Abstract
Legalization of Cannabidiol (CBD) products has ignited interest in clinical practice and research. One desired indication includes possible pain-relieving effects of CBD. The purposes of this manuscript are to 1) clarify terminology relevant to cannabinoids, 2) understand the pharmacotherapeutics of CBD, 3) examine research of the current use of CBD by older adults for treating pain, 4) discuss safety considerations with using CBD products, and 5) provide best practice recommendations for clinicians as they advise their older adult patients. A review of the literature demonstrated mixed results on the efficacy of using CBD in relieving pain in the older adult. There is inconsistency in the labeling of over-the-counter CBD products that can result in safety issues and will require more federal quality control. Likewise, the gaps in knowledge regarding safety and efficacy of CBD use in older adults are vast and will require further research.
Keywords: cannabidiol (CBD), pain, older adult, practice setting, research
Background
Widespread efforts to reduce opioid medications for chronic pain (Dowell et al., 2016) markedly decreased the number of opioid prescriptions (Schieber et al., 2019) leaving older adults with unmet needs for treating their pain (Ritchie et al., 2020). In seeking other avenues for managing pain, one trending alternative is the use of cannabis and cannabidiol (CBD) (Vyas et al., 2018). A Gallup poll conducted in 2019 found one in seven Americans said they personally use CBD products. Eight percent of those over 65 years said they used CBD for pain (40%), anxiety (20%), insomnia (11%), and arthritis (8%) (Brenan et al., 2019).
In 2018, the Farm Bill federally legalized CBD products containing a concentration of less than 0.3% delta-9 THC, without distinguishing between medical and recreational use (Hawes et al., 2020; Dill & Kurkowski, 2020). CBD regulations in individual states vary based on whether the THC content in the plant or product is, for example above or below 0.3% (Hawes et al., 2020). As more states legalize CBD, access improves through convenience stores and online. Older adults with pain may consider CBD a safe pharmacotherapy, particularly because of fears and concerns related to opioid use and misuse. Healthcare providers, however, may not be aware of legal, clinical and safety issues, making them unable to educate and guide older adults in the use of CBD to manage their pain.
This article provides information to the clinician caring for the older adult population who have questions about CBD use. The purposes here are to 1) clarify terminology relevant to cannabinoids, 2) understand the pharmacotherapeutics of CBD, 3) examine current research on the use of CBD by older adults for treating pain, 4) discuss safety considerations with using CBD products in older adults, and 5) provide best practice recommendations for clinicians as they advise their older adult patients and caregivers.
Terminology
The variety of names used in reference to cannabis products can be confusing. These terms are cannabis plants, phytocannabinoids, endogenous cannabinoids, and synthetic cannabinoids. The following explanation and Figure 1 illustrate how CBD is positioned among the armamentarium of cannabis products.
Figure 1.
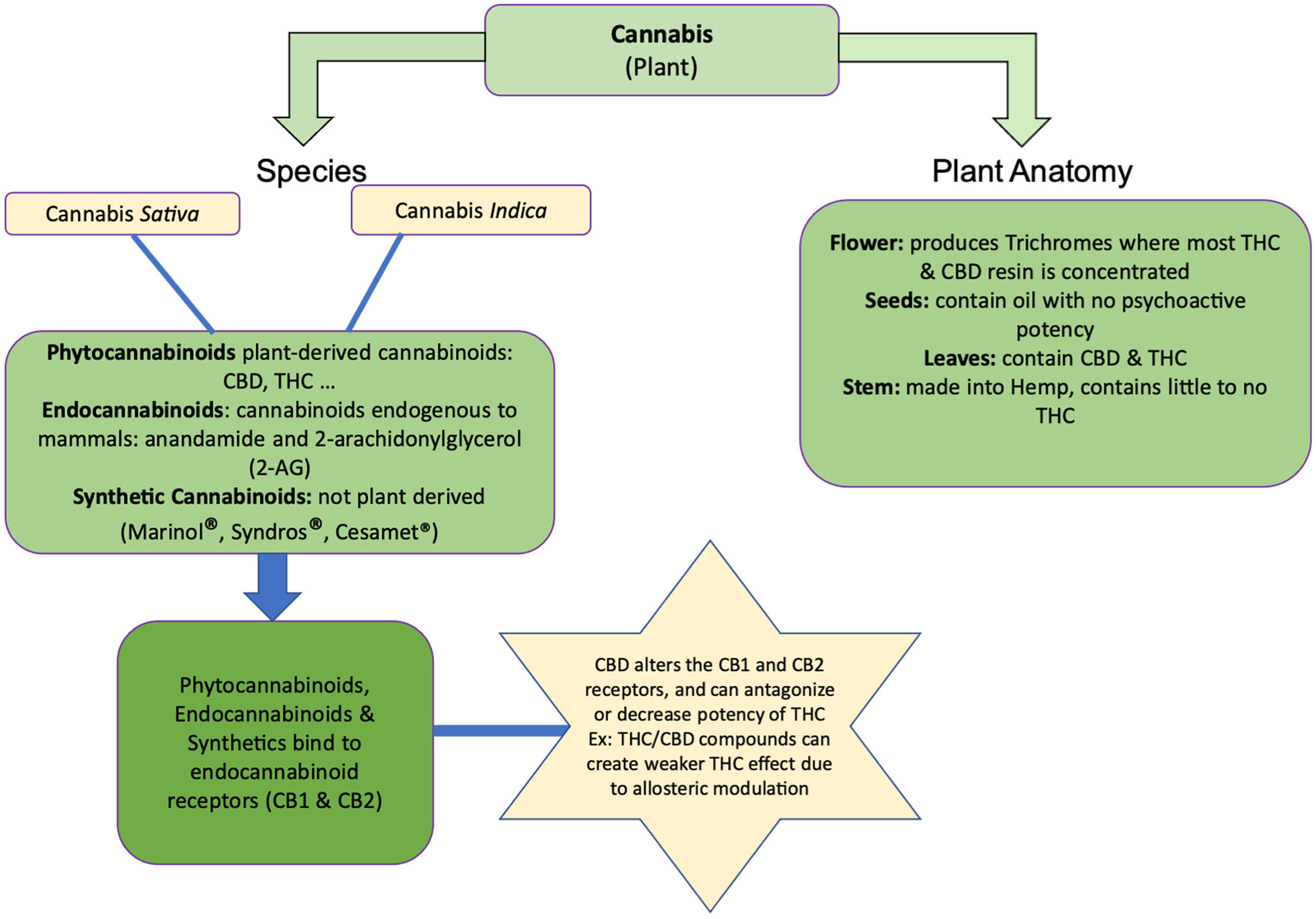
(Provides a visual breakdown of terminology related to cannabis and cannabinoids, and related cannabis plant anatomy. Delivers a concise description of how cannabinoids act in the body.)
