Abstract
Background:
With the recent interest in personalized medicine for cancer patients and immune therapy, the field of cancer vaccines has been resurrected. Previous autologous, whole cell tumor vaccine trials have not produced convincing results due, in part, to poor patient selection and inactivation methods that are harsh on the cells-methods that can alter protein structure and antigenic profiles making vaccine candidates ineffective in stimulating immune response to autochthonous tumor cells.
Materials and Methods:
We investigated a novel method for inactivating tumor cells that uses UVA/UVB light and riboflavin (vitamin B2) (RF+UV). RF+UV inactivates the tumor cells’ ability to replicate, yet preserves tumor cell integrity and antigenicity.
Results:
Our results demonstrate that proteins are preserved on the surface of RF+UV inactivated tumor cells, and that they are immunogenic via induction of dendritic cell maturation, increase in IFNγ production, and generation of tumor cell-specific IgG. Moreover, when formulated with an adjuvant (“Innocell vaccine”) and tested in different murine tumor primary and metastatic disease models, decreased tumor growth, decreased metastatic disease, and prolonged survival was observed. In addition, immune cells obtained from tumor tissue following vaccination had decreased exhausted and regulatory T cells, suggesting that activation of intra-tumoral T cells may be playing a role leading to reduced tumor growth.
Conclusions:
This data suggests that the RF+UV inactivation of tumor cells may provide an efficacious method for generating autologous whole tumor cell vaccines for use in cancer patients.
Keywords: Cancer, vaccine, autologous, immunogenic, tumor, metastatic disease
Introduction:
Cancer immunotherapy is currently an important treatment option for many patients with cancer. Over the years, various cancer vaccines have been developed and tested. Success in these studies has been hampered by how the cancer vaccines were generated1. Given our understanding of immune suppression, better technology is now available to induce response to a cancer vaccine, either alone, or more likely in combination with additional immune therapies2.
Inactivated vaccines can be prepared by a variety of approaches, including chemicals, such as paraformaldehyde, or radiation. Gamma and ultraviolet irradiation have also been used directly with success to inactivate white cells and prevent their replication 3–5. These processes do not work by selective means. Chemical approaches attack protein, enzymatic, lipid and nucleic acid components of the cells. Wavelengths of ultraviolet and gamma irradiation are absorbed by cellular nucleic acids and by cellular proteins components. The ultimate fate of the treated cells is death, but the manner in which that death occurs varies. This variability is crucial in developing approaches which use the cellular products derived from these processes for antigen presentation.
Riboflavin is a component of the B2 vitamin complex and is present in aerobic organisms6. It has found use as a photosensitizer in processes for the inactivation of pathogens in blood products by inactivating the ability of pathogens and white blood cells to replicate following treatment, while providing adequate retention of blood cell and protein functionality and integrity. Products treated by this process have been used in the blood bank setting for over 10 years for use in routine transfusion medicine practice 7.
The goal of our work was to study the feasibility and applicability of generating autologous, whole cell tumor vaccines using RF+UV to inactivate tumor cells.
Materials and Methods
Cell lines.
Mouse breast carcinoma cell line 4T1, colon carcinoma CT26, and murine Lewis Lung Carcinoma (LLC) were purchased from ATCC. Mouse breast carcinoma cell line, PyMT, was a generous gift from Dr. Douglas Graham at the University of Colorado Anshutz Medical Campus. Human CRL-2577 and HepG2 cells were purchased from ATCC (Manassas, VI). All cell lines are treated on a regular basis with BM Cyclin (Roche, Bradford, CT) and validated for species and tumor type.
Animals.
Balb/c mice were purchased from Envigo (Denver, CO) and C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in microisolator cages in the laboratory animal facility at Colorado State University. All animal experiments were approved by the Institutional Animal Care and Use Committee at Colorado State University.
Tumor Induction and measurements.
C57BL/6 mice were inoculated with LLC cells in 100 ul phosphate buffered saline, in the flank, s.c. while under isofluorane anesthesia. Other C57Bl/6 mice were inoculated with PyMT cells in 100 μl phosphate buffered saline in the mammary fat pad while under isofluorane. Balb/c mice were inoculated with 4T1 tumor cells in 100 μl phosphate-buffered saline in the mammary fat pad under anesthesia. Tumor measurements were performed (every 2–3 days) using calipers and calculated using the longest tumor diameter multiplied by the tumor measurement 90 degrees to the longest tumor diameter and represented as mm2.
RF+UV inactivation.
Tumor cells are processed into a single cell suspension and placed in cell-appropriate media + 20% FBS, without antibiotics. Cells are transferred to a Mirasol™ blood collection bag (Terumo BCT, Lakewood, CO) and 35 mls of riboflavin is mixed with the cells, for a final concentration of 50 μmol/L. The cells are inactivated at 300 Joules UV, which takes ~ 2 minutes. Cells are then sterilely collected and either used to prepare the Innocell vaccine or aliquoted and frozen in FBS + DMSO.
Proliferation assays.
Tumor cells or spleen cells were placed in culture for 24–72 hours. During the last 24 hours of the assay, EdU (Click-it assay, Thermo Fisher, Waltham, MA) was added to the cultures. After 24 hours, the cells were surface stained and/or permeabilized and stained with sulfo-cyanine5-azide (Lumiprobe, Hunt Valley, MD). Cell data was collected on a Gallios Flow Cytometer (Beckman Coulter, Brea, CA) and analyzed using FlowJo Software (FlowJo, Ashland, OR).
Surface antigen staining.
Tumor cells were placed in 96-well plates and blocked with either normal mouse serum + anti-FcγIII + human IgG or normal human serum and then stained with either CD44, Sca1, EpCam, CD24, CD34, CD117, or CD90 (ThermoFisher, mouse) or GLUT-1 or HLA (ThermoFisher, human). Cells were washed in FACs buffer (2% FBS in PBS + azide) and then run on the Gallios and analyzed using FlowJo software.
