SUMMARY
Virgin females of many species conduct distinctive behaviors, compared with post-mated and/or pregnant individuals. In Drosophila, this post-mating switch is initiated by seminal factors, implying that the default female state is virgin. However, we recently showed that loss of miR-iab-4/8-mediated repression of the transcription factor Homothorax (Hth) within the abdominal ventral nerve cord (VNC) causes virgins to execute mated behaviors. Here, we use genomic analysis of mir-iab-4/8 deletion and hth-microRNA (miRNA) binding site mutants (hth[BSmut]) to elucidate doublesex (dsx) as a critical downstream factor. Dsx and Hth proteins are highly complementary in CNS, and Dsx is downregulated in miRNA/hth[BSmut] mutants. Moreover, virgin behavior is highly dose sensitive to developmental dsx function. Strikingly, depletion of Dsx from very restricted abdominal neurons (SAG-1 cells) abrogates female virgin conducts, in favor of mated behaviors. Thus, a double-negative regulatory pathway in the VNC (miR-iab-4/8 ⫞ Hth ⫞ Dsx) specifies the virgin behavioral state.
Graphical abstract
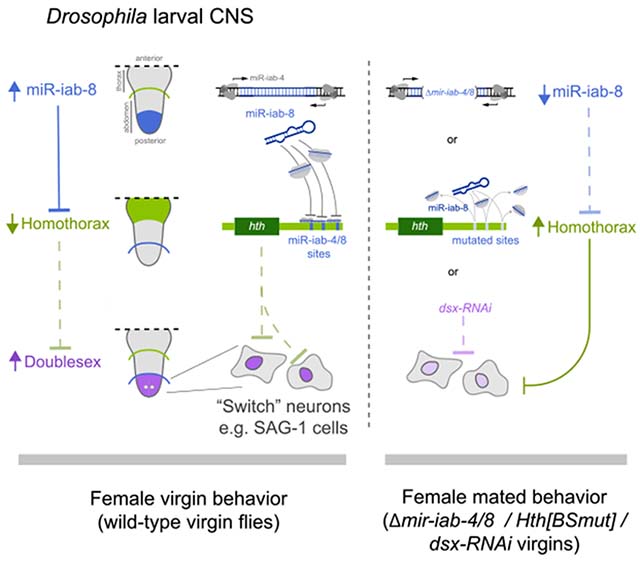
In brief
Garaulet et al. use transcriptomic analysis to reveal new downstream elements in a post-transcriptional cascade, via miR-iab-4/8 and Homothorax, that affects patterning of the CNS. This genetic circuit regulates the accumulation of a secondary target (Doublesex), whose level in specific neurons determines the behavior of adult virgin flies.
INTRODUCTION
Females of diverse invertebrate and vertebrate species coordinate multiple behavioral programs with their reproductive state. Mature female virgins are receptive to male courtship and copulation, but following mating and/or pregnancy, they decrease sexual activity and modulate behaviors to generate and foster their children. Behavioral remodeling associated with the female reproductive state includes increased aggression and nest building in avians and mammals (Ogawa and Makino, 1984; Svare et al., 1982) and decreased male acceptance, increased egg-laying, and appetitive/metabolic changes in insects (Anholt et al., 2020). The genetic and neurological control of this process has been intensively studied in fruit flies, where sexual activity induces the post-mating switch, a host of behavioral changes collectively known as post-mating responses (PMRs) (Anholt et al., 2020).
In Drosophila, as in other species, “virgin” is typically considered the default behavioral state, because factors that induce PMRs are transferred in seminal fluids during copulation. Among these, Sex Peptide (SP) is necessary and sufficient to drive most female post-mated behaviors (Kubli and Bopp, 2012). SP signals via uterine SP sensory neurons (SPSNs) (Feng et al., 2014; Häsemeyer et al., 2009; Yang et al., 2009). Some SPSN+ neurons contact abdominal interneurons in the ventral nerve cord (VNC) that express myoinhibitory peptide (Jang et al., 2017), which input into a restricted population of ascending neurons (SP abdominal ganglion [SAG] neurons) that project to the posterior brain, including pC1 neurons (Feng et al., 2014; Soller et al., 2006; Wang et al., 2020b). This outlines an ascending flow of information for how a seminal fluid peptide can alter female brain activity. The brain integrates this with auditory and visual cues to coordinate diverse behaviors mediated by distinct lineages of descending neurons and VNC populations that modulate specific behaviors according to internal state and external stimuli (Mezzera et al., 2020; Wang et al., 2020a, 2020b, 2021).
Recently, we found that post-transcriptional suppression of the homeobox gene homothorax (hth) within the VNC is critical to implement the virgin behavioral state (Garaulet et al., 2020). Of note, deletion of the Bithorax Complex (BX-C) locus mir-iab-4/8, point mutations of their binding sites in hth, or deletion of the hth neural-specific 3′ UTR extension bearing many of these microRNA (miRNA) sites all cause mutant female virgins to perform mated behaviors. Thus, the failure to integrate two post-transcriptional regulatory inputs at a single target gene prevents females from appropriately integrating their sexual internal state with external behaviors.
Our recognition of the transcription factor Hth as a target of regulatory circuits for virgin behavior implies that downstream loci may serve as a functional output for this process. Here, we used molecular genetic profiling to identify a critical requirement for Doublesex (Dsx) to implement the female virgin behavioral state. Dsx has been well studied with respect to differentiation of sexually dimorphic traits (Kopp, 2012), but its roles in post-mitotic neurons are little known. We find that expression of Dsx in the VNC mediates virgin behavior, and that modulation of Dsx in only a few abdominal VNC neurons is sufficient to convert the suite of female virgin behaviors into mated conducts.
RESULTS
VNC-iab-8 domain transcriptomes of BX-C miRNA and hth-miRNA binding site mutants
The bidirectionally transcribed BX-C miRNA locus encodes distinct miRNAs, mir-iab-4 and mir-iab-8, which are expressed in adjacent Hox-like patterns along the embryonic anterior-posterior axis. GFP sensors and in situ hybridization reveal these miRNAs are active in adjacent domains of the abdominal VNC from embryo to adult, with miR-iab-8 deployed in segments posterior to A7 (Bender, 2008; Garaulet et al., 2014; Gummalla et al., 2012; Tyler et al., 2008) Figure 1A, referred hereafter as the iab-8 domain). Expression of the BX-C Hox gene abd-A largely overlaps miR-iab-4 and demarcates the anterior miR-iab-8-5p activity domain (Figure 1B). In flies deleted for mir-iab-4/8 (trans-heterozygous Δ/C11 mutants), ectopic Hth proteins accumulate in both miRNA domains from larval stages (Figure 1C) to adult (Garaulet et al., 2014, 2020). Specific mutations of all miR-iab-4/8 binding sites in the 3′ UTR of the homeodomain-encoding isoform of homothorax (hth[BSmut]) also derepress Hth protein (Figure 1C). Although genetic evidence indicates both miRNAs contribute to the Δmir-iab-4/8 phenotype in female virgins, ectopic Hth in hth[BSmut] is most overt within the miR-iab-8 domain (Figures 1C and 1H) and is sufficient to induce PMRs in virgin females (Garaulet et al., 2020).
Figure 1. Cytological and transcriptomic analysis of a key miRNA/target regulon in the larval ventral nerve cord (VNC).
