Abstract
Perturbed maternal diet and prenatal exposure to air pollution affects the fetal brain, predisposing to postnatal neurobehavioral disorders. Glucose transporters (GLUT) are key in fueling neurotransmission, deficiency of the neuronal isoform GLUT3 culminates in autism spectrum disorders. Along with the different neurotransmitters, serotonin (5-HT) and oxytocin (OXT) are critical for the development of neural connectivity. Serotonin transporter (SERT) modulates synaptic 5-HT levels, while OXT receptor (OXTR) mediates OXT action. We hypothesized that perturbed brain GLUT1/GLUT3 regulated 5-HT-SERT imbalance, serves as a contributing factor to postnatal neuropsychiatric phenotypes, with OXT/OXTR providing a counterbalance. Employing maternal diet restricted (IUGR), high fat (HF) dietary modifications, and prenatal exposure to simulated air pollution (AP), fetal (E19) murine brain 5-HT was assessed by ELISA with SERT and OXTR being localized by immunohistochemistry, and measured by quantitative Western blot analysis. IUGR with lower head weights led to a 48% reduction in male and female fetal brain GLUT3 with no change in GLUT1, when compared to age- and sex-matched controls, with no significant change in OXTR. In addition, a ~50% (p=0.005) decrease in 5-HT and SERT concentrations was displayed in fetal IUGR brains. In contrast, despite emergence of microcephaly, exposure to a maternal high fat diet or air pollution caused no significant changes. We conclude that in the IUGR during fetal brain development, reduced GLUT3 is associated with an imbalanced 5-HT-SERT axis. We speculate that these early changes may set the stage for altering the 5HT-SERT neural axis with postnatal emergence of associated neurodevelopmental disorders.
Keywords: environmental exposure, fetal brain, intrauterine growth restriction, neurodevelopment, neurotransmitters
Introduction
The developing brain is highly dependent on energy metabolism that is fueled predominantly by glucose as the substrate [1, 2]. During fetal life, the brain is a major utilizer of glucose that is primarily derived from the mother [3]. Entry into the brain occurs by facilitative diffusion, via the glucose transporter isoform 1 (GLUT1) expressed in the blood-brain barrier [4]. This is followed by transport via the GLUT3 expressed in progenitors and neuronal cells [5]. While lack of GLUT1 resulted in developmental anomalies [6, 7], lack of GLUT3 led to features consistent with autism spectrum disorders postnatally [8, 9], with both conditions causing microcephaly and electroencephalographic seizures [10, 8, 9]. In-vitro studies have demonstrated a role for GLUT1 in vascular development of the brain [11], while GLUT3 serves glutamate induced neuronal excitotoxic tolerance via 5’-adenosine monophosphate protein kinase (AMPK) activation [12]. Further, associations between increased GLUT3 mediated glucose transport and serotonin have been reported in N2A neuroblastoma cells and non-neuronal cells such as peripheral mononuclear and skeletal muscle cells [13, 14]. In animal models of autism, disruption of the brain serotonin axis plays a major role in its pathophysiology [15, 16]. In addition, the oxytocin axis in the brain has displayed a serotonin-counteracting effect, modulating immediate and long term consequences [17, 18, 16]. Based on this cumulative information related to GLUT3, serotonin and oxytocin axes, we questioned the early relationship between fetal brain glucose transporters and the serotonin and oxytocin axes.
Serotonin (5-HT) modulates multiple physiological functions such as gastrointestinal motility, cardiovascular function, memory and mood in adult humans. Among the multiple roles of serotonin, the most intriguing is its developmental role [19–21]. A role for serotonin in neurodevelopmental disorders, such as autism or anxiety/attention deficit hyperactivity disorder, led to the recognition of its importance during early life development [22, 23, 19, 24, 21]. During embryonic and postnatal development, serotonin modulates neuron cell division, migration and differentiation, axonal and dendritic elaboration, connectivity and myelination [19]. Serotonin neurons in rodents appear at ~10.5 days gestation [19, 25, 26]. Perturbed brain serotonin concentrations during critical windows of development have long-lasting effects on brain function, contributing towards the subsequent development of anxiety in the presence of high serotonin or depression when serotonin concentrations are low [23, 20]. The placenta has been identified as an important exogenous source of serotonin [26] in early pregnancy prior to the ability of the embryonic brain to produce de novo serotonin. During this early gestational period, the placenta plays a major role in maternal-fetal transfer of tryptophan, an essential amino acid that is converted to serotonin by the placental tryptophan hydroxylase (TPH) enzyme. Hence the fetal brain serotonin axis is highly dependent on maternal diet at least during the early phase of pregnancy and continues to some extent during the latter part of pregnancy as well.
Serotonin transporter (SERT or 5-HTT) is a major regulator of serotonin neurotransmission and it has been implicated in the etiology of autism spectrum disorders (ASDs) [15] as well. [21]. SERT is first expressed during prenatal murine brain development at E12, and by E18 it is found in all subcellular neuronal compartments [27, 21]. During the course of embryonic development, SERT density peaks with advancing gestation, subsequently declining postnatally (P7) [28, 21]. During critical stages of brain development, serotonin concentrations can influence the expression or function of key regulators of serotonin neurotransmission, including SERT [29]. SERT expression and function are influenced by a large and diverse array of intrinsic and extrinsic factors. Intrinsic factors include genetic variants, auto- and hetero-dimerization, cytokines as well as hormones. Extrinsic factors such as diet, environmental stressors and drugs can also have prominent effects on SERT expression and function. Less is known about how these factors might come into play during the prenatal critical phases of brain development [21, 29].
In contrast, oxytocin (OXT) expression in placenta increases with gestation [30–32] and is also expressed within the fetal hypothalamic paraventricular nucleus (PVN). OXT expression is detected as early as E14.5, following proliferation and migration of the hypothalamic neurons [33]. OXT’s neural connections extend into the hippocampus and cerebral cortex where they interact with OXTRs [34, 35]. OXTRs first appear at E18.5 and mediate the anti-stress response and sociability postnatally [33]. Studies in rodents have revealed OXT’s anti-seizure activity [36–40], with increasing inhibition [41], potentially curbing the imbalanced excitatory to inhibitory neurotransmission [41] characteristic of autism spectrum disorders. Studies in OXTR (−/−) mice reveal a lack of cognitive flexibility as seen in autism spectrum disorders [33]. These mice reveal postnatal feeding difficulties and lack of sociability subsequently in adult life [33]. These investigations along with the purported interaction with serotonin have provided a potential for therapeutic possibilities in autism spectrum disorders [17, 18, 16].
Maternal dietary perturbations affecting early life neurodevelopment have led to altered adult neurobehaviors. In particular a hypercaloric cafeteria or western diet has led to either anxiety or depression associated with increased or decreased brain serotonin axis in primates and rodents respectively [42, 43]. On the other hand, maternal protein restricted diet led to a reduction in brain serotonin receptors [44]. Maternal caloric restriction was associated with adult onset anxiety in female rodents [45]. In lieu of dietary modifications, environmental exposures that modify maternal glucose and/or fat metabolism [46, 47] also have the propensity of affecting neurodevelopment postnatally..One such prenatal exposure is to traffic related air pollution gauged by living close to traffic heavy highways in humans or instillation of mixtures of air pollutants in rodents mimicking that generated by heavy traffic [48–50]. Changes in glucose and fat metabolism with associated emergence of autism spectrum disorders [48–50] have been reported. However, in all these studies, while early perturbations in diet or air pollutant exposures were associated with the ultimate behavioral changes during adult life, there is not much focus on fetal brain changes and its immediate impact on neurodevelopment. To this end, we have previously developed maternal dietary modified murine models. Maternal caloric restriction introduced beginning at E10 through gestation led to significant changes in neurodevelopment [51] associated with long term emergence of anxiety [45]. On the other hand, a maternal high fat dietary model previously reported by us led to glucose and fat metabolic changes in the mother and placenta [52]. Others have employed SRM1649 (a mixture of traffic related air pollutants consisting of organic and inorganic substances with a PM2.5–10 range) [53] in-vitro which we combined with an instillation in-vivo model [54] producing gestational exposure to traffic-related air pollutants.
Given that alterations in maternal diet, whether composed of calorie restriction or exposure to a high fat diet, and traffic-related air pollutants lead to long term changes in neurobehavior, we questioned the role of maternal exposures, both dietary and traffic-related air pollution, upon fetal brain expression of key molecules essential for the normal development of neural axes and function. We hypothesized that prenatal exposures to calorie restriction, high fat containing diet, or air pollution will perturb late gestation embryonic brain glucose transporters which in turn will prove detrimental to the serotonin-SERT axis, with the OXT-OXTR axis potentially counter-balancing this effect. We tested this hypothesis in our established pregnant mouse models.
