Abstract
Tumor progression relies on the ability of cancer cells to effectively invade surrounding tissues and propagate. Among the many mechanisms that contribute to tumor progression is the epithelial-to-mesenchymal transition (EMT), a phenotypic plasticity phenomenon that increases the cancer cells’ motility and invasiveness and influences their surrounding microenvironment by promoting the secretion of a variety of soluble factors. One such factor is IL-8, a chemokine with multiple pro-tumorigenic roles within the tumor microenvironment (TME), including stimulating proliferation or transformation of tumor cells into a migratory or mesenchymal phenotype. Further, IL-8 can increase tumor angiogenesis or recruit larger numbers of immunosuppressive cells to the tumor.
Prognostically, observations in many tumor types show that patients with higher levels of IL-8 at baseline experience worse clinical outcomes. Additionally, studies have shown that the chemokine directly contributes to the development of resistance to both chemotherapy and molecularly targeted agents. More recently, clinical studies evaluating levels of IL-8 in patients receiving immune checkpoint inhibition (ICI) therapy deduced that myeloid tumor infiltration driven by IL-8 contributes to resistance to ICI agents and that peripheral IL-8 can predict outcomes to ICI therapy. Further, pre-clinical data demonstrate that targeting IL-8 or its receptors enables improved tumor killing by immune cells, and treatment strategies combining blockade of the IL-8/IL-8R axis with ICI ultimately improve anti-tumor efficacy. Based on these results and the prognostic capacity of IL-8, there are a number of ongoing clinical trials evaluating the addition of IL-8 targeting strategies to immune-based therapies.
Keywords: IL-8, tumor progression, therapeutic resistance, tumor microenvironment
1. Introduction
Tumor development is a complex, multi-step process that occurs over time, and although the biology of tumors within unique tissues can be quite distinct, all tumors possess some common characteristics which aid in their survival. Namely, tumor cells are known to acquire the ability of sustained proliferation while altering their localized microenvironment to support their growth and resist destruction by the host immune system (Hanahan & Weinberg, 2011). As cancer cells grow and acquire the ability to metastasize to new sites of the body, a phase termed tumor progression, tumors also become more difficult to target by the host immune system and with therapeutics.
In order for tumor progression to occur, cancer cells must gain the ability to disseminate as well as to support their growth within the new location(s) of metastasis (Roussos, et al., 2011). One of the mechanisms used by cancer cells to acquire motility and invasiveness is the epithelial-to-mesenchymal transition (EMT) (Brabletz, et al., 2018; Derynck & Weinberg, 2019; Yuan, et al., 2019). This phenomenon, most commonly observed during embryogenesis, involves the loss of epithelial cell-to-cell contacts and the increase in expression of various mesenchymal proteins that mediate motility (vimentin, fibronectin) or the degradation of the basal membrane and extracellular matrix (matrix metalloproteinases). This combination increases the ability of cancer cells to effectively disseminate. Tumor cells undergoing these phenotypic changes or “phenotypic plasticity” can also influence their surrounding microenvironment by secreting a milieu of soluble factors that can increase angiogenesis and promote immune suppression.
We and others have extensively studied the plasticity of tumor cells undergoing EMT and the immune suppressive features this process upregulates (Anestakis, et al., 2015; David, et al., 2017; David, et al., 2016; Dominguez, et al., 2017a; Hamilton, et al., 2014; Horn, et al., 2020a; Junttila & de Sauvage, 2013). Factors such as TGF-β, IL-8, IL-6, VEGF, collagens, and others are described as key molecules affecting numerous aspects of tumor progression. In this review we will focus on the cytokine IL-8, or CXCL8, a pro-inflammatory chemokine which binds to two G-protein coupled receptors, CXCR1 and CXCR2, and is responsible for attracting neutrophils to sites of injury and inflammation. IL-8 can be secreted by macrophages and endothelial cells while its counterpart receptors exhibit expression on monocytes, granulocytes, and endothelial cells (Waugh & Wilson, 2008). IL-8 production is dependent on upstream NF-kB signaling, and the chemokine itself triggers downstream activation of PI3K and MAPK signaling cascades (Liu, et al., 2016). Within the context of tumors, IL-8 has been described to have dual pro-tumorigenic roles, including directly impacting tumor cells themselves and modifying the composition of the tumor microenvironment (TME) (David, et al., 2016). Furthermore, increased levels of IL-8 have been shown to correlate with poor prognosis in many cancers as well as the development of resistance to therapies (Cheng, Y., et al., 2019). Herein, we will review the effects of IL-8 in tumor biology, the ways that IL-8 affects responses to current therapies, and the novel therapeutics targeting the IL-8/IL-8R axis that are currently in development.
2. The Role of IL-8 in Tumor Biology
IL-8 has been shown to influence the biology of numerous types of cancer, including melanoma, prostate, colon, pancreatic, breast, and lung (Cheng, Y., et al., 2019). The overarching functions of the chemokine within these cancers can be broadly divided into two categories, direct and indirect effects. IL-8 directly influences tumors by altering features of the tumor cells themselves through the transition of tumor cells to a mesenchymal phenotype, into a migratory cell state, or by promoting proliferation. In one study, it was shown that hepatocellular carcinoma (HCC) cells are able to produce IL-8 in response to the neuropeptide neurotensin (previously correlated with poor prognosis (Yu, et al., 2013)). IL-8 production promoted a transition of tumor cells to a mesenchymal phenotype and functionally resulted in increased tumor cell migration and invasion in vitro as well as the development of metastases in vivo. Importantly, these changes were found to be reversible with the use of CXCR1/2 antagonist therapy (Xiao, et al., 2018). In the context of breast cancer, it was shown that the induction of EMT within tumor cells subsequently increases the activity of the IL-8/IL-8R cytokine axis. The IL-8 secreted from mesenchymal tumor cells was able to induce EMT in surrounding epithelial cells, and this induction of IL-8 was essential for the acquisition and maintenance of the metastatic phenotype of tumor cells. Importantly, blockade of IL-8 receptors significantly decreased the invasiveness of breast cancer cells in vitro (Fernando, et al., 2011). Numerous pre-clinical studies have shown that activation of NF-kB signaling, through various upstream molecules, results in tumor progression precipitated by IL-8 (Geng, et al., 2017; Zhang, B., et al., 2015; Zhang, T., et al., 2019). In one specific example, activation of NF-kB signaling was shown to maintain the mesenchymal phenotype of glioma cells as well as induce cytoskeletal rearrangement allowing for increased chemotaxis and invasion. In this model the activity of IL-8 was described to work in an autocrine fashion as it bound CXCR1 on tumor cells to trigger the signaling cascade resulting in increased NF-kB activity (Zhang, B., et al., 2015).