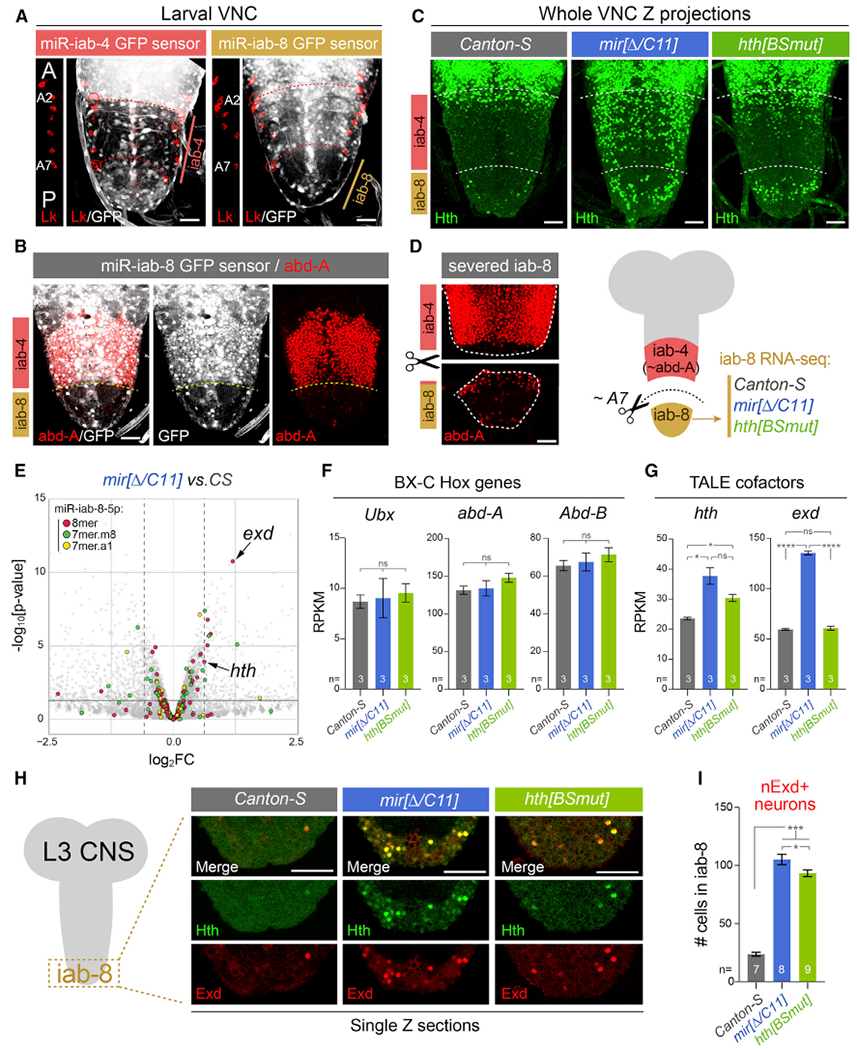
All stainings depict third instar larval VNC.
(A) The Bithorax Complex (BX-C) miRNA locus yields mir-iab-4 and mir-iab-8 from opposite strands. Their activity is associated with repression of ubiquitously transcribed tub-GFP-miRNA sensors, revealing distinct domains in abdominal segments, whose registers are marked by segmental marker Leukokinin (Lk; red).This places miR-iab-4-5p activity in segments A2–A7 and miR-iab-8-5p in A8–A9. A portion of the Lk staining is shown to the left of each image to reference the segment locations.
(B) The posterior limit of abd-A (A7a/p) corresponds to the miR-iab-4/miR-iab-8 border.
(C) Homothorax (Hth) is largely absent throughout the abdominal VNC in wild-type flies but is derepressed in mir-iab-4/8 and hth[BSmut] mutants, most overt within the iab-8 domain.
(D) Validation of dissection strategy to prospectively isolate A8–A9 VNC domain (iab-8 region) for transcriptomics. Stainings show two pieces of an individual VNC, which were imaged separately for this montage.
(E) Volcano plot of transcripts >1 reads per kilobase of transcript, per million mapped reads (RPKM) showing that TALE cofactors hth and exd are among the most significantly derepressed miR-iab-8-5p targets in the BX-C miRNA mutant VNC; most other direct targets were unaffected. Black horizontal line demarcates the 0.05 cutoff for p value; dotted vertical lines indicate the 1.5 cutoff for fold change.
(F and G) Comparison of BX-C Hox genes and TALE cofactors expression in wild-type, miRNA mutant, and hth[BSmut] iab-8 domain VNCs. exd is derepressed in the miRNA mutant, but not in hth[BSmut].
(H) Single-plane confocal images demonstrating nuclear colocalization of Hth and Exd in the iab-8 domain of mir-iab-4/8 and hth[BSmut] VNCs.
(I) Quantification of ectopic nuclear Exd cells in the iab-8 domain of VNCs of the indicated genotypes.
Statistical significance was evaluated using unpaired t test with Welch’s correction (F and G) and Mann-Whitney non-parametric test (I). Three biological replicates per genotype were used for transcriptome analysis shown in (E)–(G). *p < 0.05, ***p < 0.001, ****p < 0.0001; ns, not significant. Error bars, SEM. Scale bars, 25 μm (A–D); 40 μm (H). A, anterior; p, posterior.
Based on this, we sought to collect transcriptome data from the female iab-8 VNC domain. Because we lack markers that permit positive selection of this region, we opted for manual separation. We analyzed larval VNC, which owing to its more extended morphology than adult VNC, was more amenable to microdissection. With practice, we could reproducibly sever the VNC at the A7 pair of nerves, posterior to the major domain of abd-A as assessed by post hoc immunostaining (Figure 1D), thus liberating the iab-8 region of the VNC (segments A8 and A9). We prepared triplicate RNA sequencing (RNA-seq) samples from this region from the three genotypes (Figure 1D; Figure S1).
Although both mutants were reproducibly distinct from Canton-S control, they exhibited limited overall changes in gene expression (Figure S1). We queried Δmir-iab-4/8 data with respect to different classes of conserved seed matches for miR-iab-8-5p (http://www.targetscan.org/vert_72/). Among genes expressed at a minimum level (>1 RPKM), the strong majority bearing target sites were unchanged (Figure 1E; Figure S1), and only modestly more targets were upregulated than downregulated (at 1.5-fold change, 10 up and 6 down; Figure 1E). Bulk tissue sequencing might underestimate target responses if they were heterogeneous on a cell-by-cell basis. For example, we did not observe significant changes in validated BX-C Hox gene targets Ubx and abd-A (Tyler et al., 2008) (Figure 1F), which detectably express ectopic, although sporadic, proteins within the iab-8 domain of BX-C miRNA mutants (Bender, 2008; Garaulet et al., 2014; Gummalla et al., 2012). In any case, because the effects of BX-C miRNA deletion on their targets in abdominal VNC were limited, it was notable that two of the highest and most significantly upregulated miR-iab-8-5p targets were hth and extradenticle (exd) (Garaulet et al., 2014) (Figures 1E and 1G). Consistent with detection of ectopic Hth protein, the iab-8 region of hth [BSmut] VNC also derepressed hth but did not upregulate exd, whose levels were changed only in Δmir-iab-4/8 (Figure 1G; Figure S1).
Hth and Exd are heterodimeric TALE-homeodomain proteins that act as Hox gene cofactors but also have independent functions. Hth is a spatially patterned nuclear factor, and in the VNC, the anterior boundary of iab-4 activity is normally coincident with the loss of Hth (Figure 1C) (Garaulet et al., 2020). Exd is expressed more broadly but remains cytoplasmic in the absence of Hth, which serves as its nuclear escort (Pai et al., 1998; Rieckhof et al., 1997). Because of this, nuclear Exd is very sparse in wild-type abdominal VNC segments. In contrast, BX-C miRNA mutants broadly exhibit ectopic nuclear Exd within both iab-4 and iab-8 domains (Figure 1H) (Garaulet et al., 2014). If this required joint release of both genes from miRNA control, we might expect a different pattern of abdominal Exd in hth[BSmut]. However, the nuclear intensity of ectopic Exd colocalized to that of Hth and was similar between the two mutants, despite the fact that exd RNA increased only in mir[Δ/C11] and not hth[BSmut] mutant VNC (Figures 1G and 1H). We quantified the iab-8 domains of the three genotypes of overtly Exd+ nuclei and observed comparable, strong increases in both mir[Δ/C11] and hth [BSmut] mutants (Figure 1I). This suggests that even though Exd is a prominent miR-iab-4/8 target (Figure 1E) (Garaulet et al., 2014), it is not limiting for derepressed Hth to exert phenotypic or regulatory effects in these mutants.