Materials and Methods
Animal Models
Wild-type C57/BL6 female mice purchased from the UCLA Division of Laboratory Animal Medicine (obtained from The Jackson Lab, Bar Harbor, ME) were housed in 12-h light, 12-h dark cycles, and care or treatment was provided according to the UCLA Animal Research Committee’s approved protocol in accordance with the guidelines set by the National Institutes of Health regarding the Care and Use of Laboratory Animals.
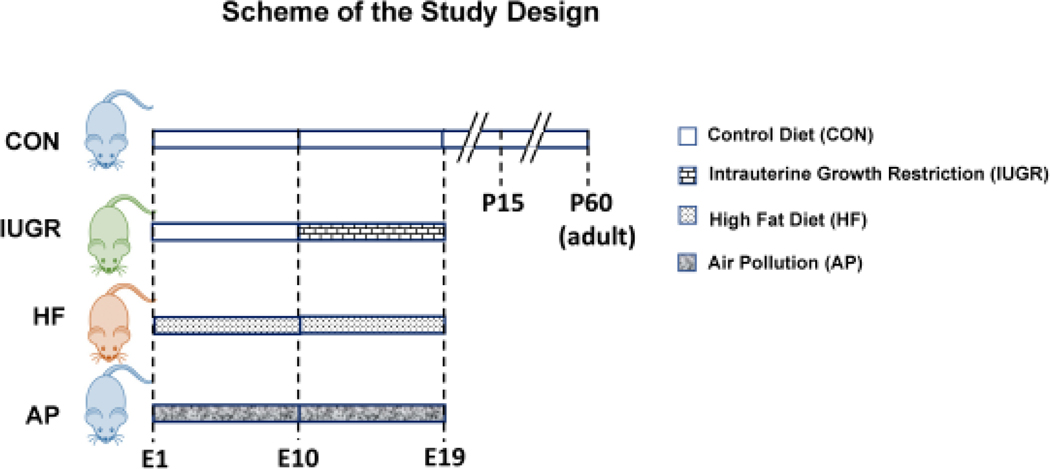
Dietary Modifications: We employed three different animal groups for our studies: Control group on regular chow diet, maternal calorie restriction (IUGR) and a high fat (HF) hyper-caloric group (Figure 1).
Traffic-related air pollution: Two groups were engaged, where one group received nasal instillation of SRM1649 suspension (obtained from the National Institute of Standards and Technology [Gaithersburg, MD, USA]) and the other group saline.
Figure 1. Experimental Design:
Scheme demonstrates the experimental design: 1) Compared to CON mothers reared on regular chow diet, 2) intrauterine growth restriction (IUGR) group mothers received reduced regular chow diet from gestational day 10 to 19. 3) In the High fat diet (HF) group mothers received HF diet for 8 weeks prior to pregnancy plus during gestation day 1 to 19. 4) AP group mothers received SRM1649 (air pollution) intra-nasally while being reared on a regular chow diet from E1 to E18, while the respective CON received intra-nasal saline.
Wild type C57/BL6 female mice (two or three months old) in all groups were mated with male counterparts. Presence of a vaginal plug was designated as gestational day 1.
A. Maternal Caloric Restriction (IUGR) studies.
At gestational day 10, pregnant mice were arbitrarily assigned to the Control group (CON) with ad libitum access to a standard rodent chow diet (TD. 06414, Herlan Teklad Laboratories, Indianapolis, IN; composition: carbohydrate 63.9%, fat 4.5% and protein 14.5%) and water. Intrauterine growth restriction (IUGR) was produced by maternal dietary restriction (~50% calories from gestational day 10 to day 19, calculated based on daily chow intake/day by ad lib fed pregnant mice considered to be 100%) [55] (Figure 1).
B. Hyper-caloric High Fat (HF) studies
Wild-type C57/BL6 female mice were housed with ad libitum access to a standard rodent chow diet and water till four weeks of age. During the fifth week of life female mice were given a high fat diet (TD 88137, Herlan Teklad Laboratories, Indianapolis, IN composition: carbohydrate 45%, fat 42% and protein 13%). At twelve weeks of life the high fat (HF) fed females were mated with regular chow fed male counterparts. After confirming the presence of a vaginal plug, pregnant females were given HF diet until E19 [52] (Figure 1).
C. Traffic-related Air Pollution Exposure Studies:
Female mice from gestational day 1 to 18 received daily either SRM1649 suspended in 20 μl of sterile saline (Air pollution – AP group) or an equal volume of sterile saline (CON) intra-nasally (divided between the two nares) under light restrain. SRM1649 (15μg/μl) (obtained from the National Institute of Standards and Technology [Gaithersburg, MD, USA]) was suspended in sterile saline and sonicated for 15 min to ensure uniform suspension of dissoluble PAHs, PCBs and inorganic constituents with a heterogeneous PM size reduced to a range between ~PM2.5 to ~PM10 ( Figure 1), thereby simulating traffic-related air pollutants.
All the experimental groups, at gestational day 19 underwent hysterotomy under inhalational isoflurane (4% for induction, and 1.25% to 1.5% for maintenance). Upon delivery of the embryos, they were weighed. Heads were separately weighed, brains collected after craniotomy, then snap-frozen immediately in liquid nitrogen, and stored in −80°C until further analyses. All embryos from a single pregnancy constituted an n=1.
Antibodies
Mouse anti-serotonin transporter (Frontier, Hokkaido, Japan), rabbit anti-synaptophysin (Millipore, Temecula, CA), rabbit anti-Glut1 (Abcam, Burlingame, CA), rabbit anti-Glut3 (gift from Dr. Takata in Japan), rabbit anti-oxytocin receptor (Thermo Fisher Scientific, Waltham, MA), mouse anti-vinculin and anti-β–actin (Sigma Chemical Co., St. Louis, MO.) antibodies were employed in our studies (Table 1).
Table 1:
Details of Antibodies Employed in the Study
| Antibody | Manufacturer details | Description | Concentration/Data |
|---|---|---|---|
| Glut1 | Abcam Cat No: ab652 RRID: AB_305540 Species: Rabbit Polyclonal | Synthetic peptide conjugated to KLH, corresponding to amino acids 478–492 of Human Glucose Transporter GLUT1 | Rabbit/1:1,000/ Western blot; IHC |
| Glut3 | Gift from Dr. Takata in Japan RRID:AB_2631293 Species: Rabbit Polyclonal | Synthetic peptide corresponding to amino acids 449–458 (C-terminus) of the deduced amino-acid sequence of rat GLUT3 | Rabbit/1:1,000/ Western blot; IHC |
| Serotonin transporter | Frontier Institute Co. Ltd. Cat NO: HTT-Rb-AF560 RRID: AB_2571775 Species: Rabbit Polyclonal | Antigen: mouse HTT, 1–77 aa | Rabbit/1:500/Western blot, IHC |
| Synaptophysin | Millipore Cat No: 04–1019 RRID: AB_1977519 Species: Rabbit Polyclonal | Synaptophysin (C-term) clone YE269 | Rabbit/1:1,000/IHC |
| Oxytocin receptor | Thermo Fisher Cat No: PA5–77764 RRID: AB_2736284 Species: Rabbit Polyclonal | Synthetic peptide conjugated to KLH derived from within residues 346–358 of rat Oxytocin receptor | Rabbit/1:400/Western blot |
| β-Actin | Cell Signaling Technology, Cat No: 8457 RRID: AB_10950489 Species: Rabbit Polyclonal | A synthetic peptide corresponding to residues near the amino terminus of human β-actin protein. | Rabbit/1:20,000/Western blot |
| Vinculin | Sigma-Aldrich Cat No: V9131 RRID: AB_477629 Species: Mouse Monoclonal | Derived from the hVIN-1 hybridoma produced by the fusion of mouse myeloma cells and splenocytes from immunized BALB/c mice. | Mouse/1:60,000/Western blot |
The source, antigen against which antibody was generated, and the host and dilution of antibodies employed in the study are presented.
Immunohistochemistry:
Mouse brains were collected following craniotomy and embedded in O.T.C. Compound (Tissue-Tek, Torrance, CA). Tissue samples were snap frozen in liquid nitrogen and stored in −80°C. Frozen serial coronal whole brain sections (10 μm) were obtained using a cryostat (Leica Microsystems, CM1850, Nussloch, Germany), and these sections were mounted on to slides. Sections were fixed in 100% ethanol at −20°C for 20 min followed by washing three times with PBS for 5 min, each.
