, a review. J Rehabil Res Dev. 2000;37:189–199. [PubMed] [Google Scholar]
- 23.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. Bone Joint Surg Am. 1984;66:397–402. [PubMed] [Google Scholar]
- 24.Hillam RA, Skerry TM. Inhibition of bone resorption and stimulation of formation by mechanical loading of the modeling rat ulna in vivo. J Bone Miner Res. 1995;5:683–689. doi: 10.1002/jbmr.5650100503. [DOI] [PubMed] [Google Scholar]
- 25.Yoshiya S. The effects of exercise and sports activities on bone and joint morbidities. Clin Calcium. 2017;27:39–43. (In Japanese) [PubMed] [Google Scholar]
- 26.Singh MA. Physical activity and bone health. Aust Fam Physician. 2004;33(125) [PubMed] [Google Scholar]
- 27.Santos L, Elliott-Sale KJ, Sale C. Exercise and bone health across the lifespan. Biogerontology. 2017;18:931–946. doi: 10.1007/s10522-017-9732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang WG, Koh JT, Kim EJ. Tunicamycin negatively regulates BMP2-induced osteoblast differentiation through CREBH expression in MC3T3E1 cells. BMB Rep. 2011;44:735–740. doi: 10.5483/BMBRep.2011.44.11.735. [DOI] [PubMed] [Google Scholar]
- 29.Beck GJ Jr, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci USA. 2000;97:8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahalingam CD, Datta T, Patil RV, Kreider J, Bonfil RD, Kirkwood KL, Goldstein SA, Abou-Samra AB, Datta NS. Mitogen-activated protein kinase phosphatase 1 regulates bone mass, osteoblast gene expression, and responsiveness to parathyroid hormone. J Endocrinol. 2011;211:145–156. doi: 10.1530/JOE-11-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Li RX, Wan ZM, Xu C, Li JY, Hao QX, Guo Y, Liu L, Zhang XZ. Counter-effect of constrained dynamic loading on osteoporosis in ovariectomized mice. J Biomech. 2013;46:1242–1247. doi: 10.1016/j.jbiomech.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Zhang P, Dai Q, Yang X, Fu R, Jiang L, Fang B. Effect of mechanical stretch on the proliferation and differentiation of BMSCs from ovariectomized rats. Mol Cell Biochem. 2013;382:273–282. doi: 10.1007/s11010-013-1744-1. [DOI] [PubMed] [Google Scholar]
- 33.Cong Q, Jia H, Li P, Qiu S, Yeh J, Wang Y, Zhang ZL, Ao J, Li B, Liu H. P38α MAPK regulates proliferation and differentiation of osteoclast progenitors and bone remodeling in an aging-dependent manner. Sci Rep. 2017;7(45964) doi: 10.1038/srep45964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenblatt MB, Shim JH, Glimcher LH. Mitogen-activated protein kinase pathways in osteoblasts. Annu Rev Cell Dev Biol. 2013;29:63–79. doi: 10.1146/annurev-cellbio-101512-122347. [DOI] [PubMed] [Google Scholar]
- 35.Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int. 2013;24:2377–2386. doi: 10.1007/s00198-013-2313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo JK, Choi SJ, Kim JK. Expression profiles of subtracted mRNAs during cellular senescence in human mesenchymal stem cells derived from bone marrow. Exp Gerontol. 2013;5:464–471. doi: 10.1016/j.exger.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Ye X, Xiao H, Zhu N, Wei C, Sun X, Wang L, Wang B, Yu X, Lai X, et al. PTPN21 overexpression promotes osteogenic and adipogenic differentiation of bone marrow-derived mesenchymal stem cells but inhibits the immunosuppressive function. Stem Cells Int. 2019;2019(4686132) doi: 10.1155/2019/4686132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liedert A, Nemitz C, Haffner-Luntzer M, Schick F, Jakob F, Ignatius A. Effects of estrogen receptor and Wnt signaling activation on mechanically induced bone formation in a mouse model of postmenopausal bone loss. Int J Mol Sci. 2020;21(8301) doi: 10.3390/ijms21218301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen J, Tang SY, Nguyen D, Alliston T. Load regulates bone formation and Sclerostin expression through a TGFβ-dependent mechanism. PLoS One. 2013;8(e53813) doi: 10.1371/journal.pone.0053813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Huang W, Liu H, Zheng Y, Liao L. Mechanical stretching of pulmonary vein stimulates matrix metalloproteinase-9 and transforming growth factor-β1 through stretch-activated channel/MAPK pathways in pulmonary hypertension due to left heart disease model rats. PLoS One. 2020;15(e0235824) doi: 