Abstract
Peripheral nerve sheath tumors such as neurofibroma, comprise 5% of all benign soft tissue tumors and usually occur due to an underlying neurofibromatosis. A plexiform neurofibroma, which is a tumor occurring exclusively in neurofibromatosis1, is a rare entity and is an uncommon variant of neurofibroma. We report the clinical and imaging features of plexiform neurofibroma in two young male patients, in whom the imaging diagnosis was confirmed after biopsy. The report not only aims to highlight the characteristic imaging features of plexiform neurofibroma but we also emphasize the ultrasound appearances which are significantly characteristic and can effectively lead to the correct diagnosis at the preliminary stage of investigation. The tumors which originate from nerve sheath, are large, lobulated masses and demonstrate typical imaging features of simultaneous involvement of subcutaneous and cutaneous tissues along with infiltrative invasion of deeper structures. The tumors characteristically display fat and fluid contents and a “target sign’ on evaluation by ultrasound, CT and MRI. Imaging plays an important role in confirming the diagnosis, delineating involved structures, excluding simulating conditions and forewarning a possible malignant transformation.
Keywords: Peripheral nerve sheath tumor, Neurofibromatosis, Plexiform neurofibroma, Ultrasound, Target sign, CT, MRI
Introduction
Peripheral nerve sheath tumors, namely neurofibroma, comprise 5% of all benign soft tissue tumors [1]. Neurofibromas may be single or multiple and majority of these occur due to underlying neurofibromatosis (NF), the etiology for which is a mutation in the NF1 gene (2-4). A plexiform neurofibroma, which is a tumor occurring exclusively in NF I, is an uncommon variant of a benign neurofibroma, which arises by a disorderly proliferation of all neural elements of a peripheral nerve. On histology, the endoneural matrix is found to be increased, with separation of nerve fascicles and proliferation of Schwann cells [2,3]. The nomenclature aptly describes not only the pathology of this entity but also its origin. The term “plexus” or plexiform (one with features of a plexus) denotes a complex network of interlacing or interwoven blood vessels or nerves, as exist in this entity. The term "plexiform" is derived from the Latin verb "plex", which means to interweave or braid or plait together.
Neurofibromas are known to manifest most frequently as localized lesions, less frequently as a diffuse form, and rarely as a plexiform variety [1,2]. The localized form, which is the most common manifestation, is familiar to Radiologists, as the imaging appearances have been extensively reported and documented [1]. However, the plexiform variety which is defined as one arising from multiple nerves and their branches and infiltrating the surrounding soft tissue and skin, has been less frequently described in radiology literature. The clinical concern in a plexiform neurofibroma is not only towards the significant cosmetic disfigurement, and compression of the adjoining vital structures, but also due to its potential towards malignant transformation, which occurs in approximately 10% of cases [2].
We report the clinical and imaging features of two young male patients with biopsy proven plexiform neurofibroma (PNF). The report not only aims to highlight the characteristic imaging features of PNF but we also emphasize the ultrasound appearances, which are significantly characteristic and can effectively lead to the correct diagnosis at the preliminary stage of investigation.