The slides containing sections were first incubated for 1 hour with 5% normal donkey serum containing 0.2% triton-X100 and 1% gelatin, followed by incubation at room temperature for 1 hour with different primary antibodies: (mouse anti-SERT, 1:500 dilution, [Frontier, Hokkaido, Japan]; or rabbit anti-synaptophysin, 1:500, [Millipore, Temecula, CA]; rabbit anti-OXTR, 1:200 [Thermo Fisher Scientific, Waltham, MA]; and rabbit anti-Glut3 [Dr. Takata in Japan]). Following washing with PBS three times for 5 min each, corresponding secondary antibodies carrying the fluorescence detection tags (Alexa Fluor 488 or Alexa Fluor 594, [Jackson Immuno-Research Laboratories, West Grove, PA]) were incubated at room temperature for 1 hour at 1:500 dilution. 4′,6-diamidino-2-phenylindole (DAPI, 1:1000 dilution) was used to stain the cellular nuclei. Tissue sections were visualized using a Nikon E-600 microscope (Nikon, Melville, NY) equipped with a cooled, charge-coupled device camera (CoolSNAP HQ Monochrome; Roper Scientific, Tucson, AZ) and the images captured by the Metamorph software (www.moleculardevices.com, RRID: SCR_002368) [9].
ELISA: Brain Serotonin
Brain serotonin concentrations were measured in duplicates within supernatants (50 μg protein measured by the Bio-Rad assay) of brain tissue homogenates by an ELISA kit (Serotonin High Sensitive ELISA, Eagle Biosciences, Nashua, NH) that has previously been validated [56]. The range of measurements is from 0 to 100 pg/50 μg. Sensitivity is 0.39 pg/sample and the specificity is ~100%, 0.22% for tryptamine, 0.025% for 5-methoxytryptamine, 0.0021% for 5-hydroxytryptophan, <0.001 for melatonin and 5-HIAA and <0.0001 for L-tryptophan. The intra–assay coefficient of variation ranged from 6.6% to 8.7%.
Western Blot Analyses:
Cerebral tissues were homogenized and sonicated in RIPA buffer (20mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA 1 mM EGTA 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4 1μg/ml leupeptin (Pierce, Waltham, WA) with protease inhibitors (Thermo Scientific, Canoga Park, CA) including 2 mM PMSF as previously subscribed [9, 51]. The resulting homogenate was centrifuged at 10,000g at 4°C for 10 min, and the protein content was measured by the Bio-Rad protein assay (Bio-Rad Laboratories, Irvine, CA) and subjected to Western blot analysis. Briefly, homogenates (30 μg of protein) were subjected to sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and the separated proteins transferred to nitrocellulose membrane filters (Bio-Rad, Hercules, CA). Membranes were washed with PBST three times for 5 min each and blocked in 3% bovine serum albumin for 1 hour, followed by overnight incubation at 4°C with the specific primary antibodies, mouse anti-serotonin transporter antibody (1:500 dilution), rabbit anti-Glut1 (1/1,000 dilution), anti-Glut3 (1/1,000 dilution) or anti-oxytocin receptor (1/200 dilution). Membranes were subsequently washed in PBST six times for 5 min each and incubated at room temperature over 45 min with the appropriate secondary horseradish peroxidase-conjugated antibody (Pierce, Waltham, WA). The proteins were visualized in ChemiDoc Imaging System (BioRad, Hercules, California) or Typhoon Scanner (GE Healthcare, Pasadena, CA) by blotting with the enhanced chemiluminescence (ECL) plus detection kit (GE Healthcare BioSciences Corp., Piscataway, NJ) following horseradish peroxidase-labeled anti-rabbit IgG for anti-Glut1, anti-Glut3, anti-β-actin (1:20,000 dilution) and anti-oxytocin receptor or anti-mouse IgG for anti-vinculin (1:60,000 dilution) (Sigma, St. Louis, MO). Each protein was quantified by using Image Lab software (Bio-Rad, Hercules, California) or Image Quant software (GE Healthcare, Pasadena, CA), normalized to either vinculin or β-actin as an internal control (each one chosen based on the molecular weight of the primary protein, to prevent overlap in visualization/quantification of the primary protein versus the respective internal control) as an internal control, and expressed as a percent of respective CON values.
Sex as a biological variable
Intersex difference was explored between E19 CON and IUGR as well as at developmental time points E19, P15, and adult. A total of 120 males and 120 females were included in the study. The remaining data analyses, again denoted in the results and figure legends, were not subanalyzed as animals were not sexed prior to obtaining samples.
Genotyping for sex determination
Genetic sex determination of E19 embryos was performed as previously described [51]. Briefly, for genetic sex determination, extracted tail DNA fragments of either the X-chromosomal Xlr gene (X-linked lymphocyte regulated complex) or the Y-chromosomal Sly gene (Sycp3-like Y-linked) were amplified using gene specific PCR primers [57, 51]. The PCR conditions consisted of denaturation at 95°C for 2 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s. Finally, extension at 72°C for 5 min was done. Sex at P15 and adult stages was determined by visualization of the external genitalia.
Data Analyses
Data are expressed as mean ± standard error of the mean. Sample size was predetermined by conducting a power analysis employing the Stat-mate software at a power of 80% and a p value of 0.05. Analysis of variance models were used to compare various treatment groups and statistical significance established by Prism (7th edition) software (www.graphpad.com, RRID:SCR_002798). Inter-group differences were determined post-hoc by either the Tukey’s or Sidak’s multiple comparison test. When only two experimental groups were compared, the Student’s unpaired t-test was employed. Significance was assigned when the p value was <0.05.
Results
Fetal mouse body and head weights
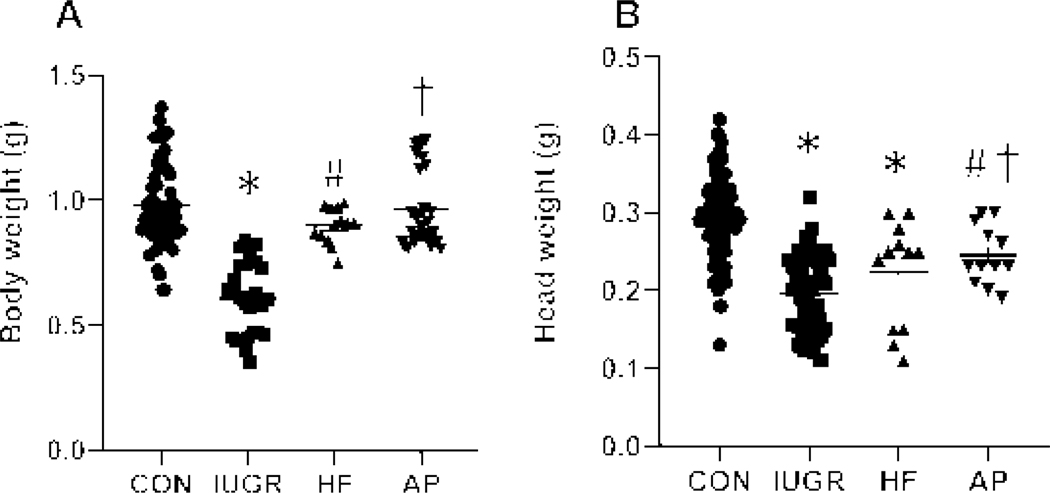
Examination of fetal (E19) body weights revealed a reduction only in the IUGR group (p<0.0001) compared to the other groups (Figure 2A). Similarly, a decrease in head weight was also seen in the IUGR group (p=0.0001) when compared to CON (Figure 2B). Additionally, a reduction in head weights emerged in the high fat (HF) (p=0.001) and air pollution (AP) (p=0.0217) groups when compared to CON.
Figure 2. Fetal (E19) Body weights (A) and Head weights (B).
Fetal (E19) Body (A) and Head (B) Weights are depicted in all four experimental groups. Body weights (A), one-way ANOVA, F-statistic: 3, 125=39.18, p<0.0001, by Sidak’s multiple comparisons post-hoc test, *p<0.0001 vs CON, #p<0.0001 or †p<0.0001 vs IUGR; n=61 in CON, n=27 in IUGR, n=13 in HF and n=28 in AP. Head weights (B), one-way ANOVA, F-statistic: 3, 165=40.06, p<0.0001, by Sidak’s multiple comparisons post-hoc test, *p=0.0001 or #p=0.0217 vs CON, †p=0.0213 vs IUGR; n=92 in CON, n=53 in IUGR, n=12 in HF and n=12 in AP. Data are shown as means ± standard error of the mean.