Given the restricted effects of BX-C miRNA loss on direct targets in the iab-8 VNC, we examined features of presumably indirect expression changes. If derepressed Hth was responsible for some of these effects, they might be associated with overlapping responses between miRNA deletion and hth[BSmut] VNC. Intriguingly, we observe substantial overlaps between their up- and downregulated gene sets, with relatively few genes exhibiting discordant behavior (Figure 2A; Table S1). Co-regulated loci included neuronal receptors, channels, and peptide hormones (Figure S2). Taken together, these data were consistent with the notion that deregulated Hth may drive aberrant gene expression downstream of BX-C miRNA loss.
Figure 2. A double-negative gene-regulatory circuit: BX-C miRNAs prevent Hth from excluding Dsx in the VNC.
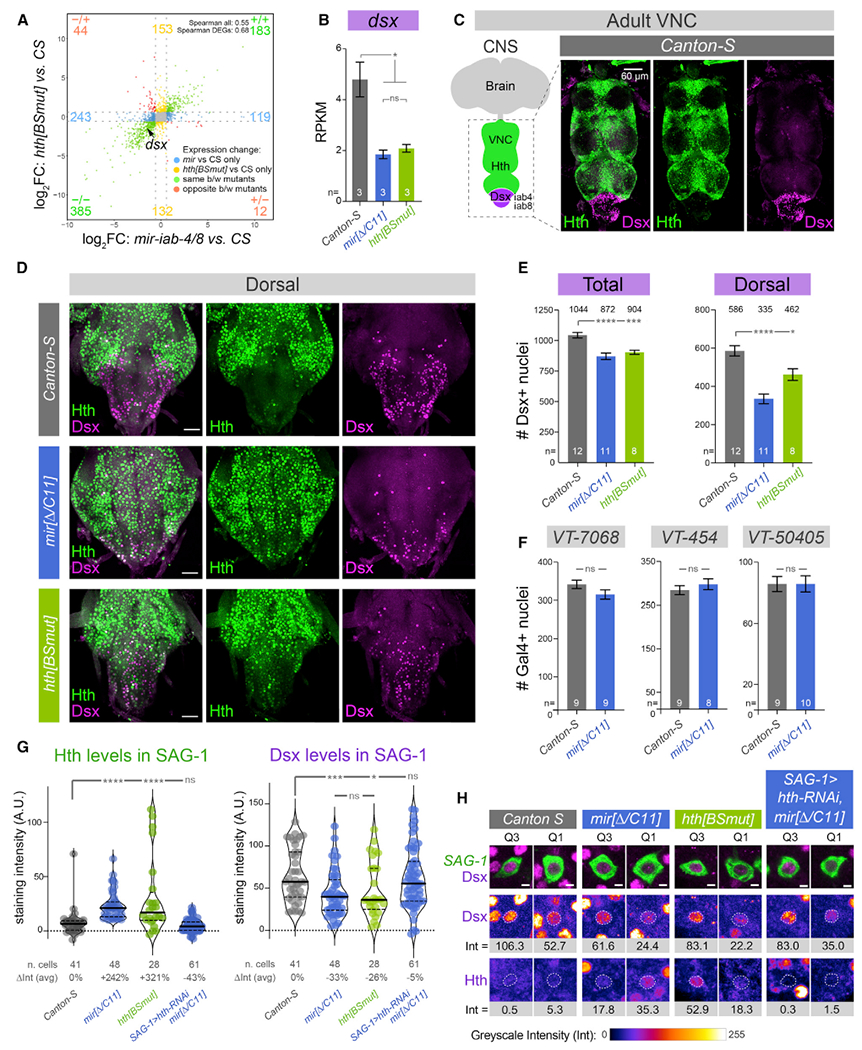
(A) Transcriptome analysis reveals substantial similarity in the larval iab-8 domain of the BX-C miRNA and hth[BSmut] mutants, compared with Canton-S (three biological replicates per genotype). Only genes > 1 RPKM and p < 0.05 are plotted. Dotted gray lines indicate fold change = 1.5.
(B) A notable downregulated transcript in both mutants is the sex-specific differentiation factor doublesex (dsx).
(C) Hth and Dsx proteins are spatially complementary in the adult VNC.
(D) Derepression of Hth in BX-C miRNA mutant (mir[Δ/C11]) and hth[BSmut] compromises accumulation of Dsx in abdominal segments of adult VNCs, especially in dorsal planes as shown here.
(E) Quantification of Dsx+ nuclei in wild-type, BX-C miRNA mutant, and hth[BSmut] VNC; total VNC and dorsal half are shown. The number of Dsx+ neurons is compromised in the mutant conditions.
(F) Conversely, the number of other identified abdominal subpopulations that mediate PMRs (VT-switch lines) are not affected by miRNA deletion.
(G) Quantification of Hth and Dsx protein levels in individual abdominal neurons relevant to the female post-mating switch (abdominal SAG-1 neurons). Depression of Hth correlates with reduction of Dsx within identified neurons.
(H) Representative GFP-labeled SAG-1 neurons from higher (Q3) and lower (Q1) quartiles of Dsx, as quantified in (G), with corresponding Dsx and Hth levels. The nuclear levels of each factor (Int) were calculated by subtracting the signals in cytoplasm (marked in green) from the corresponding nucleus (dotted line) for each antigen; samples were co-stained and imaged in parallel within the linear range.
t test with Welch’s correction (B, E, and F); Mann-Whitney non-parametric test (G). *p < 0.05, ***p < 0.001, ****p < 0.0001; ns, not significant. Error bars, SEM. Scale bars, 60 μm (C); 30 μm (D); 2 μm (H).
Spatial complementarity of Hth and Dsx is disrupted by loss of miRNA regulation
Among genes co-regulated by BX-C miRNA loss and deletion of their binding sites from hth, we were particularly intrigued by doublesex (dsx), which was ~2-fold lower in both mutants (Figures 2A and 2B). This transcription factor is widely studied for its central role in sex determination locus, and its vertebrate homolog DMRT1 similarly controls sex-specific differentiation (Kopp, 2012). However, less is known about its functions in post-mitotic neurons. We were intrigued by the highly spatially complementary pattern of Dsx and Hth proteins in the nervous system. In the VNC, Dsx protein is restricted to the posterior abdominal ganglion and abuts the domain of Hth, located more anteriorly (Figure 2C); very few neurons co-express these proteins (Figure S3). The reciprocal pattern of Dsx and Hth also extends to the brain. Although Dsx accumulates more sparsely in this setting, only rarely is Dsx colocalized with Hth, even among closely apposed cells (Figure S3).
Immunostaining of BX-C miRNA mutants revealed a decrease in Dsx+ neurons in the abdominal ganglion of both miRNA and hth[BSmut] mutants, which was most visually evident in their dorsal regions (Figure 2D). We quantified all Dsx+ neurons throughout the volume of the VNC and observed ~150 fewer Dsx+ neurons in both mutant conditions (Figure 2E), but the difference was greater in the dorsal half (Figure 2E). We recently showed that repression of hth by BX-C miRNAs that is relevant for female PMRs occurs in specific abdominal VNC neurons, marked by VT-7068, VT-454, and VT-50405 Gal4 drivers (i.e., Vienna Tiles [VT]-switch lines) (Feng et al., 2014; Garaulet et al., 2020). Because the numbers of neurons labeled by these drivers (~80–300) were not substantially affected in miRNA mutants (Figure 2F), loss of Dsx reactivity in mutants was not due to loss of abdominal neurons that mediate the post-mating switch per se.