Case reports
Case 1
[This patient was seen and evaluated at Author(1)’s prior Institute]
A 14 year old boy presented with a 3-year history of progressively increasing hirsutism, skin darkening and disfiguring ‘bulges’ over the right occipital, temporal, facial, cervical and supraclavicular regions. He also complained of an abnormal bony protrusion over the sternal region. Clinical examination revealed extensive ‘cafe-au-lait’ spots involving the entire involved regions (Fig. 1). On palpation the mass was reported as feeling like a ‘bag of worms’. The clinical examination suggested a diagnosis of neurofibromatosis with neural and vascular abnormalities. The patient was referred for ultrasound evaluation to assess the varying components of the soft tissue tumor. On sonographic evaluation, there was a mixed echogenicity tumor located in the subcutaneous and cutaneous planes, which was extending from the occipital, pre-auricular, parotid, submandibular into the cervical, supraclavicular and shoulder regions. The tumor was comprised of multiple hypoechoic tubulars, serpiginous, and spherical foci on a hyperechoic background and a few of them showed a hypoechoic focal nodule surrounded by a hyperechoic periphery (“target sign”) (Fig. 2). On color Doppler, there was peripheral and central vascularity, with normally tapering vessels, high resistance flow. The tubular and spherical hypoechoic foci of the tumor did not demonstrate vascularity (Fig. 2). A CT scan was obtained for further evaluation, revealing a lobulated tumor, which was hypoattenuating relative to muscle and located in the subcutaneous and cutaneous planes of the right preauricular, parotid, cervical, supraclavicular, retrosternal, and shoulder regions (Fig. 3). There was associated remodeling of the right clavicle and right aspect of the manubrium. (Fig. 3). On MRI, the T2W and STIR sequences revealed a heterogeneously hyperintense, infiltrating, soft tissue tumor in the in the subcutaneous and cutaneous planes of the right infra temporal fossa, facial region, cervical region, supraclavicular and shoulder region and was displacing the rotator cuff muscles infero-laterally (Fig. 4). The tumor comprised of multiple, scattered, spherical and tubular foci as internal components (Fig. 4). Coronal scans revealed the tumor components to involve/originate from a number of branches and fascicles of the right C2 to C6 nerves (Fig. 4). A large number of the round foci showed a typical “target sign” with a central hypo-intensity surrounded by a hyper-intense signal (Fig. 5). On T1 W sequences, the lesion showed a mixed pattern of hyperintense background with internal foci showing hypointense signal to the adjoining muscles (Fig. 5). The gamut of imaging appearances was characteristic for a plexiform neurofibroma and an imaging guided biopsy confirmed the same. The histopathology examination revealed multiple areas of collagen around nerve bundles and multiple tightly packed serpentine nuclei, which were diagnostic of the entity (Fig. 6). The patient was advised multi stage neurosurgical resection with cosmetic reconstruction and genetic screening for self and family. Unfortunately, despite adequate counselling, the parents refused to accept any further intervention and further follow up was therefore not possible.
Fig. 1.
Clinical appearance of patient number 1 (a boy of 14 y), shows excessive hirsutism on the right facial and neck regions (white arrows in A, B), with café au lait pigmentation over the preauricular, facial and shoulder regions on the right side (blue arrows in a, red arrows in C). The manubrium is protuberant (green arrow in B) (Color version of figure is available online.)
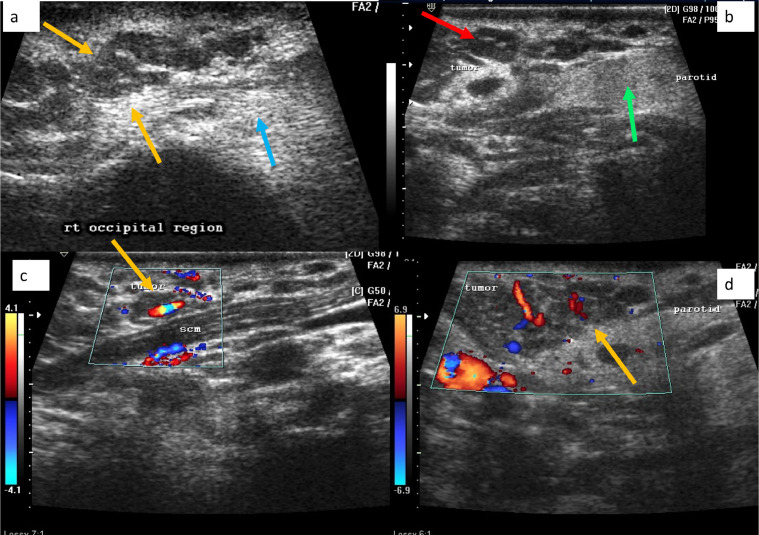
Fig. 2.
Ultrasound of right head and neck region in patient no 1, shows an ill-defined hypo-echoeic tumor, comprising of tubular, serpiginous foci (yellow arrow in A), surrounded by an echogenic background (blue arrow in A), located in the cutaneous and subcutaneous regions. The tumor was abutting the parotid gland (green arrow in B) and a target sign was seen (red arrow in B). Color Doppler evaluation revealed good vascularity within the tumor (yellow arrows in C, D) (Color version of figure is available online.)
