Abstract
Objectives:
To determine the rate of second primary lung cancer (SPLC) and describe the clinical characteristics and radiological findings in individuals with a prior history of cancer presenting to a low-dose computed tomography (LDCT) lung cancer screening program at a tertiary cancer centre.
Methods:
Patients with a previous history of malignancy, a life expectancy ≥5 years referred for CT lung cancer screening between May 2, 2011, to November 28, 2018, were included. Demographics regarding risk factors including smoking history and prior history of thoracic radiation were collected. CT scan features assessed nodule size, morphologic features, and number. The Lung-CT Reporting and Data System (Lung-RADS) scoring system was retrospectively applied to studies performed before October 2014 and prospectively applied to remainder of studies. Data was collected in a Health Insurance Portability and Accountability Act (HIPAA)-compliant manner.
Results:
A total of 543 patients were studied (mean age of 66 years). All had a previous history of cancer, most commonly breast cancer 205 (38%), head and neck cancer 105 (19%), and lung cancer 87 (16%). Of screening CTs performed, 17.5% were positive screening study results as per Lung-RADS scoring system. SPLC was diagnosed in 35 patients (6.4%) with 21 prevalence cancers detected and 14 interval cancers detected in subsequent screening rounds.
Conclusions:
The rate of screen-detected SPLC in patients with prior malignancy is higher than reported rates seen in historical prospective screening studies. Our study suggests the need for prospective research to evaluate any mortality benefit that screening may have in this population.
Keywords: Screening; Lung cancer; X-ray computed tomography; Neoplasms, Second Primary
Introduction
Cancer survivorship has increased over the last 30 years. The population of cancer survivors in the United States is currently 17 million, approximately 5% of the total population, and is projected to exceed 21 million by 20291. The current number of cancer survivors in Europe is unknown due to heterogenicity of cancer registries across countries2, however there are more than 3 million new cases of cancer diagnosed in Europe each year3 with approximately half of patients surviving for 10 years or more2. Therefore cancer survivorship is a growing public health issue.
It has been estimated that cancer survivors have a 14% higher risk of developing a second primary malignancy than the general population4, with lung cancer being the most common diagnosis5. Lung cancer occurring as a second primary malignancy represents 8–14% of all lung cancer diagnoses6–8, and the diagnosis of lung cancer is frequently the primary driver of future life expectancy9,10.
Data from previous lung cancer screening trials have provided evidence that low-dose computed tomography (CT) screening can reduce lung cancer mortality; notably, the National Lung Screening Trial (NLST) in United States demonstrated a 20% relative reduction in lung cancer deaths in individuals aged 55 to 74 years with a smoking history of 30 pack-years or greater who underwent annual low-dose CT screening11. Recently, the Dutch-Belgian Randomised Lung Cancer Screening Trial (Dutch acronym: NELSON) demonstrated a 26% reduction in lung cancer deaths at 10 years follow-up in high-risk asymptomatic men who underwent CT lung cancer screening12. These findings have led to more national and international professional associations recommending low-dose CT screening for lung cancer13,14.
Patients with risk factors, aside from age and smoking exposure, have been inconsistently included in lung cancer screening trials to date. Additional risk factors include occupational exposure to carcinogens, and a history of certain cancers (including smoking-related cancers)13,15. In recognition of the potential increased risk in these patients, the National Cancer Comprehensive Network (NCCN) lung cancer screening guidelines have included a second high-risk group for whom screening is recommended: individuals ≥50 years with a smoking history of ≥20 pack-years and with additional risk factors that increase the risk of lung cancer to ≥1.3%13.
There has also been an inconsistent approach to the inclusion of patients with a previous history of malignancy in lung cancer screening studies, some trials excluding them, and others placing limits on their participation. Of note, some previous studies have found that the incidence of second primary lung cancer (SPLC) in survivors of certain cancers is higher than that of the control arm in the NLST, suggesting that these populations may also benefit for inclusion in screening5,16,17.
The objective of this study was to review our institutional experience of lung cancer screening in patients with a previous history of malignancy, to evaluate the clinical characteristics and radiological findings in these patients and to assess the incidence of lung cancer in this population.
Methods
This was a retrospective, single-centre study reviewing our experience with low-dose CT lung screening in a population of patients with a previous history of malignancy. The Institutional Review Board (IRB) approved retrospective analysis of data for the time period of the study. All studies were performed as part of a lung cancer screening (LCS) program to which the patients were referred clinically. Previous CT studies performed as a part of patient’s clinical workup for previous malignancy were not analyzed. The need for written consent was waived by the IRB.
Patients:
All patients referred for lung cancer screening with low-dose CT between May 2, 2011, and November 28, 2018, and who met the following inclusion criteria were included in the study: (i) previous history of malignancy, with (ii) life expectancy of ≥5 years (life expectancy of >5 years is a requirement for entry into the clinical lung cancer screening program within the institution and is determined by referring clinicians based on clinical factors at the time of screening referral, including initial stage of treated tumor, time since initial diagnosis, and age), and (iii) met NCCN guidelines criteria for lung cancer screening (Table 1). Patients with evidence of active malignancy or a history of metastatic disease were excluded.
Table 1.
NCCN high-risk groups recommended for CT lung cancer screening
| Group 1 | Group 2 |
|---|---|
| Age 55–74 years | Age ≥ 50 years |
| ≥ 30 pack-year smoking history | ≥ 20 pack-year smoking history |
| Smoking cessation < 15 years | Additional risk factors (excluding second hand smoke) |
| • Cancer historya | |
| • Family history of lung cancer in 1st degree relatives | |
| • History of COPD or pulmonary fibrosis | |
| • Radon or occupational exposureb | |
Lung cancer, head and neck cancer, smoking-related cancers, and lymphoma
Carcinogens affecting the lungs e.g., asbestos, silica, beryllium, diesel fumes, and coal smoke.