Dendritic cell cultures.
Bone marrow was collected from C57Bl/6 mice, washed and placed in culture with recombinant IL-4 and GM-CSF. Media was changed every few days and when the culture was 6 days old, the DCs were removed from the culture and co-incubated with either media or RF+UV inactivated 4T1 ex vivo tumor cells for 48 hours. Flow cytometry was used to measure activated DCs, using antibodies against CD11c, CD11b, CD80, CD86, CD40 and MHC Class II (ThermoFisher).
IFNγ production.
IFNγ was measured in culture supernatants using a Mouse IFNγ ELISA (DuoSet, R&D Systems, Minneapolis, MN).
Vaccine preparation.
Inactivated tumor cells were quantitated immediately after inactivation and frozen in 107 live cells. One vial of thawed cells was used to generate vaccines for 10 mice. Thawed cells were washed in PBS and added to a cationic-lipid DNA complex (CLDC)-based adjuvant8. A 1% carboxymethyl cellulose was added to the vaccine and mice were vaccinated by a s.c. injection in the skin above their radius bone, on both sides, while the mice were under isofluorane anethesia. This site drains to the axillary lymph nodes (data not shown). Vaccines were given in 200ul volumes, 100ul to each side. Following vaccination, mice received 100ul, i.p., of 60 mg/kg losartan in order to block recruitment of immune suppressive myeloid cells to the site of vaccine injection 9. Mice received 2 subsequent doses of 60 mg/kg losartan 24 and 48 hours after their first dose. Mice also received a booster dose of 100 μl CLDC i.p. 24 hours after the vaccines were given to further enhance immune activation.
Metastatic disease model.
Balb/c mice were injected with luciferase-expressing 4T1 tumor cells in the mammary fat pad in PBS. When tumors reached an average area of ~51 mm2 the tumors were surgically excised and tumor tissue was collected in media and stored at 4C overnight. The next day, the tumor tissues were minced, treated with collegenase (Clostridium histolyticum, Sigma, St Louis, MO) and filtered to remove undigested pieces of tumor. The single cell suspension was inactivated as described above. Mice were then vaccinated as described above. Starting 2 days after surgery (1 day post the first Innocell vaccine), mice were injected with D-luciferin (GoldBio, St Louis, MO), rested for 10 minutes, then imaged on an IVIS Imaging System (Perkin Elmer, Akron, OH) under anesthesia. Photon flux was calculated using the IVIS Imaging Software package.
Tumor Infiltrating Lymphocyte staining.
Tumors were excised from mice and collagenase digested into a single cell suspension. Cell number was quantitated and 500,000 cells per 96-well were plated. Cells were stained for CD45, CD3, CD4, CD8, PD-1, Lag3, Tim3 and CD25 (Thermo Fisher).
Statistical Analysis.
Differences between two groups were analyzed using a one or two-tailed Student’s t-test. Differences between multiple groups were analyzed using a One-way ANOVA. Differences between tumor growth over time was analyzed using a Two-way ANOVA, with repeated measures. Overall survival data was analyzed using a Log-rank Mantel-Cox test). A p-value of 0.05 or less was considered statistically significant. All statistical analysis was performed using Prism v7 or v8 software (GraphPad Software, San Diego, CA).
Results
Cells inactivated using RF+UV do not proliferate.
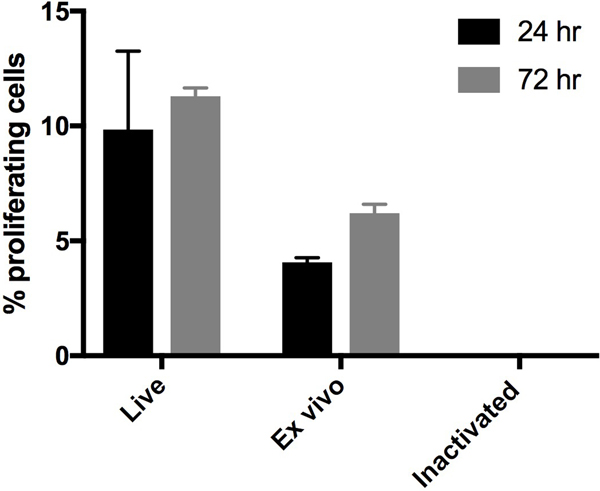
We evaluated treatment of tumor cells with RF+UV to induce complete inactivation. One million RF+UV treated cells obtained10 from ex vivo PyMT tumor tissue were injected s.c. into C57Bl6 mice once a week for 3 weeks. The mice were monitored for 160 days after injection and no tumors were observed (data not shown). Inactivated PyMT ex vivo tumor cells were also injected into immune-deficient NOD/SCID mice. These mice also had no evidence of tumor formation 250 days after injection of the inactivated cells (data not shown). Visual observation of RF+UV inactivated cells in culture did not indicate active proliferation (data not shown). To confirm this objectively, we inactivated the fast-growing murine 4T1 breast carcinoma cell line. RF+UV inactivated 4T1 cells were monitored for proliferation at 24 and 72 hours following inactivation (Figure 1). No proliferation of the tumor cells in culture was observed following inactivation.
Figure 1.
4T1 tumor cells were injected into the mammary fat pad of Balb/c mice. When tumors reached ~10mm in diameter, the mice were euthanized and tumors were removed, and processed into a single cell suspension. Some of the tumor cells were inactivated using RF+UV (“Inactivated”). Some of the tumor cells were placed directly in culture (“Ex vivo”). The “Ex vivo” and “Inactivated cells,” along with the original 4T1 tumor cell line (“Live”) were incubated at 37C for 24 or 72 hours. Proliferation was measured using EdU staining, where the EdU was added during the last 24 hours of culture.