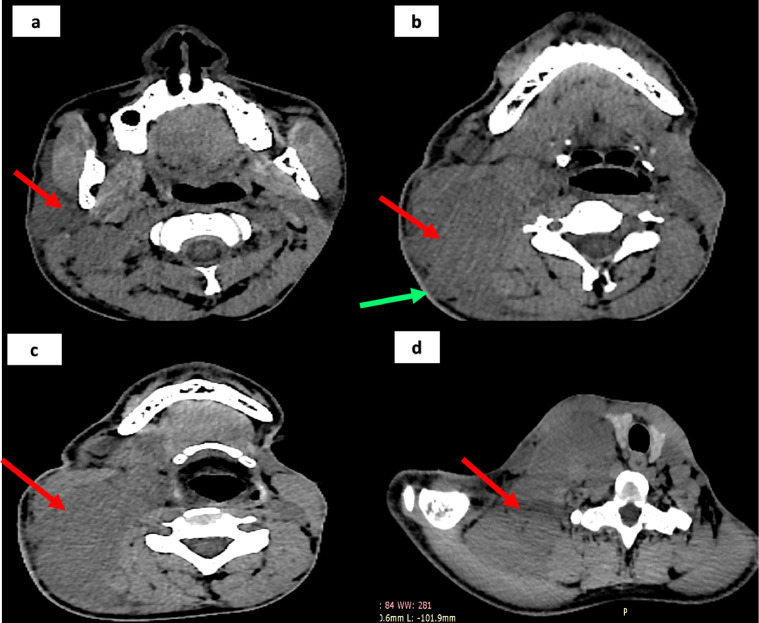
Fig. 3.
CT scan of the skull base, head and neck region, in patient no 1, shows a lobulated, hypodense tumor in the occipital, facial and neck regions, which is largely cutaneous and subcutaneous in location (yellow arrow in a, green arrows in B). The tumor at the thoracic inlet, insinuates behind the clavicle (red arrow in C). the sternum and clavicle show modelling deformities (white arrows in D, green arrows in E) (Color version of figure is available online.)
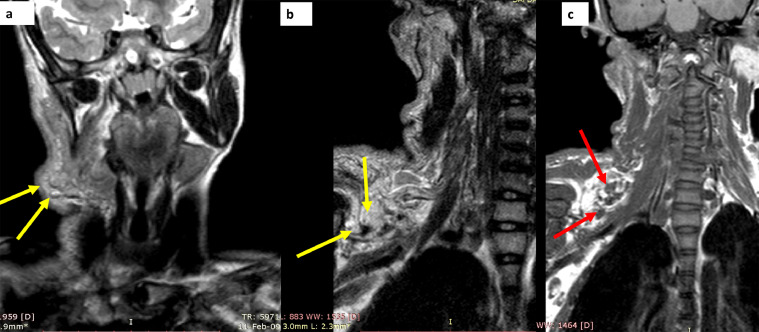
Fig. 4.
MRI study in patient no 1, obtained as T2 W sequences in the neck and brachial plexus region, shows a homogenously hyper-intense, lobulated tumor over the occipital, submandibular and cervical region (yellow arrows in A, B). the tumor is both superficial and deep to the sternocleidomastoid and shows a target sign in (green arrows in B). The tumor appears to be arising from the C3 to C5 nerve roots (red arrows in C) (Color version of figure is available online.)
Fig. 5.
MRI study in patient no 1, obtained as T2 W sequences in the neck and brachial plexus region, shows a homogenously hyper-intense, lobulated tumor over the occipital, submandibular and cervical regions, which is largely subcutaneous and cutaneous in location. The tumor has multiple spherical foci which display a target sign (yellow arrows in A, B). The tumor is hypointense to muscle on T1W sequences (red arrows in C) (Color version of figure is available online.)
Fig. 6.
Biopsy image low power (A) and high power (B) of patient number 1, using H and E stain shows nerve bundles (blue arrows) surrounded by collagenous tissue (black arrow). Multiple tightly packed serpentine nuclei are also seen (green arrows). Conforming the diagnosis of plexiform neurofibroma (Color version of figure is available online.)