Cannabis plants.
Cannabis is a plant used for pharmacological effect. The terms marijuana and cannabis are interchangeable. There are over 500 separate chemicals in the cannabis plant. Two subspecies of cannabis are cannabis indica and cannabis sativa (Lee et al., 2018). Within the cannabis plant, cannabinoids are found in high concentrations in the buds, with lower concentrations in the leaves (see Figure 1). Cannabinoids are not necessarily solely for medicinal purposes and can be categorized into three types: phytocannabinoids, endocannabinoids, and synthetic cannabinoids.
Phytocannabinoids.
Phytocannabinoids are exogenous (i.e., not found in the human body) cannabinoids isolated from the cannabis plant; and, when ingested, they bind to cannabinoid receptors (CB1 and CB2). One prescription phytocannabinoid, nabiximols (Sativex®), comes in an oromucosal spray of whole cannabis plant extract and is approved in Canada. Sativex® contains a one-to-one concentration of delta-9-tetrahydrocannabinol (THC) to CBD (National Academies of Sciences, Engineering, and Medicine, 2017; Jensen et al., 2015). Each microliter spray of Sativex® contains 2.7 mg THC and 2.5 mg CBD for adjunctive treatment of spasticity in patients with multiple sclerosis (Sativex® Product Monograph, 2019). THC is primarily responsible for the spray’s psychoactive effects of “euphoria.”
CBD is a non-psychoactive phytocannabinoid (Jensen, et al., 2015) found in approximately 40% of cannabis extracts, and has analgesic and anti-inflammatory properties (Gazendam et al, 2020). Hemp is the fiber extracted from the stem of the cannabis plant and is useful in making rope, textiles, building material, paper, and packaging. Hemp contains little to no THC, with less than 0.3% THC in hemp extracts (Hilderbrand, 2018), whereas hemp concentrations of CBD are higher (Hilderbrand, 2018). Hemp CBD products became available over the counter in 2018 when it became legal to sell CBD in the United States. Systematic reviews found CBD used in various oral formulations such as oral capsules or solutions, and sublingual oil with doses ranging from 50 mg/day to 1,000 mg/day (Wong, Chan, Cheung, 2020; Stockings et al., 2018; Larsen & Shahinas, 2020). This vast range of doses for CBD products found in the literature depicts the need for establishing safe dosing parameters in the older adult populations. One CBD-based prescription medication, Epidiolex®, was approved by the U.S. Food and Drug Administration (FDA) (2018) for treatment of seizures (VanDolah et al., 2019). Notably, this FDA approved CBD-based prescription medication is the only CBD product where purity, dose, and composition is known and regulated.
Endogenous cannabinoids.
Endocannabinoids are endogenous cannabinoids (i.e., found in the human body). There are two types: anandamide and 2-arachidonylglycerol (2-AG). These endocannabinoids bind to cannabinoid receptors (CB1 and CB2) in various parts of the body. CB1 receptors are found in the brain and peripherally (e.g., intestines, liver, adipose, immune cells), whereas CB2 receptors are in the spleen, tonsils, and immune cells (Mastinu et al., 2020). The endocannabinoid system modulates appetite, memory, motor responses and posture, creating some of the common effects associated with the cannabis use (National Academies of Sciences, Engineering, and Medicine, 2017), and making it a potential target site for future pain intervention strategies (Donvito et al., 2018; Manzanares et al., 2006).
Synthetic cannabinoids.
Synthetic cannabinoids are non-prescription or prescription, with many synthetic, non-prescription cannabinoids (such as K2 or Spice) considered illegal and federally banned in many states. Non-prescription synthetic cannabinoids are consumed through vaping, smoking, or drinking tea. Synthetic cannabinoids number in the hundreds; and non-prescription preparations lack manufacturing standards, so the potency and concentration details are not provided, which can create risk for the consumer. Nevertheless, consumers perceive these as safe and legal, making them popular. Synthetic cannabinoids bind to cannabinoid receptors but may affect the brain in unpredictable ways compared to THC (Centers for Disease Control, 2021). The FDA has only approved synthetic cannabinoids containing delta-9-THC analogues for prescriptions. These synthetic cannabinoids are Dronabinol (Marinol®, Syndros®) and nabilone (Cesamet®), and are indicated for nausea and vomiting, and appetite stimulation, but are also often used off-label for pain management (Mack & Joy, 2000; Berlach et al., 2006). Synthetic cannabinoids have a high affinity for the CB1 receptor and prescription synthetic cannabinoids have purity and composition with therapeutic value (National Academies of Sciences, Engineering, and Medicine, 2017). The focus of this manuscript is oral CBD alone, with an aim of understanding the pharmacotherapeutics of CBD without the psychoactive properties of THC. While topical products of CBD are available in the marketplace, permeation rates and skin retention studies are only beginning (Casiraghi, Musazzi, Centin, Franzè, Minghetti, 2020). One may hear of Cannabidiolic Acid (CBDA) which is the raw, unheated CBD that has not progressed beyond animal research and not pertinent for the older adult at this time (Formato et al., 2020).
Pharmacotherapeutics
By understanding human age-related changes, guidance can be provided to clinicians on interindividual variabilities among older adults based on their coexisting co-morbidities and medications. The key features of absorption, volume of distribution, metabolism, elimination, and mechanism-of-action of CBD are discussed. Furthermore, older adults may be prescribed multiple medications so drug to drug interactions are reviewed. These aspects will highlight the importance of critically evaluating the risk and benefits of CBD in older adults.