RF+UV inactivated tumor cells maintain surface protein expression and are immunogenic.
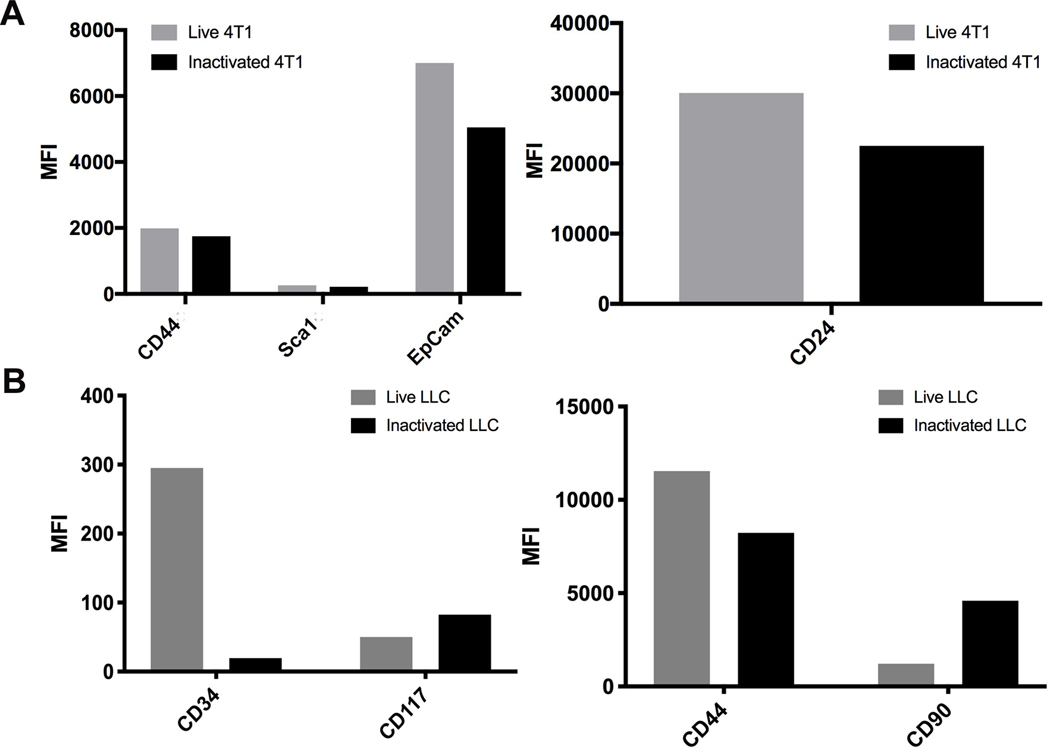
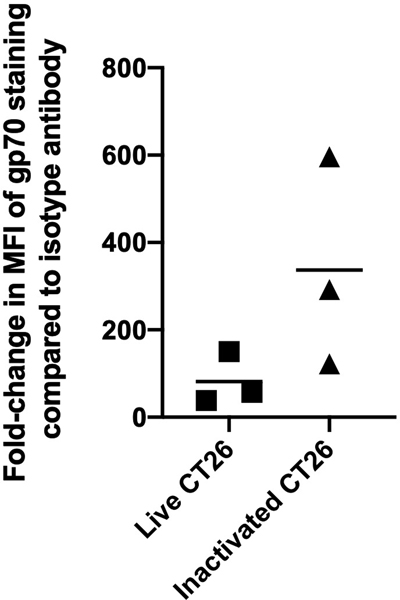
To determine if tumor cell protein expression was affected by the RF+UV inactivation process, we tested murine tumor cells lines for the expression of proteins prior to and following inactivation. As shown in Figure 2, expression of multiple tumor-associated surface proteins was not affected by the inactivation process, as demonstrated using two different murine tumor cell lines, 4T1 and the mouse lung carcinoma line, LLC. We also looked at expression of the known tumor-associated antigen, gp70, on a third mouse tumor cell line, CT26 mouse colon carcinoma 11. Surface expression of gp70 was maintained in CT26 tumor cells inactivated with RF+UV (Figure 3).
Figure 2.
(A). Murine breast carcinoma cell line, 4T1, or (B). murine lung carcinoma cell line, LLC, were inactivated using RF+UV. Surface expression of various proteins was measured using flow cytometry.
Figure 3.
CT26 tumor cells were inactivated using RF+UV and then stained for the known tumor antigen, gp70. Gp70 expression was maintained or increased on these cells. Shown are results of 3 independent assays.