Case no 2
[This patient was seen and evaluated at Author (1)’s current institute]
A 20-year-old male, presented with the complaints of a progressively enlarging soft tissue mass, over the right facial, neck and axillary regions for the last 2 years. The patient was referred for sonographic evaluation with a clinical diagnosis of extensive cervical lymphadenopathy. Ultrasound performed over the involved regions, using a high frequency linear probe, revealed a large tumor, which comprised of multiple, lobulated, soft tissue masses with infiltrative margins, which had a hyperechoeic background intermixed with hypoechoic foci of spherical shape, located largely in the cutaneous and sub cutaneous plane (Fig. 7). There was negligible vascularity on color Doppler flow imaging, even at the lowest scale and also on power Doppler, which therefore ruled out a vascular malformation. The sonographic appearances suggested a complex soft tissue tumor and the possibility of a neurogenic origin was considered. A CT scan was obtained for further evaluation. The study revealed a hypodense tumor involving the right infra temporal, parotid, submandibular, cervical, supra clavicular and axillary regions. In the neck the tumor was both superficial and deep to the sternocleidomastoid muscle. At the thoracic inlet, the tumor had infiltrated the superior mediastinal compartment and involved the para-tracheal and para-vertebral regions. The trachea and adjoining great vessels were displaced to the left side by the tumor. There was no evidence of bony involvement or remodeling (Fig. 8). Further characterization was done by MRI. On T2W, STIR and T2 SPAIR sequences a large, hyperintense, lobulated and infiltrating, multi-loculated tumor was seen, involving the brachial plexus and adjoining the trunk, cord and branches, from C2 to C7 right sided nerve roots. A “target sign” was evident in a few foci of the tumor in the neck region. The tumor had an isointense signal to adjacent (muscles) on T1WI (Figs. 9 and 10). The imaging features were consistent with plexiform neurofibroma. A biopsy of the lesion showed spindle cells with elongated serpentine nuclei deposited in a collagen matrix and fibroblasts, around a nerve. Occasional mast cells were also seen. Mitotic activity and nuclear atypia were absent with features consistent with neurofibroma (Fig. 11). S 100 testing was subsequently performed and found to be positive. As in the previous patient, this patient was also advised multi stage neurosurgical resection with cosmetic reconstruction and genetic screening for self and family. However, despite vigorous counselling, the patient refused to accept any further intervention and was lost to further follow up.
Fig. 7.
Ultrasound examination over the head and neck region in patient no2, shows a multilobulated tumor comprising of spherical and geographic hypoechoeic foci (red arrows in A, B) on a hyperechoeic background (blue arrow in A). The tumor is deep to sternocleidomastoid (green arrow in B) (Color version of figure is available online.)
Fig. 8.
NCCT of head, neck and upper thorax in patient number 2, shows a homogenously hypodense tumor, located in the parotid, oropharyngeal, para-pharyngeal, thoracic inlet regions (red arrows in A, B, C and D). the tumor has a lobulated surface (green arrows in B). it displaces the thyroid and trachea across the midline (D) (Color version of figure is available online.)
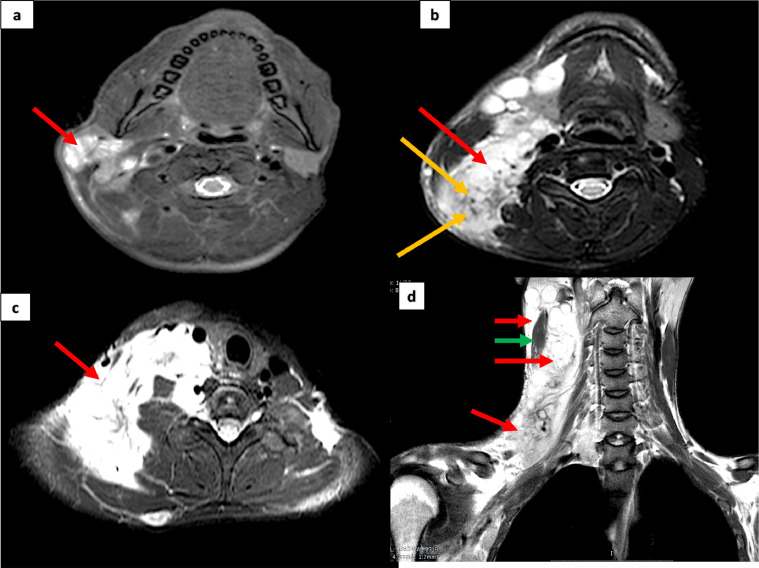
Fig. 9.