Absorption.
Older adults tend to have slower drug absorption due to a reduced gastric acid, slowing of gastric emptying, decreased gastric blood flow, reduced absorption capacity of the small intestine, and in some older adults a decreased motility of the GI system (Dumic et al., 2019). This situation is potentiated with the lipophilic properties of oral CBD (i.e. “edibles”) which cause CBDs to precipitate in the GI tract, slowing the absorption rate and resulting in an estimated bioavailability of only about 6% (Millar et al., 2020). Moreover, CBD is highly lipid soluble and so accumulates in the subcutaneous body fat typical in older adults. This accumulation in body fat delays release and reduces bioavailability and the overall effect (Mechoulam et al., 2020). Efforts have been made to increase the bioavailability of oral ingestion by formulating CBD in oil or alcohol (Millar et al., 2020) and formulating for better water solubility. Alternatively, administering CBD with meals can improve bioavailability and lower interindividual variability (Birnbaum et al., 2019; Silmore et al., 2021),
Volume of distribution.
Age-related changes include decreased total-body water volume and lean body mass, and increased body fat. These changes increase the volume of distribution in lipid-soluble drugs, prolong half-life, and amplify the side effect profiles of any lipid-soluble medication, including CBD. Furthermore, lower levels of serum albumin, under periods of acute illness or malnutrition, can impact protein binding, creating higher levels of unbound drug. This is significant in CBD preparations intended for oral use, as 94% of the metabolites are protein bound. Theoretically, if older adults have reduced protein-binding capacity, then higher levels of unbound drug will be available in their system, creating a stronger pharmacologic effect. This could put older adults at risk for falls, sedation, and other central nervous system (CNS) effects. When there are compounds of THC/CBD unwanted CNS effects can occur such as dizziness, insomnia, confusion, hallucinations (Velayudhan et al., 2021; Johnson et al., 2013; Portenoy et al., 2012). Clinical decision-making considerations in the older adult would be to reduce the dose of CBD and lengthen the time between repeated doses (Landmark & Brandi, 2020).
Metabolism.
Metabolism transforms drugs to more water-soluble compounds or metabolites. Metabolism slows with aging due to reduced liver size and hepatic blood flow but can also be slowed by heart failure and drugs that induce or inhibit cytochrome P-450 (CYP450) enzymes. Age-related changes reduce the hepatic capacity of phase 1 metabolism in breaking down and converting CBD to metabolites, prolonging their clearance. Additionally, dose comparison studies on Epidiolex® showed a three-fold elevation of transaminase in 17% of patients on 20 mg/kg/day doses, compared to 1% dosed with 10 mg/kg/day (Greenwich Biosciences, 2018). Even while individualizing dose requirements, a decrease in dose was required in all of these conditions (Chesney et al., 2020).
Elimination.
Elimination occurs when the drug and its active and inactive metabolites are excreted primarily through the renal system, through passive glomerular filtration, active tubular secretion, and partial reabsorption. Older adults have reduced glomerular filtration rate which prolongs the clearance of many medications and can result in side effects or toxicity. Serum creatinine level is less accurate for predicting elimination of medications in the older adult therefore a creatinine clearance is used to predict toxicity for many medications including CBD (American Geriatric Society, 2019; McLachlan & Pont, 2012).
Mechanism of action.
CBD affects the endocannabinoid system through mechanisms distinct from those affected by THC. The endocannabinoid receptors are CB1 and CB2, and THC binds to CB1 and CB2 with higher affinity than CBD. Consequently, CBD has less psychoactive properties than THC (Peres et al., 2018). However, when CBD formulations also contain THC (for example, Sativex® has a one-to-one concentration of CBD and THC) CBD inhibits the reuptake of THC and the main endocannabinoid 2-arachidonylglycerol (2-AG). This is due to a negative allosteric modulation that allows CBD to bind to the CB receptor and change it so THC or 2-AG are less likely to bind. Thus, formulations combining THC and CDB give less psychoactive effects (Laprairie et al., 2015).
Drug to Drug Interactions
Understanding interactions between CBD and conventional medications is challenging due to lack of research regarding drug interactions and variability in labeling the CBD product ingredients. However, applying what is known about federally approved products in the U.S. (Epidiolex®) and Canada (Sativix®) can inform drug interactions. Metabolic inhibition and induction of CBD occurs through CYP450 isoform activity including 3A4 and 2C19, creating drug-to-drug interactions (DDIs).
CYP3A4.
Co-administration of CBD with CYP3A4 inhibitors, such as ketoconazole, loperamide, nefazodone, amiodarone, verapamil, cimetidine, eprepitant, imatinib, and protease inhibitors can increase bioavailability of CBD increasing risk for adverse effects, so a reduction of CBD dose is recommended (Chesney et al., 2020; Coggins, 2020; Brown, Winterstein, 2019). Co-administration of CBD with CYP3A4 inducers, such as enzalutamide, phenytoin, carbamazepine, topiramate, phenobarbital, rifampicin, efavirenz, or pioglitazone will decrease bioavailability of CBD resulting in decreased effectiveness of CBD so dose may need to be increased (Brown, Winterstein, 2019).
CYP2C19.
Co-administration of CBD with CYP2C19 inhibitors, such as fluvoxamine, fluoxetine, cimetidine, ketoconazole, fluconazole, efavirenz can increase bioavailability of CBD increasing risk for adverse effects, so a reduction of CBD dose is recommended (Chesney et al., 2020; Winterstein, 2019). Co-administration of CBD with CYP2C19 inducers, such as rifampin, carbamazepine, phenobarbital, phenytoin, and St. John’s Wort will decrease bioavailability of CBD resulting in decreased effectiveness of CBD so dose may need to be increased (Chesney et al., 2020; Brown, Einterstein, 2019). Of note, CBD is a known inhibitor of CYP2C19, an enzyme necessary to convert clopidogrel into its active thiol metabolite. Inhibition of this enzyme can therefore lead to subtherapeutic concentrations of the active form of clopidogrel.