Glut1 and Glut3 protein in E19 fetal CON, IUGR, HF and AP mouse brain
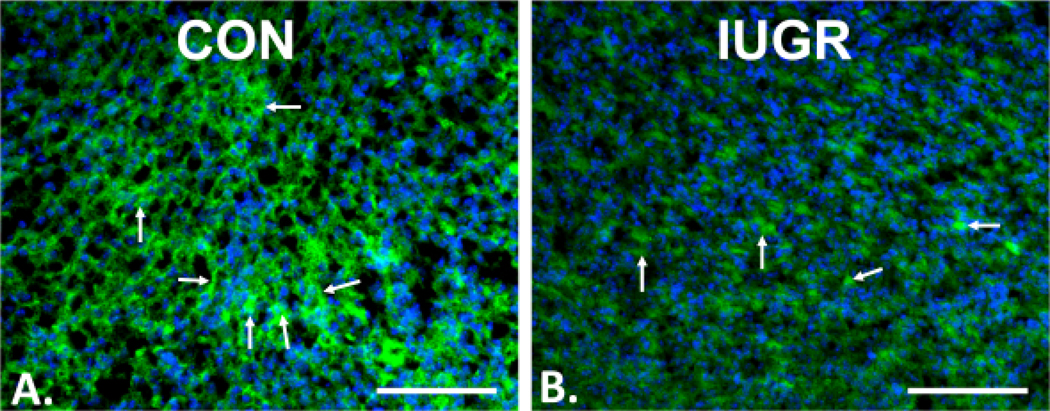
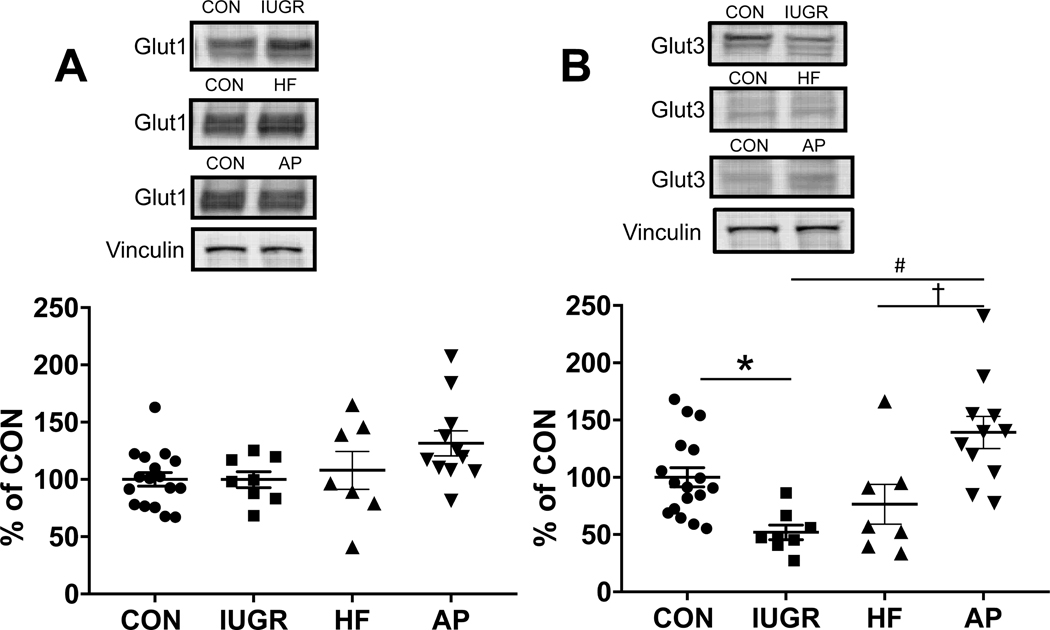
Immunofluorescence localization of Glut3 with Alexa 488, is shown in neural membranes within E19 CON (Figure 3A) and IUGR (Figure 3B) brain sections. The intensity of Glut3 immunostaining was decreased in the cortex of IUGR compared to CON (Figure 3). In addition, by Western blot analysis of E19 fetal CON, IUGR, HF and AP brains (Figure 4), while there was no difference among the CON, IUGR, HF and AP Glut1 concentrations (Figure 4A) (one-way ANOVA, F-statistic: 3, 39=2.651, p=0.0621, n=17 for CON, n=8 for IUGR, n=7 for HF and n=11 for AP), Glut3 concentrations were significantly decreased to 48% in IUGR when compared to CON at E19 (Figure 4B). The pattern of reduction was similar in males and females. (Supplementary Figure 1). However, in the AP group, brain Glut3 concentrations were 2.7 fold-increased or 1.8 fold-increased when compared to IUGR or HF respectively (Figure 4B) (one-way ANOVA, F-statistic: 3, 39=8.981, p=0.0001, n=17 for CON, n=8 for IUGR, n=7 for HF and n=11 for AP; Sidak’s multiple comparisons post-hoc test, *p=0.0318, CON vs. IUGR; #p<0.0001, IUGR vs. AP; †p=0.009, HF vs. AP).
Figure 3. Immunofluorescence localization of Glut3.
Alexa 488, green in neural membranes, shown by arrows in the E19 fetal brain (cortex) of CON (A) and IUGR (B) groups, with DAPI nuclear stain (blue). The expression of Glut3 protein was decreased in the cortex of IUGR compared to CON. Scale bar = 100 μM.
Figure 4. Western blot analysis of Glut1 (A) & Glut3 (B).
Top panels show representative blots with Glut1 & Glut3 shown above and vinculin as the internal loading control shown below. Glut1 and Glut3 protein concentrations quantified by Western Blot analysis in IUGR, HF and AP vs. CON. Glut1 expression in the CON, IUGR, HF and AP groups (A). There are no significant differences between groups (one-way ANOVA, F-statistic: 3, 39=2.651, p=0.0621, n=17 for CON, n= 8 for IUGR, n=7 for HF and n=11 for AP). However, Glut3 concentrations were significantly decreased to 48% in IUGR when compared to CON (B). In addition, in the AP group, brain Glut3 concentrations were 2.7 fold-increased or 1.8 fold-increased when compared to IUGR or HF respectively (B) (one-way ANOVA, F-statistic: 3, 39=8.981, p=0.0001, n=17 for CON, n= 8 for IUGR, n=7 for HF and n=11 for AP; Sidak’s multiple comparisons post-hoc test, *p=0.0318, CON vs. IUGR; #p<0.0001, IUGR vs. AP; †p=0.009, HF vs. AP). Data are shown as means ± standard error of the mean.
Validation of the SERT antibody using Immunohistochemistry
The specificity of the mouse and rat SERT antibodies was validated in adult mouse brain frontal cortical sections by immunohistochemistry as shown in Figure 5. SERT was reported to be located in presynaptic 5-HT nerve terminals and 5-HT cell bodies [21]. No co-localization of SERT with synaptophysin, which is a marker of pre-synaptic neuronal axons, was seen (Figure 5), supporting the expression of SERT on neuronal bodies that lack synaptophysin immunostaining.
Figure 5. SERT and synaptophysin immunolocalization:
Dual immunofluorescence localization of SERT (Alexa 594, arrows) and synaptophysin (Alexa 488, arrowheads) in mouse E19 fetal cerebral cortex is demonstrated. DAPI was used as the nuclear stain. Scale bar = 100 μm.
Serotonin and SERT protein in E19 fetal mouse brain
Serotonin concentrations decreased by 60% (One way ANOVA, F-statistic: 3,36=4.605 p =0.0079 Tukey’s post-hoc test *p=0.0037) (Figure 6A) and SERT protein expression (Figure 6B) decreased by 46% (One way ANOVA, F-statistic: 3,48=12.41, p <0.0001. Tukey’s test *p<0.0001) in response to maternal calorie restriction (IUGR) (n=8) when compared to age-matched CON (n=15). In contrast, no statistically significant change was observed in fetal brain (E19) serotonin and SERT concentrations in response to maternal high fat dietary exposure (HF) (n=7) versus CON (n=15), despite a decreasing trend observed in brain serotonin concentrations in the HF group versus CON (Figure 6A and B). Similarly, no difference in serotonin and SERT concentrations was evident following maternal exposure to simulated air pollution (Figure 6A and B).
Figure 6. Quantification of serotonin and SERT:
The graphs demonstrate E19 brain serotonin concentrations quantified by ELISA (A) and SERT concentrations quantified by Western Blot analysis (B), in IUGR, HF and AP vs. CON. Serotonin: Fetal murine brain serotonin concentrations (A) decreased in the IUGR but not in HF or AP groups when compared to CON. (one-way ANOVA, F-statistic: 3, 36=4.605, p=0.0079, n=15 for CON, n=7 for HF, n=8 for IUGR, and n=10 for AP; Tukey’s post-hoc test demonstrates *p=0.0037 in IUGR vs CON). When comparing IUGR to HF and AP groups, no significant differences emerged, although the AP group trended higher than IUGR (p=0.0974). SERT protein: (B) Top panels display representative blots of SERT shown above and β-actin as the internal loading control shown below. The bottom graphs depict quantification of Western blots demonstrating decreased SERT in the IUGR group when compared to age-matched CON (*p<0.0001), HF (#p=0.0005) and AP (*p<0.0001) groups (one-way ANOVA, F-statistic: 3, 48=12.41, p<0.0001, n=25 for CON, n=7 for HF, n=8 for IUGR, and n=12 for AP; Tukey’s post-hoc test, *p<0.0001 vs CON or AP; #p=0.0005 vs HF). Data are shown as means ± standard error of the mean.