The regulatory intersection of VT-7068 and VT-50405 (as a split-Gal4 combination) labels a handful of neurons in the entire CNS of female flies, with typically four “SAG-1” abdominal neurons found in the iab-8 domain (Figure S4)(Feng et al., 2014). These project to the central brain (Figure S4) and constitute a minimal set of VNC cells whose enforced activation can partially induce virgin behaviors in mated females (Feng et al., 2014). We employed these abdominal SAG-1 neurons for quantitative analysis of Hth and Dsx, taking great care to stain wild-type and mutant VNC in the same wells and to image them in parallel using identical settings (see STAR Methods). We reliably observed elevated Hth in identified SAG-1 neurons, in both miRNA deletion and hth[BSmut] mutants, concomitant with downregulation of Dsx (Figures 2G and 2H). Dsx exhibits a moderately bimodal distribution in wild-type SAG-1 neurons (Figures 2G and 2H). However, deletion of BX-C miRNAs eliminates most of the Dsx-high class, and total levels of Dsx are significantly lower in both mir-iab-4/8 and hth[BSmut] mutants (Figure 2G). Therefore, loss of miRNA-mediated regulation of Hth results in its derepression in abdominal VNC neurons, which in turn is associated with decreased Dsx.
To understand if the decrease in abdominal Dsx protein was promoted by elevated Hth, we misexpressed hth in SAG-1 neurons and analyzed Dsx in abdominal SAG-1 nuclei. This reduced Dsx slightly, although the difference with wild-type was not significant (Figure S4). Conversely, ectopic Dsx decreased Hth moderately (Figure S4). However, these experiments are difficult to interpret due to high levels of overexpressed Hth or Dsx, which are likely non-physiological (Figure S4). Therefore, we utilized a different approach to deplete hth in mir-iab-4/8 mutants. In SAG-1>hth-RNAi; mir[Δ/C11] virgins, Dsx levels and distribution were restored to wild-type values (Figures 2G and 2H). Thus, the decrease in Dsx proteins in mutants requires elevation of endogenous Hth. In other words, there is a double-negative relationship extending from the miRNA to Hth to Dsx.
Dsx is highly dose sensitive for female virgin behavior
dsx is necessary for male courtship (McRobert and Tompkins, 1985), as well as to specify the female circuitry necessary for the post-mating switch (Rezával et al., 2012; Rideout et al., 2010; Robinett et al., 2010). However, despite the fact that dsx-expressing neurons are required for female reproductive behaviors, the function of Dsx in female behavior has received comparably little attention.
We initially examined loss-of-function alleles of dsx. Null mutants display inter-sex cuticular features, including male and female elements on their genitalia and sex combs (Nothiger et al., 2009), but dsx heterozygotes develop normal genitalia and normal numbers and appearance of sex combs on male forelegs (Figure S5). Thus, a single copy of dsx is sufficient for gross anatomy.
We then examined female behavior. The two most commonly monitored aspects of the post-mating switch are egg-laying and receptivity. Female virgins are highly receptive to male courtship and lay few eggs in the first few days after eclosion (Figures 3A and 3B). Following copulation, females become refractory to further copulation attempts for several days and increase egglaying substantially (Anholt et al., 2020) (Figures 3A and 3B). Surprisingly, dsx heterozygosity compromises both behaviors: egg-laying is increased and receptivity is decreased compared with wild-type virgins. Importantly, both readouts were similarly affected in independent dsx alleles, compared with their control siblings (Figures 3A and 3B). Together, these slight but genetically robust differences in egg-laying and receptivity seemed to indicate a partial transition to the mated state in pre-inseminated virgins, triggered by dsx heterozygosity. To confirm this hypothesis, we tested two additional behaviors associated with female internal states. Vaginal plates opening are mostly performed by receptive virgins and very rarely are observed in the early days after copulation (Wang et al., 2021) (Figure 3C). Conversely, mated females reject actively courting males by extruding their ovipositor (Mezzera et al., 2020; Wang et al., 2020a)(Figure 3D). For both of these performances, independent dsx heterozygotes also differ from canonical wild-type virgins. Together, dsx heterozygosity attenuates virgin behaviors while enhancing mated-specific PMRs, suggesting subjective induction of the post-mated state (Figures 3A–3D).
Figure 3. Endogenous Dsx is essential for females to interpret the virgin behavioral state.
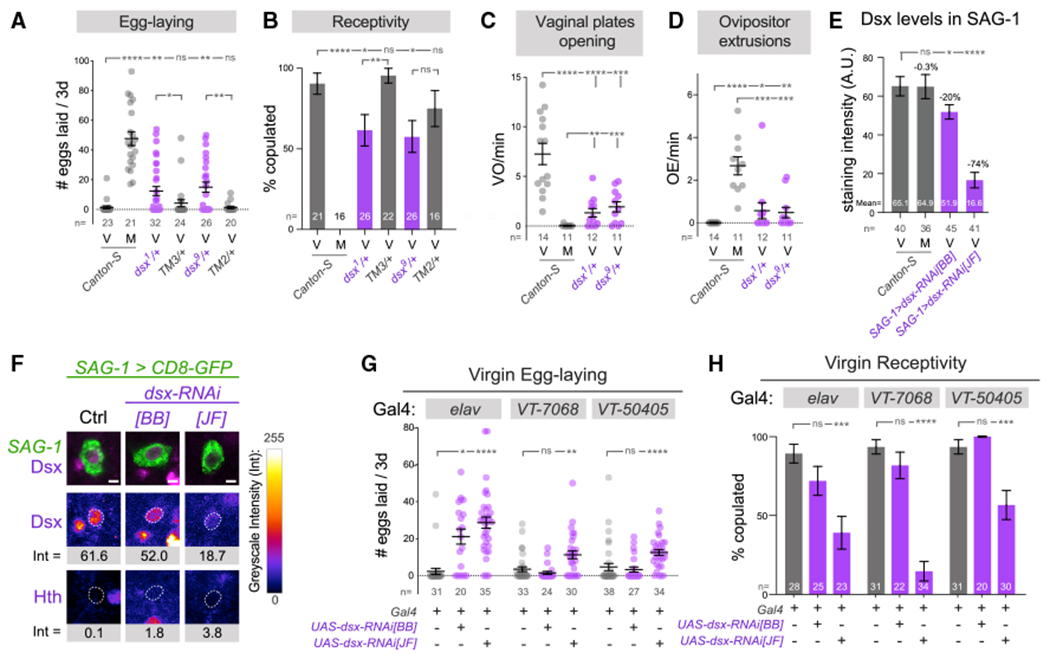
(A–D) Comparison of female behaviors in wild-type virgins, mated females, and doublesex heterozygotes, illustrating a partial transition to the mated state in the latter.
(E) Analysis of Dsx levels in abdominal SAG-1 neurons pre- and 24 h post-insemination and in virgins using independent dsx-RNAi transgenes. dsx-RNAi[JF] has a stronger effect on Dsx in SAG-1 neurons.
(F) Representative GFP-labeled abdominal SAG-1 neurons from average Dsx levels are shown, with corresponding Dsx and Hth staining. The dotted line corresponds to the nucleus of each neuron.
(G and H) Pan-neuronal knockdown of dsx results in increased egg-laying and decreased receptivity by female virgins. Restricted knockdown using VT-switch lines and dsx-RNAi[JF] transgene also induces virgin egg-laying and reduces receptivity.
Mann-Whitney non-parametric test (A, C–E, and G); Fisher’s exact test (B and H). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, not significant. Error bars, SEM. Scale bar, 2 μm (F). Eggs were collected over 3 days for all virgin genotypes and over the first 24 h after copulation in mated flies (A and G). M, mated females; V, virgin females.
Given that female virgin performance depends on dsx dosage, we wondered if PMRs in wild-type females could be triggered by a dsx decrease as a putative effect of mating. We analyzed Dsx staining in abdominal SAG-1 neurons, but Dsx levels were similar between virgins and females 24 h post-insemination (Figure 3E). Although this does not preclude that other neuronal lineages might experience Dsx fluctuation, it suggests Dsx is not an active component of the switch.