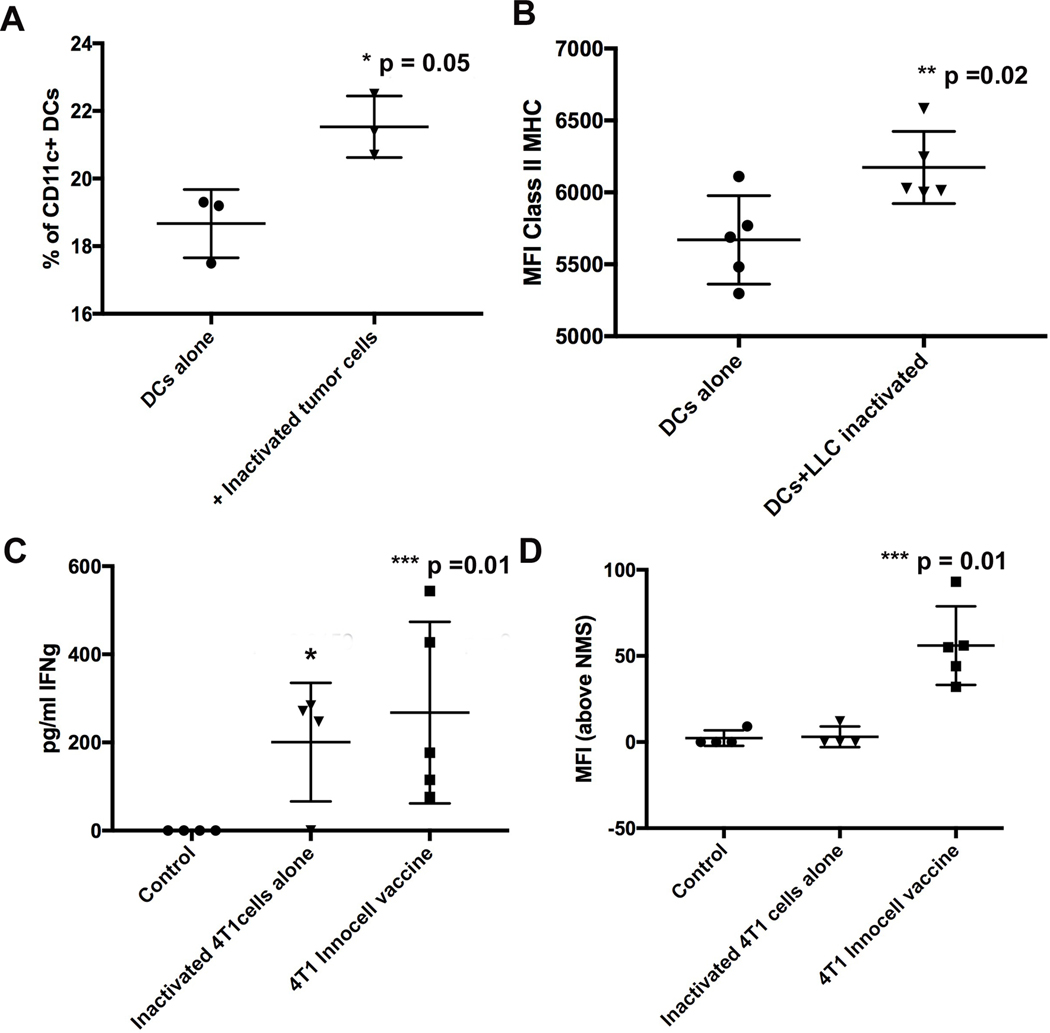
To determine if the RF+UV inactivated tumor cells were immunogenic, inactivated, ex vivo, LLC tumor cells, obtained from excision of LLC tumors from C57Bl/6 mice, were co-cultured with immature, C57Bl/6 bone-marrow derived dendritic cells (DCs) for 24 or 48 hours. We saw that the co-culturing of the inactivated LLC cells with the immature DCs resulted in an increased percentage of activated DCs as measured by surface expression of CD80, CD86 and CD40 (Figure 4A), and an increase in the geometric mean fluorescence intensity (MFI) of MHC Class II expression (Figure 4B).
Figure 4.
Immature dendritic cells (DCs) were derived from bone marrow of healthy C57Bl/6 mice and then cultured for 48 hours with RF+UV inactivated 4T1 ex vivo tumor cells. Mature DCs were measured by flow cytometry and defined as CD11c+ CD80+ CD86+ CD40+ cells and shown as percentage of CD11c+ cells (A) and by the increased MFI of Class II MHC on the CD11c+ DCs (B). (C). Spleen cells from these mice were re-stimulated in culture with inactivated 4T1 tumor cells for 72 hours and supernatants were screened for IFNγ by ELISA. (D). Serum was obtained from these mice and was tested at a 1:1000 dilution for binding to inactivated 4T1 cell line-derived tumor cells.
Next, tumor cells obtained from ex vivo excision of 4T1 tumors grown in the mammary fat pad of Balb/c mice were RF+UV inactivated and combined with CpG ODN adjuvant and injected into healthy, naïve Balb/c mice. The mice were boosted 2 weeks later. One-week post-boost, spleen cells, upon ex vivo re-stimulation with RF+UV inactivated 4T1 tumor cells, showed increased production of IFNγ (Figure 4C). Moreover, 4T1 tumor cell-specific IgG antibodies were detected in the serum of the vaccinated mice (Figure 4D).
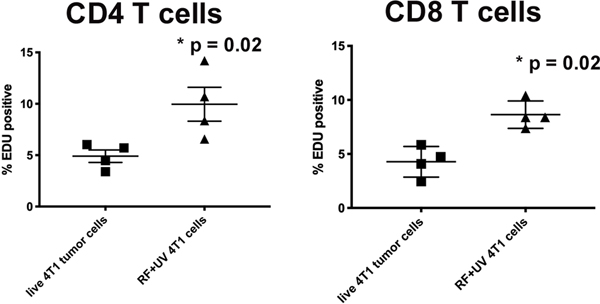
Lastly, we challenged spleen cells derived from untreated, 4T1-tumor bearing mice against both live 4T1 cell-line derived tumor cells and RF+UV-inactivated 4T1 cell-line derived tumor cells and measured T cell proliferation. As shown in Figure 5, there was an increase in proliferation of both subsets (CD4 and CD8) of T cells incubated with the inactivated 4T1 cells compared to live 4T1 tumor cells.
Figure 5:
RF+UV inactivated cells induce proliferation of T cells from spleens from 4T1-tumor bearing mice. Spleen cells were collected from Balb/c mice with orthotopic 4T1 tumors that were not treated. The cells were co-cultured with either live 4T1 tumor cells or RF+UV-inactivated tumor cells for 72 hours and proliferation of CD4+ and CD8+ T cells was measured using EdU.