OXTR in mouse brain
We initially employed immunostaining to determine the specificity of the OXTR antibody (Figure 7). Employing postnatal brain sections, we noted the presence of OXTR in the cortex (Figure 7A & B) and hippocampus (Figure 7C & D). Following these analyses, we next confirmed the presence of OXTR in mouse cortex that is enriched in neuronal Glut3. We initially analyzed the temporal expression of OXTR at different developmental stages, E19, P15 and adults in both males (Figure 8A) and females (Figure 8B) by Western blot analysis (Figure 8A–C). In males, .the expression of OXTR significantly increased at P15 and adult stages compared to E19 (one-way ANOVA, F-statistic: 2, 9=10.64, p=0.0043, n=4 each group; Sidak’s multiple comparisons post-hoc test, *p=0.05 vs. E19, #p=0.0041 vs. E19). In contrast, no difference among the three developmental stages in females was seen. When males and females were combined (Figure 8C), OXTR amounts significantly increased at the adult stage when compared to E19 (one-way ANOVA, F-statistic: 2, 21=7.145, p=0.0043, n=8 each group; Sidak’s multiple comparisons post-hoc test, *p=0.0035 vs. E19).
Figure 7. Immunofluorescence localization of oxytocin receptor (OXTR):
Alexa 488, green in the hypothalamus (A & B) and hippocampus (C & D) of postnatal day 15 brain (cortex) with DAPI nuclear stain (A & C, blue). The OXTR protein was expressed in neural membranes (arrows). Scale bar = 100 μM.
Figure 8. Quantification of OXTR:
Ontogeny studies: Examination of oxytocin receptor (OXTR) at E19, PN (postnatal) 15 and adult (2 months old) mouse cortices. Western blot analysis showing representative blots in insets that depict cortical OXTR (~43 kD) (top panels) with vinculin (vin; bottom panels, internal loading control), and quantification expressed as a percent of the E19 value (A-C) or of the E19 CON value (D), depicted in graphs. Males (A), females (B) and males and females combined (C) are demonstrated. In males (A), the expression of OXTR significantly increased in PN15 and adult compared to E19 (one-way ANOVA, F-statistic: 2, 9=10.64, p=0.0043, n=4 each group; Sidak post-hoc test, *p=0.05 vs E19, #p=0.0041 vs E19). In females (B), there was no differences among the three groups of females (one-way ANOVA, F-statistic: 2, 9=2.877, p=0.1081, n=4 each group; Sidak post-hoc test; P15 vs E19, p=0.4027: adult vs E19, p=0.1219: adult vs P15, p=0.8170). However, in the combined groups of males and females (C), the amount of OXTR significantly increased in adult compared to E19 (one-way ANOVA, F 2, 21=7.145, p=0.0043, n=8 each group; Sidak post-hoc test, P15 vs E19, p=0.09: adult vs E19, *p=0.0035: adult vs P15, p=0.4078). In the E19 brain OXTR concentrations of CON, IUGR, HF and AP groups (D), there was no significant difference observed despite a trend towards a 16% decrease in the AP group when compared to CON (one-way ANOVA, F-statistic: 3, 39=0.7615, p=0.5225, n=17 for CON, n= 8 for IUGR, n=7 for HF and n=11 for AP). Data are shown as means ± standard error of the mean.
Since we had observed a reduction in E19 brain Glut3 concentrations in IUGR compared to CON (Figure 3 & Figure 4), we next investigated brain OXTR concentrations in CON, IUGR, HF and AP groups (Figure 8D). There was no significant difference observed despite a trend towards a 16% decrease in the AP group when compared to CON (one-way ANOVA, F-statistic: 3, 39=0.7615, p=0.5225, n=17 for CON, n= 8 for IUGR, n=7 for HF and n=11 for AP). In addition, a trend towards a decrease was also evident in the IUGR male group (t6=1.51, p=0.1818, n=4 each) compared to the sex-matched CON (Supplementary 2A). No difference in the female IUGR group (Supplementary 2B) or the combined male and female IUGR group (Supplementary 2C) versus the respective CON group was observed.
Discussion
We have demonstrated that maternal caloric restriction induced IUGR reduced late gestation fetal brain Glut3 expression, while not significantly affecting Glut1. This decrease of ~50% reflects prior studies of the classical glut3 heterozygous (glut3+/−) embryonic null mice [58] where genetic modification achieved the same end result as IUGR. The glut3+/− embryos went on to subsequently develop neurobehavioral changes [8]. These neurobehavioral changes consisted of abnormal spatial learning, working memory and abnormal cognitive flexibility, perturbed social behavior with reduced vocalization and presence of stereotypies at a low frequency [8]. Similarly, the IUGR offspring also developed neurobehavioral perturbations as an adult [45]. These changes may have their roots in altered embryonic development of neural cellular processes [51], which may have resulted or in turn have affected the neuronal glucose transport mediated by Glut3. While it is difficult to quantify the neuronal glucose transport function in-vivo, separate from the blood-brain barrier and glial Glut1 [6, 59, 7], one can surmise from in-vitro experiments that a reduction in neuronal Glut3 which activates AMPK leads to diminution of ATP interfering with neurotransmission [12].
While the development of multiple neurotransmittory pathways is essential to effect normal neurodevelopment, for the purposes of this report, we focused primarily on serotonin that is derived from the essential amino-acid tryptophan. We and others have previously demonstrated a reduction in tryptophan when maternal dietary/protein restriction is imposed [60, 61]. Maternal tryptophan is essential for the early gestational serotonin-dependent neural networks [62]. Subsequently, the fetal brain innately produces serotonin, which is independent of maternal amino-acid metabolism [62]. Our present investigation demonstrates that fetal brain serotonin concentrations are also reduced in the presence of maternal caloric restricted IUGR. Both Glut3 and serotonin expression may be reduced in the IUGR due to changes in progenitor cell proliferative processes that have previously been observed to result in microcephaly at E19 [51]. Similarly, in our present study we observed a reduction in head weights consistent with microcephaly. This diminution in serotonin was mirrored by a decrease in SERT concentrations, supporting a lack of maturation in the late gestation embryo of the adaptive upregulation that is seen subsequently [63].
Maternal high fat dietary exposure has also been observed to result in a relative amino-acid deficiency [64]. We noted that while maternal high fat diet did not cause significant changes in serotonin and SERT concentrations in the embryonic brain, the trend towards a decrease particularly in serotonin along with a reduction in head weights (microcephaly) was concerning. We also assessed the impact of gestational exposure to air pollutants mimicking conditions of heavy traffic. Previous studies have revealed changes in fatty acid metabolism [65, 66] along with certain studies suggesting an association with diabetes mellitus and other related chronic disorders [67–69] upon close proximity to heavy traffic related roadways. Furthermore, recent associations have revealed gestational exposure to air pollution reduced the ultrasound assessed human fetal bi-parietal diameter [70–72], with the offspring subsequently developing autism spectrum disorders [73–75]. Murine studies have demonstrated that exposure to volatile organic compounds perturbs adult brain serotonin concentrations affecting neurobehavior [76]. However, in our gestational mouse model, no significant effect was observed upon embryonic brain serotonin and SERT concentrations, although again a trend towards lower serotonin concentrations emerged. This observation suggests that perhaps other mechanisms besides serotonin alone are in play for changes related to gestational air pollution exposures, given that reduced fetal head weights (microcephaly) emerged.
In contrast, given that OXT is only produced by the hypothalamic paraventricular nucleus (PVN), we concentrated on OXTR that mediates inhibitory neurotransmission [77], opposing the excitatory activity of serotonin-SERT. Initially, similar to prior studies with cortical Glut3 [5, 78] and that of others with brain serotonin-SERT investigations [79, 27, 19], cortical OXTR also increased with development peaking at a postnatal stage of P15, remaining constant thereafter into the adult stage. While E19 cortical OXTR was low, in a combination of IUGR males and females no change in OXTR concentrations was seen. Teasing out males from females, albeit underpowered, revealed IUGR males to be more vulnerable than females. These results suggest that perhaps unlike the case of Glut3 in adult brain [59], here E19 IUGR males and females when separated showed similar changes in brain Glut3. However, in IUGR brain serotonin-SERT, the possibility exists that the males are more vulnerable when compared to females. Since we prospectively did not separate the males from the females in serotonin-SERT analyses in particular, this is a limitation of our present study. It is quite possible that even in the HF and air pollution exposures, males were more affected than females, responsible for the overall decreasing trend in serotonin observed in the combination of sexes.