In a more indirect manner, IL-8 also carries out functions which alter the surrounding TME in order to promote tumor development and metastasis. IL-8 increases angiogenesis within the tumor and alters the immune microenvironment by recruiting higher numbers of infiltrating immune suppressive cells. In pre-clinical models of mouse and human colorectal cancer (CRC) in IL-8 transgenic mice, IL-8 enhanced tumor growth by inducing a significant increase in CD31+ peritumoral vasculature. In the instance of syngeneic CXCR2 KO mouse models, tumor growth was significantly inhibited, presumably due to the lack of increased tumor vasculature in the absence of IL-8 signaling (Lee, et al., 2012). Further evidence for the role of IL-8/CXCR2 in tumor angiogenesis was described in studies demonstrating in vitro that IL-8 is the primary cytokine responsible for disrupting cell junctions, resulting in greater permeability of endothelial cells in glioblastoma (Dwyer, et al., 2012).
Not only does IL-8 affect the cell phenotype of the tumor and endothelial cells in the TME, but it also changes the immune composition within the tumor site. IL-8 preferentially recruits pro-tumorigenic immune cells which are able to dampen the anti-tumor immune response of cytotoxic immune cells. Most notably, it has been described that the conversion of resident monocytes into tumor-associated macrophages (TAM) or the recruitment of myeloid derived suppressor cells (MDSC) to the TME enables suppression of the anti-tumor efficacy of cytotoxic T cells (Ostrand-Rosenberg & Fenselau, 2018), a process in which IL-8 signaling is highly implicated. In studies using serum and PBMCs from patients with gastric cancer, it was shown that IL-8 enhances PI3K/Akt signaling to increase levels of arginase I in MDSC, which promotes MDSC suppression of CD8+ T cell activity (Mao, et al., 2018). Many studies have also assessed the influence of IL-8 in TAM in different types of cancers; in one pre-clinical example of pancreatic adenocarcinoma, it was observed that tumor cell conditioned media promoted the differentiation of monocytes into M2-like TAM secreting high levels of IL-8 that, in turn, controlled multiple pro-tumor effects (Chen, et al., 2018). While MDSC and TAM are some of the most notable subsets of cells discussed in an immunosuppressive TME, they are far from the only immune cells affected by IL-8. Neutrophils present within the TME can also exert pro- or anti-tumor effects. Naturally, neutrophils are an early subset of innate immune responders in the case of injury or infection; however, through polarization, these cells can take on suppressive features, becoming tumor-associated neutrophils (Wang, et al., 2018). These suppressive neutrophils that traffic to the TME promote tumorigenesis through suppression of tumor infiltrating lymphocytes (Germann, et al., 2020). Upon arrival to an injury or tumor site, neutrophils produce extracellular traps which are primarily composed of DNA and proteolytic enzymes used to target pathogens (Kaplan & Radic, 2012). In a study using peripheral blood from cancer patients, it was observed that IL-8 not only chemoattracted MDSC but also stimulated the formation of neutrophil extracellular traps from the granulocytic MDSC population (Alfaro, et al., 2016).
Another subset of immune cells which provides important anti-tumor efficacy is NK cells. A study on oesophageal squamous cell carcinoma found that STAT3 signaling was activated in tumor cells via the IL-8 and IL-6 pathways; this activation led to a decrease of the expression of NK-activating receptors Nkp30 and NKG2D, rendering NK cells ineffective. The blockade of STAT3 signaling was able to reverse these effects (Wu, et al., 2019).
Ultimately, it is important to remember that both direct and indirect effects of IL-8 on individual cell types can result in cascading alterations to the TME. The phenotypic changes of the tumor cells influence the TME while the changes in the vessels, extracellular matrix, presence of immune cells, etc. further influence the tumor. In the study of pancreatic adenocarcinoma described above, the newly differentiated M2-TAM further secreted IL-8, enhancing tumor cell motility in vitro and promoting tumor EMT via the upregulation of the transcription factor Twist, resulting in increased metastasis in vivo (Chen, et al., 2018). In another example of a pro-tumor feedback loop, authors used an ovarian cancer model to demonstrate that IL-8 is able to induce stemness in cell lines as well as polarize macrophages towards an M2 phenotype; blocking either IL-8 or STAT3 signaling reversed these phenotypic changes in the cells. Co-culture with ovarian cancer stem-like cells and macrophages also suggested that this interaction can produce further IL-8, creating a situation in which IL-8 production ultimately stimulates either the production of more IL-8 by other cells or recruits/modifies the TME to have a greater proportion of cells that would produce IL-8 such as MDSC/TAM (Ning, et al., 2018).
3. IL-8 as a Prognostic Factor
Many studies have assessed the value of IL-8 as a prognostic marker in numerous types of cancer. In a cohort of 68 patients with pancreatic cancer, for example, it was observed that IL-8 was strongly associated with poor survival (Feng, et al., 2018). Similarly, in ovarian cancer, an analysis of serum from 26 patients described that high IL-8 was associated with a worse disease prognosis (Sanguinete, et al., 2017). In another study focusing on angiogenic markers, one of which was IL-8, authors evaluated the serum of 64 patients with non-small cell lung cancer (NSCLC) for both prognostic and predictive effects. IL-8 levels were significantly raised prior to therapy compared to controls; this was true for both adenocarcinoma and squamous cell carcinoma. When comparing responders and non-responders treated with current standard of care therapy, the responders demonstrated significantly lower levels of baseline IL-8 (Keskin, et al., 2019). Furthermore, in multiple clinical studies, IL-8 has been described as a predictive marker in breast cancer. In a phase 2 clinical trial enrolling 58 patients with metastatic breast cancer, patients who had lower levels of IL-8 (<16.6 pg/mL) throughout the duration of therapy had a significantly higher rate of overall survival with a median of 47.5 months compared to 20.5 months for those with higher IL-8 (>16.6 pg/mL) in a trajectory analysis (Tiainen, et al., 2019). In another study, samples from 346 patients with breast cancer were assessed for the expression of IL-8 and TGF-β and their potential influence on the development of chemoresistance, denoted by the level of the MRP1 (multidrug resistance-associated protein 1) gene. Evaluating samples before and at up to 4 time points throughout the course of chemotherapy, it was identified that MRP1 gene expression increased over time and was strongly correlated with increases in IL-8 and TGF-β levels detected in the peripheral blood (Zhuang & Wang, 2018). While chemotherapeutic regimens may not be directly comparable for their efficacy across different tumor types, one clear pattern is that the non-responders or those patients who experience poor outcomes generally have higher levels of IL-8 present in their serum, and presumably at the site of their tumor. Each of the cases discussed provides examples of the utility of IL-8 as a prognostic and predictive biomarker, but the data remain correlative. In order to evaluate these findings in a more causative and mechanistic fashion, many groups have studied resistance to a variety of therapies in the context of high-IL-8 expressing tumors in pre-clinical models.