MRI examination of patient number 2, over the head, neck and brachial plexus region, using T2W sequences, shows a large homogenously hyper-intense tumor occupying the examined areas, in the superficial as well as deeper planes (red arrows in A, B, C, D). A target sign is visible in B (yellow arrows). The tumor is both superficial and deep to sternocleidomastoid (green arrow in D) (Color version of figure is available online.)
Fig. 10.
MRI examination of patient number 2, over the head, neck and brachial plexus region, using STIR sequences, shows a large homogenously hyper-intense tumor in the superficial as well as deeper planes of the examined areas and extending into the axilla (red arrows in A, B). A target sign is seen in the supraclavicular region (green arrows in A) (Color version of figure is available online.)
Fig. 11.
Biopsy images of patient no 2, using H and E stain, the low power (A), high power view (B, C) shows: serpentine nuclei marked with green arrow (in B), with a collagenous background seen around the nerve bundles (green arrows in C). Appearances are consistent with neurofibroma (Color version of figure is available online.)
Discussion
Neurofibromatosis (NF) is an autosomal dominant phakomatoses or neurocutaneous syndrome which is clinically diagnosed by the typical presence of café-au-lait macules, intertriginous freckling, Lisch nodules and neural tumors such as neurofibromas and gliomas [4], [5], [6]. The disorder occurs in 1/4,000 births and is inherited or may occur by spontaneous genetic mutation [3]. There are two distinctly recognized varieties of neurofibromatosis, NF I and NF II, based on differences in clinical and genetic features [4,6,7]. Type I disease, also known as von Recklinghausen's disease, which occurs due to loss of function of the NF I gene, presents with neurofibromas, café au lait spots, freckling and optic gliomas. NF type II, occurs due to loss of function of the NF II gene, is characterized by bilateral vestibular schwannomas and meningiomas [4].
Neurofibromas which are the most prevalent benign nerve sheath tumors, occur in three forms: localized, diffuse and plexiform. The former usually presents as a skin-colored papule or a subcutaneous nodule and arises from the endoneurium and connective tissue of peripheral nerve sheaths [1,2]. Unlike a cutaneous localized neurofibroma, which typically presents during preadolescent life, a plexiform neurofibroma is usually present at birth and grows during early childhood and adolescent period. Approximately 50 percent of them are known to occur in the head, neck and facial regions [3]. On palpation, a plexiform neurofibroma is classically described to have a consistency like a ``bag of worms'' due to the presence of both soft and hard nodular components, comprised of deformed connective tissue and skin folds. [1,[7], [8], [9]. The major concern in affected patients is extensive cosmetic disfigurement and mass effect on organs and vital structures. PNFs can cause ocular defects if they involve the orbits, dysphagia, and dyspnoea due to local mass effect if they involve the neck region, and motor deficits in the limb distal to the nerves they involve. Malignant change which reportedly occurs in 8%-12% of plexiform neurofibromas, is another impending complication.
The role of imaging is important for a variety of reasons, including delineating the extent of involvement and effect on adjacent structures, exposing associated anomalies and last but not least, for predicting possible malignant transformation. The invaluable role of sonography in evaluation of soft tissue tumors and especially plexiform neurofibroma remains rather under-scored in the radiology literature. The role of sonography as a primary modality remains to be recognized not only for the exclusion of simulating conditions at the earliest instance of imaging, but also because a radiation free technique is preferable in the younger population.