Furthermore, if there are higher levels of THC than is known to the patient or the healthcare provider due to inaccurate labelling of CBD product, the likelihood of adverse effects is increased. Caution is warranted for susceptible older adults with complex medication protocols that could predispose them to drug-drug interactions.
Research of Cannabidiol Uses for Pain in the Older Adult
A recent systematic review of randomized controlled trials could not identify studies on the analgesic properties of CBD (Fisher et al., 2021) but CBD use was described and investigated in small studies treating rheumatic pain, cancer pain, fibromyalgia, neurogenic pain from multiple sclerosis, neuropathic pain, and non-specified chronic pain (Capano et al., 2020; Urits et al., 2020; Good et al., 2020; Ueberall et al., 2019; Van de Donk et al., 2019; Fitzcharles et al., 2016; Serpell et al., 2014; Portenoy et al., 2012; Johnson et al., 2010; Wade et al., 2003). The quality of evidence on safety and efficacy is weak, results are mixed, and few studies included the older adult (Levy et al., 2020; Kim et al., 2017). For example, two studies examined CBD alone for cancer-related pain and transplant pain, but reductions in pain were inconclusive (Good et al., 2020; Cuñetti et al., 2018). In contrast, in a study of 97 people with chronic pain who were on opioids at least one year, CBD use resulted in a significant reduction of opioid use, improvement of pain, greater enjoyment and general activity (PEG scale), an improved quality of life, but notably, their pain disability index did not improve (Capano et al., 2020). The mixed results of studies like these indicate a need for comprehensive investigations using randomized controlled trials on the safety and efficacy of CBD-only products.
Products containing both THC and CBD have been studied for a variety of pain conditions and also showed mixed results. In some studies, THC/CBD was demonstrated to be effective against pain from cancer, fibromyalgia, neuropathy, and non-specified chronic pain (Capano et al., 2020; Urits et al., 2020; Ueberall et al., 2019; Van de Donk et al., 2019; Portenoy et al., 2012; Johnson et al., 2010). Other studies, however, found THC/CBD did not significantly improve pain in rheumatic diseases, nociceptive pain, or peripheral neuropathy (Ueberall et al., 2019; Fitzcharles et al., 2016; Serpell et al., 2014). For example, nabiximols contain THC and CBD and licensed in Israel and Canada for neuropathic pain in multiple sclerosis. Studies gave conflicting evidence for nabiximols in improvement of pain in patients with chronic neuropathic or non-cancer pain, with no studies focusing on pain in older adults (Beedham et al., 2020). A Cochrane review recommended, based on small to moderate evidence of benefit, to consider THC/CBD as a 3rd or 4th tier treatment in chronic neuropathic pain syndromes, after anticonvulsant agents and antidepressant agents fail (Mücke et al., 2018).
Driving while using CBD and combined THC/CBD is a public-safety concern. In driving-impairment studies, differences were found in the effects from inhaled CBD-dominant, THC-dominant, and THC/CBD equivalents (Arkell et al., 2020). THC-dominant and THC/CBD equivalent cannabis produced significant impairment and lane deviations while driving 40 to 100 minutes following vaporization (p<0.001), but no statistically significant impairment and lane deviation findings for CBD only (Arkell et al., 2020). The population studied were young healthy adults and not older adults, however, it did highlight the differences in impairment effects of THC/CBD and THC compared with CBD only. Older adults operating heavy machinery while using CBD therapy, especially with higher doses, require clear and direct warning of increased risk of impairment and lane deviation that can result in accidents and fatalities (Arkell et al., 2020; Rubin, 2020).
All of these studies show evidence of a gap in knowledge concerning CBD and how it might affect the older adult. Levy et al. (2020) reported fewer than 250 older adults have been included in cannabis studies. The complexity of care in older adults with chronic pain cannot be ignored. Therefore, funding and implementing randomized CBD trials in the older adult population is necessary before CBD is deemed efficacious for pain treatment in the older adult (Fick et al., 2020).
Safety
Patient safety is a concern with CBD use. The U.S. FDA expressed uncertainty regarding the safety and quality of available CBD products (The Gerontological Society of America, 2021). Safety concerns include lack of patient information and healthcare clinicians’ advice available when products have thorough investigations and approval through the FDA. Without the usual safety monitors by the FDA and other regulating bodies, the risk of harm increases. For example, poison phone calls for CBD have increased from 3 in 2014 to 2,218 calls in 2020 (The Gerontological Society of America, 2021). Additionally, the focus of CBD studies have occurred with healthy volunteers and those with seizure activity or mood disorders with demonstrated safety of oral administration of 300 mg/day up to 1500 mg/day (Zuardi et al., 2010; Trembly & Sherman, 1990; Cunha et al., 1980). Higher dosages can be potentially harmful for the older adult; therefore direct applicability to older adults with cognitive and physiological challenges remain unknown (Sherman et al., 2018). The safety appraisals evaluated in the following paragraphs will be product variability, contraindications, and adverse effects.
Product Variability
Clinicians need to know how to inform and guide their older adult patients on the safe use of CBD (Highet et al., 2020; Manning & Bouchard, 2021). Among countries across the globe and between states in the US, laws concerning cannabis and CBD products vary widely (see Table 1) and product labelling is not reliable (Hawes et al., 2020). Two examples reveal the labelling discrepancies particularly well. One was found in the United Kingdom where 84 CBD products were tested and only 31% were accurately labeled for CBD content (within 10% of advertised content). Also, THC was detected in 18 of those samples with a mean level of 0.45%, above U.S. regulated THC level of 0.3% (Liebling et al. 2020). The other example is regarding CBD sold online in the U.S., where 26% contained less CBD than the label stated and THC was detected in up to 21.43% of the unlabeled products (Bonn-Miller et al., 2017).
Table 1.