COPD = Chronic Obstructive Pulmonary Disease.
Image Interpretation:
CT lung cancer screening examinations were performed without intravenous contrast on 16-row or 64-row multidetector CT scanners (GE Medical Systems) at 120kVp and either 40mA or 80mA value depending on patient chest diameter with a mean dose length product (DLP) of 89.9mGy*cm. Axial images were obtained from lung apices to the lung bases with a slice thickness of 1.25mm. Clinical image interpretation was performed by one of six thoracic radiologists on the institutional Picture Archiving and Communication System (PACS) (GE Healthcare). Studies were compared to prior imaging if available at time of interpretation, including non-screening CTs performed during the period of study.
For examinations performed between September 25, 2014, and November 28, 2018, the Lung-CT Reporting and Data System 1.0 (Lung-RADS1.0) score applied at the time of clinical reporting was recorded. Examinations performed between May 2, 2011, and September 24, 2014, prior to the use of Lung-RADS1.0 in our institution, were retrospectively reviewed by one thoracic radiologist with two years of experience who applied the Lung-RADS 1.0 scoring system (Table 2). A thoracic radiologist with more than 20 years of experience adjudicated in cases of disagreement with the clinical interpretation, which occurred in five cases.
Table 2.
Schematic of patient selection
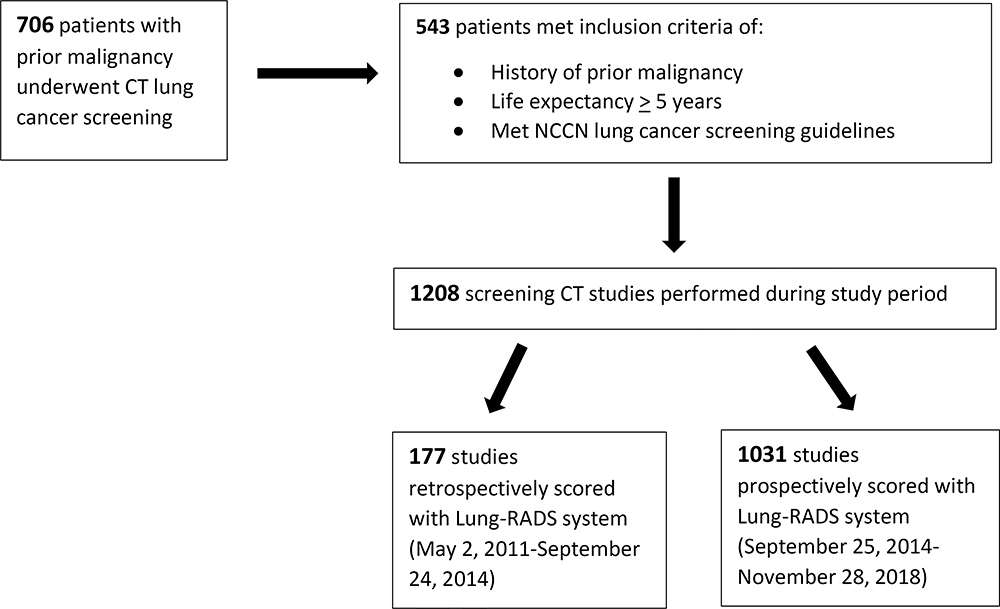 |
For the total study period, clinical CT reports were issued using a structured reporting system which included size, morphology, and number of nodules. From May 2, 2011, to September 24, 2014, structured reports were based on the NCCN Clinical Practice Guidelines in Oncology for Lung Cancer Screening (version 1.2012); according to the algorithm, the screening examination was determined to be positive if (i)a solid nodule without benign features was ≥4 mm or (ii)a groundglass nodule was ≥5 mm, requiring 1 year follow-up imaging. From September 25, 2014, to November 28, 2018, the structured reports were based on Lung-RADS1.0 scoring system, a quality assurance tool created by American College of Radiology (ACR) to standardised lung cancer screening CT reporting and outcomes. For solid nodules, the examination was determined to be positive if the nodule was (i)≥6 mm, (ii)new and ≥4 mm, or (iii)growing (Figure 1). For groundglass nodules, the examination was determined to be positive if the nodule was (i)≥20 mm on baseline CT or (ii)≥20 mm and new. For part-solid nodules, the examination was determined to be positive if the nodule was (i)new, (ii)≥6 mm total diameter, or (iii)had new or growing solid component. Lung nodules were measured manually with the mean of bidirectional diameter recorded, and with growth defined as increase in size of >1.5mm when compared to most recent prior study as per LUNG-RADSv1.0 guidelines18. Screening intervals were performed as per recommendation of guidelines used at the time of reporting.
Figure 1.