4. IL-8 Plays a Central Role in the Development of Resistance to Therapies
a. Induction of Chemoresistance
Within the context of chemotherapy, extensive research substantiates that IL-8 plays a crucial role in the generation of chemoresistant tumor cells. In two pre-clinical studies, doxorubicin (Dox)-resistant cell lines were developed in vitro utilizing models of CRC (Du, et al., 2018) and osteosarcoma (Cheng, M., et al., 2018). In both studies, IL-8 was the only cytokine exhibiting increased levels in Dox-resistant cells, and mechanistically both studies implicated the downstream ABCB1/MDR1 pathway. ABCB1 is a key ATP-binding cassette subfamily B member and MDR1 is multidrug resistant 1, a product produced as a result of ABCB1 activation; this pathway has been previously linked with drug-resistance. In the CRC cell line models, IL-8 increases downstream phosphorylation of p65, which binds to the ABCB1 promoter. By targeting IL-8 with siRNA or inhibitors of either CXCR1/2 or NF-kB signaling, downregulation of ABCB1 and decreases in MDR1 mRNA and protein were observed (Du, et al., 2018). In the context of osteosarcoma, IL-8 also upregulates the downstream ABCB1, and further upstream analysis demonstrated that HDAC6 was involved in the increase in IL-8 expression in Dox-resistant osteosarcoma cells. Targeting p65 or HDAC6 enabled regulation of the expression of ABCB1 or IL-8 in osteosarcoma cells, respectively (Cheng, M., et al., 2018). Importantly, in each of these cases, inhibition of IL-8 re-sensitized Dox-resistant cells to doxorubicin again.
In a study in gastric cancer the same p65/ABCB1 mechanism was again found to mediate chemoresistance, this time to cisplatin. Importantly, the clinical portion of this research deduced that not only is high serum IL-8 predictive of poor response to platinum-based chemotherapy but also that the high levels of IL-8 in chemoresistant patients originated from cancer-associated fibroblasts rather than tumor cells (Zhai, et al., 2019). Further, in malignant pleural mesothelioma it was observed that ABCB5 was upregulated particularly in the tumor initiating cell population. Within these cells either IL-8/IL-1β or Wnt/β-catenin signaling was upregulated in order to achieve higher levels of ABCB5, and ultimately, cisplatin resistance (Milosevic, et al., 2020). Beyond those described here, there are numerous additional studies demonstrating that increases in IL-8 enable chemoresistance within many cancers, including pancreatic, colorectal, breast, melanoma, and others (Dabkeviciene, et al., 2015; Imafuji, et al., 2019; Sootichote, et al., 2018). Collectively, these findings imply that disruption of IL-8 may be able to improve the activity of chemotherapy in many types of cancer.
b. Inciting Escape from Molecular Targeted Therapies
As chemotherapy does not provide long-term efficacy to all patients, the field has moved towards the investigation of more targeted therapies. At this time some of these therapeutics are only available within the context of tumor recurrence while others are incorporated as adjuvant therapy in the front-line setting. However, as these therapies become more widely used there is a growing problem of tumor escape from them as well. Prior research from our group and others has shown that IL-8 is also involved in this resistance to targeted therapies.
In the context of HCC, the role of PI3K/Akt/mTOR pathway inhibition in tumor resistance to therapy was assessed. Specifically, the study focused on the outgrowth of liver cancer stem cells as one of the key mechanisms by which resistance is acquired. They found that, in vitro, HCC cells treated with sorafenib or regorafenib, multikinase inhibitors frequently used in the clinic, developed drug resistance, exhibited an increased proportion of liver cancer stem cells, and expressed high levels of IL-8. In screens of additional PI3K/Akt/mTOR pathway inhibitors which could be used in combination with sorafenib, it was observed that the mTOR inhibitor rapamycin was able to decrease the level of IL-8 expression; treatment with rapamycin followed by sorafenib led to tumor regression via increased sensitivity to sorafenib (Kahraman, et al., 2019).
As IL-8 has played a role in tumor resistance at many levels, there have also been numerous therapeutic agents designed to target IL-8, the IL-8 receptors, or the many diverse features involved in IL-8 signaling. A recent review summarized the current options for targeting the various CXCLs which bind to the CXCR2 receptor in the context of both cancer and inflammatory diseases (Cheng, Y., et al., 2019). In the HCC study discussed above, the authors found that knock down of IL-8 via siRNA or inhibition with the CXCR1/2 inhibitor reparixin, enhanced sensitivity to sorafenib, decreased the number of liver cancer stem cells, and resulted in improved tumor regression (Kahraman, et al., 2019). Just as described in HCC, in pre-clinical models of ovarian cancer, sorafenib-resistant cells reportedly exhibited higher levels of IL-8 expression. In these studies, sorafenib-resistant tumors treated with CXCR2 inhibitor SB225002 exhibited improved anti-tumor efficacy compared to those treated with either drug alone (Devapatla, et al., 2015).
In a pre-clinical study of NSCLC (EGFR WT and mutant) our group demonstrated that lung cancer cells which developed resistance to erlotinib, an EGFR-targeted small molecule inhibitor, exhibited a more mesenchymal cell phenotype and upregulation of p38 MAPK signaling, which resulted in high levels of IL-8 production and secretion. Therapeutic blockade of IL-8 with an anti-IL-8 antibody improved the susceptibility of the cancer cells to erlotinib and was able to reverse some of the mesenchymal features that had been taken on by the resistant cells (Fernando, et al., 2016). As evidenced by each of these studies, IL-8 plays an important role in resistance to molecularly targeted therapeutics.