RF+UV inactivated tumor cells formulated as part of an adjuvanted vaccine reduces primary and metastatic tumor cell growth
Primary Tumor Growth Suppression Studies
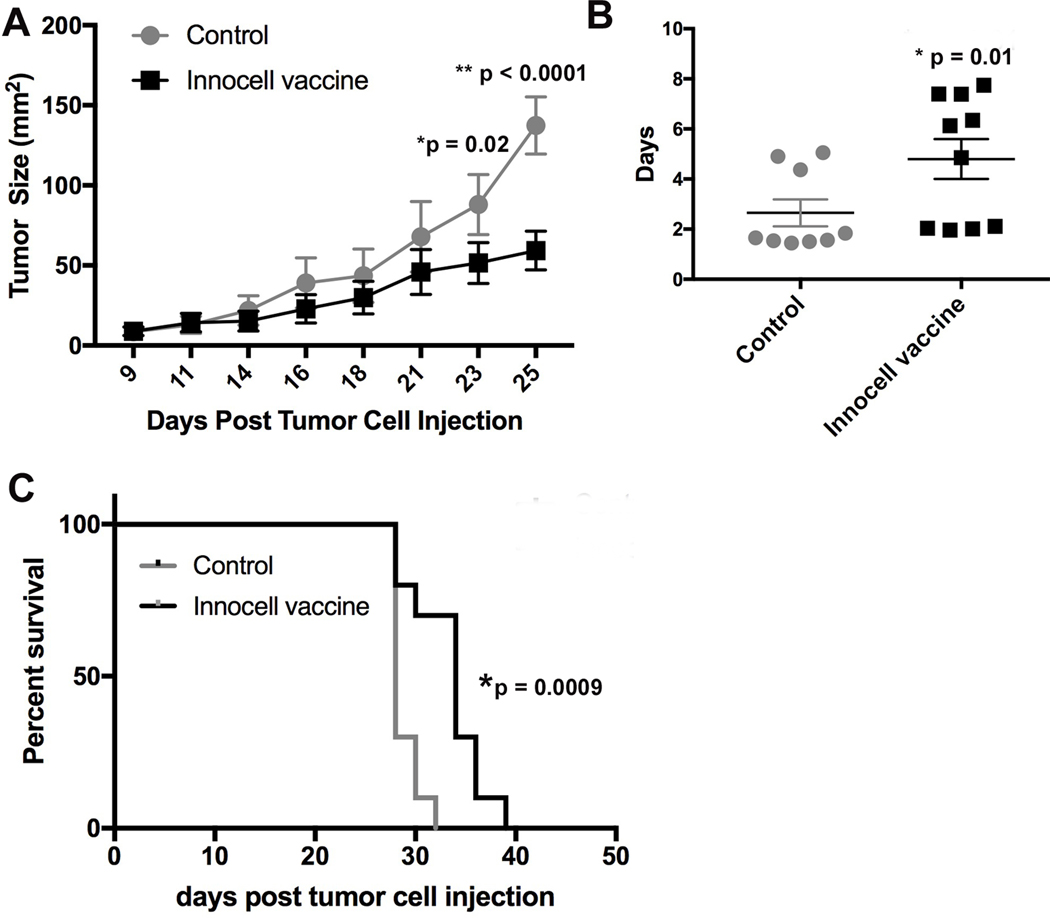
To evaluate the immunogenicity of the RF+UV inactivated tumor cells as part of a vaccine, we combined the inactivated tumor cells with cationic liposome DNA complexes (CLDC) adjuvant and gave the vaccine as a once a week, s.c. injection in tumor-bearing mice. Initial studies looked at the effect of the Innocell vaccine on primary mammary tumor growth. PyMT tumor cells were orthotopically implanted into the mammary fat pad of C57Bl/6 mice. Once tumors measured ~1 cm in diameter, tumor tissue was surgically collected and the isolated cells were RF+UV inactivated. Other mice were injected with PyMT cells in the mammary fat pad and three days later were vaccinated with the PyMT Innocell vaccine, s.c. into the caudal area of both forelimbs to target the vaccine to the axillary draining lymph nodes (LNs), as vaccinating farther away from the location of a primary tumor produces a more robust immune response 12. Mice also received 3 daily doses of losartan, initiated on the same day as the vaccine. Losartan, which has CCR2 antagonistic properties, was shown to reduce recruitment of inflammatory monocytes to vaccine-draining LNs9,13. As shown in Figure 6A, mice that received weekly Innocell vaccination had significantly reduced tumor growth compared to control mice. While the tumors continued to grow, the doubling time of the tumors from the vaccinated mice was significantly reduced compared to control mice (Figure 6B) and the vaccinated mice took longer to reach their euthanasia endpoint (tumors exceeding 15 mm in diameter, Figure 6C). A similar protocol was used in a C57Bl/6 mouse model of lung cancer (LLC) and produced similar results (Figure 7).
Figure 6:
C57Bl/6 mice were injected with PyMT tumor cells in the mammary fat pad. Three days later, the mice received their first vaccine consisting of RF+UV inactivated tumor cells (derived from ex vivo PyMT tumors) admixed with CLDC adjuvant and concurrent 3 day treatment with i.p. losartan (60 mg/kg), repeated weekly. (A). Tumor growth was measured and reported as tumor size (area in mm2). (B). Doubling time of the tumor area was significantly prolonged in mice treated with the Innocell vaccine (p =0.01, two-tailed t-test). (C). Overall survival of mice was significantly increased in mice treated with the Innocell vaccine (p = 0.0009, Log-rank Mantel-Cox test).