In our present study, we have demonstrated an imbalance between cerebral cortical serotonin and SERT concentrations early in life in response to maternal dietary modifications. These changes have the ability to affect brain plasticity and adaptation of the serotonergic neural networks [80] during their formative stages of embryonic development, with a lasting impact on adult neurobehavioral expression. In addition, to these early life changes, we did not see any sex-specific changes in Glut3, but have observed sex-specific changes in OXTR expression as early as during the embryonic phase of development. Our present embryonic investigations utilized the murine species since it was necessary to ensure that the role of the placenta was maintained intact in modulating the development of the embryonic glucose transporter and serotonergic pathways. The murine placenta which is hemochorial in nature is akin to that of the human placenta and has been widely characterized by us and others [81, 82]. Further, it is known that besides expressing Glut1, Glut3 and OXTR, placental tryptophan hydroxylase is essential for providing serotonin to the developing embryo [26]. We had examined the E19 placental SERT concentrations in response to maternal caloric restriction and observed undetectable amounts even in CON placentas (negative data was not shown).
The maternal calorie restriction model for producing IUGR is well established [83, 84, 82]. This model results in the chronicity typical of human IUGR and as shown by us demonstrates a chronic reduction in utero-placental blood flow [82]. In addition, this murine model has demonstrated a reduction in trans-placental macronutrient fetal supply, culminating in growth restricted embryos [82]. This calorie restriction is initiated during mid-gestation when brain de-novo serotonin synthesis occurs, rather than early in gestation when the embryonic brain is fully reliant on placental transport and tryptophan hydroxylase conversion of tryptophan to an embryonic serotonin supply [26]. At near term (E19), embryonic brain serotonin and SERT concentrations were reduced suggestive of reduced de-novo synthesis of 5-HT which in turn may have downregulated SERT expression levels. Such a change may adversely affect neural connectivity and plasticity of the developing mammalian brain [19, 21]. In contrast, intra-uterine exposure to a high fat diet in the murine model led to enhanced intra-placental fatty acid (via CD36 protein) and glucose transport (GLUT1 protein) without much change in sub-types of amino acid transporters (SNATs and LAT2 proteins) [52]. Under such circumstances, embryonic brain serotonin and SERT concentrations remained unchanged with no significant perturbation, despite the decreasing trend in serotonin observed.
In various adult IUGR rodent studies, cerebral cortical concentrations of serotonin and SERT were found to normalize [85, 86, 45], the perturbations experienced during the critical embryonic window of development are not seen. Thus the impact of these embryonic perturbations on serotonin-dependent neural connectivity may have long lasting effects upon the brain [21], without displaying any neurotransmitter perturbations. While in this study we did not undertake adult studies particularly defining neurobehavior, many others have studied the neurobehavior of the adult IUGR offspring or high fat exposed offspring, and observed anxiety and hyperactivity in the former [87, 88, 45] and depressive behaviors in the latter [89]. While there may be multiple factors contributing towards this ultimate neurobehavioral phenotype, our studies demonstrate a major role of maternal diet upon the early life determinants of this ultimate phenotype. In addition, despite the normalization of either serotonin or SERT in the adult brain, various other perturbations related to activation of enhancers/modifiers, suppression of endogenous inhibitors, or other epigenetic factors can alter the serotonin-SERT pathway resulting in altered availability or action of serotonin for neurotransmission [21, 29]. Our observations where maternal dietary modifications perturb the 5-HT-5-HTT balance is reminiscent of the opposite impact of drugs such as selective serotonin reuptake inhibitors (SSRI) that increase the availability of synaptic serotonin for neurotransmission. Maternal SSRIs can easily cross the placenta and alter fetal brain serotonin availability [90], opposing what we detected with maternal dietary modifications.
Genetic mutations of the serotonin receptor isoforms (5-HT1B and 5-HT1D) have led to changes in fetal neural connections causing aberrant neuro-behavior [25, 62]. While we have not assessed serotonin receptors in our present study, non-genetic environmental alterations in either serotonin or SERT or both impact the synaptic serotonin availability and function. Particularly during embryonic development (~E10.5), serotonin availability is necessary for neurogenesis, migration and axonal development [19]. We have shown that dietary environments can cause an early 5-HT-5-HTT imbalance with the possibility of deranging neural/axonal development and synaptic connections. These derangements come with the propensity of long-term implications that adversely affect plasticity and adult neuro-behavior [91]. More recently, mutations of SERT have been implicated in autism spectrum disorders [15, 92], and circulating maternal serotonin concentrations have been associated with ASD in their offspring [93]. However, serotonin fails to cross the placenta [62], suggesting other indirect effects of these changes having implications for the developing embryo. It is known that males predominate in developing ASD [94], while females have a higher incidence of anxiety [95].
In conclusion, we have demonstrated that maternal dietary modifications (either low calories or high calories) alter fetal cerebral cortical neuronal glucose transporter, serotonin and/or SERT concentrations, with not much of an effect on OXTR. While prenatal exposure to air pollution and maternal high fat diet did not show significant changes, teasing out males from females may be important in future studies. These embryonic brain changes may underlie the development of anxiety and hyperactivity in the adult IUGR offspring [87, 88]. The molecular mechanisms tying early introduction of dietary modifications with the developing offspring’s brain neurotransmitters is of significant importance, necessary for unraveling the pathogenesis of neurodevelopmental and mental health disorders. Such information may assist in targeted therapeutic interventions for these disorders after the fact, and prevention prior to the fact focused on optimizing maternal diet during pregnancy.
Supplementary Material
Acknowledgments
We thank Drs. James Waschek, Ph.D. and Carlos Cepeda, Ph.D. (Dept. of Psychiatry and Biobehavioral Sciences, UCLA) for their ongoing review and valuable insights.
Funding Sources
This work was supported by grants from the National Institutes of Health HD-81206 and HD-41230 (to SUD).
Footnotes
Staterment of Ethics
The authors, reviewers, and editors affirm that in accordance with the policies set by the Developmental Neuroscience Journal this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
Animal care or treatment was provided according to the UCLA Animal Research Committee’s approved protocol in accordance with the guidelines set by the National Institutes of Health regarding the Care and Use of Laboratory Animals.
Conflict of Interest Statement
The authors declare no competing financial interests.