CBD Legalization by State
| Categories of legalization | State where legalization occurred |
|---|---|
| Allow the sale of cannabis for medical and recreational purposes | Maine, Massachusetts, Michigan, Vermont, Washington DC, Alaska, California, Colorado, Nevada, Oregon, and Washington |
| Allow the sale of cannabis with or without THC for only medical purposes | Connecticut, Delaware, Florida, Illinois, Maryland, New Hampshire, New Jersey, New York, Ohio, Pennsylvania, Rhode Island, West Virginia, Arizona, Arkansas, Hawaii, Louisiana, Minnesota, Missouri, Montana, New Mexico, North Dakota, Oklahoma, Utah |
| Allow the sale of CBD for medical purposes only | Alabama, Georgia, Indiana, Mississippi, North Carolina, South Carolina, Tennessee, Virginia, Wisconsin, Iowa, Kansas, Kentucky, Texas, Wyoming |
| No legal form of CBD | Idaho, Nebraska, South Dakota |
For updated information on state cannabis product legalization status, please access the following link: https://disa.com/map-of-marijuana-legality-by-state.
Warning patients and their families that package labelling may be inaccurate is important for two reasons: mislabeled products may lack therapeutic value, and unintentionally administered THC might cause adverse effects, particularly in older adults (Fick et al., 2020; Bonn-Miller et al., 2017). The call to action for clinicians is to be cognizant of the contents of products they are recommending. Clinicians may need to contact the manufacturer to gather accurate information.
Contraindications.
Over-the-counter CBD products have little to no contraindications listed on the label. As a guide, a list of contraindications found on the Epidiolex® packet insert (Greenwich Biosciences, Inc., 2018) which is a CBD-only FDA-approved product, include hypersensitivity to cannabidiol and sesame seed oil, and suicidal ideation and behavior. Animal studies show Epidiolex® may cause fetal harm. Concomitant use with central nervous system depressants including alcohol and hepatotoxic agents must be with caution. Additionally, as with many other drugs, it is best not to abruptly stop taking CBD (Greenwich Biosciences, Inc., 2018). Prior to initiating CBD treatment evaluate hepatic function (e.g., ALT, AST, ALP, and total bilirubin) and monitor these lab tests at intervals of 1 month, 3 months, and 6 months after the initiating dose. Studies have shown Epidiolex® can cause dose-related hepatic injury (Greenwich Biosciences, Inc., 2018). Patients with hepatic impairment will require lower dosages. Lung function should be considered with CBD therapy when inhalation is the chosen delivery method. While more studies on inhaled CBD are needed, one randomized control trial reported no clinically significant effects from inhaled cannabis in participants with advanced chronic obstructive pulmonary disease (COPD) (Abdallah et al., 2018). However, the American Lung Association warns of health risk for inhalation of cannabis for people with COPD (2020).
Adverse effects
CBD, THC/CBD compounds, and administering oral and inhaled routes were investigated for adverse effects. For oral CBD only, the most common adverse drug effects reported were drowsiness and symptoms of gastrointestinal upset, such as nausea, vomiting, diarrhea, and abdominal pain (Chesney et al., 2020; Good et al., 2020). Dizziness was not reported in studies of CBD only (Chesney et al., 2020; Good et al., 2020); however, in studies using a combination of THC/CBD, dizziness was reported along with drowsiness and gastrointestinal upset symptoms such as nausea, vomiting, diarrhea, and abdominal pain (Cuñetti et al., 2018; Langford et al., 2013; Portenoy et al., 2012; Johnson et al., 2013). Furthermore, many studies demonstrated an increase in appetite only when ingesting a combination of THC/CBD not CBD alone, suggesting THC not CBD might increase the appetite (Ueberall et al., 2019; Brunt & van Genugten et al., 2014; Wan et al., 2017).
Dizziness was an adverse effect with inhalation of CBD compounds, a concern in the older adult population who may be at risk for falls (Beedham et al., 2020; Abuhasira et al., 2018). Van de Donk et al. (2019) prepared four different concentrations of THC/CBD and adverse effects were coughing, drug high, bad taste, headache, dizziness, sore throat, nausea, vomiting, as well as sleepiness at concentrations of 9% CBD and less than 1% THC. Thus, adverse effects should be considered when patients are using inhaled or orally delivered CBD or THC/CBD.
Recommendations for Clinicians
Clinicians have a crucial role as a resource to patients who want information about using CBD, whether it is prescribed or not (see Table 1). Older adults may use CBD to help manage their chronic conditions and tend to prefer oral ingestion over other routes of administration (Haug et al., 2017).
Key clinical components for clinicians to consider are:
1) Consider the role of CBD in the overall pain-treatment plan and interactions with other treatment options. Terminology is confusing to patients and clinicians alike. Understanding whether patient questions concern CBD or THC/CBD compounds can help the clinician to incorporate appropriate pharmacotherapeutics language into the discussion. Awareness of the influence marketing has on the patient is important when CBD products are directly targeted to the consumer, since the patient’s perception of why they are using CBD for pain may be distorted or over-sold. Identify preconceived notions of cannabis products and explore expectations the patient may have about CBD and their condition (Highet et al., 2020). If quality of life is decreased, or first- and second-tier medication options have been ineffective, clinicians could consider CBD for pain management (Croker et al., 2021).
2) Educate patients about the use, risks, anticipated effects and adverse effects, and dosing of CBD for pain management (see Table 2). Discuss the risk for adverse drug effects, such as hepatotoxicity and the need to use lower doses due to accumulation and prolonged effect. Prior to initiating CBD, determine the drug-to-drug interactions with the patient’s current medications, especially medications that depress the central nervous system. Determine when patients are taking CBD with respect to meals, since food may impact CBD levels depending on the route of admission. Patients can be instructed to take CBD with meals for improved bioavailability and a stable dose response.
Table 2.
Patient education key points
| Avoid central nervous system depressants and alcohol while on CBD therapy to avoid excess sedative effects |
| Tell your doctor about any other medications or supplements you are taking as they may interact with CBD |
| Your healthcare provider may need to run various lab tests (blood and urine) while you are on CBD therapy. Providers can monitor these labs to prevent any toxicity due to age related metabolism and excretion changes. |
| Patients initiating CBD therapy should avoid activities that require mental acuity until the effects of CBD are experienced, e.g., driving, operating heavy machinery, legal discussions |
| Understand that the route of administration matters. For example, the onset of action after inhalation will be quick in comparison to oral delivery |
3) Discuss safety issues or concerns, since accuracy of product labeling of CBDs is problematic. A phone call to the product manufacturer may be required to clarify the ingredients. Counsel the patient on the lack of uniformity of product content, as this may impact their response and adverse effects. Regulations from the states are not consistent, and policies on CBD and THC/CBD compounds need further revision. Keep in mind, studies show that lower doses of THC/CBD were generally better tolerated than higher doses (Johnson et al., 2010; Portenoy et al., 2012). Finally, understand the legality of CBD use in your state, as states vary on the kind of access available to the public or clinicians.