5. The Incorporation of IL-8 into Immunotherapy Treatments
a. IL-8 as a Therapeutic Target: Pre-clinical Evidence
As mentioned previously, the EGFR-TKI erlotinib provides efficacy in the treatment of lung cancer, however, many tumors develop erlotinib-resistance. Building on earlier studies, Dominguez et al. observed that short-term erlotinib therapy improved the sensitivity of tumor cells to both immune cell killing via NK cells and antigen-specific T cells, however, this was not true of long-term erlotinib therapy. In this scenario tumor cell plasticity transitioned cells to a more mesenchymal phenotype, and, in turn, made them resistant to immune-mediated therapy. The neutralization of IL-8 was able to again sensitize tumor cells to the combination therapy of erlotinib and NK cell lysis. This and the study by Fernando et al. corroborate a role for IL-8 to be added to both molecularly targeted and immune-mediated therapeutic regimens. Furthermore, these findings demonstrate that the timing of each therapy within a combination regimen may be important as the plasticity of the tumor cells and the composition of the TME change over time (Dominguez, et al., 2016).
In the context of the difficult to treat triple negative breast cancer, it is known that high levels of IL-8 contribute to therapy resistance and the development of a highly mesenchymal tumor phenotype. Our group previously tested the blockade of IL-8 in combination with cellular therapy with promising results. The neutralizing antibody, HuMax-IL8, was shown to shift the tumor cell plasticity away from being highly mesenchymal as the tumor cells lost mesenchymal fibronectin and gained epithelial E-cadherin when incubated with HuMax-IL8. Similarly, in vivo, HuMax-IL8 antibody therapy decreased mesenchymal and stemness features of the tumor as well as recruited fewer polymorphonuclear MDSC to the tumor site. Finally, tumor lysis by NK cells and antigen-specific T cells were both improved with the addition of HuMax-IL8 treatment in vitro (Dominguez, et al., 2017b). Another study, here in the context of head and neck squamous cell carcinoma, investigated the role of MDSC in the effectiveness of NK cell therapy. Results showed that blockade of IL-8 signaling via SX-682, a small molecule inhibitor targeting CXCR1/2, diminished the amount of MDSC which trafficked to the tumor site. This lack of MDSC allowed for significantly enhanced anti-tumor efficacy of adoptive cell therapy using NK cells (Greene, et al., 2020).
Altogether, in multiple pre-clinical studies, the blockade of IL-8 has demonstrated significant improvements in the efficacy of immune-mediated therapies such as NK cell or antigen-specific T-cell mediated lysis. While immunotherapies such as these cell-based therapies, vaccines, and others, have revolutionized the treatment of cancer in recent years, tumor plasticity continues to allow tumors to evade the immune system not only through the up-regulation of IL-8 and recruitment of MDSC but also through expression of other suppressive molecules such as TGF-β or PD-L1. As such, numerous antibodies targeting immune checkpoint molecules such as PD-L1, PD-1, or CTLA-4 have been developed and are now widely used across oncology therapy, in some cases as approved neo-adjuvant or second-line therapies and in other cases as experimental agents in the setting of disease relapse (Horn, et al., 2020a; Moujaess, et al., 2019).
b. The Significance of IL-8 in Immune Checkpoint Therapy
As immune checkpoint inhibition (ICI) therapy becomes widespread across many tumor types, with clear groups of patients who are responders and non-responders, the field continues to search for methods by which response to ICI can be predicted. As IL-8 has become recognized as a potential predictor of poor outcomes in the context of chemotherapy or molecularly targeted therapeutics, it became of interest to assess its potential role in both predicting the response of patients to ICI and in its potential ability to initiate escape from these agents. Accordingly, two studies aimed to discover additional biomarkers which could be used in conjunction with PD-1/PD-L1 expression. In the first study, serum of 44 patients with melanoma and 19 with NSCLC were evaluated over time. Patients in the trial were treated with nivolumab (α-PD-1) or pembrolizumab (α-PD-1) monotherapy or the combination of nivolumab and ipilimumab (α-PD-1 plus α-CTLA-4). Evaluations found that within the group of patients responding to therapy, the amount of IL-8 in the serum significantly decreased at the time of the best response when compared to baseline. Analogously, IL-8 levels rose in non-responders at the time of disease progression. These findings were validated across the two diseases studied as well as across the varied α-PD-1/α-CTLA-4 treatment agents (Sanmamed, et al., 2017). In a second study, 38 patients with advanced NSCLC who were treated with nivolumab were evaluated; serum was assessed for any novel proteins that may separate responders from non-responders and serve as useful biomarkers in the future. Results reported that those who achieved a durable clinical benefit exhibited high levels of BMP-9, a ligand belonging to the TGF-β family, and low levels of IL-8, IP-10, TNFΑ, and follistatin at baseline (Oyanagi, et al., 2019). More recently, two independent research studies evaluated the level of IL-8 in patients treated with ICI on a large scale. Schalper et al. evaluated serum from patients across four phase 3 clinical trials which treated renal cell carcinoma, NSCLC, or melanoma with nivolumab (α-PD-1) or ipilimumab (α-CTLA-4), everolimus (mTOR inhibitor), or docetaxel (chemotherapy) across 1,344 total patients. Using clinical measures of ORR, OS, and PFS, correlations between baseline IL-8 were assessed. In all four trials the OS of patients with lower serum IL-8 at baseline was associated with significantly improved survival probability. Importantly, this observation was seen across patients treated with ICI, mTOR inhibition, and chemotherapy. Additionally, this analysis determined a quantifiable concentration of IL-8 within the serum, with ≥ 23 pg/mL resulting in worse survival outcomes. The authors went on to then assess the correlation of circulating IL-8 to IL-8 and other markers present within the tumor itself; high IL-8 in the serum was positively correlated with IL-8 gene expression within the tumor. Furthermore, high circulating IL-8 correlated with an increase in neutrophils and monocytes at the tumor site while fewer IFN-γ+ T cells were detected. Finally, no correlation was found between the levels of IL-8 in the serum and PD-L1 expression in the tumor, and a minimal correlation was found between the level of IL-8 gene expression and tumor mutational burden in data assessed from the TCGA database (Schalper, et al., 2020). This research has shown that IL-8 status within the serum is capable of providing a glimpse into the immune suppressive environment at the site of the tumor and that, independent of the type of therapy employed, patients with high levels of IL-8 may benefit from an initial reduction in IL-8 in order to increase the chances of additional therapeutic agents providing efficacy.