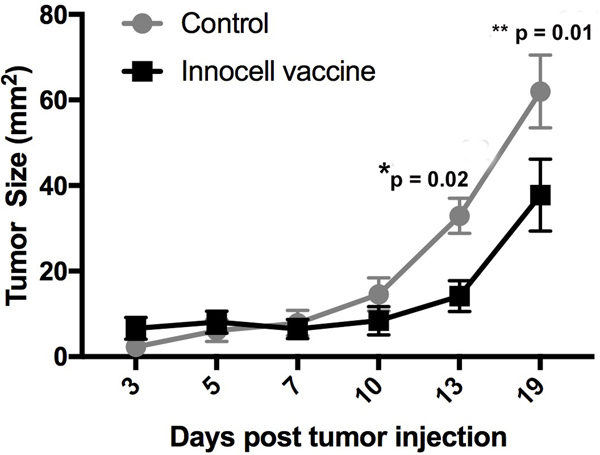
Figure 7:
Innocell vaccine reduces tumor growth in a murine lung carcinoma model. C57bl/6 mice were injected with LLC cells s.c. in the flank. Three days after tumor cell injection, mice were vaccinated with an LLC-based Innocell vaccine, consisting of RF+UV inactivated, LLC tumor cells admixed with CLDC adjuvant and co-administered with 3 daily doses of losartan (60 mg/kg).
Metastatic Tumor Growth Suppression Studies
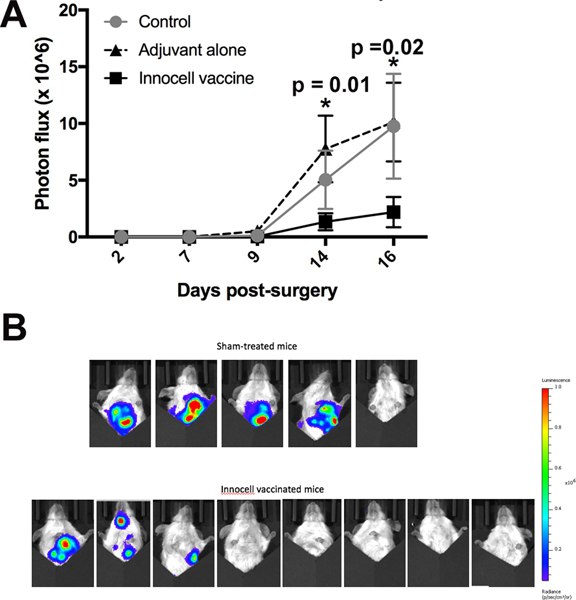
To study our vaccine in a more clinically relevant setting, we utilized primary tumors excised from 4T1 tumor-bearing mice to generate the RF+UV inactivated cells. In this case, the mice that the tumors were excised from were either treated with vehicle control, adjuvant alone (CLDC + losartan), or the Innocell vaccine generated from their autologous tumors and then monitored for the development and progression of metastatic disease. Specifically, Balb/c mice bearing orthotopic, luciferase-expressing 4T1 breast carcinoma tumors underwent surgical removal of their primary tumors when the average tumor size was approximately 50 mm2. It is known that when tumors reach this size, 100% of the mice will develop metastases in their lungs14. The excised tumors were then combined and the cells were treated with RF+UV. One day after the surgical removal of the tumors, mice received their first Innocell vaccine, and were revaccinated weekly thereafter. Mice were monitored for metastatic disease using IVIS imaging. There was a decrease in the metastatic burden of mice treated with the 4T1 Innocell vaccine compared to the other two groups of mice (Figure 8A and B).
Figure 8:
Metastatic disease development was monitored in mice using luciferase and IVIS imaging. (A). Mice receiving the Innocell vaccine had a significantly reduced luciferase photon flux on days 14 (adjuvant alone vs Innocell vaccine, p = 0.0157, two-way ANOVA with repeated measures) and day 16 (Control vs Innocell vaccine, p = 0.0119 and adjuvant vs Innocell vaccine, p = 0.0021 by two-way ANOVA with repeated measures). (B). IVIS images of the sham-treated mice vs the Innocell vaccinated mice on day 14.
Despite these observations, a delay in the development of metastatic disease was not seen. This may have been due in part to the small number of mice per group. On day 14, 4 of the 5 sham-treated mice had metastatic disease, while 3 of the 8 Innocell treated mice had evidence of disease (Figure 8B).
Innocell vaccine causes reduction in T regulatory cells and exhausted T cells.
One facet of cancer is its ability to elicit immune suppression. Tumor cells can generate immune suppression through mechanisms such as direct contact with T cells via expression of checkpoint molecules, such as PD-L1, or through recruitment of immune suppressive cells, including T regulatory cells 15–17. To assess the functional state of the T cells in tumors of the Innocell vaccinated mice, LLC tumor-bearing mice received three Innocell vaccines, one week apart. Three days after the third vaccine, Innocell-vaccinated and sham-treated mice were euthanized and tumor tissue was collected for flow cytometry analysis of T cell functional status and T regulatory cells. There was a decrease in the percentage of CD4+ T cells that expressed CD25, a marker of T regulatory cells 18 (Figure 9A). In addition, there was a decrease in CD4+ and CD8+ T cells expressing Lag3 and Tim3, two immune checkpoint molecules 19,20 (Figure 9B and C). While there was not a difference in percentages of CD4+ or CD8+ T cells expressing PD-1, there was a difference in the amount of PD-1 expressed per CD4+ T cell as measured by mean fluorescence intensity (MFI, Figure 9D).
Figure 9.
LLC tumor bearing mice were treated weekly with the Innocell vaccine. On day 20 post-tumor cell injection, the mice were euthanized and tumor tissue collected for analysis by flow cytometry. (A). Innocell vaccinated mice had significantly less CD4+CD25+ T regulatory cells (*p = 0.004, two-tailed t-test). (B). CD4+ T cells and (C). CD8+ T cells were analyzed for expression of the immune checkpoint molecules PD-1, Lag3 and Tim3. (D). CD4+ T cells in the tumor had a significantly reduced expression level of PD-1 as determined by measuring MFI on the intra-tumor CD4+ T cells. *p-value = 0.04, two-tailed t-test).