4) Monitor the older adult for potential adverse effects from CBD. Screen initial hepatic function prior to initiating the dose, as CBD use may elevate transaminase and is potentially hepatotoxic. Effectiveness in pain management for older adults requires repeated evaluation. Creatinine clearance is valuable to determine if prolonged elimination of CBD will create issues. Determine if other medications may become toxic when used concurrently with CBD. Until further studies are conducted on CBD in the older adult populations, this content may help ensure patient safety in the older adult population hoping to treat their pain with CBD.
Conclusion
Legalization of CBD has increased the popularity of the product among older adults; however, research on its benefits and adverse side effects in this population is in its infancy. Interest in CBD use for pain management has been bolstered by the challenges of managing pain in older adults with minimal potential for adverse effects, but healthcare providers who are asked for guidance by older patients may have limited education related to CBD. Moreover, key information is unknown. More research would inform our understanding of CBD and its action and effects in the older adult population.
Evidence from studies in adults suggests THC/CBD combinations provide mild to moderate alleviation of pain in a variety of conditions, e.g., cancer related pain, fibromyalgia, non-specified chronic pain, and neuropathic pain. CBD has minimal psychoactive effects and therefore may be preferred in the older adult. In these instances, dosing adjustments might be needed due slower metabolism and excretion in the older patient. Future research is likely to reveal further that CBD is a relatively safe alternative to opioids and other analgesic agents for pain management in older adults.
Future Research
Throughout this manuscript, gaps in knowledge and labelling regulations are noted (see Table 3). Funding of additional research is crucial in this area as legalization of CBD continues to grow across the United States. Currently, only limited information is available regarding CBD use in the older adult, including safety and efficacy.
Table 3.
Call to action
| Safety and efficacy trials on CBD use of pain management are needed for older adult and ethnic and racial minority populations. |
| Package labelling of CBD products needs to be accurate and enforced across the nation. |
| Uniformity in CBD ingredients and dosing across states. |
| Clinicians must be cognizant of the contents of the products they recommend. |
Going forward, the work needs to address several key issues. Racial and ethnic minority populations must be included in any pain management studies to reduce health disparities in research, and these older adult and ethnic and racial minority populations require over-recruitment. Community advisory boards for studies have been influential in setting effective recruitment procedures that can also be used for CBD research (Manning & Bouchard, 2021). Finally, product labelling needs to be studied and evidence-based regulation should be put in place, nationally, to protect the public and older adults who use CBD. Funding for a research agenda in this area is a priority.
Contributor Information
Brooke Porter, University of Iowa, College of Nursing.
Barbara St. Marie, University of Iowa, College of Nursing.
Gary Milavetz, University of Iowa, College of Pharmacy.
Keela Herr, University of Iowa, College of Nursing.
References
- Abdallah SJ, Smith BM, Ware MA, Moore M, Li PZ, Bourbeau J, & Jensen D (2018). Effect of Vaporized Cannabis on Exertional Breathlessness and Exercise Endurance in Advanced Chronic Obstructive Pulmonary Disease. A Randomized Controlled Trial. Annals of the American Thoracic Society, 15(10), 1146–1158. 10.1513/AnnalsATS.201803-198OC [DOI] [PubMed] [Google Scholar]
- Abuhasira R, Schleider LB, Mechoulam R, & Novack V (2018). Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. European Journal of Internal Medicine, 49, 44–50. 10.1016/j.ejim.2018.01.019 [DOI] [PubMed] [Google Scholar]
- American Geriatric Society 2019 Beers Criteria Update Expert Panel (2019). American Geriatrics Society 2019 Updated Beers Criteria for potentially inappropriate medication use in older adults. Journal of American Geriatric Society, 67, 674–694. [DOI] [PubMed] [Google Scholar]
- American Lung Association (2020). Marijuana and lung health. Accessed from: https://www.lung.org/quit-smoking/smoking-facts/health-effects/marijuana-and-lung-health
- Arkell TR, Vinckenbosch F, Kevin RC, Theunissen EL, McGregor IS, & Ramaekers JG (2020). Effect of Cannabidiol and Δ9-Tetrahydrocannabinol on Driving Performance: A Randomized Clinical Trial. JAMA, 324(21), 2177–2186. 10.1001/jama.2020.21218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beedham W, Sbai M, Allison I, Coary R, & Shipway D (2020). Cannabinoids in the Older Person: A Literature Review. Geriatrics (Basel, Switzerland), 5(1), 2. 10.3390/geriatrics5010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlach DM, Shir Y, Ware MA (2006). Experience with the synthetic cannabinoid nabilone in chronic noncancer pain. Pain Medicine, 7(1), 25–29. 10.1111/j.1526-4637.2006.00085.x [DOI] [PubMed] [Google Scholar]
- Birnhaum AK, Karanam A, Marino SE, Barkley CM, Remmel RP, Roslawski M, Gramling-Aden M, Leppik IE (2019). Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia, 60(8), 1586; doi: 10.1111/epi.16093 [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R (2017). Labeling accuracy of cannabidiol extracts sold online. JAMA, 318(17), 1708–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenan M (2019). 14% of Americans say they used CBD products. Gallup World Headquarters, 901 F Street, Washington, D.C. Accessed from Gallup https://news.gallup.com/poll/263147/americans-say-cbd-products.aspx [Google Scholar]
- Brunt TM, van Genugten M, Höner-Snoeken K, van de Velde MJ, & Niesink RJ (2014). Therapeutic satisfaction and subjective effects of different strains of pharmaceutical-grade cannabis. J Clin Psychopharmacol, 34(3), 344–349. 10.1097/jcp.0000000000000129 [DOI] [PubMed] [Google Scholar]