10.1371/journal.pone.0235824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balooch G, Balooch M, Nalla RK, Schilling S, Filvaroff EH, Marshall GW, Marshall SJ, Ritchie RO, Derynck R, Alliston T. TGF-beta regulates the mechanical properties and composition of bone matrix. Proc Natl Acad Sci USA. 2005;102:18813–18818. doi: 10.1073/pnas.0507417102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54:182–190. doi: 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Yan Y, Wang L, Ge L, Pathak JL. Osteocyte-mediated translation of mechanical stimuli to cellular signaling and its role in bone and non-bone-related clinical complications. Curr Osteoporos Rep. 2020;18:67–80. doi: 10.1007/s11914-020-00564-9. [DOI] [PubMed] [Google Scholar]
- 44.Morrell AE, Brown GN, Robinson ST, Sattler RL, Baik AD, Zhen G, Cao X, Bonewald LF, Jin W, Kam LC, Guo XE. Mechanically induced Ca2+ oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Res. 2018;6(6) doi: 10.1038/s41413-018-0007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eichholz KF, Woods I, Riffault M, Johnson GP, Corrigan M, Lowry MC, Shen N, Labour MN, Wynne K, O'Driscoll L, Hoey DA. Human bone marrow mesenchymal stem/stromal cell behaviour is coordinated via mechanically activated osteocyte-derived extracellular vesicles. Stem Cells Transl Med. 2020;9:1431–1447. doi: 10.1002/sctm.19-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin Y, Peng Y, Zhao W, Pan J, Ksiezak-Reding H, Cardozo C, Wu Y, Divieti PP, Bonewald LF, Bauman WA, Qin W. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J Biol Chem. 2017;292:11021–11033. doi: 10.1074/jbc.M116.770941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng W, Chen Q, Zhang Y, Xia R, Gu X, Hao Y, Yu Z, Sun X, Hu D. BMP9 promotes osteogenic differentiation of SMSCs by activating the JNK/Smad2/3 signaling pathway. J Cell Biochem. 2020;121:2851–2863. doi: 10.1002/jcb.29519. [DOI] [PubMed] [Google Scholar]
- 48.Walker LM, Publicover SJ, Preston MR, Said Ahmed MA, El Haj AJ. Calcium-channel activation and matrix protein upregulation in bone cells in response to mechanical strain. J Cell Biochem. 2000;79:648–661. doi: 10.1002/1097-4644(20001215)79:4<648::aid-jcb130>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 49.Uda Y, Azab E, Sun N, Shi C, Pajevic PD. Osteocyte mechanobiology. Curr Osteoporos Rep. 2017;15:318–325. doi: 10.1007/s11914-017-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazur CM, Woo JJ, Yee CS, Fields AJ, Acevedo C, Bailey KN, Kaya S, Fowler TW, Lotz JC, Dang A, et al. Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Res. 2019;7(34) doi: 10.1038/s41413-019-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lerner UH, Ohlsson C. The WNT system: Background and its role in bone. J Intern Med. 2015;277:630–649. doi: 10.1111/joim.12368. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Mohammed A, Oubaidin M, Evans CA, Zhou X, Luan X, Diekwisch TG, Atsawasuwan P. Cyclic stretch and compression forces alter microRNA-29 expression of human periodontal ligament cells. Gene. 2015;566:13–17. doi: 10.1016/j.gene.2015.03.055. [DOI] [PubMed] [Google Scholar]
- 53.Pozio A, Palmieri A, Girardi A, Cura F, Carinci F. Titanium nanotubes activate genes related to bone formation in vitro. Dent Res J (Isfahan) 2012;9 (Suppl 2):S164–S168. doi: 10.4103/1735-3327.109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasui M, Shiraishi Y, Ozaki N, Hayashi K, Hori K, Ichiyanagi M, Sugiura Y. Nerve growth factor and associated nerve sprouting contribute to local mechanical hyperalgesia in a rat model of bone injury. Eur J Pain. 2012;16:953–965. doi: 10.1002/j.1532-2149.2011.00094.x. [DOI] [PubMed] [Google Scholar]
- 55.Shi J, Wang Z, Guo X, Shen J, Sun H, Bai J, Yu B, Wang L, Zhou W, Liu Y, et al. Spirin inhibits osteoclast formation and wear-debris-induced bone destruction by suppressing mitogen-activated protein kinases. J Cell Physiol. 2020;235:599–2608. doi: 10.1002/jcp.29164. [DOI] [PubMed] [Google Scholar]
- 56.Vriend J, Reiter RJ. Melatonin, bone regulation and the ubiquitin-proteasome connection: A review. Life Sci. 2016;145:152–160. doi: 10.1016/j.lfs.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto Y, Rottapel R. Bone dynamics and inflammation: Lessons from rare diseases. Immunol Med. 2020;43:61–64. doi: 10.1080/25785826.2020.1720104. [DOI] [PubMed] [Google Scholar]