Expression of Dsx in restricted abdominal neurons is required for virgin behavior
With knowledge that virgin behavior is highly sensitive to dsx dosage, we next interrogated where and when Dsx is required in the nervous system for female responses. We used a dsx-RNAi stock published by Bruce Baker’s lab (dsx-RNAi[BB]) Robinett et al., 2010) and an independent TRiP-JF line (dsx-RNAi[JF]). Although the dsx-RNAi[BB] stock actually contains two transgene copies, it was documented to induce only weak dsx knockdown at 25°C (Robinett et al., 2010); dsx-RNAi[JF] has not been directly assessed for Dsx suppression. We compared their efficacies when activated at 25°C using SAG-1-Gal4, which allowed us to quantify Dsx alteration in defined abdominal SAG-1 cells. We found that dsx-RNAi[BB] induced mild but significant reduction of Dsx protein, within the range observed in the miRNA and hth[BSmut] mutants, while dsx-RNAi[JF] caused stronger depletion (Figures 3E and 3F, compare with Figures 2G and 2H). Consistent with this hierarchy, dsx-RNAi[JF], but not dsx-RNAi [BB], could reduce male sex comb bristles at 25°C (Figure S5). Nevertheless, dsx-RNAi[JF] induced only a mild cuticular defect by comparison with the viable trans-allelic combination of dsx [1]/[9], which yields full loss of differentiated male sex combs (Figure S5), as well as general inter-sex features. Thus, we have inducible genetic reagents that reproduce mild suppression of Dsx protein and function, within a range relevant to disruption of Dsx levels seen in BX-C miRNA and hth[BSmut] mutants.
We then used egg-laying and receptivity as readouts to screen a potential shift to a subjective post-mated state. Upon induction of either dsx-RNAi transgene with pan-neuronal elav-Gal4, both transgenes reliably increased the egg-laying capacity of young virgins (Figure 3G), in line with the results obtained for dsx heterozygotes. Similarly, receptivity was significantly compromised (Figure 3H). We additionally tested fertility and climbing in these genetic combinations to rule out general, non-specific effects of dsx depletion, undesired side effects of RNAi machinery activation, or possibly off-targeting. Both male and female fertility and locomotion in knockdowns were indistinguishable from controls, emphasizing the specificity of dsx function for virgin behaviors, as well as its dose sensitivity compared with cuticular structures, which remain unperturbed under similar dsx depletion (Figure S5).
Restricting dsx knockdown to specific VT-switch lineages, using VT-7068 and VT-50405 Gal4 lines, induced similar effects in both egg-laying and receptivity (Figures 3G and 3H). The effects with dsx-RNAi[JF] were stronger than with dsx-RNAi[BB], further highlighting the sensitivity of virgin behaviors to dsx dosage. Moreover, when dsx-RNAi was limited to the intersection of these drivers, the sparser SAG-1 lineage, both RNAi lines induced aberrant egg-laying in young virgins, and dsx-RNAi[JF] also substantially compromised virgin receptivity (Figures 4A and 4B). These phenotypes were in line with those observed in mir-iab-4/8 and hth[BSmut] mutants (Garaulet et al., 2020) (Figures 4A and 4B), suggesting that Dsx decrease may be causal to behavioral defects found in the absence of hth regulation by miR-iab-4/8. To investigate if depletion of dsx affected neuronal differentiation, we analyzed dendritic (VNC) and axonal (brain) patterns of SAG-1 abdominal neurons in wild-type and both knockdowns. We did not observe any gross anatomical defects upon dsx depletion (Figure S5). Altogether, our experiments indicate that virgin behaviors and PMRs are specifically sensitive to Dsx levels. We note that the split-Gal4 system may confer enhanced activity over the individual VT-switch lines. But the fact that the knockdown occurs in very restricted neurons, including the four ascending neurons (Figure 3E), and is only a partial knockdown supports a strong requirement for Dsx in these cells for female behavior.
Figure 4. SAG-1 neurons specifically require Dsx for a suite of female virgin behaviors.
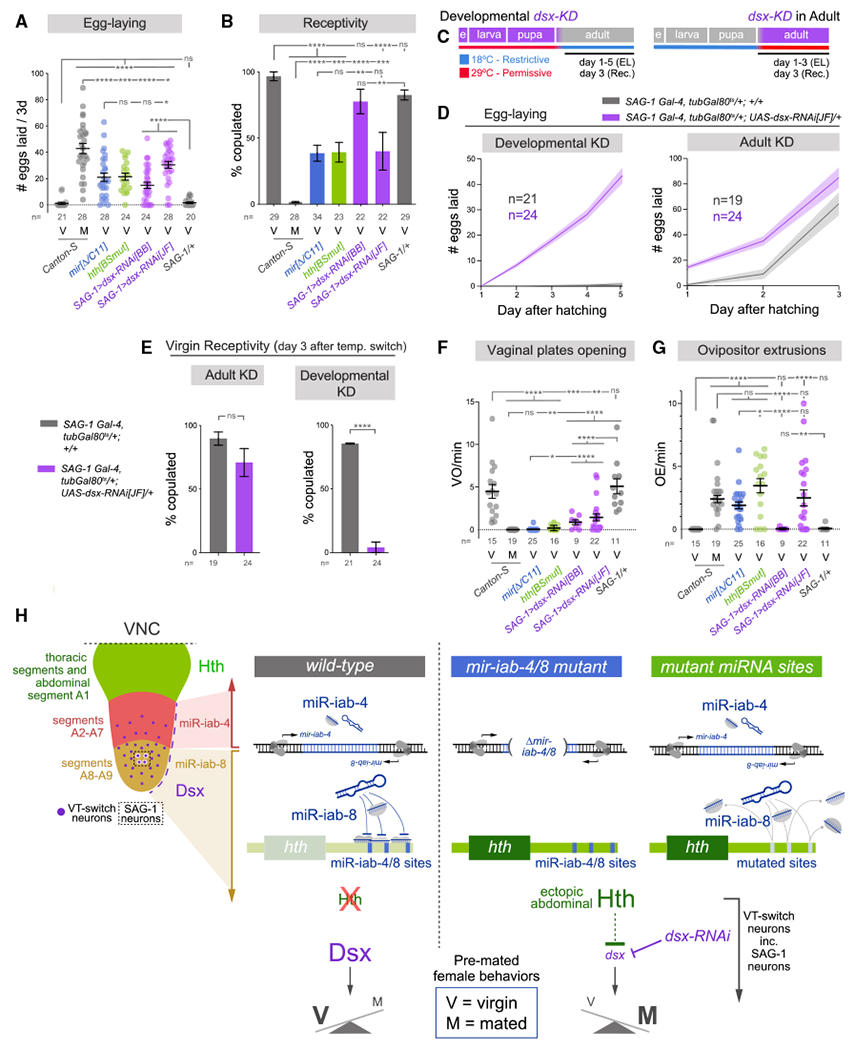
(A and B) Suppression of Dsx within the SAG-1 lineage enhances egg-laying and compromises virgin receptivity, similar to whole-animal mir-iab-4/8 deletion and hth[BSmut] mutants. dsx-RNAi[JF] has a stronger effect.
(C) Strategy for temporal depletion of dsx. EL, egg-laying; Rec., receptivity.
(D and E) Analysis of egg-laying (D) and receptivity (E) in flies with developmental or adult knockdown of dsx, highlighting the developmental role of dsx for virgin adult behaviors.
(F and G) Modulation of Dsx levels also decreases vaginal plates opening (F) and induces ovipositor extrusions (G), comparable with mir-iab-4/8 deletion and hth [BSmut] mutants.
(H) Model for the genetic and spatial control of female virgin behavior in the VNC. Upper left, larval VNC illustrates the protein domains of Hth (in thoracic segments and A1) and Dsx (in A2–A9), and RNA domains of mir-iab-4 (A2–A7) and mir-iab-8 (A8–A9). Restricted neural populations that govern the female post-mating switch are distributed within A2–A9 (VT-switch neurons), including four SAG-1 neurons within the mir-iab-8 domain. In wild-type abdominal VNC, the activity of mir-iab-4/8 binding sites on hth-30 UTR, or depleted of dsx within VT-switch neurons (and in as few as those of highly restricted SAG-1 neurons) exhibit a switch from virgin to post-mated behaviors.
Mann-Whitney non-parametric test (A, F, and G); Fisher’s exact test (B and E). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, not significant. Error bars, SEM. Eggs were collected over 3 days for all virgin genotypes and over the first 24 h after copulation in mated flies. V, virgin; M, mated.