RF+UV inactivation of human tumor cells results in cells that are metabolically active, but not able to proliferate.
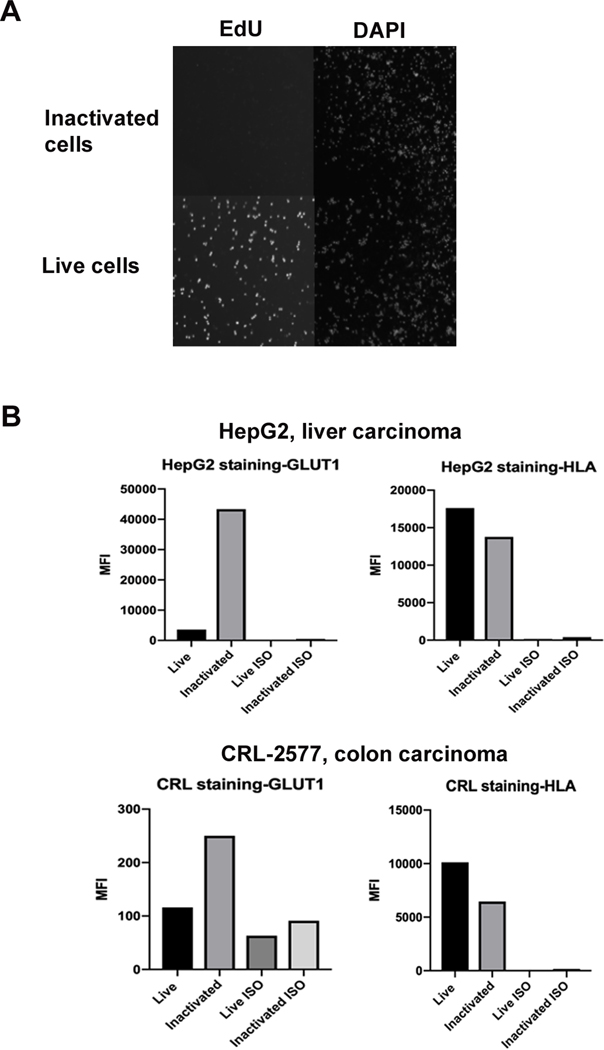
We wanted to evaluate if the RF+UV would inactivate human tumor cells while preserving protein expression as observed in animal cell lines. The human colon carcinoma line, CRL-2577 was RF+UV inactivated and compared to non-inactivated cells. Figure 10A shows that while the untreated CRL-2577 bound to EdU, the riboflavin/UV light inactivated tumor cells had no EdU-associated fluorescence. Cell adhesion to plastic was not observed with visual inspection of the cultured, RF+UV inactivated cells (data not shown).
Figure 10.
Human tumor cells inactivated with RF+UV do not proliferate and maintain surface antigen expression. (A). Human CRL-2577 cells were inactivated with RF+UV, rested in culture overnight, and then labeled with EdU for 6 hours before being stained with DAPI and imaged. Both live and inactivated cells stained with DAPI, while only the live cells stained with EdU, indicating that RF+UV inactivation inhibited proliferation of the human tumor cells. (B). CRL-2577 and the human liver carcinoma line, Hep G2, were stained for expression of GLUT-1 and HLA prior to and following RF+UV inactivation.
Next, CRL-2577 and the human liver carcinoma cell line, Hep G2, were stained for expression of GLUT-1 and HLA. HepG2 expressed high levels of GLUT-1 and HLA, while the CRL-2577 cells expressed low levels of GLUT-1 and high levels of HLA (Figure 10B). Nonetheless, expression of both antigens following RF+UV inactivation was observed, with a noted increase in GLUT-1 by both cell lines after inactivation.
Discussion
Cell-based immune therapies have recently become important therapeutic options for certain cancers, particular hematologic neoplasias and chimeric-antigen receptor (CAR) T cell therapies. These therapies consist of targeting single, or limited tumor antigens, and work well for blood-derived tumors, where elimination of healthy cells in addition to neoplastic cells, is not detrimental to the patient. However, similar approaches to generating CAR T cells for solid tumors have been challenging. Thus, there is a need for better cell-based immune therapies for these tumors. Many groups have investigated the use of cancer vaccines to augment the anti-tumor immune response.
Many cancer vaccines are designed to target either one or a limited number of tumor-associated antigens. However, it is known that tumor cells can mutate those antigens, rendering them yet again, invisible to the immune system. Thus, vaccines designed to target multiple antigens will likely provide greater success than those that target a limited set of antigens. Moreover, a majority of passenger mutations in cancer patients are unique to their tumor, so cancer vaccines that are personalized to the individual will likely have a greater success. For example, there have been demonstrated successes using neoantigen-based peptide cancer vaccines 21–23.
Our preliminary data presented here suggests that RF+UV inactivation prevents tumor cell proliferation of both mouse and human tumor cells, prevents growth of the RF+UV inactivated tumor cells in vivo, while maintaining protein expression. Moreover, this inactivation process may make tumor cells more immunogenic by increasing DC maturation and IFNγ production in spleen cells for mice vaccinated with either inactivated tumor cells only, or the Innocell vaccine (and re-stimulated with RF+UV inactivated 4T1 cells in culture). We also noted the induction of CD4+ and CD8+ T cell proliferation in tumor-bearing mice spleen cells, ex vivo following exposure to the vaccine.
Platelets treated by this process have been shown to demonstrate up-regulation of protein kinases such as p38 MAPK and expression of cell signaling agents24. These modifications in tumor cells may be responsible for the enhanced antigen presentation observed in treated products reported here. The effect that this may have relative to enhanced interaction of treated tumor cells with immune response cells in vivo requires further evaluation and study. Current studies are underway to investigate changes in RNA and protein expression following RF+UV inactivation.