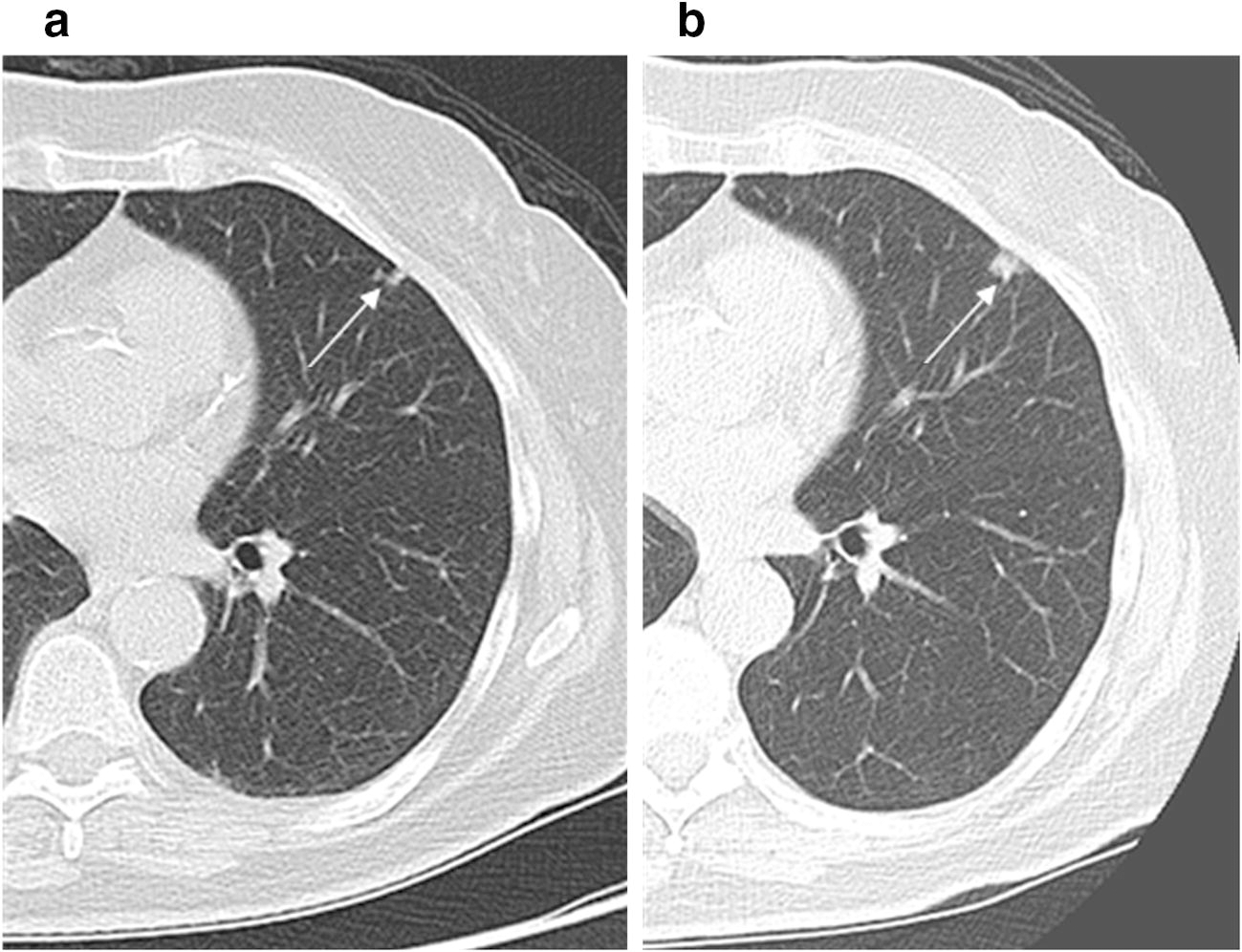
64-year-old female ex-smoker with a previous history of breast cancer enrolled in the lung cancer screening program. (a) Low-dose CT lung cancer screen study (screen 5) demonstrating a 6 mm left upper lobe solid nodule, slowly growing from prior screening studies, Lung-RADS 2. (b) Subsequent low-dose CT lung cancer screen study (screen 6) performed 1 year later as per guidelines demonstrating growth of left upper lobe solid nodule, now 11 mm, Lung-RADS 4b. This was subsequently resected and confirmed as squamous cell carcinoma.
Clinical Data:
Clinical data was obtained from the Electronic Medical Record and included smoking history, history of prior thoracic radiation, as well as subsequent investigations including biopsies and pathology.
Results
Demographics:
Between May 2, 2011, and November 28, 2018, 706 patients with a previous history of malignancy underwent CT lung cancer screening; 543 patients met the inclusion criteria for the study. Of the included patients, 231(43%) were male and 312(57%) were female. The mean age was 66 years (range, 50−91 years). The most common previous cancers were breast cancer (n=205 (38%)), head and neck cancer (n=105 (19%)), and lung cancer (n=87 (16%)). Additional cancers are summarised in Table 3. The median interval between diagnosis of prior of cancer and inclusion in the screening programme was 6 years.
Table 3.
Subtypes of cancer in patients undergoing lung cancer screening
| Overall Cohort | SPLC Patients | ||
|---|---|---|---|
| Cancer | No. of Patients (%) | No. of Patients (%) | Rate of SPLC within cancer type |
| Breast | 205 (37.8%) | 11 (30.5%) | 5.4% |
| Head and Neck | 105 (19.3%) | 7 (19%) | 6.7% |
| Lung | 87 (16.0%) | 7 (19%) | 8.0% |
| Prostate | 66 (12.2%) | 3 (8%) | 4.5% |
| Renal cell carcinoma | 41 (7.6%) | 1 (3%) | 2.4% |
| Thyroid | 27 (5.0%) | 2 (5.5%) | 7.4% |
| Bladder | 22 (4.1%) | 1 (3%) | 4.5% |
| Colorectal | 22 (4.1%) | 2 (5.5%) | 9.0% |
| Melanoma | 18 (3.3%) | 3 (8%) | 16.7% |
| Lymphoma | 11 (1.8%) | 1 (3%) | 9.1% |
| Esophagus | 6 (1.1%) | 0 (0%) | 0% |
| Ovarian | 4 (0.7%) | 0 (0%) | 0% |
| Gastric | 3 (0.6%) | 0 (0%) | 0% |
| Cervix | 3 (0.6%) | 0 (0%) | 0% |
| Chronic Lymphocytic Leukemia | 2 (0.4%) | 0 (0%) | 0% |
| Myeloma | 1 (0.2%) | 0 (0%) | 0% |
Percentages are ≥100% as 112 patients had more than 1 cancer diagnosis.