Having identified a minimal set of neurons where dsx is necessary for egg-laying and receptivity, we sought the timing of Dsx activity to regulate these behaviors. Our RNA-seq analysis demonstrates differential expression of dsx in miRNA and hth[BSmut] mutants in late third instar larvae, prior to the establishment of the adult circuitry controlling PMRs. We temporally restricted dsx knockdown by including temperature-sensitive Gal80 (tub-Gal80ts) in SAG-1>dsx-RNAi[JF] flies and switching them from the restrictive (18°C) to permissive (29°C) temperatures and vice versa right after at eclosion (Figure 4C). These temperature shifts allowed us to discern between developmental or adult functions of Dsx in SAG-1 neurons in relation to virgin behaviors and PMRs. Monitoring egg-laying and receptivity, we found that adult knockdown induced subtle but statistically significant differences in these two behaviors. Nonetheless, the effects of developmental knockdown were dramatic for virgin performance: while the studied behaviors were unaffected by this temperature regimen in controls (little egg-laying and full receptivity), experimental individuals laid eggs profusely and remained completely refractory to male courtship (Figures 4D and 4E).
Our previous work showed that disruption of miR-iab-4/8 regulation of hth in the VNC suppresses virgin behaviors and induces a switch to a subjective mated state (Garaulet et al., 2020). Does depletion of Dsx similarly cause a broad switch in the behavioral output of virgin females toward a subjective mated state? In our Anal tests, we extended our analyses to other behaviors, comparing SAG-1>dsx-RNAi with wild-type virgin and mated females, as well as with mir-iab-4/8 and hth[BSmut] mutant virgins. By analyzing other virgin behaviors that are defective in mutants (e.g., opening of vaginal plates), as well as mated behaviors that are ectopically gained by mutant virgins (e.g., ovipositor extrusion), we gain greater confidence that depletion of dsx reflects a switch in behavioral state. Indeed, these analyses demonstrate that SAG-1>dsx-RNAi females qualitatively fail to coordinate virgin status with virgin behavioral programs and instead act as mated animals (Figures 4A, 4B, 4F, and 4G). For most readouts using SAG-1 > dsx-RNAi[JF], the effects observed were also quantitatively comparable with mir-iab-4/8 deletion and hth[BSmut] mutants. Although we do not imply that SAG-1 is the only lineage affected in these mutants, these data reveal that Dsx function in these specific cells is pertinent for normal behavior. In summary, a double-negative regulatory axis within the CNS is critical for virgin females to appropriately coordinate their external behaviors with their internal state (Figure 4H).
DISCUSSION
We recently established how miRNA mediated suppression of the transcription factor Hth to safeguard the virgin female behavioral state (Garaulet et al., 2020). Using engineered alleles and spatio-temporal hth manipulations, we demonstrated a developmental requirement for post-transcriptional regulation of Hth within the abdominal ganglion of the CNS for female behavior. However, Hth was not required in otherwise wild-type VT-switch neurons for execution of virgin behaviors, implying that expression of Hth in the abdominal VNC must normally be prevented. This involves integration of two mechanisms: a high density of BX-C miRNA binding sites (miR-iab-4/8) within the hth-HD 3′ UTR, as well as neural-specific 3′ UTR elongation, which unveils many of these sites only on neural hth isoforms.
Here, we extend this regulatory axis by showing that loss of BX-C miRNAs, acting through derepressed Hth, leads to downregulation of the Dsx in the abdominal VNC. Dsx is well-known as a master sex determination transcription factor (Kopp, 2012), and it shows localized expression in specific CNS domains. However, although the activity of Dsx-expressing neurons per se has been implicated in the switch in females (Feng et al., 2014; Häsemeyer et al., 2009; Yang et al., 2009), the functions of Dsx in post-mitotic neurons are less well defined. Our work reveals that Dsx itself is a central component in specifying virgin behavior, because its restricted suppression in as few as four (SAG-1+) neurons is sufficient to induce post-mated behaviors. It remains to be better defined how SAG-1 neurons are affected by depletion of Dsx. We did not see overt differentiation defects, but we cannot rule out an effect of masculinization. Otherwise, our recent work suggests an activity defect in a general population of switch neurons in the miRNA mutant (Garaulet et al., 2020), but more direct analysis of dsx-depleted SAG-1 neurons awaits.
Altogether, in contrast with highly branched regulatory networks that are bioinformatically inferred to lie downstream of individual miRNAs, we reveal a linear, double-negative regulatory cascade comprising miRNAs and two transcription factors (Figure 4H). These findings provide impetus to assess possible direct regulation of Dsx by Hth, as well as to elucidate Dsx targets that are relevant to female behavioral control. Overall, we expand a genetic hierarchy that is essential for females to couple the virgin internal state with appropriate behaviors.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Eric Lai (laie@mskcc.org, tel: 212-639-5578).
Materials availability
Transgenic flies generated in this study are available from the corresponding authors on request.
Data and code availability
The original behavior data for Figures 1, 3, 4, and S5 in the paper are available from the corresponding authors. The raw RNA-seq data reported in this study were deposited in the NCBI Gene Expression Omnibus under accession GEO: GSE166562.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
This study used male and female flies of wild-type and genetically engineered strains of Drosophila melanogaster. Virgin and mated parameters refer to assays of female behavioral performances.
Fly strains and maintenance
Larval and adult flies were raised on cornmeal/molasses media recipe: 83.8% water, 0.6% agar, 4.6% cornmeal, 2.3% dried yeast, 7.8% molasses solids, 0.3% propionic acid, 0.1% tegosept, 0.5% ethanol. They were kept at 25°C (unless mentioned otherwise), 55% humidity and under 12h:12h LD cycles.
Drosophila lines used in this study
mir[Δ] (Bender, 2008), mir [C11] and hth[BSmut] (Garaulet et al., 2020), Canton-S (gift of Karla Kaun), VT-lines and SAG-1 split Gal4 line (Feng et al., 2014), UAS-hth-RNAi (Vienna Drosophila RNAi Center), 2xUAS-dsx-RNAi[BB] (Robinett et al., 2010), tub-GFP-mir-iab-4 and -iab-8 sensors (Tyler et al., 2008). The following lines were obtained from Bloomington Drosophila Stock Center: elav-Gal4 [C-155] (BDSC #458), UAS-mCD8-GFP (BDSC #5137), UAS-Red-Stinger (BDSC #8547), tubGal80ts (BDSC #7108), UAS-dsx-RNAi [JF] (BDSC #26716), dsx[1] (BDSC #1679), dsx[9] (BDSC #44223), UAS-dsxF (BDSC #44223).
All the lines used in this study have been backcrossed at least 8 generations to the Canton-S wild-type strain.
METHOD DETAILS
RNA extraction and sequencing
Female larvae were dissected on ice for a maximum of 30 minutes (30-40 larvae). The posterior third of larvae was removed with forceps, the remaining was turned inside down. Using 2mm curved blade spring scissors (Fine Science Tools #15000-04), VNC were severed at the level of the A7 pair of nerves, and immediately placed into TRIzol (Thermo Fisher Scientific #15596018). After dissections, samples in TRIzol were stored at −80°C. Each biological replicate pooled severed VNCs of 120-150 larvae, dissected in 4-5 periods of 30 min. 3 replicates were generated per genotype. RNA extraction was performed with TRIzol.
500 ng of total RNA per dissected VNC sample was used for TruSeq stranded mRNA library preparation (Illumina) by the Integrated Genomics Operation (IGO) core at MSKCC. Libraries were sequenced on Illumina HiSeq-1000 sequencer with PE-100 mode. The raw sequence data are available from GEO accession number: GEO: GSE.166562.