In an additional study, Yuen et al. assessed circulating IL-8 as well as IL-8 gene expression within matched PBMC and tumor samples from trials collectively treating 1,445 patients with either metastatic urothelial carcinoma or renal cell carcinoma with atezolizumab therapy (α-PD-L1). Consistent with Schalper et al. results, the authors found that high levels of plasma IL-8 (pIL-8), here defined as ≥ 15 pg/mL, at baseline are correlated with worse OS and decreased ORR. Importantly, this remains true even in cases which exhibited high T cell effector (Teff) signatures indicative of an inflamed tumor, which generally is thought to be a promising indicator of response to ICI therapy. Consequently, patients with a high Teff score and low IL-8 had the best outcomes. The poor OS outcomes, stratified by high pIL-8, were observed across varied treatment regimens assessed, including atezolizumab, chemotherapy, or atezolizumab and bevacizumab (α-VEGF) or sunitinib (broad α-RTK), although they were only statistically significant in the cases of atezolizumab or chemotherapy. An additional evaluation of changes in pIL-8 levels after 6 weeks on therapy determined that increases in pIL-8 resulted in significantly worse OS and ORR in atezolizumab-treated patients (Yuen, et al., 2020). These two independent studies evaluating large cohorts of patients treated with ICI agents came to remarkably similar conclusions. First, IL-8 has a potential role in driving therapeutic resistance to ICI therapy, most likely mediated through myeloid tumor infiltration. Second, both studies concluded that peripheral IL-8 can be used as a biomarker to reasonably predict clinical outcomes to ICI therapy and that, if patients were to be stratified by pre-therapy levels of IL-8, or a panel of markers including IL-8, it may be advantageous to first target the IL-8 pathway in those who express high IL-8 before advancing on to ICI therapy.
c. Bringing it All Together: The Case for Combination of ICI and IL-8/IL-8R Therapy
As IL-8/IL-8R monotherapy has shown to be effective at reversing some therapeutic resistance and IL-8 has been shown to be a biomarker within patients who do not respond well to ICI therapy, the ensuing question is to ask whether the combination of IL-8 therapy and ICI therapy could provide further improvements in anti-tumor efficacy. In our lab, we recently studied whether combinatorial inhibition of CXCR1/2, via SX-682, along with a therapeutic bifunctional immune checkpoint molecule which blocks PD-L1 and TGF-β, bintrafusp alfa (formerly M7824), could provide a therapeutic advantage. We found that this combination therapy provided the best tumor control in murine models of lung and breast cancer and observed that these improvements in efficacy were attributable, at least in part, to an increase in activated T cells within the tumor as well as a decrease in the population of granulocytic MDSC at the tumor site (Horn, et al., 2020b). Complementary to these findings, additional pre-clinical studies have found that reduction in the number of immunosuppressive cells at the tumor site results in improvements in immunotherapy efficacy. In mouse models of oral squamous cell carcinoma and lung cancer, SX-682 inhibited the trafficking of neutrophilic MDSC and improved the efficacy of the combination of ICI and adoptively transferred T cells (Sun, et al., 2019). Analogously, in mouse models of pancreatic cancer, preventing the recruitment of M2-TAM to the tumor allowed α-PD-1 therapy to be more successful. In these studies the pancreatic cancer was described to express high levels of IL-8, however, the addition of IFN-γ to PD-1 blockade was able to suppress the levels of IL-8 signaling, resulting in the decreased TAM trafficking, and ultimately, improved ICI efficacy observed (Zhang, M., et al., 2020).
d. Combinatorial ICI and IL-8/IL-8R Therapies in the Clinic
There are a number of ongoing clinical trials evaluating the use of either CXCR1/2 inhibitors or an anti-IL-8 monoclonal antibody (Table 1). Bilusic et al. reported results from a phase I clinical trial in which 15 patients with advanced solid tumors were treated with HuMax-IL8 (currently BMS-986253). In the trial, HuMax-IL8 was considered safe without any observation of dose-limiting toxicities, serum IL-8 levels were significantly reduced in patients during therapy, and 73% of patients achieved stable disease (Bilusic, et al., 2019). Ongoing studies are now evaluating HuMax-IL8 therapy in combination with nivolumab (α-PD-1) in NSCLC or HCC (NCT04123379), or with nivolumab and androgen deprivation therapy in hormone-sensitive prostate cancer (NCT03689699).
Table 1.
Clinical studies for the treatment of cancer with IL-8 and IL-8 receptor antagonist agents
| IL-8 Agent | Title of Clinical Trial | Clinical Trial Number* | Status |
|---|---|---|---|
| IL-8 | |||
| HuMax-IL8 (BMS-986253) |
Nivolumab and BMS-986253 for Hormone-Sensitive Prostate Cancer (MAGIC-8) | NCT03689699 | R |
| HuMax-IL8 (BMS-986253) |
A Phase II, Randomized, Controlled Trial of Nivolumab in Combination With BMS-986253 or Cabiralizumab in Advanced Hepatocellular Carcinoma (HCC) Patients | NCT04050462 | R |
| HuMax-IL8 (BMS-986253) |
Neoadjuvant Nivolumab With CCR2/5-inhibitor or Anti-IL-8) for Non-small Cell Lung Cancer (NSCLC) or Hepatocellular Carcinoma (HCC) | NCT04123379 | R |
| HuMax-IL8 (BMS-986253) |
An Investigational Immuno-Therapy Study of Experimental Medication BMS-986253 Given in Combination With Nivolumab in Patients With Advanced Cancers | NCT03400332 | A |
| HuMax-IL8 (BMS-986253) |
HuMax-IL8 (Interleukin-8) in Patients With Advanced Malignant Solid Tumors | NCT02536469 | C |
| IL-8 Receptor | |||
| SX-682 | SX-682 Treatment in Subjects With Myelodysplastic Syndrome Who Had Disease Progression or Are Intolerant to Prior Therapy | NCT04245397 | R |
| SX-682 | SX-682 Treatment in Subjects With Metastatic Melanoma Concurrently Treated With Pembrolizumab | NCT03161431 | R |
| SX-682 | A Study to Evaluate the Safety and Tolerability of SX-682 in Combination With Nivolumab as a Maintenance Therapy in Patients With Metastatic Pancreatic Ductal Adenocarcinoma | NCT04477343 | NR |
| AZD5069 | Combination Study of AZD5069 and Enzalutamide | NCT03177187 | R |
| AZD5069 | Study to Assess MEDI4736 With Either AZD9150 or AZD5069 in Advanced Solid Tumors & Relapsed Metastatic Squamous Cell Carcinoma of Head & Neck | NCT02499328 | A |
| AZD5069 | Phase Ib/II Study of MEDI4736 Evaluated in Different Combinations in Metastatic Pancreatic Ductal Carcinoma | NCT02583477 | C |
| Navarixin | Efficacy and Safety Study of Navarixin (MK-7123) in Combination With Pembrolizumab (MK-3475) in Adults With Selected Advanced/Metastatic Solid Tumors (MK-7123-034) | NCT03473925 | A |
www.clinicaltrials.gov; R= recruiting; NR= not yet recruiting; A= active; C= completed as of August 6, 2020.