SPLC = second primary lung cancer
All included patients had a current or prior smoking history; over 86% of patients (468) had a smoking pack-year history of ≥30 years. 288 (53%) patients had a history of radiation to the neck or thorax.
SPLC Diagnoses:
SPLC occurred in 35(6.4%) of 543 patients over the duration of the study period. Of the 35 patients with SPLC, 23(66%) patients had adenocarcinoma, 7(20%) had squamous cell carcinoma, 3(9%) had small cell carcinoma, and 2(6%) had carcinoid tumor. Additionally, 4 cases of metastases and one case of radiation-associated high-grade chest sarcoma were detected in the screened population. SPLC was diagnosed in 17 men (49%) and 18 women (51%), with an average age of 69 years.
The median number of CT screening rounds was 1 (range, 1–7). For the 35 SPLC patients, 21(60%) patients received a diagnosis at the prevalence (baseline) screening study; 14(40%) received a diagnosis of interval cancer in the subsequent screening rounds (4 in round two, 3 in round three, 2 in round four, 3 in round five, and 1 patient in rounds six and seven, respectively). The median time between first screening chest CT and subsequent lung cancer diagnosis was 426 days (range, 10−2162).
Regarding the prior history of these patients, 11(32%) had a history of breast cancer, 7(20%) had a history of lung cancer, and 7(20%) had a history of head and neck cancer. Additional previous cancers are summarised in Table 3. 32(91%) patients had a smoking history of ≥30 pack-years: 8 were active smokers and 27 were ex-smokers. 9(26%) patients had previous thoracic radiation, (7 thoracic wall; 2 neck), and of these patients, 3 SPLCs arose within prior thoracic wall radiation fields.
Of these patients, 33(94%) were stage IA/IB at diagnosis. 26 patients(74.3%) were treated with surgical resection, 6 patients (17.1%) were treated with stereotactic body radiotherapy, 2 patients were treated with chemotherapy, and 1 patient was placed on active radiological surveillance according to patient’s preference. Using the PLCOm2012 calculator and based on baseline characteristics of the subset of patients who subsquently developed SPLC, the mean 6 year risk of developing lung cancer was 6.2%.
Imaging Characteristics
Overall, 1208 screening low-dose CT scans were performed with a maximum number of 7 screens performed per patient. In addition, 168 standard dose chest CTs were performed in the group during the period. 131 patients were lost to follow-up, of whom 14 were lost following a positive screen. Screening is ongoing in 266 patients.
386 (71.1%) patients had prior CT imaging for comparison. 70% of baseline screening CTs had multiple nodules. The dominant nodule was assigned a Lung-RADS score as per the Lung-RADS1.0 criteria. The number of positive scans (Lung-RADS 3 or greater) in round one was 120(22%) compared to the expected population prevalence 9% (additional details on Lung-RADS1.0 categories are provided in Table 4). The imaging characteristics of the SPLCs are summarized in Table 5. Of the SPLCs, 26 were solid lesions, 4 were part-solid lesions, and 5 were groundglass lesions. The majority of SPLCs occurred on a background of multiple additional pulmonary nodules. The majority of SPLCs were within the upper lobes (74.2%); 15 in the left upper lobe, 4 in the left lower lobe, 11 in the right upper lobe, 1 in the right middle lobe, and 4 in the right lower lobe.
Table 4.
Summary of Lung-RADS assessment categories after each round of CT screening
| Lung-RADS score | Screen 1 (n=543) | Screen 2 (n=319) | Screen 3 (n=187) | Screen 4 (n=92) | Screen 5 (n=45) | Screen 6 (n=18) | Expected Population Prevalence as per Lung-RADS guidelines |
|---|---|---|---|---|---|---|---|
| ≤ 2 | 78% | 85% | 91% | 87% | 78% | 78% | 90% |
| 3 | 11% | 7% | 2% | 3% | 7% | 5% | 5% |
| 4A/4B | 11% | 8% | 7% | 10% | 15% | 17% | 4% |
Screen 7: n = 2 (Lung-RADS 2; Lung-RADS 4B)
Table 5.
Characteristics of malignant pulmonary nodules identified on lung cancer screening CT
| Feature | Number of patients (%) |
|---|---|
| Nodule Consistency | |
| Groundglass | 5 (14.2%) |
| Part solid | 4 (11.4%) |
| Solid | 26 (74.3%) |
| Lung-RADS score* | |
| 1 | 0 (0%) |
| 2 | 5 (14.3%) |
| 3 | 0 (0%) |
| 4A | 6 (17.1%) |
| 4B | 24 (68.6%) |
| Pathology | |
| Adenocarcinoma | 22 (62.9%) |
| Adenocarcinoma in situ | 1 (2.8%) |
| Small Cell Carcinoma | 3 (8.6%) |
| Squamous Cell Carcinoma | 7 (20.0%) |
| Carcinoid | 2 (5.7%) |
| Stage at diagnosis | |
| 1 | 33 (94.3%) |
| 2 | 0 (0%) |
| 3 | 0 (0%) |
| 4 | 2 (5.7%) |
Refers to the Lung-RADS score on the screening study immediately before pathological diagnosis
7.9%(43/543) of patients underwent percutaneous CT-guided biopsy. Of the biopsies, 3 demonstrated non-malignant histology (1 nodular scar, 1 focal organizing pneumonia, and 1 atypical pneumocyte hyperplasia) (Figure 2). 2.4%(13/543) of patients underwent surgical resection without a presurgical biopsy. Of these, 3 resected nodules demonstrated non-malignant histology (1 focal organizing pneumonia, 1 necrotizing granulomatous inflammation, and 1 organizing infarct). This accounts for an overall false positive rate of 14.2% (7.0% in biopsy group and 23.0% in surgical group).