Bioinformatic analysis
RNA-seq data were aligned to the Drosophila melanogaster reference genome (Version r6.21) using HISAT2 software with standard parameters (Kim et al., 2015). We used featureCounts in the Rsubread package to compute features and read numbers for each bam file (Liao et al., 2019). The read counts per gene were then normalized to obtain RPKM values using edgeR package from R Bioconductor (Robinson et al., 2010) and extracting transcript length from Biomart (Durinck et al., 2005). Fold changes between samples were calculated using edgeR applying no filter. Genes targeted by miR-iab-8-5p were identified using conserved TargetScanFly predictions (Agarwal et al., 2018).
Immunohistochemistry, imaging, and image quantification analysis
Larval and adult CNS were dissected in cold PBS and fixed for 1h in 4% paraformaldehyde + 0.1% Triton. Primary and secondary antibodies were incubated for > 36h at 4°C in wash buffer (PBS +1% BSA) and mounted in Vectashield (Vector Labs). Antibodies used were mouse anti-abd-A (gift of Ian Duncan), rabbit and guinea pig anti-Hth (Salvany et al., 2009), rat anti-Dsx (Sanders and Arbeitman, 2008) and Alexa- 488, –555, –647 conjugated goat and/or donkey antibodies from Thermo Fisher Scientific.
Imaging was performed in a Leica TCS SP5 confocal microscope. Each VNC was typically scanned in 55 planes (Z step ~2 μm). When image quantification or comparison was performed (Figures 2 and 3), all different genotypes used were dissected at the same time, fixed and incubated together in the same well. To identify the genotype of each VNC while mounting, different parts of the head (eyes, proboscis, antennae, etc.) were left attached or removed from the VNC during dissection. Then, the same number of VNCs from different genotypes were arranged in a known fashion per slide, to avoid differences in the quantification due to the mounting process. Laser power and offset were maintained identically for all the samples being compared. Gain was slightly adjusted to an internal control in each case.
Image quantification analysis was performed using FIJI (Schindelin et al., 2012). To obtain the values of Dsx intensity, each nuclei was identified on the GFP channel, its Dsx/Hth signal measured, and individually normalized by subtracting the background signal of a similar area in the cytoplasmic region of the same cell. All adult VNC images are 0-24 hr old females.
Behavioral assays
We collected virgin males and females after eclosion and kept them isolated in vials at 25°C, 55% humidity and 12h:12h LD cycles until utilized for behavior assays. All tests were performed at ZT 7-11 and at least at four different occasions. Vaginal plate opening, ovipositor extrusions and receptivity were assayed at day 3 after eclosion in custom 18-multiplex mating arenas (chamber size: 10 mm diameter). From eclosion to day 3, individual male and female virgins were kept isolated in vials. At day 3, single males and females were placed in a half of each arena and allowed to acclimate for 5 min before the assay. Then, they were allowed to interact and recorded for 10 minutes. Ovipositor extrusions and vaginal plates openings were analyzed during the first 4 minutes after courtship initiation or until mating. Counts of either behavior were normalized to time (min). Receptivity was calculated as the cumulative proportion of animals mated at 10 min. Egg-laying was calculated as the number of eggs laid in the first 3 days after eclosion (for virgins of all genotypes), and during the first 24h after copulation (for mated females).
For mated behaviors, virgin females were kept isolated in individual vials. At day 3, they were allowed to mate to CS males, and immediately separated from males after copulation. Then, they were placed individually in single vials. Egg-laying in mated females is the number of eggs laid per female during the first 24h after mating. At 24h after mating, mated females were assayed for vaginal plate opening, ovipositor extrusions and receptivity with fresh males, following the protocol detailed above.
For temperature shifts, flies carrying tub-Gal80ts were placed at either restrictive (18°C) or permissive (29°C) temperature during development, and shifted to the new temperature immediately after eclosion. 55% humidity and 12h:12h LD cycles were maintained.
Negative geotaxis was estimated in male flies as the average time required to climb a height of 9 cm inside a fly vial. Flies were house kept in vials in groups of as many as 5 flies right after eclosion. After 3-5 days, wings were manually clipped under CO2 flow, and returned to the vial for additional 48h. Then, they were transferred to an experimental vial with no food and recorded for 2-3 minutes after 3 taps. Each fly was monitored for three trials.
Fertility was measured as the proportion of flies giving rise to viable progeny. Individual males and females were crossed to 3 flies of the opposite sex in single vials. Progeny was screened in these vials one week after.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical significance was evaluated using Fisher’s exact test for receptivity (Figures 3 and 4) and fertility (Figure S5); Mann-Whitney non parametric test for egg-laying (Figures 3 and 4), ovipositor extrusions (Figures 3 and 4), vaginal plates openings (Figures 3 and 4), fluorescence intensity (Figures 2 and 3; Figure S4), number of nuclei (Figure 1), number of sex combs (Figure S5), and climbing (Figure S5); and unpaired t test with Welch’s correction for differential gene expression analysis (Figures 1 and 2) and number of neurons (Figure 2). Ns = not significant, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Error bars in Figures 1, 2, 3, 4, and S5 represent SEM. All n values are displayed on the figures.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit anti-Hth (1:500) | Salvany et al., 2009 | N/A |
| guinea pig anti-Hth (1:500) | Salvany et al., 2009 | N/A |
| Mouse anti-Abd-A (1:500) | Gift of Ian Duncan | Department of Biology. Washington University in St. Louis |
| Rat anti-Dsx | Sanders and Arbeitman, 2008 | RRID: AB_2569439 |
| Donkey anti-rabbit Alexa fluor 488 | Thermo Fisher Scientific | Cat# A32790; RRID: AB_2762833 |
| Goat anti-rabbit Alexa fluor 647 | Thermo Fisher Scientific | Cat# A32733; RRID: AB_2633282 |
| Donkey anti-mouse Alexa fluor 594 | Thermo Fisher Scientific | Cat# A32744; RRID: AB_2762826 |
| Donkey anti-mouse Alexa fluor 647 | Thermo Fisher Scientific | Cat# A32787; RRID: AB_2762830 |
| Goat anti-guinea pig Alexa fluor 488 | Thermo Fisher Scientific | Cat# A-11073; RRID: AB_2534117 |
| Donkey anti-rat Alexa fluor 594 | Thermo Fisher Scientific | Cat# A-21209; RRID: AB_2535795 |
| Chemicals, peptides, and recombinant proteins | ||
| Gel Loading Buffer II | Invitrogen | AM8547 |
| AccuPrime Pfx DNA Polymerase | Thermo Fisher | 12344024 |
| Trizol reagent | Life Technologies | 15596018 |
| Oligo d(T)25 Magnetic Beads | New England Biolabs | S1419S |
| Millennium RNA Markers | Thermo Fisher | AM7150 |
| Amersham Megaprime DNA Labeling System | Cytiva | RPN1606 |
| VECTASHIELD_ Vibrance Antifade Mounting Medium without DAPI | Vector Laboratories | H-1700-2 |
| VECTASHIELD_ Antifade Mounting Medium with DAPI | Vector Laboratories | H-1500-10 |
| Experimental models: Organisms/Strains | ||
| mir[C11] | Garaulet et al., 2020, Eric Lai lab. | N/A |
| mir[Δ] | Bender, 2008, Gift of Welcome Bender, Harvard University | N/A |
| Canton-S | Gift of Karla Kaun, Department of Neuroscience, Brown University. | N/A |
| VT-lines and SAG-1 split Gal4 line | Feng et al., 2014, Gift of Barry Dickson, Janelia Farm Research Campus. | N/A |
| tub-GFP-sensors for miR-iab-4 and miR-iab-8 | Tyler et al., 2008, Eric Lai lab | N/A |
| hth[BSmut] | Garaulet et al., 2020, Eric Lai lab. | N/A |
| 2xUAS-dsx-RNAi[BB] | Robinett et al., 2010 | N/A |
| UAS-hth-RNAi | Vienna Drosophila RNAi Center | 100630 |
| tubGal80ts | Bloomington Drosophila Stock Center | 7108 |
| elav-Gal4[C-155] | Bloomington Drosophila Stock Center | 458 |
| UAS-mCD8-GFP | Bloomington Drosophila Stock Center | 5137 |
| UAS-Red-Stinger | Bloomington Drosophila Stock Center | 8547 |
| UAS-dsx-RNAi[JF] | Bloomington Drosophila Stock Center | 26716 |
| dsx[1] | Bloomington Drosophila Stock Center | 1679 |
| dsx[9] | Bloomington Drosophila Stock Center | 44223 |
| UAS-dsxF | Bloomington Drosophila Stock Center | 44223 |
| UAS-hth | Gift of Richard S. Mann, Columbia University. Casares and Mann, 1998 | N/A |
| Deposited data | ||
| VNC RNA-seq data | This study. | NCBI GEO: GSE166562 |
| Software and algorithms | ||
| Prism 7 for Mac OS X | GraphPad | https://www.graphpad.com |
| Fiji ImageJ 2.0.0-rc-68/1.52e | N/A | https://imagej.nih.gov/ij |
| Rstudio Version 1.2.1335 | Rstudio | https://www.rstudio.com/ |
| IGV_2.6.3 | Broad Institute | http://software.broadinstitute.org/software/igv/ |
Highlights.