When tested as part of a vaccine with adjuvant, the RF+UV tumor cells elicited reduced percentages of regulatory CD4+ T cells and immune exhausted T cells in the tumor microenvironment. These changes in the profile of tumor-infiltrating lymphocytes resulted in reduced primary tumor growth and reduced metastatic disease in mouse tumor models. This method is currently under investigation in a veterinary clinical setting in canine patients that spontaneously develop cancer. This setting can serve as a model for human disease.
Acknowledgements.
The authors would like to thank Mrs. Lori Bentsen for her technical assistance in measuring and imaging the mice for the vaccine studies under blinded sample identities. RG and AG designed experiments and conducted data analysis and also led writing of the manuscript equally. CG, MG, HP performed laboratory studies indicated and assembled data.
REFERENCES
- 1.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019;18: 197–218. [DOI] [PubMed] [Google Scholar]
- 2.Morse MA, Lyerly HK. Checkpoint blockade in combination with cancer vaccines. Vaccine 2015;33: 7377–85. [DOI] [PubMed] [Google Scholar]
- 3.Chu EH. Effects of ultraviolet radiation on mammalian cells. I. Induction of chromosome aberrations. Mutat Res 1965;2: 75–94. [DOI] [PubMed] [Google Scholar]
- 4.Daly MJ. Death by protein damage in irradiated cells. DNA Repair (Amst) 2012;11: 12–21. [DOI] [PubMed] [Google Scholar]
- 5.Chapman JD, Stobbe CC, Gales T, Das IJ, Zellmer DL, Biade S, Matsumoto Y. Condensed chromatin and cell inactivation by single-hit kinetics. Radiat Res 1999;151: 433–41. [PubMed] [Google Scholar]
- 6.Jiang HN, Li Y, Cui ZJ. Photodynamic Physiology-Photonanomanipulations in Cellular Physiology with Protein Photosensitizers. Front Physiol 2017;8: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yonemura S, Doane S, Keil S, Goodrich R, Pidcoke H, Cardoso M. Improving the safety of whole blood-derived transfusion products with a riboflavin-based pathogen reduction technology. Blood Transfus 2017;15: 357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melander A, Olsson J, Lindberg G, Salzman A, et al. 35th Annual Meeting of the European Association for the Study of Diabetes : Brussels, Belgium, 28 September-2 October 1999. Diabetologia 1999;42: A1–A330. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell LA, Hansen RJ, Beaupre AJ, Gustafson DL, Dow SW. Optimized dosing of a CCR2 antagonist for amplification of vaccine immunity. Int Immunopharmacol 2013;15: 357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruane PH, Edrich R, Gampp D, Keil SD, Leonard RL, Goodrich RP. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion 2004;44: 877–85. [DOI] [PubMed] [Google Scholar]
- 11.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, Pardoll DM, Jaffee EM. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci U S A 1996;93: 9730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohlfest JR, Andersen BM, Litterman AJ, Xia J, Pennell CA, Swier LE, Salazar AM, Olin MR. Vaccine injection site matters: qualitative and quantitative defects in CD8 T cells primed as a function of proximity to the tumor in a murine glioma model. J Immunol 2013;190: 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell LA, Henderson AJ, Dow SW. Suppression of vaccine immunity by inflammatory monocytes. Journal of Immunology 2012;189: 5612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnard RA, Regan DP, Hansen RJ, Maycotte P, Thorburn A, Gustafson DL. Autophagy Inhibition Delays Early but Not Late-Stage Metastatic Disease. J Pharmacol Exp Ther 2016;358: 282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allegrezza MJ, Conejo-Garcia JR. Targeted Therapy and Immunosuppression in the Tumor Microenvironment. Trends Cancer 2017;3: 19–27. [DOI] [PubMed] [Google Scholar]
- 16.Gasser S, Lim LHK, Cheung FSG. The role of the tumour microenvironment in immune therapy. Endocr Relat Cancer 2017. [DOI] [PubMed] [Google Scholar]
- 17.Ibanez-Vea M, Zuazo M, Gato M, Arasanz H, Fernandez-Hinojal G, Escors D, Kochan G. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment: Current Knowledge and Future Perspectives. Arch Immunol Ther Exp (Warsz) 2017. [DOI] [PubMed] [Google Scholar]
- 18.Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J 2012;26: 2253–76. [DOI] [PubMed] [Google Scholar]
- 19.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res 2014;2: 393–8. [DOI] [PubMed] [Google Scholar]
- 20.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009;10: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aurisicchio L, Salvatori E, Lione L, Bandini S, Pallocca M, Maggio R, Fanciulli M, De Nicola F, Goeman F, Ciliberto G, Conforti A, Luberto L, Palombo F. Poly-specific neoantigen-targeted cancer vaccines delay patient derived tumor growth. J Exp Clin Cancer Res 2019;38: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547: 217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwasa S, Yamada Y, Heike Y, Shoji H, Honma Y, Komatsu N, Matsueda S, Yamada A, Morita M, Yamaguchi R, Tanaka N, Kawahara A, Kage M, Shichijo S, Sasada T, Itoh K. Phase I study of a new cancer vaccine of ten mixed peptides for advanced cancer patients. Cancer Sci 2016;107: 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schubert P, Coupland D, Culibrk B, Goodrich RP, Devine DV. Riboflavin and ultraviolet light treatment of platelets triggers p38MAPK signaling: inhibition significantly improves in vitro platelet quality after pathogen reduction treatment. Transfusion 2013;53: 3164–73. [DOI] [PubMed] [Google Scholar]