The earliest description of the sonographic appearances of plexiform neurofibroma was perhaps by Reuter et al, who described these tumors as comprising of hypoechoic nodules, which needed to be distinguished from an abscess and a vascular malformation [10]. However, a more definitive description was emphasized by Hong et al, who described this entity as a poorly marginated tumor, comprising of multiple hypoechoic nodules on a hyperechoic background with significant vascularity [7]. This appearance described by Hong et al was seen in both of our patients. We further add from the experience in our reported cases, that the description should also include the features of the tumor being multi lobulated, largely located in the cutaneous and subcutaneous planes and that the hypoechoic foci may be serpiginous, spherical or geographic in shape. Furthermore, that the vascular pattern shows a pattern of peripherally attenuating vessels which distinguish this tumor from a vascular malformation. A vascular malformation can also be excluded as this tumor does not show augmentation of out flow and in flow by compression and release respectively, nor does it show low resistance flow.
The CT features of plexiform neurofibroma have been described as being typically low attenuation due to the myelin-lipid content, fat entrapment and high-water content in endoneurial myxoid tissue [11,12]. A similar low attenuation lesion was seen in the subcutaneous and cutaneous planes in both our patients. Moreover, osseous remodeling, a typical feature of neurofibromatosis was revealed in our first patient, who had a deformed right clavicle and manubrium. The role of CT is important to assess bony involvement.
MRI is the reference standard modality for evaluating neural tissues and also for delineating the parent nerve in cases of tumors of neural origin. In plexiform neurofibromas, the tumor has been described as being characteristically lobulated with a hyperintense signal on T2W imaging. A “target sign” has been described as being pathognomonic for plexiform neurofibroma and each “target” focus is believed to depict the individual involved nerve fascicle. The lesion has a central low intensity surrounded by a rim of high intensity, especially when oriented in the longitudinal direction of the nerve [9,11,12]. The central low intensity is due to the fibrous component and the surrounding myxoid elements lend the hyperintense signal. A “reverse target sign” has also been described in plexiform neurofibromas which is characteristically seen on contrast enhanced scans [1]. A similar appearance of a lobulated tumor with multiple foci showing target sign was seen in both our patients on T2W and on fat suppression sequences. The appearance reported on T1W sequences is that of a tumor hypointense to adjoining muscles, which was also seen in both our patients. There is a distinct role for contrast enhanced MRI, to demonstrate peripheral enhancement, if the tumor is suspected to be undergoing malignant transformation [11]. The latter is indicated on non- contrast studies by perilesional edema, intratumoral cystic changes and heterogeneity on T1W images [11]. In both the reported cases there were no such tell-tale signs, therefore contrast studies were not performed.
In cases with MRI signs suggestive of malignant transformation into a malignant peripheral nerve sheath tumor (MPNST), PET–CT is valuable in guiding the site for biopsy as well as for confirming malignancy [11].
Since the clinical stigmata of NF1 and the imaging features of plexiform neurofibroma are characteristic, a differential diagnosis may need to be considered only in those patients who lack overt clinical features. On ultrasound, the tumor may be mistaken for a vascular malformation and the distinguishing features include absence of a normal arborizing pattern and presence of a low resistance flow in the latter. The other entities which need to be excluded are infiltrative soft tissue sarcomas, such as a dermatofibrosarcoma protuberans, plexiform fibrohistiocytic tumor and desmoplastic melanoma [2]. In our experience, on imaging all these tumors will characteristically lack a preponderance of fat and fluid, which is typical in a plexiform neurofibroma.
The recommended treatment is surgical resection and a subtotal approach aimed at debulking of massive tumors is preferred over total excision, for the reasons of morbidity and functional neurological impairment associated with the latter [3,12]. In unresectable and progressive tumors, interferon therapy has been tried [8].
Conclusion
Plexiform neurofibromas are a subset of neural tumors which occur characteristically in patients with NF1. The tumors which originate from nerve sheath, are large, lobulated masses and demonstrate characteristic imaging features of simultaneous involvement of subcutaneous and cutaneous tissues along with infiltrative invasion of deeper structures. The tumors typically display fat and fluid contents on evaluation by ultrasound, CT and MRI due to the fat and fluid content of myelin in the nerve sheath. Imaging plays an important role in confirming the diagnosis, delineating involved structures, excluding simulating conditions and malignant transformation.