In addition to direct targeting of IL-8, the targeting of CXCR1 and CXCR2 are also being pursued in the clinic. The CXCR1/2 inhibitor SX-682 is being evaluated in combination with pembrolizumab (α-PD-L1) in metastatic melanoma (NCT03161431). The CXCR1/2 antagonist Navarixin is being tested in combination with pembrolizumab in the treatment of advanced solid tumors (NCT03473925), and the CXCR2 antagonist AZD5069 is being assessed in combination with durvalumab (α-PD-L1) in advanced head and neck squamous cell carcinoma (NCT02499328). As clinical trial results are published employing these IL-8/IL-8R targeted therapies we will learn whether this strategy can improve efficacy across a broad spectrum of malignancies for those patients who have not responded well to prior therapies.
6. Conclusion
While the chemokine IL-8 is able to affect the biology of tumors in many different ways, it is clear that its overarching role is in promoting tumor progression (Figure 1). Research has shown that across many tumor types, levels of IL-8 in the peripheral blood can serve as a prognostic marker for patients being treated with therapeutics from chemotherapy to more targeted drugs, including the recent immune checkpoint inhibitor therapies. Not only can IL-8 serve as a prognostic factor but much pre-clinical research has shown that IL-8 plays a direct role in mechanisms driving resistance to chemotherapy, molecularly targeted therapy, and ICI therapy. As IL-8 is able to aid the tumor in evading both the natural immune response and therapeutics via so many different mechanisms of action, including driving an EMT within the tumor cells, promoting tumor migration and proliferation, increasing tumor angiogenesis, and altering the tumor immune microenvironment, it stands to reason that an effective approach may be to target multiple aspects of this tumor progression. As cancer therapy advances, numerous multi-modal therapeutic approaches are being employed in both sequential and concurrent combination treatment strategies. Based on the many pro-tumorigenic roles of IL-8 and its recognition as a predictor of poor clinical outcomes, it is logical that IL-8/IL-8R specific targeting may provide further clinical benefit in a variety of tumor malignancies when combined with current treatment regimens.
Figure 1.
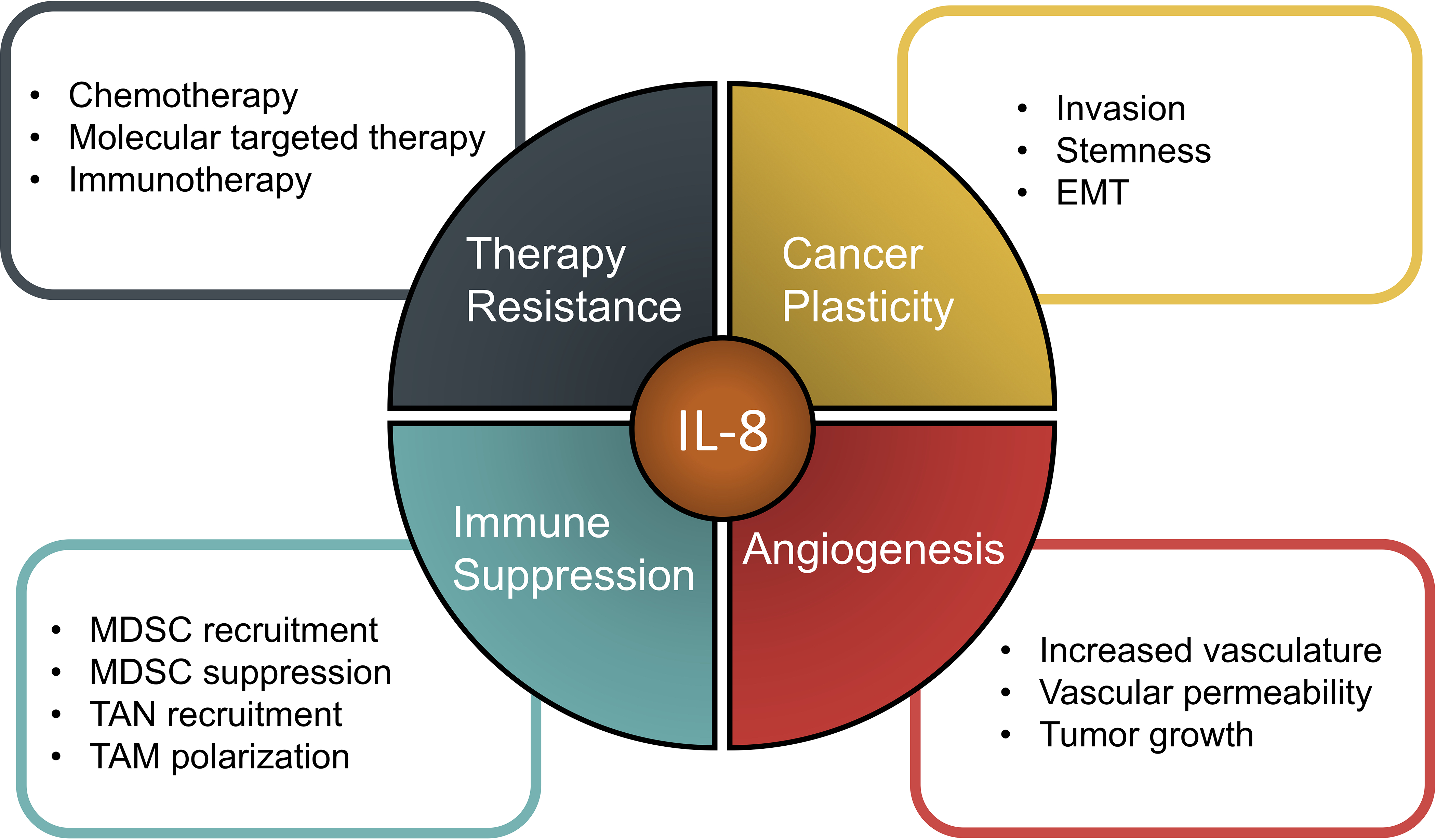
The role of IL-8 in various aspects of tumor progression.