Figure 2.
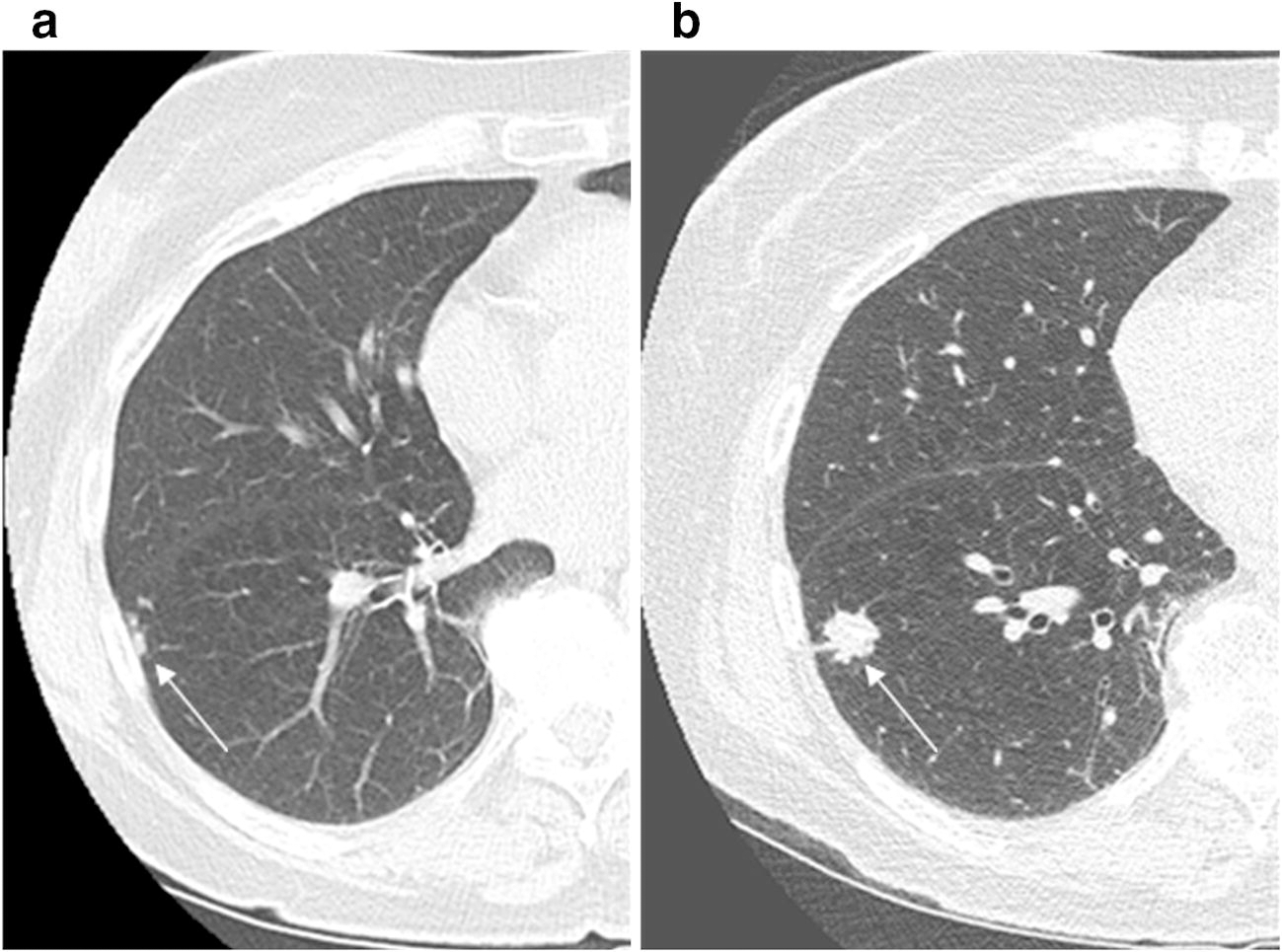
70-year-old female current smoker with a previous history of breast cancer, enrolled in lung cancer screening program. (a) Low-dose CT lung cancer screen study (screen 2) demonstrating new right lower lobe new tree in bud nodules, Lung-RADS 4a, probably infectious or inflammatory in aetiology. (b) Subsequent repeat short interval CT (3 months) demonstrated 15 mm right lower lobe spiculated solid nodule, Lung-RADS 4b, at site of previous tree in bud nodules, again favoured to be infectious or inflammatory. This lesion was subsequently biopsied and was confirmed as organizing pneumonia and resolved on follow-up imaging.
Discussion
This study describes the clinical characteristics and radiological findings in a cohort of patients with a history of malignancy undergoing lung cancer screening. We detected SPLCs at a greater rate in patients with a history of malignancy undergoing CT lung cancer screening than previously reported in high-risk individuals without a history of malignancy undergoing lung cancer screening19.
Our incidence of 6.4% of SPLC is higher than the detection rates previously reported for lung cancer screening trials. The low-dose CT control arm of NLST in the United States reported a detection rate of 2.4% in patients after a positive CT screening study11. The NELSON trial in Europe reported a detection rate of 3.2%20. The European Multicentric Italian Lung Detection trial (MILD) reported a detection rate of 2.0%21. Our current study adds to a previously published retrospective study performed in our institution where a smaller cohort of 139 patients were analysed with SPLC detected in 5% patients with a prior history of malignancy undergoing lung cancer screening17. Our current study also detected greater prevalence (3.9%) and incidence (1.3–2.2%) rates than those from comparative screening rounds in both the NLST (prevalence 1.1% and incidence 0.3–0.6%)19,22and the NELSON trial (prevalence 0.9% and incidence 0.8–1.1%)20. Our cohort had a broadly comparible smoking pack year history to both NLST and NELSON cohorts11,20. Our cohort does however differ with median age of the cohort of 66 years compared to 58 years in NELSON trial and a slight predominence of females, converse of both NLST and NELSON, which is probably explained by the large number of patients with history of breast cancer referred for LCS in our institution.