Patient consent
The authors confirm that a written informed consent in the local language was obtained from both patient parties for publication of the case report, on the conditions of maintaining anonymity of identity.
Author Contribution
Dr Shabnam Bhandari Grover: Study Design, Data Collection, Review of Literature, Manuscript writing; Dr. Rohit Kundra: Study Design, Data Collection, Review of Literature, Manuscript writing; Dr. Hemal Grover: Review of Literature, Manuscript writing; Dr. Vishal Gupta: Manuscript writing; Dr. Rishab: Manuscript writing.
Footnotes
Funding: The authors confirm that no funding was required or obtained for this study.
Competing interests: None of the authors have any conflicts of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2021.06.025.
Appendix. Supplementary materials
References
- 1.Yilmaz S, Ozolek JA, Zammerilla LL, Fitz CR, Grunwaldt LJ, Crowley JJ. Neurofibromas with imaging characteristics resembling vascular anomalies. AJR Am J Roentgenol. 2014;203(6):W697–W705. doi: 10.2214/AJR.13.12409. PMID: 25415736. [DOI] [PubMed] [Google Scholar]
- 2.Messersmith L, Krauland K. Neurofibroma. 2020 Aug 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. PMID: 30969529.
- 3.Wise JB, Cryer JE, Belasco JB, Jacobs I, Elden L. Management of head and neck plexiform neurofibromas in pediatric patients with neurofibromatosis type 1. Arch Otolaryngol Head Neck Surg. 2005;131(8):712–718. doi: 10.1001/archotol.131.8.712. PMID: 16103304. [DOI] [PubMed] [Google Scholar]
- 4.Le C, Bedocs PM. Neurofibromatosis. 2020 Aug 10. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 29083784.
- 5.Becker B, Strowd RE., 3rd. Phakomatoses. Dermatol Clin. 2019;37(4):583–606. doi: 10.1016/j.det.2019.05.015. PMID: 31466597. [DOI] [PubMed] [Google Scholar]
- 6.Feng Y, Yang ZG, Chen T, Wang Q, Deng W. Giant plexiform neurofibroma with hemorrhage in cranio-maxillofacial region as depicted on CT and MRI. Eur J Med Res. 2010;15(2):84–87. doi: 10.1186/2047-783x-15-2-84. PMID: 20452890PMCID: PMC3352051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong R.-B., Wang T.-G., Chang Y.-L., Wang C.-L., Hsieh F.-J. Sonographic appearance of plexiform neurofibroma of the foot: report of a case. J Med Ultrasound. 2002;10(3):141–145. doi: 10.1016/s0929-6441(09)60032-1. [DOI] [Google Scholar]
- 8.Tchernev G, Chokoeva AA, Patterson JW, Bakardzhiev I, Wollina U, Tana C. Plexiform neurofibroma: a case report. Medicine (Baltimore) 2016;95(6):e2663. doi: 10.1097/MD.0000000000002663. PMID: 26871793PMCID: PMC4753888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand R, Yadav DS, Yadav V, Yadav D, Bhatia D. Plexiform neurofibroma in a 16-year-old girl. Radiol Case Rep. 2015 Dec 7;7(3):708. doi: 10.2484/rcr.v7i3.708. PMID: 27326300PMCID: PMC4899673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuter KL, Raptopoulos V, DeGirolami U, Akins CM. Ultrasonography of a plexiform neurofibroma of the popliteal fossa. J Ultrasound Med. 1982;1(5):209–211. doi: 10.7863/jum.1982.1.5.209. PMID: 6820385. [DOI] [PubMed] [Google Scholar]
- 11.Gosein M, Ameeral A, Banfield R, Mosodeen M. Plexiform neurofibroma of the wrist: imaging features and when to suspect malignancy. Case Rep Radiol. 2013 doi: 10.1155/2013/493752. 2013:493752Epub 2013 Apr 4. PMID: 23691413PMCID: PMC3638521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senhaji G., Gallouj S., Assenhaji I., Baybay H., Fz M. Giant plexiform neurofibroma of the pelvic region: unusual presentation in an uncommon location (about a case) J Dermat Cosmetol. 2018;2(1):20–23. doi: 10.15406/jdc.2018.02.00030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.