References
- 1.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013. October;36(10):587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dienel GA. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol Rev. 2019. January 1;99(1):949–1045. [DOI] [PubMed] [Google Scholar]
- 3.Mitanchez D. [Ontogenesis of glucose regulation in neonate and consequences in neonatal management]. Arch Pediatr. 2008. January;15(1):64–74. [DOI] [PubMed] [Google Scholar]
- 4.Takata K, Hirano H, Kasahara M. Transport of glucose across the blood-tissue barriers. Int Rev Cytol. 1997;172:1–53. [DOI] [PubMed] [Google Scholar]
- 5.Khan JY, Rajakumar RA, McKnight RA, Devaskar UP, Devaskar SU. Developmental regulation of genes mediating murine brain glucose uptake. The American journal of physiology. 1999. March;276(3):R892–900. [DOI] [PubMed] [Google Scholar]
- 6.Zheng PP, Romme E, van der Spek PJ, Dirven CM, Willemsen R, Kros JM. Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Annals of neurology. 2010. December;68(6):835–44. [DOI] [PubMed] [Google Scholar]
- 7.Tang M, Gao G, Rueda CB, Yu H, Thibodeaux DN, Awano T, et al. Brain microvasculature defects and Glut1 deficiency syndrome averted by early repletion of the glucose transporter-1 protein. Nat Commun. 2017. January 20;8:14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Fung C, Shin D, Shin BC, Thamotharan S, Sankar R, et al. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry. 2010. March;15(3):286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin BC, Cepeda C, Estrada-Sanchez AM, Levine MS, Hodaei L, Dai Y, et al. Neural Deletion of Glucose Transporter Isoform 3 Creates Distinct Postnatal and Adult Neurobehavioral Phenotypes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018. October 31;38(44):9579–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vivo DC, Leary L, Wang D. Glucose transporter 1 deficiency syndrome and other glycolytic defects. Journal of child neurology. 2002. December;17 Suppl 3:3S15–23; discussion 3S24–5. [PubMed] [Google Scholar]
- 11.Tang M, Park SH, De Vivo DC, Monani UR. Therapeutic strategies for glucose transporter 1 deficiency syndrome. Annals of clinical and translational neurology. 2019. September;6(9):1923–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisova P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009. March 4;29(9):2997–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajduch E, Rencurel F, Balendran A, Batty IH, Downes CP, Hundal HS. Serotonin (5-Hydroxytryptamine), a novel regulator of glucose transport in rat skeletal muscle. J Biol Chem. 1999. May 7;274(19):13563–8. [DOI] [PubMed] [Google Scholar]
- 14.Stapel B, Gorinski N, Gmahl N, Rhein M, Preuss V, Hilfiker-Kleiner D, et al. Fluoxetine induces glucose uptake and modifies glucose transporter palmitoylation in human peripheral blood mononuclear cells. Expert Opin Ther Targets. 2019. October;23(10):883–91. [DOI] [PubMed] [Google Scholar]
- 15.Muller CL, Anacker AMJ, Veenstra-VanderWeele J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience. 2016. May 3;321:24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagano M, Takumi T, Suzuki H. Critical roles of serotonin-oxytocin interaction during the neonatal period in social behavior in 15q dup mice with autistic traits. Sci Rep. 2018. September 12;8(1):13675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolen G. Autism: Oxytocin, serotonin, and social reward. Social neuroscience. 2015;10(5):450–65. [DOI] [PubMed] [Google Scholar]
- 18.Hirosawa T, Kikuchi M, Ouchi Y, Takahashi T, Yoshimura Y, Kosaka H, et al. A pilot study of serotonergic modulation after long-term administration of oxytocin in autism spectrum disorder. Autism Res. 2017. May;10(5):821–28. [DOI] [PubMed] [Google Scholar]
- 19.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003. December;4(12):1002–12. [DOI] [PubMed] [Google Scholar]
- 20.Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–74. [DOI] [PubMed] [Google Scholar]
- 21.Daws LC, Gould GG. Ontogeny and regulation of the serotonin transporter: providing insights into human disorders. Pharmacol Ther. 2011. July;131(1):61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73(1–2):19–29. [DOI] [PubMed] [Google Scholar]
- 23.Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001. November 15;56(5):479–85. [DOI] [PubMed] [Google Scholar]
- 24.Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? Int J Dev Neurosci. 2005. February;23(1):75–83. [DOI] [PubMed] [Google Scholar]
- 25.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007. May;10(5):588–97. [DOI] [PubMed] [Google Scholar]
- 26.Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011. April 21;472(7343):347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruning G, Liangos O, Baumgarten HG. Prenatal development of the serotonin transporter in mouse brain. Cell Tissue Res. 1997. August;289(2):211–21. [DOI] [PubMed] [Google Scholar]
- 28.Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E, et al. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res. 2000. February 7;119(2):251–7. [DOI] [PubMed] [Google Scholar]
- 29.Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience. 2017. February 7;342:212–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petraglia F, Imperatore A, Challis JR. Neuroendocrine mechanisms in pregnancy and parturition. Endocrine reviews. 2010. December;31(6):783–816. [DOI] [PubMed] [Google Scholar]
- 31.Kim SC, Lee JE, Kang SS, Yang HS, Kim SS, An BS. The regulation of oxytocin and oxytocin receptor in human placenta according to gestational age. Journal of molecular endocrinology. 2017. October;59(3):235–43. [DOI] [PubMed] [Google Scholar]
- 32.Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Frontiers in physiology. 2018;9:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinevich V, Desarmenien MG, Chini B, Tauber M, Muscatelli F. Ontogenesis of oxytocin pathways in the mammalian brain: late maturation and psychosocial disorders. Front Neuroanat. 2014;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013. December 3;253:155–64. [DOI] [PubMed] [Google Scholar]
- 35.Freeman SM, Smith AL, Goodman MM, Bales KL. Selective localization of oxytocin receptors and vasopressin 1a receptors in the human brainstem. Social neuroscience. 2017. April;12(2):113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erbas O, Yilmaz M, Korkmaz HA, Bora S, Evren V, Peker G. Oxytocin inhibits pentylentetrazol-induced seizures in the rat. Peptides. 2013. February;40:141–4. [DOI] [PubMed] [Google Scholar]
- 37.Dobolyi A, Kekesi KA, Juhasz G, Szekely AD, Lovas G, Kovacs Z. Receptors of peptides as therapeutic targets in epilepsy research. Current medicinal chemistry. 2014;21(6):764–87. [DOI] [PubMed] [Google Scholar]
- 38.Erfanparast A, Tamaddonfard E, Henareh-Chareh F. Intra-hippocampal microinjection of oxytocin produced antiepileptic effect on the pentylenetetrazol-induced epilepsy in rats. Pharmacological reports : PR. 2017. August;69(4):757–63. [DOI] [PubMed] [Google Scholar]
- 39.Cai Q, Feng L, Yap KZ. Systematic review and meta-analysis of reported adverse events of long-term intranasal oxytocin treatment for autism spectrum disorder. Psychiatry and clinical neurosciences. 2018. March;72(3):140–51. [DOI] [PubMed] [Google Scholar]
- 40.Panaitescu AM, Isac S, Pavel B, Ilie AS, Ceanga M, Totan A, et al. Oxytocin Reduces Seizure Burden and Hippocampal Injury in a Rat Model of Perinatal Asphyxia. Acta endocrinologica. 2018. Jul-Sep;14(3):315–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uzunova G, Pallanti S, Hollander E. Excitatory/inhibitory imbalance in autism spectrum disorders: Implications for interventions and therapeutics. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2016. April;17(3):174–86. [DOI] [PubMed] [Google Scholar]
- 42.Thompson JR, Valleau JC, Barling AN, Franco JG, DeCapo M, Bagley JL, et al. Exposure to a High-Fat Diet during Early Development Programs Behavior and Impairs the Central Serotonergic System in Juvenile Non-Human Primates. Front Endocrinol (Lausanne). 2017;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreton E, Baron P, Tiplady S, McCall S, Clifford B, Langley-Evans SC, et al. Impact of early exposure to a cafeteria diet on prefrontal cortex monoamines and novel object recognition in adolescent rats. Behav Brain Res. 2019. May 2;363:191–98. [DOI] [PubMed] [Google Scholar]
- 44.Ye W, Pitlock MD, Javors MA, Thompson BJ, Lechleiter JD, Hensler JG. The long-term effect of maternal dietary protein restriction on 5-HT1A receptor function and behavioral responses to stress in adulthood. Behav Brain Res. 2018. September 3;349:116–24. [DOI] [PubMed] [Google Scholar]
- 45.Tomi M, Zhao Y, Thamotharan S, Shin BC, Devaskar SU. Early life nutrient restriction impairs blood-brain metabolic profile and neurobehavior predisposing to Alzheimer’s disease with aging. Brain Res. 2013. February 7;1495:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi L, Wei C, Fan W. Fine-particulate matter (PM2.5), a risk factor for rat gestational diabetes with altered blood glucose and pancreatic GLUT2 expression. Gynecol Endocrinol. 2017. August;33(8):611–16. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Zeng X, Du X, Pan K, Song L, Song W, et al. Parental PM2.5 Exposure-Promoted Development of Metabolic Syndrome in Offspring Is Associated With the Changes of Immune Microenvironment. Toxicol Sci. 2019. August 1;170(2):415–26. [DOI] [PubMed] [Google Scholar]
- 48.Costa LG, Chang YC, Cole TB. Developmental Neurotoxicity of Traffic-Related Air Pollution: Focus on Autism. Current environmental health reports. 2017. June;4(2):156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang YC, Cole TB, Costa LG. Prenatal and early-life diesel exhaust exposure causes autism-like behavioral changes in mice. Part Fibre Toxicol. 2018. April 20;15(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nephew BC, Nemeth A, Hudda N, Beamer G, Mann P, Petitto J, et al. Traffic-related particulate matter affects behavior, inflammation, and neural integrity in a developmental rodent model. Environmental research. 2020. April;183:109242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baldauf C, Sondhi M, Shin BC, Ko YE, Ye X, Lee KW, et al. Murine maternal dietary restriction affects neural Humanin expression and cellular profile. J Neurosci Res. 2020. May;98(5):902–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganguly A, Devaskar SU. High-fat diet affects pregestational adiposity and glucose tolerance perturbing gestational placental macronutrient transporters culminating in an obese offspring in wild-type and glucose transporter isoform 3 heterozygous null mice. J Nutr Biochem. 2018. December;62:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chauhan V, Breznan D, Goegan P, Nadeau D, Karthikeyan S, Brook JR, et al. Effects of ambient air particles on nitric oxide production in macrophage cell lines. Cell Biol Toxicol. 2004. July;20(4):221–39. [DOI] [PubMed] [Google Scholar]