Lung cancer screening trials with large cohorts have inconsistently included patients with a prior history of malignancy. For example, the NLST excluded any person with a history of lung cancer or a diagnosis of any other cancer within last 5 years (except non-melanomatous skin cancer or carcinoma in situ). The NELSON trial excluded any person with a history of lung cancer within the previous 5 years or who was still undergoing treatment, as well as a person with a history of renal, breast cancer or melanoma. The lack of representation of cancer survivors in previous screening trials is at odds with the growing number of cancer survivors worldwide23,24. Survivors of common cancers have an overall risk of 8.1% for developing a second primary malignancy (SPM), most commonly lung cancer5. This rate is greater than our detected rate of malignancy, however it pertains to risk of SPM overall not just risk of SPLC. Additionally, this study demonstrated colorectal and prostate cancer were the second and third most common detected SPM respectively5, for which there are established screening programs in which patients with history of prior malignancy are not routinely excluded25,26. Some authors have discussed the possibility of including patients with prior malignancy in lung cancer screening programs27, however to date the lack of data pertaining to this patient cohort has limited the widespread applicability of current screening guidelines to cancer survivors.
Donin et al. have shown that the incidence of SPLC varies when stratified according to the primary cancer site, with survivors of head and neck, bladder, and esophageal cancers developing SPLC at higher rates than other cancers16, with the cumulative incidence of SPLC in patients with prior head and neck cancer exceeding that of the NLST control arm and the cumulative incidence of SPLC in patients with prior bladder and esophageal cancers comparable to that of NLST control arm.
Due to the variable number of different cancers in our cohort, which is comparatively smaller than in the above population-based trials, we were unable to determine if specific cancers had statistically increased risk of developing a SPLC. However, we did note that patients with a history of a smoking-related cancer (lung, head and neck, or bladder) accounted for 15/35 (43%) SPLC diagnoses and there is a well-established link between increased smoking intensity/duration and lung cancer risk28. Also, our SPLC rate of 8% in patients with a previous history of lung cancers mirrors the previously published rate of 7% in non-screened early-stage lung cancer survivors in our institution29. This potentially suggests that there are subgroups of cancer survivors which may be at increased risk of development of SPLC, possibly relating to prior or current smoking exposure, although we recognize that larger cohort prospective studies are needed in this population to further support this hypothesis.
However, smoking history is not the only risk factor for the development of lung cancer. Genetic predisposition and carcinogenic effects of previous cancer treatments, e.g., thoracic radiation30, have also been hypothesized as potential mechanisms of clustering of SPLC with certain types of cancer. In our group, of the patients who were diagnosed with a SPLC, one third developed the cancer in an area of lung which had previously been included in a radiation field, however due to the small number of cases it is hard to determine the significance of this finding in our cohort.
Our study demonstrates a higher rate of screen-detected lung cancer than has been seen in historical controls, reassuringly the majority of these were early stage cancers, however we cannot comment on any impact that lung cancer screening may have on the mortality in this cohort. The detection of lung cancer during screening does not prove that screening is beneficial. For example, 60% of cancers detected in our cohort were prevalence cancers: the majority were adenocarcinoma, some of which may have had an indolent biology. In addition, our cohort of cancer survivors may not have a baseline life expectancy comparable to historical controls without a prior cancer history. No study has demonstrated a survival benefit of lung cancer screening in patients with a prior history of cancer. A randomised study by Westeel et al. comparing follow-up in patients post resection of early-stage NSCLC with examination and chest radiograph versus examination, chest radiograph and CT chest has not yet met the primary endpoint of overall survival benefit but has found that SPLC were more frequently asymptomatic and amenable to curative treatment in patients followed in CT arm31,32.
The available retrospective data shows the heterogenous nature of survival in this patient population. Studies have found that the median survival of patients with head and neck cancer33 and Hodgkin’s lymphoma or chronic lymphocytic leukaemia34 who subsequently developed SPLC was significantly lower than that of patients with de-novo lung cancer. Conversely, patients with breast cancer and SPLC diagnosis have been found to be diagnosed at an earlier stage and have slightly increased overall survival compared to lung cancer patients without breast cancer35. The heterogeneity of survival may reflect variability in patient age, comorbidities, and prior cancer treatment.
Our study reflects the radiological challenges of screening this complex population. Our false positive rate was 14.2%, which is slightly higher than the ACR reported rate of 10.4%36. Our positive screen rate of 9–22% in all rounds of screening was greater than or equal to the expected general population prevalence of 9% as per ACR guidelines. Within the Lung-RADS3 category, our cohort had a rate of 11% within the first screening round, which is significantly higher than the reported rate of 5% per ACR guidelines. Additionally, 10.3% of our cohort underwent invasive procedures (image-guided biopsy or surgical resection) which is significantly higher than the previously published rate of 2.5% in a non-oncological population36. Furthermore, there was a 7% false positive rate within the biopsied group and a 23% false positive rate within the surgical resection group compared with rates of 1.5% and 1.0%, respectively, in a non-oncological screening population36. This probably reflects the sequela of prior cancer treatments, including radiation with resultant parenchymal abnormalities which makes these studies more difficult to interpret. Interestingly we only detected 4 cases of metastatic disease within the cohort; this low number may be due to the fact that patients which are referred to the screening programme must not have evidence of active malignancy or history of metastatic disease and as such are ostensibly deemed treated of their primary cancer by referring physicians.