Acknowledgments
Financial Support: This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health (NIH)
Abbreviations:
- CRC
colorectal cancer
- Dox
doxorubicin
- EMT
epithelial-to-mesenchymal transition
- HCC
hepatocellular carcinoma
- ICI
immune checkpoint inhibition
- MDSC
myeloid derived suppressor cells
- NSCLC
non-small cell lung cancer
- TAM
tumor-associated macrophages
- TME
tumor microenvironment
Footnotes
Conflicts of Interest: The authors declare that there are no conflicts of interest.
References
- Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, et al. (2016). Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin Cancer Res, 22, 3924–3936. [DOI] [PubMed] [Google Scholar]
- Anestakis D, Petanidis S, Kalyvas S, Nday CM, Tsave O, Kioseoglou E, et al. (2015). Mechanisms and applications of interleukins in cancer immunotherapy. Int J Mol Sci, 16, 1691–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilusic M, Heery CR, Collins JM, Donahue RN, Palena C, Madan RA, et al. (2019). Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Immunother Cancer, 7, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T, Kalluri R, Nieto MA, Weinberg RA (2018). EMT in cancer. Nat Rev Cancer, 18, 128–134. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Lian GD, Li JJ, Zhang QB, Zeng LJ, Yang KG, et al. (2018). Tumor-driven like macrophages induced by conditioned media from pancreatic ductal adenocarcinoma promote tumor metastasis via secreting IL-8. Cancer Med, 7, 5679–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Cai W, Huang W, Chen Y, Wu Z, Luo P, et al. (2018). Histone deacetylase 6 regulated expression of IL-8 is involved in the doxorubicin (Dox) resistance of osteosarcoma cells via modulating ABCB1 transcription. Eur J Pharmacol, 840, 1–8. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Ma XL, Wei YQ, Wei XW (2019). Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer, 1871, 289–312. [DOI] [PubMed] [Google Scholar]
- Dabkeviciene D, Jonusiene V, Zitkute V, Zalyte E, Grigaitis P, Kirveliene V, et al. (2015). The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116. Med Oncol, 32, 258. [DOI] [PubMed] [Google Scholar]
- David JM, Dominguez C, Palena C (2017). Pharmacological and immunological targeting of tumor mesenchymalization. Pharmacol Ther, 170, 212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JM, Dominguez C, Hamilton DH, Palena C (2016). The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance. Vaccines (Basel), 4, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Weinberg RA (2019). EMT and Cancer: More Than Meets the Eye. Dev Cell, 49, 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devapatla B, Sharma A, Woo S (2015). CXCR2 Inhibition Combined with Sorafenib Improved Antitumor and Antiangiogenic Response in Preclinical Models of Ovarian Cancer. PLoS One, 10, e0139237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C, Tsang KY, Palena C (2016). Short-term EGFR blockade enhances immune-mediated cytotoxicity of EGFR mutant lung cancer cells: rationale for combination therapies. Cell Death Dis, 7, e2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C, David JM, Palena C (2017a). Epithelial-mesenchymal transition and inflammation at the site of the primary tumor. Semin Cancer Biol, 47, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C, McCampbell KK, David JM, Palena C (2017b). Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight, 2, e94296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, He Y, Li P, Wu W, Chen Y, Ruan H (2018). IL-8 regulates the doxorubicin resistance of colorectal cancer cells via modulation of multidrug resistance 1 (MDR1). Cancer Chemother Pharmacol, 81, 1111–1119. [DOI] [PubMed] [Google Scholar]
- Dwyer J, Hebda JK, Le Guelte A, Galan-Moya EM, Smith SS, Azzi S, et al. (2012). Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2. PLoS One, 7, e45562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Qi Q, Wang P, Chen H, Chen Z, Meng Z, et al. (2018). Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer. J Int Med Res, 46, 5228–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C (2011). IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res, 71, 5296–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando RI, Hamilton DH, Dominguez C, David JM, McCampbell KK, Palena C (2016). IL-8 signaling is involved in resistance of lung carcinoma cells to erlotinib. Oncotarget, 7, 42031–42044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng R, Tan X, Wu J, Pan Z, Yi M, Shi W, et al. (2017). RNF183 promotes proliferation and metastasis of colorectal cancer cells via activation of NF-kappaB-IL-8 axis. Cell Death Dis, 8, e2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann M, Zangger N, Sauvain MO, Sempoux C, Bowler AD, Wirapati P, et al. (2020). Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFbeta. EMBO Mol Med, 12, e10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, et al. (2020). Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin Cancer Res, 26, 1420–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DH, Huang B, Fernando RI, Tsang KY, Palena C (2014). WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Res, 74, 2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Horn LA, Fousek K, Palena C (2020a). Tumor Plasticity and Resistance to Immunotherapy. Trends Cancer, 6, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn LA, Riskin J, Hempel HA, Fousek K, Lind H, Hamilton DH, et al. (2020b). Simultaneous inhibition of CXCR1/2, TGF-beta, and PD-L1 remodels the tumor and its microenvironment to drive antitumor immunity. J Immunother Cancer, 8, e000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imafuji H, Matsuo Y, Ueda G, Omi K, Hayashi Y, Saito K, et al. (2019). Acquisition of gemcitabine resistance enhances angiogenesis via upregulation of IL8 production in pancreatic cancer. Oncol Rep, 41, 3508–3516. [DOI] [PubMed] [Google Scholar]
- Junttila MR, de Sauvage FJ (2013). Influence of tumour micro-environment heterogeneity on therapeutic response. Nature, 501, 346–354. [DOI] [PubMed] [Google Scholar]