RNA-seq analysis reveals genes downstream of miR-iab-4/8/homothorax regulon
miR-iab-4/8 regulation of homothorax determines Doublesex levels in Drosophila female CNS
Developmental control of miR/Hth/Dsx circuit regulates female behavior
ACKNOWLEDGMENTS
We thank Welcome Bender, Barry Dickson, Natalia Azpiazu, Carlos Ribeiro, Carolina Rezaval, Stephen Goodwin, Karla Kaun, Michelle Arbeitman, Richard Mann, Ian Duncan, and the Bloomington Drosophila Stock Center for fly strains, plasmids, and antibodies used in this study. We thank Ernesto Sánchez-Herrero and Paloma Martín for help and support. Work in E.C.L.’s group was supported by the NIH (R01-GM083300 and R01-NS083833) and MSK Core grant P30-CA008748.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.109335.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Agarwal V, Subtelny AO, Thiru P, Ulitsky I, and Bartel DP (2018). Predicting microRNA targeting efficacy in Drosophila. Genome Biol. 19, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt RRH, O’Grady P, Wolfner MF, and Harbison ST (2020). Evolution of Reproductive Behavior. Genetics 214, 49–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W (2008). MicroRNAs in the Drosophila bithorax complex. Genes Dev. 22, 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares F, and Mann RS (1998). Control of antennal versus leg development in Drosophila. Nature 392, 723–726. [DOI] [PubMed] [Google Scholar]
- Durinck S, Moreau Y, Kasprzyk A, Davis S, De Moor B, Brazma A, and Huber W (2005). BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 21, 3439–3440. [DOI] [PubMed] [Google Scholar]
- Feng K, Palfreyman MT, Häsemeyer M, Talsma A, and Dickson BJ (2014). Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron 83, 135–148. [DOI] [PubMed] [Google Scholar]
- Garaulet DL, Castellanos MC, Bejarano F, Sanfilippo P, Tyler DM, Allan DW, Sánchez-Herrero E, and Lai EC (2014). Homeotic function of Drosophila Bithorax-complex miRNAs mediates fertility by restricting multiple Hox genes and TALE cofactors in the CNS. Dev. Cell 29, 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet DL, Zhang B, Wei L, Li E, and Lai EC (2020). miRNAs and Neural Alternative Polyadenylation Specify the Virgin Behavioral State. Dev. Cell 54, 410–423.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummalla M, Maeda RK, Castro Alvarez JJ, Gyurkovics H, Singari S, Edwards KA, Karch F, and Bender W (2012). abd-A regulation by the iab-8 noncoding RNA. PLoS Genet. 8, e1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häsemeyer M, Yapici N, Heberlein U, and Dickson BJ (2009). Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61, 511–518. [DOI] [PubMed] [Google Scholar]
- Jang YH, Chae HS, and Kim YJ (2017). Female-specific myoinhibitory peptide neurons regulate mating receptivity in Drosophila melanogaster. Nat. Commun 8, 1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, and Salzberg SL (2015). HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A (2012). Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. 28, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E, and Bopp D (2012). Sexual behavior: how Sex Peptide flips the postmating switch of female flies. Curr. Biol 22, R520–R522. [DOI] [PubMed] [Google Scholar]
- Liao Y, Smyth GK, and Shi W (2019). The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 47, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobert SP, and Tompkins L (1985). The effect of transformer, doublesex and intersex mutations on the sexual behavior of Drosophila melanogaster. Genetics 111, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzera C, Brotas M, Gaspar M, Pavlou HJ, Goodwin SF, and Vasconcelos ML (2020). Ovipositor Extrusion Promotes the Transition from Courtship to Copulation and Signals Female Acceptance in Drosophila melanogaster. Curr. Biol 30, 3736–3748.e5. [DOI] [PubMed] [Google Scholar]
- Nothiger R, Leuthold M, Andersen N, Gerschwiler P, Grüter A, Keller W, Leist C, Roost M, and Schmid H (2009). Genetic and developmental analysis of the sex-determining gene ‘double sex’ (dsx) of Drosophila melanogaster. Genet. Res 50, 113–123. [Google Scholar]
- Ogawa S, and Makino J (1984). Aggressive behavior in inbred strains of mice during pregnancy. Behav. Neural Biol 40, 195–204. [DOI] [PubMed] [Google Scholar]
- Pai CY, Kuo TS, Jaw TJ, Kurant E, Chen CT, Bessarab DA, Salzberg A, and Sun YH (1998). The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, extradenticle, and suppresses eye development in Drosophila. Genes Dev. 12, 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, and Goodwin SF (2012). Neural circuitry underlying Drosophila female postmating behavioral responses. Curr. Biol 22, 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout EJ, Dornan AJ, Neville MC, Eadie S, and Goodwin SF (2010). Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat. Neurosci 13, 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, and Mann RS (1997). Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 91, 171–183. [DOI] [PubMed] [Google Scholar]
- Robinett CC, Vaughan AG, Knapp JM, and Baker BS (2010). Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 8, e1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, and Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvany L, Aldaz S, Corsetti E, and Azpiazu N (2009). A new role for hth in the early pre-blastodermic divisions in Drosophila. Cell Cycle 8, 2748–2755. [DOI] [PubMed] [Google Scholar]
- Sanders LE, and Arbeitman MN (2008). Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev. Biol 320, 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller M, Haussmann IU, Hollmann M, Choffat Y, White K, Kubli E, and Schäfer MA (2006). Sex-peptide-regulated female sexual behavior requires a subset of ascending ventral nerve cord neurons. Curr. Biol 16, 1771–1782. [DOI] [PubMed] [Google Scholar]
- Svare B, Mann MA, Broida J, and Michael SD (1982). Maternal aggression exhibited by hypophysectomized parturient mice. Horm. Behav 16, 455–461. [DOI] [PubMed] [Google Scholar]
- Tyler DM, Okamura K, Chung WJ, Hagen JW, Berezikov E, Hannon GJ, and Lai EC (2008). Functionally distinct regulatory RNAs generated by bidirectional transcription and processing of microRNA loci. Genes Dev. 22, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang K, Forknall N, Parekh R, and Dickson BJ (2020a). Circuit and Behavioral Mechanisms of Sexual Rejection by Drosophila Females. Curr. Biol 30, 3749–3760.e3. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang K, Forknall N, Patrick C, Yang T, Parekh R, Bock D, and Dickson BJ (2020b). Neural circuitry linking mating and egg laying in Drosophila females. Nature 579, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wang F, Forknall N, Yang T, Patrick C, Parekh R, and Dickson BJ (2021). Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature 589, 577–581. [DOI] [PubMed] [Google Scholar]
- Yang CH, Rumpf S Xiang Y, Gordon MD, Song W, Jan LY, and Jan YN (2009). Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61, 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original behavior data for Figures 1, 3, 4, and S5 in the paper are available from the corresponding authors. The raw RNA-seq data reported in this study were deposited in the NCBI Gene Expression Omnibus under accession GEO: GSE166562.