The main limitation of this study is that it is a retrospective analysis of our experience. We also recognize the challenge of selecting the appropriate screening candidates in a population with a prior cancer history, as determining life expectancy ≥5 years can be challenging and subjective. In our institution we relied on the expertise of our referring physicians to select appropriate patients.
We recognise the potential limitations of using Lung-RADS scoring system, a diameter-based model, to predict nodules at risk of developing into lung cancer. Tammemagi et al. developed the PLCOm2012 risk calculator15 based on patient demographics and smoking history and probability of a diagnosis of lung cancer in a 6-year period, which has been shown to be more sensitive than the NLST criteria for lung-cancer detection15. Interestingly, the PLCOm2012 calculator includes history of prior malignancy as a risk factor. When we analyzed our subset of patients who subsequently developed SPLC based on their baseline characteristics, the mean 6-year risk of developing lung cancer was 6.2%; however, if the risk factor of prior malignancy was removed from the calculation in the same subset of patients, the mean risk decreased to 4.3%. We believe that this supports our hypothesis that patients with prior history of malignancy are potentially at an increased risk of SPLC. Another risk calculator, the Brock University Calculator37 which uses patient and nodule specific characteristics, has also been shown to outperform Lung-RADS in NLST data38, especially with subsolid nodules39. Volumetric-based NELSON and British Thoracic Society algorithms have been shown to have higher accuracy and reproducibility compared to diameter measurements40. With the advent of artificial intelligence in clinical practice, volumetric models combined with risk models are likely to shape screening programs, however we again need prospective data in patients with history of malignancy to determine the benefit of screening this population.
Conclusion
In conclusion, we have demonstrated higher rates of screen-detected SPLC in patients with prior malignancy than reported rates seen in historical prospective screening studies which did not consistently include patients with prior malignancy. We have also demonstrated higher rates of positive screening studies and higher rates of invasive diagnostic procedures in patients with prior malignancy, underlining the complexity of screening this complex cohort. Our study suggests the need for prospective research to evaluate any mortality benefit that screening may have in this population.
Key points:
The rate of screen-detected second primary lung cancer in patients with prior malignancy is higher than reported rates seen in historical prospective randomised lung cancer screening studies in a general screened population.
Patients with a prior malignancy undergoing lung cancer screening have higher rates of positive screening studies and higher rates of invasive diagnostic procedures than those reported in a general screening population.
Prospective research is required to evaluate if screening offers a mortality benefit in this population.
Acknowledgements
We thank Joanne Chin for her Editorial Support of this manuscript.
Funding information
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Guarantor:
The scientific guarantor of this publication is Michelle S. Ginsberg, MD.
Abbreviations:
- NELSON
Dutch-Belgian Randomised Lung Cancer Screening Trial
- NLST
National Lung Screening Trial
- PACS
Picture Archiving and Communication System
- SPLC
second primary lung cancer
Footnotes
Conflict of Interest:
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry:
No complex statistical methods were necessary for this paper.
Informed Consent:
Written informed consent was waived by the Institutional Review Board.
Ethical Approval:
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap:
Some study subjects or cohorts have been previously reported in J Thorac Oncol. 2016 Sep;11(9):1447–52.
Methodology
• retrospective
• observational
• performed at one institution
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 4.Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA FJJ. New Malignancies Among Cancer Survivors - SEER Cancer Registries, 1973–2000 - SEER Publications. Bethesda, MD. doi:NIH Publ. No. 05–5302 Accessed August 20, 2019. [Google Scholar]
- 5.Donin NM, Filson CP, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992–2008. Cancer. 2016;122(19):3075–3086. doi: 10.1002/cncr.30164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinmuth N, Stumpf P, Stumpf A, et al. Characteristics of lung cancer after a previous malignancy. Respir Med. 2014;108(6):910–917. doi: 10.1016/j.rmed.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 7.Quadrelli S, Lyons G, Colt H, Chimondeguy D SC. Lung cancer as a second primary malignancy: increasing prevalence and its influence on survival. Ann Surg Oncol. 16:1033–10. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann H-S, Neef H, Schmidt P. Primary lung cancer and extrapulmonary malignancy. Eur J Cardiothorac Surg. 2007;32(4):653–658. doi: 10.1016/j.ejcts.2007.06.024 [DOI] [PubMed] [Google Scholar]
- 9.Milano MT, Peterson CR, Zhang H, Singh DP, Chen Y. Second primary lung cancer after head and neck squamous cell cancer: Population-based study of risk factors. Head Neck. 2012;34(12):1782–1788. doi: 10.1002/hed.22006 [DOI] [PubMed] [Google Scholar]
- 10.Del Rey J, Placer J, Vallmanya F, et al. Are patients with non-muscle-invasive bladder cancer a suitable population for a lung cancer screening trial? BJU Int. 2009;106(1):49–52. doi: 10.1111/j.1464-410X.2009.09081.x [DOI] [PubMed] [Google Scholar]
- 11.National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Koning HJ, Van Der Aalst CM, De Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 13.Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2018;16(4):412–441. doi: 10.6004/jnccn.2018.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kauczor H-U, Bonomo L, Gaga M, et al. ESR/ERS white paper on lung cancer screening. Eur Radiol. 2015;25(9):2519–2531. doi: 10.1007/s00330-015-3697-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tammemägi MC, Katki HA, Hocking WG, et al. Selection Criteria for Lung-Cancer Screening. N Engl J Med. 2013;368(8):728–736. doi: 10.1056/NEJMoa1211776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donin NM, Kwan L, Lenis AT, Drakaki A, Chamie K. Second primary lung cancer in United States Cancer Survivors, 1992–2008. Cancer Causes Control. 2019;30(5):465–475. doi: 10.1007/s10552-019-01161-7 [DOI] [PubMed] [Google Scholar]
- 17.Halpenny DF, Cunningham JD, Long NM, Sosa RE, Ginsberg MS. Patients with a Previous History of Malignancy Undergoing Lung Cancer Screening: Clinical Characteristics and Radiologic Findings. J Thorac Oncol. 2016;11(9):1447–1452. doi: 10.1016/j.jtho.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LungRADS v 1.0| ACR. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads. Accessed February 19, 2020. [Google Scholar]
- 19.National Lung Screening Trial Research Team TNLSTR, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980–1991. doi: 10.1056/NEJMoa1209120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol. 2014;15(12):1342–1350. doi: 10.1016/S1470-2045(14)70387-0 [DOI] [PubMed] [Google Scholar]
- 21.Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21(3):308–315. doi: 10.1097/CEJ.0b013e328351e1b6 [DOI] [PubMed] [Google Scholar]
- 22.Patz EF, Greco E, Gatsonis C, Pinsky P, Kramer BS, Aberle DR. Lung cancer incidence and mortality in National Lung Screening Trial participants who underwent low-dose CT prevalence screening: a retrospective cohort analysis of a randomised, multicentre, diagnostic screening trial. Lancet Oncol. 2016;17(5):590–599. doi: 10.1016/S1470-2045(15)00621-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the "Silver Tsunami": Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. doi: 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15(1):23–34. doi: 10.1016/S1470-2045(13)70546-1 [DOI] [PubMed] [Google Scholar]
- 25.NCCN Colorectal Screening Guidelines v2019.2 https://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf Accessed April 4, 2020.