- 58.Hamamura K, Swarnkar G, Tanjung N, Cho E, Li J, Na S, Yokota H. RhoA-mediated signaling in mechanotransduction of osteoblasts. Connect Tissue Res. 2012;53:398–406. doi: 10.3109/03008207.2012.671398. [DOI] [PubMed] [Google Scholar]
- 59.Rosselli-Murai LK, Almeida LO, Zagni C, Galindo-Moreno P, Padial-Molina M, Volk SL, Murai MJ, Rios HF, Squarize CH, Castilho RM. Periostin responds to mechanical stress and tension by activating the MTOR signaling pathway. PLoS One. 2013;8(e83580) doi: 10.1371/journal.pone.0083580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorentzon M, Eriksson AL, Nilsson S, Mellström D, Ohlsson C. Association between physical activity and BMD in young men is modulated by catechol-O-methyltransferase (COMT) genotype: The GOOD study. J Bone Miner Res. 2007;22:1165–1172. doi: 10.1359/jbmr.070416. [DOI] [PubMed] [Google Scholar]
- 61.Chen B, Lin T, Yang X, Li Y, Xie D, Cui H. Intermittent parathyroid hormone (1-34) application regulates cAMP-response element binding protein activity to promote the proliferation and osteogenic differentiation of bone mesenchymal stromal cells, via the cAMP/PKA signaling pathway. Exp Ther Med. 2016;11:2399–2406. doi: 10.3892/etm.2016.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin C, Zhang Y, Hu L, Tian Y, Chen Z, Li D, Zhao F, Su P, Ma X, Zhang G, et al. Mechanical unloading reduces microtubule actin crosslinking factor 1 expression to inhibit β-catenin signaling and osteoblast proliferation. J Cell Physiol. 2018;233:5405–5419. doi: 10.3892/etm.2016.3177. [DOI] [PubMed] [Google Scholar]
- 63.Olivares-Navarrete R, Hyzy S, Wieland M, Boyan BD, Schwartz Z. The roles of Wnt signaling modulators Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state in osteogenesis on microstructured titanium surfaces. Biomaterials. 2010;313:2015–2024. doi: 10.1016/j.biomaterials.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leijten JC, Bos SD, Landman EB, Georgi N, Jahr H, Meulenbelt I, Post JN, van Blitterswijk CA, Karperien M. GREM1, FRZB and DKK1 mRNA levels correlate with osteoarthritis and are regulated by osteoarthritis-associated factors. Arthritis Res Ther. 2013;15(R126) doi: 10.1186/ar4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galea GL, Paradise CR, Meakin LB, Camilleri ET, Taipaleenmaki H, Stein GS, Lanyon LE, Price JS, van Wijnen AJ, Dudakovic A. Mechanical strain-mediated reduction in RANKL expression is associated with RUNX2 and BRD2. Gene X. 2020;5(100027) doi: 10.1016/j.gene.2020.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szczesniak AM, Gilbert RW, Mukhida M, Anderson GI. Mechanical loading modulates glutamate receptor subunit expression in bone. Bone. 2005;37:63–73. doi: 10.1016/j.bone.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Lin TH, Yang RS, Tang CH, Wu MY, Fu WM. Regulation of the maturation of osteoblasts and osteoclastogenesis by glutamate. Eur J Pharmacol. 2008;589:37–44. doi: 10.1016/j.ejphar.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 68.Eleniste PP, Huang S, Wayakanon K, Largura HW, Bruzzaniti A. Osteoblast differentiation and migration are regulated by dynamin GTPase activity. Int J Biochem Cell Biol. 2014;46:9–18. doi: 10.1016/j.biocel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naghii MR, Torkaman G, Mofid M. Effects of boron and calcium supplementation on mechanical properties of bone in rats. Biofactors. 2006;28:195–201. doi: 10.1002/biof.5520280306. [DOI] [PubMed] [Google Scholar]
- 70.Nakano Y, Forsprecher J, Kaartinen MT. Regulation of ATPase activity of transglutaminase 2 by MT1-MMP: Implications for mineralization of MC3T3-E1 osteoblast cultures. Cell Physiol. 2010;223:260–269. doi: 10.1002/jcp.22034. [DOI] [PubMed] [Google Scholar]
- 71.Sun D, Junger WG, Yuan C, Zang W, Bao Y, Qin D, Wang C, Tan L, Qi B, Zhu D, et al. Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem Cells. 2013;31:1170–1180. doi: 10.1002/stem.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The datasets generated and/or analyzed during the current study are available in the Gene Expression Omnibus repository, [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE179199 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE179201].