- 54.Kurtz ML, Astort F, Lezon C, Ferraro SA, Maglione GA, Orona NS, et al. Oxidative stress response to air particle pollution in a rat nutritional growth retardation model. J Toxicol Environ Health A. 2018;81(20):1028–40. [DOI] [PubMed] [Google Scholar]
- 55.Ganguly A, Collis L, Devaskar SU. Placental glucose and amino acid transport in calorie-restricted wild-type and Glut3 null heterozygous mice. Endocrinology. 2012. August;153(8):3995–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015. April 9;161(2):264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McFarlane L, Truong V, Palmer JS, Wilhelm D. Novel PCR assay for determining the genetic sex of mice. Sex Dev. 2013;7(4):207–11. [DOI] [PubMed] [Google Scholar]
- 58.Ganguly A, McKnight RA, Raychaudhuri S, Shin BC, Ma Z, Moley K, et al. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab. 2007. May;292(5):E1241–55. [DOI] [PubMed] [Google Scholar]
- 59.Dai Y, Zhao Y, Tomi M, Shin BC, Thamotharan S, Mazarati A, et al. Sex-Specific Life Course Changes in the Neuro-Metabolic Phenotype of Glut3 Null Heterozygous Mice: Ketogenic Diet Ameliorates Electroencephalographic Seizures and Improves Sociability. Endocrinology. 2017. April 1;158(4):936–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes. 2009. March;58(3):559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calkins KL, Thamotharan S, Dai Y, Shin BC, Kalhan SC, Devaskar SU. Early dietary restriction in rats alters skeletal muscle tuberous sclerosis complex, ribosomal s6 and mitogen-activated protein kinase. Nutrition research. 2018. June;54:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011. December 1;197:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Versteeg RI, Schrantee A, Adriaanse SM, Unmehopa UA, Booij J, Reneman L, et al. Timing of caloric intake during weight loss differentially affects striatal dopamine transporter and thalamic serotonin transporter binding. FASEB J. 2017. October;31(10):4545–54. [DOI] [PubMed] [Google Scholar]
- 64.Edlow AG, Guedj F, Sverdlov D, Pennings JLA, Bianchi DW. Significant Effects of Maternal Diet During Pregnancy on the Murine Fetal Brain Transcriptome and Offspring Behavior. Frontiers in neuroscience. 2019;13:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salim SY, Kaplan GG, Madsen KL. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut microbes. 2014. Mar-Apr;5(2):215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller DB, Ghio AJ, Karoly ED, Bell LN, Snow SJ, Madden MC, et al. Ozone Exposure Increases Circulating Stress Hormones and Lipid Metabolites in Humans. American journal of respiratory and critical care medicine. 2016. June 15;193(12):1382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bateson TF, Schwartz J. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology. 2004. March;15(2):143–9. [DOI] [PubMed] [Google Scholar]
- 68.Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, et al. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012. February 14;125(6):767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho J, Choi YJ, Suh M, Sohn J, Kim H, Cho SK, et al. Air pollution as a risk factor for depressive episode in patients with cardiovascular disease, diabetes mellitus, or asthma. Journal of affective disorders. 2014. March;157:45–51. [DOI] [PubMed] [Google Scholar]
- 70.Smarr MM, Vadillo-Ortega F, Castillo-Castrejon M, O’Neill MS. The use of ultrasound measurements in environmental epidemiological studies of air pollution and fetal growth. Current opinion in pediatrics. 2013. April;25(2):240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clemens T, Turner S, Dibben C. Maternal exposure to ambient air pollution and fetal growth in North-East Scotland: A population-based study using routine ultrasound scans. Environment international. 2017. October;107:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malmqvist E, Liew Z, Kallen K, Rignell-Hydbom A, Rittner R, Rylander L, et al. Fetal growth and air pollution - A study on ultrasound and birth measures. Environmental research. 2017. January;152:73–80. [DOI] [PubMed] [Google Scholar]
- 73.Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environmental health perspectives. 2013. March;121(3):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weisskopf MG, Kioumourtzoglou MA, Roberts AL. Air Pollution and Autism Spectrum Disorders: Causal or Confounded? Current environmental health reports. 2015. December;2(4):430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kerin T, Volk H, Li W, Lurmann F, Eckel S, McConnell R, et al. Association Between Air Pollution Exposure, Cognitive and Adaptive Function, and ASD Severity Among Children with Autism Spectrum Disorder. Journal of autism and developmental disorders. 2018. January;48(1):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang F, Fangfang Z, Guo X, Chen W, Yao W, Liu H, et al. Effects of volatile organic compounds and carbon monoxide mixtures on learning and memory, oxidative stress, and monoamine neurotransmitters in the brains of mice. Toxicol Ind Health. 2018. March;34(3):178–87. [DOI] [PubMed] [Google Scholar]
- 77.Leonzino M, Busnelli M, Antonucci F, Verderio C, Mazzanti M, Chini B. The Timing of the Excitatory-to-Inhibitory GABA Switch Is Regulated by the Oxytocin Receptor via KCC2. Cell reports. 2016. April 5;15(1):96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thamotharan S, Stout D, Shin BC, Devaskar SU. Temporal and spatial distribution of murine placental and brain GLUT3-luciferase transgene as a readout of in vivo transcription. Am J Physiol Endocrinol Metab. 2013. February 1;304(3):E254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruning G, Liangos O. Transient expression of the serotonin transporter in the developing mouse thalamocortical system. Acta histochemica. 1997. March;99(1):117–21. [DOI] [PubMed] [Google Scholar]
- 80.Sbrini G, Brivio P, Bosch K, Homberg JR, Calabrese F. Enrichment Environment Positively Influences Depression- and Anxiety-Like Behavior in Serotonin Transporter Knockout Rats through the Modulation of Neuroplasticity, Spine, and GABAergic Markers. Genes (Basel). 2020. October 23;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 2014. April;27(1):11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ganguly A, Touma M, Thamotharan S, De Vivo DC, Devaskar SU. Maternal Calorie Restriction Causing Uteroplacental Insufficiency Differentially Affects Mammalian Placental Glucose and Leucine Transport Molecular Mechanisms. Endocrinology. 2016. October;157(10):4041–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen PY, Ganguly A, Rubbi L, Orozco LD, Morselli M, Ashraf D, et al. Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol Genomics. 2013. July 15;45(14):565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ganguly A, Chen Y, Shin BC, Devaskar SU. Prenatal caloric restriction enhances DNA methylation and MeCP2 recruitment with reduced murine placental glucose transporter isoform 3 expression. J Nutr Biochem. 2014. February;25(2):259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Kloet ER, Kovacs GL, Szabo G, Telegdy G, Bohus B, Versteeg DH. Decreased serotonin turnover in the dorsal hippocampus of rat brain shortly after adrenalectomy: selective normalization after corticosterone substitution. Brain Res. 1982. May 13;239(2):659–63. [DOI] [PubMed] [Google Scholar]
- 86.Barr JL, Forster GL. Serotonergic neurotransmission in the ventral hippocampus is enhanced by corticosterone and altered by chronic amphetamine treatment. Neuroscience. 2011. May 19;182:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lahti J, Raikkonen K, Pesonen AK, Heinonen K, Kajantie E, Forsen T, et al. Prenatal growth, postnatal growth and trait anxiety in late adulthood - the Helsinki Birth Cohort Study. Acta Psychiatr Scand. 2010. March;121(3):227–35. [DOI] [PubMed] [Google Scholar]
- 88.Mikaelsson MA, Constancia M, Dent CL, Wilkinson LS, Humby T. Placental programming of anxiety in adulthood revealed by Igf2-null models. Nat Commun. 2013;4:2311. [DOI] [PubMed] [Google Scholar]
- 89.Contu L, Hawkes CA. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int J Mol Sci. 2017. May 19;18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olivier JD, Akerud H, Kaihola H, Pawluski JL, Skalkidou A, Hogberg U, et al. The effects of maternal depression and maternal selective serotonin reuptake inhibitor exposure on offspring. Front Cell Neurosci. 2013;7:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shah R, Courtiol E, Castellanos FX, Teixeira CM. Abnormal Serotonin Levels During Perinatal Development Lead to Behavioral Deficits in Adulthood. Front Behav Neurosci. 2018;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siemann JK, Muller CL, Forsberg CG, Blakely RD, Veenstra-VanderWeele J, Wallace MT. An autism-associated serotonin transporter variant disrupts multisensory processing. Transl Psychiatry. 2017. March 21;7(3):e1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Montgomery AK, Shuffrey LC, Guter SJ, Anderson GM, Jacob S, Mosconi MW, et al. Maternal Serotonin Levels Are Associated With Cognitive Ability and Core Symptoms in Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2018. November;57(11):867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loomes R, Hull L, Mandy WPL. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry. 2017. June;56(6):466–74. [DOI] [PubMed] [Google Scholar]
- 95.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011. August;45(8):1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.