- 26.American Cancer Society Recommendations for Prostate Cancer Early Detection. | ACR; https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed April 4, 2020. [Google Scholar]
- 27.Erkmen CP, Kaiser LR, Ehret AL. Lung cancer screening: Should we be excluding people with previous malignancy? World J Respirol. 2016;6(1):1. doi: 10.5320/wjr.v6.i1.1 [DOI] [Google Scholar]
- 28.2014. Surgeon General’s Report: The Health Consequences of Smoking—50 Years of Progress | CDC. https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm#report. Accessed August 20, 2019. [Google Scholar]
- 29.Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145(1):75–82. doi: 10.1016/j.jtcvs.2012.09.030 [DOI] [PubMed] [Google Scholar]
- 30.Salminen E, Pukkala E, Teppo L. Bladder cancer and the risk of smoking-related cancers during followup. J Urol. 1994;152(5 Pt 1):1420–1423. doi: 10.1016/s0022-5347(17)32435-7 [DOI] [PubMed] [Google Scholar]
- 31.Westeel V, Barlesi F, Foucher P, et al. 1273OResults of the phase III IFCT-0302 trial assessing minimal versus CT-scan-based follow-up for completely resected non-small cell lung cancer (NSCLC). Ann Oncol. 2017;28(suppl_5). doi: 10.1093/annonc/mdx378.012 [DOI] [Google Scholar]
- 32.Westeel V, Barlesi F, Foucher P, et al. OA 16.03 Recurrences and 2nd Primary Cancers in the IFCT-0302 Trial Assessing a CT-Scan-Based Follow-Up after Lung Cancer Surgery. J Thorac Oncol. 2017;12(11):S1788–S1789. doi: 10.1016/j.jtho.2017.09.422 [DOI] [Google Scholar]
- 33.Pagedar NA, Jayawardena A, Charlton ME, Hoffman HT. Second Primary Lung Cancer After Head and Neck Cancer. Ann Otol Rhinol Laryngol. 2015;124(10):765–769. doi: 10.1177/0003489415582259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milano MT, Li H, Constine LS, Travis LB. Survival after second primary lung cancer. Cancer. 2011;117(24):5538–5547. doi: 10.1002/cncr.26257 [DOI] [PubMed] [Google Scholar]
- 35.Milano MT, Strawderman RL, Venigalla S, Ng K, Travis LB. Non–Small-Cell Lung Cancer After Breast Cancer: A Population-Based Study of Clinicopathologic Characteristics and Survival Outcomes in 3529 Women. J Thorac Oncol. 2014;9(8):1081–1090. doi: 10.1097/JTO.0000000000000213 [DOI] [PubMed] [Google Scholar]
- 36.Kaminetzky M, Milch HS, Shmukler A, et al. Effectiveness of Lung-RADS in Reducing False-Positive Results in a Diverse, Underserved, Urban Lung Cancer Screening Cohort. J Am Coll Radiol. 2019;16(4):419–426. doi: 10.1016/J.JACR.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 37.Calculator: Solitary pulmonary nodule malignancy risk in adults (Brock University cancer prediction equation) - UpToDate. https://www.uptodate.com/contents/calculator-solitary-pulmonary-nodule-malignancy-risk-in-adults-brock-university-cancer-prediction-equation. Accessed September 13, 2019.
- 38.White CS, Dharaiya E, Dalal S, Chen R, Haramati LB. Vancouver Risk Calculator Compared with ACR Lung-RADS in Predicting Malignancy: Analysis of the National Lung Screening Trial. Radiology. 2019;291(1):205–211. doi: 10.1148/radiol.2018181050 [DOI] [PubMed] [Google Scholar]
- 39.Hammer MM, Palazzo LL, Kong CY, Hunsaker AR. Cancer Risk in Subsolid Nodules in the National Lung Screening Trial. Radiology. 2019;293(2):441–448. doi: 10.1148/radiol.2019190905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heuvelmans MA, Walter JE, Vliegenthart R, et al. Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax. 2018;73(8):779–781. doi: 10.1136/thoraxjnl-2017-210770 [DOI] [PubMed] [Google Scholar]