- Kahraman DC, Kahraman T, Cetin-Atalay R (2019). Targeting PI3K/Akt/mTOR Pathway Identifies Differential Expression and Functional Role of IL8 in Liver Cancer Stem Cell Enrichment. Mol Cancer Ther, 18, 2146–2157. [DOI] [PubMed] [Google Scholar]
- Kaplan MJ, Radic M (2012). Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol, 189, 2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin S, Kutluk AC, Tas F (2019). Prognostic and Predictive Role of Angiogenic Markers in Non- Small Cell Lung Cancer. Asian Pac J Cancer Prev, 20, 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D, et al. (2012). Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer, 106, 1833–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. (2016). The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev, 31, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao FY, Zhao YL, Lv YP, Teng YS, Kong H, Liu YG, et al. (2018). CD45(+)CD33(low)CD11b(dim) myeloid-derived suppressor cells suppress CD8(+) T cell activity via the IL-6/IL-8-arginase I axis in human gastric cancer. Cell Death Dis, 9, 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic V, Kopecka J, Salaroglio IC, Libener R, Napoli F, Izzo S, et al. (2020). Wnt/IL-1beta/IL-8 autocrine circuitries control chemoresistance in mesothelioma initiating cells by inducing ABCB5. Int J Cancer, 146, 192–207. [DOI] [PubMed] [Google Scholar]
- Moujaess E, Haddad FG, Eid R, Kourie HR (2019). The emerging use of immune checkpoint blockade in the adjuvant setting for solid tumors: a review. Immunotherapy, 11, 1409–1422. [DOI] [PubMed] [Google Scholar]
- Ning Y, Cui Y, Li X, Cao X, Chen A, Xu C, et al. (2018). Co-culture of ovarian cancer stem-like cells with macrophages induced SKOV3 cells stemness via IL-8/STAT3 signaling. Biomed Pharmacother, 103, 262–271. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S, Fenselau C (2018). Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol, 200, 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagi J, Koh Y, Sato K, Mori K, Teraoka S, Akamatsu H, et al. (2019). Predictive value of serum protein levels in patients with advanced non-small cell lung cancer treated with nivolumab. Lung Cancer, 132, 107–113. [DOI] [PubMed] [Google Scholar]
- Roussos ET, Condeelis JS, Patsialou A (2011). Chemotaxis in cancer. Nat Rev Cancer, 11, 573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinete MMM, Oliveira PH, Martins-Filho A, Micheli DC, Tavares-Murta BM, Murta EFC, et al. (2017). Serum IL-6 and IL-8 Correlate with Prognostic Factors in Ovarian Cancer. Immunol Invest, 46, 677–688. [DOI] [PubMed] [Google Scholar]
- Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. (2017). Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol, 28, 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. (2020). Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med, 26, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sootichote R, Thuwajit P, Singsuksawat E, Warnnissorn M, Yenchitsomanus PT, Ithimakin S, et al. (2018). Compound A attenuates toll-like receptor 4-mediated paclitaxel resistance in breast cancer and melanoma through suppression of IL-8. BMC Cancer, 18, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, et al. (2019). Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight, 4, e126853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiainen L, Hamalainen M, Luukkaala T, Tanner M, Lahdenpera O, Vihinen P, et al. (2019). Low Plasma IL-8 Levels During Chemotherapy Are Predictive of Excellent Long-Term Survival in Metastatic Breast Cancer. Clin Breast Cancer, 19, e522–e533. [DOI] [PubMed] [Google Scholar]
- Wang X, Qiu L, Li Z, Wang XY, Yi H (2018). Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases. Front Immunol, 9, 2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh DJ, Wilson C (2008). The interleukin-8 pathway in cancer. Clin Cancer Res, 14, 6735–6741. [DOI] [PubMed] [Google Scholar]
- Wu J, Gao FX, Wang C, Qin M, Han F, Xu T, et al. (2019). IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J Exp Clin Cancer Res, 38, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao P, Long X, Zhang L, Ye Y, Guo J, Liu P, et al. (2018). Neurotensin/IL-8 pathway orchestrates local inflammatory response and tumor invasion by inducing M2 polarization of Tumor-Associated macrophages and epithelial-mesenchymal transition of hepatocellular carcinoma cells. Oncoimmunology, 7, e1440166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Ren X, Chen Y, Liu P, Wei X, Li H, et al. (2013). Dysfunctional activation of neurotensin/IL-8 pathway in hepatocellular carcinoma is associated with increased inflammatory response in microenvironment, more epithelial mesenchymal transition in cancer and worse prognosis in patients. PLoS One, 8, e56069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Norgard RJ, Stanger BZ (2019). Cellular Plasticity in Cancer. Cancer Discov, 9, 837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C, et al. (2020). High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med, 26, 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J, Shen J, Xie G, Wu J, He M, Gao L, et al. (2019). Cancer-associated fibroblasts-derived IL-8 mediates resistance to cisplatin in human gastric cancer. Cancer Lett, 454, 37–43. [DOI] [PubMed] [Google Scholar]
- Zhang B, Shi L, Lu S, Sun X, Liu Y, Li H, et al. (2015). Autocrine IL-8 promotes F-actin polymerization and mediate mesenchymal transition via ELMO1-NF-kappaB-Snail signaling in glioma. Cancer Biol Ther, 16, 898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Huang L, Ding G, Huang H, Cao G, Sun X, et al. (2020). Interferon gamma inhibits CXCL8-CXCR2 axis mediated tumor-associated macrophages tumor trafficking and enhances anti-PD1 efficacy in pancreatic cancer. J Immunother Cancer, 8, e000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Liu L, Lai W, Zeng Y, Xu H, Lan Q, et al. (2019). Interaction with tumorassociated macrophages promotes PRL3 induced invasion of colorectal cancer cells via MAPK pathwayinduced EMT and NFkappaB signalinginduced angiogenesis. Oncol Rep, 41, 2790–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Wang J (2018). Correlations of MRP1 gene with serum TGF-beta1 and IL-8 in breast cancer patients during chemotherapy. J BUON, 23, 1302–1308. [PubMed] [Google Scholar]