- Capano A, Weaver R, & Burkman E (2020). Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: a prospective cohort study. Postgrad Med, 132(1), 56–61. 10.1080/00325481.2019.1685298 [DOI] [PubMed] [Google Scholar]
- Casiraghi A, Musazzi UM, Centin G, Franzè S, Minghetti P (2020). Topical administration of cannabidiol: influence of vehicle-related aspects on skin permeation process. Pharmaceuticals, 13, 337–349. Doi: 10.3390/ph13110337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC; 2021). Health Studies – Understanding Chemical and Radiation Exposures. Access from https://www.cdc.gov/nceh/hsb/chemicals/sc/About.html
- Chesney E, Oliver D, Green A, Sovi S, Wilson J, Englund A, Freeman TP, & McGuire P (2020). Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology, 45(11), 1799–1806. 10.1038/s41386-020-0667-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker JA 3rd, Bobitt JL, Arora K, & Kaskie B (2021). Assessing Health-Related Outcomes of Medical Cannabis Use among Older Persons: Findings from Colorado and Illinois. Clin Gerontol, 44(1), 66–79. 10.1080/07317115.2020.1797971 [DOI] [PubMed] [Google Scholar]
- Cuñetti L, Manzo L, Peyraube R, Arnaiz J, Curi L, & Orihuela S (2018). Chronic Pain Treatment With Cannabidiol in Kidney Transplant Patients in Uruguay. Transplantation Proceedings, 50(2), 461–464. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, & Mechoulam R (1980). Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology, 21(3), 175–185. 10.1159/000137430 [DOI] [PubMed] [Google Scholar]
- Dill JL, & Kurkowski A (2020). CBD: Considerations for Use Within the Health System. Hospital Pharmacy, 55(1), 9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, Lichtman AH (2018). The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology Reviews, 43, 52–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R (2016). CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA, 315(15),1624–1645. Doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumic I, Nordin T, Jecmenica M, Lalosevic MS, Milosavljevic T, Milovanovic T (2019). Gastrointestinal tract disorders in older age. Canadian Journal of Gastroenterology and Hepatology, Article ID 6757524; 10.115/2019/6757524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick DM (2021). Evaluating the Safety of Cannabinoid-Based Medicines for Older Adults. JAMA network open, 4(2), e2035952. 10.1001/jamanetworkopen.2020.35952 [DOI] [PubMed] [Google Scholar]
- Fisher E, Moore RA, Fogarty AE, Finn DP, Finnerup NB, Gilron I, Haroutounian S, Krane E, Rice A, Rowbotham M, Wallace M, & Eccleston C (2020). Cannabinoids, cannabis, and cannabis-based medicine for pain management: a systematic review of randomised controlled trials. Pain, 10.1097/j.pain.0000000000001929. [DOI] [PubMed] [Google Scholar]
- Fitzcharles MA, Baerwald C, Ablin J, & Häuser W (2016). Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): A systematic review of randomized controlled trials. Schmerz, 30(1), 47–61. [DOI] [PubMed] [Google Scholar]
- Formato M, Crescente G, Scognamiglio M, Fiorentino A, Pecoraro MT, Piccolella S, Catauro M, Pacifico S (2020). Molecules,25(11), 2638–2662. DOI: 10.3390/molecules25112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazendam A, Nucci N, Gouveia K, Khalik HA, Rubinger L, Johal H (2020). Cannabinoids in the management of acute pain: a systematic review and meta-analysis. Cannabis and Cannabinoid Research, 5(4), 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good PD, Greer RM, Huggett GE, & Hardy JR (2020). An Open-Label Pilot Study Testing the Feasibility of Assessing Total Symptom Burden in Trials of Cannabinoid Medications in Palliative Care. J Palliat Med, 23(5), 650–655. 10.1089/jpm.2019.0540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwich Biosciences, Inc. (2018). Epidiolex® Reference ID: 4282447. Carlsbad, CA 92008.
- Haug NA, Padula CB, Sottile JE, Vandrey R, Heinz AJ, & Bonn-Miller MO (2017). Cannabis use patterns and motives: A comparison of younger, middle-aged, and older medical cannabis dispensary patients. Addict Behav, 72, 14–20. 10.1016/j.addbeh.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes EM, Lee CR, Brackney DE, Ensley TG, Kidd J, & Page C (2020). Cannabidiol Products: Review of the Regulatory and Clinical Considerations. Journal for Nurse Practitioners, 16(10), 747–755. 10.1016/j.nurpra.2020.07.022 [DOI] [Google Scholar]
- Highet BH, Lesser ER, Johnson PW, & Kaur JS (2020). Tetrahydrocannabinol and Cannabidiol Use in an Outpatient Palliative Medicine Population. American Journal of Hospice & Palliative Care, 37(8), 589–593. 10.1177/1049909119900378 [DOI] [PubMed] [Google Scholar]
- Hilderbrand RL (2018). Hemp & Cannabidiol: What is a medicine? Missouri Medicine, 115(4), 306–309. [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Chen J, Furnish T, & Wallace M (2015). Medical Marijuana and Chronic Pain: a Review of Basic Science and Clinical Evidence. Current Pain and Headache Reports, 19(10), 50. 10.1007/s11916-015-0524-x [DOI] [PubMed] [Google Scholar]
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, & Fallon MT (2010). Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. Journal of Pain Symptom Management, 39(2), 167–179. 10.1016/j.jpainsymman.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Johnson JR, Lossignol D, Burnell-Nugent M, & Fallon MT (2013). An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. Journal of Pain Symptom Management, 46(2), 207–218. 10.1016/j.jpainsymman.2012.07.014 [DOI] [PubMed] [Google Scholar]
- Kim J, Grobelna A (2017). Nabilone for chronic pain management: a review of clinical effectiveness and guidelines. Ottawa: CADTH. [PubMed] [Google Scholar]
- Landmark CJ, Brandi U (2020). Pharmacology and drug interactions of cannabinoids. Epileptic Disorders,22(Suppl 2), S16–S22. [DOI] [PubMed] [Google Scholar]
- Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, & Ratcliffe S (2013). A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. Journal of Neurology, 260(4), 984–997. 10.1007/s00415-012-6739-4 [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM (2015). Cannabidiol is a negative allosterid moculator of the cannabinoid CB1 receptor. British Journal of Pharmacology, 172, 4790–4805; doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C, & Shahinas J (2020). Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials. Journal of clinical medicine research, 12(3), 129–141. 10.14740/jocmr4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Grovey B, Furnish T, & Wallace M (2018). Medical Cannabis for Neuropathic Pain. Current Pain and Headache Reports, 22(1), 8. 10.1007/s11916-018-0658-8 [DOI] [PubMed] [Google Scholar]
- Levy C, Galenbeck E, & Magid K (2020). Cannabis for Symptom Management in Older Adults. The Medical Clinics of North America, 104(3), 471–489. 10.1016/j.mcna.2020.01.004 [DOI] [PubMed] [Google Scholar]
- Liebling JP, Clarkson NJ, Gibbs BW, Yates AS, O’Sullivan SE (2020). An analysis of over-the-counter cannabidiol products in the United Kingdom. Cannabis and Cannabinoid Research, 10.1089/can.2019.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack A, Joy J (2000). Marijuana as Medicine? The Science Beyond the Controversy. Washington (DC): National Academies Press (US). [PubMed] [Google Scholar]
- Manning L, & Bouchard L (2021). Medical Cannabis Use: Exploring the Perceptions and Experiences of Older Adults with Chronic Conditions. Clinical Gerontologist, 44(1), 32–41. 10.1080/07317115.2020.1853299 [DOI] [PubMed] [Google Scholar]
- Manzanares J, Julian MD, Carrascosa A (2006). Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Current Neuropharmacology, 4(3), 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastinu A, Ribaudo G, Ongaro A, Bonini SA, Memo M, & Gianoncelli A (2021). Critical Review on the Chemical Aspects of Cannabidiol (CBD) and Harmonization of Computational Bioactivity Data. Current Medicinal Chemistry, 28(2), 213–237. 10.2174/0929867327666200210144847 [DOI] [PubMed] [Google Scholar]
- McLachland AJ, Pont LG (2012). Drug metabolism in older people – a key consideration in achieving optimal outcomes with medicines. Journal of Gerontology, 67A(2), 175–180. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, & Gallily R (2002). Cannabidiol: an overview of some pharmacological aspects. Journal of Clinical Pharmacology, 42(S1), 11s–19s. 10.1002/j.1552-4604.2002.tb05998.x [DOI] [PubMed] [Google Scholar]
- Meissner H, Cascella M (2020). Cannabidiol (CBD). Treasure Island (FL) StatPearls Publishing. Access from https://www.ncbi.nlm.nih.gov/books/NBK556048/ [PubMed] [Google Scholar]
- Millar SA, Maguire RF, Yates AS, O’Sullivan SE (2020). Towards better delivery of cannabidiol (CBD). Pharmaceuticals, 13, 219; doi: 10.3390/ph13090219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mücke M, Phillips T, Radbruch L, Petzke F, & Häuser W (2018). Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews, 3(3), Cd012182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (2017). The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. Washington, DC: The National Academies Press. doi: 10.17226/24625. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, McQuade R, Wright S, & Fallon MT (2012). Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain, 13(5), 438–449. 10.1016/j.jpain.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Raman M, Middleton RJ, Kalra PA, Green D (2017). Estimating renal function in old people: an in-depth review. International Urology and Nephrology, 49(11), 1979–1988. doi: 10.1007/s11255-017-1682-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie CS, Barrett SB, Thompson N, Miaskowski C (2020). Unintended consequences of opioid regulations in older adults with multiple chronic conditions. The Gerontologist, 60(7), 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R (2020). Driving Under the Influence of CBD or THC—Is There a Difference? Jama, 324(21), 2144–2145. 10.1001/jama.2020.22181 [DOI] [PubMed] [Google Scholar]
- Sativex® Product Monograph. Product Monograph Including Patient Medication Information. Accessed from https://omr.bayer.ca/omr/online/sativex-pm-en.pdf
- Schieber LZ, Guy GP, Seth P, Young R, Mattson CL, Mikosz CA, Schieber RA (2019). Trends and patterns of geographic variation in opioid prescribing practices by state, United States, 2006–2017. JAMA Network Open,2,(3), e190665. Doi: 10.1001/jamanetworkopen.2019.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, & Ehler E (2014). A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. European Journal of Pain, 18(7), 999–1012. 10.1002/j.1532-2149.2013.00445.x [DOI] [PubMed] [Google Scholar]
- Sherman C, Ruthirakuhan M, Vieira D, Lanctôt KL, & Herrmann N (2018). Cannabinoids for the treatment of neuropsychiatric symptoms, pain and weight loss in dementia. Current Opinion in Psychiatry, 31(2), 140–146. 10.1097/yco.0000000000000399 [DOI] [PubMed] [Google Scholar]
- Silmore LH, Willmer AR, Capparelli EV, Rosania GR (2021). Food effects on the formulation, dosing, and administration of cannabidiol (CBD) in humans: a systematic review of clinical studies. Pharmacotherapy, 41(4), 405–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L (2018). Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain, 159, 1932–1954. [DOI] [PubMed] [Google Scholar]
- The Gerontological Society of America (2021). Medical Use of Cannabidiol (CBD) in Older Adults: a focused discussion on safety. Summary of Proceedings from The Gerontological Society of America. [Google Scholar]
- Trembly B, Sherman M (1990). Double-blind clinical study of cannabidiol as a secondary anticonvulsant. Paper presented at Marijuana ‘90 International Conference on Cannabis and Cannabinoids, Kolympari (Crete); July 8–11. [Google Scholar]
- Ueberall MA, Essner U, & Mueller-Schwefe GH (2019). Effectiveness and tolerability of THC:CBD oromucosal spray as add-on measure in patients with severe chronic pain: analysis of 12-week open-label real-world data provided by the German Pain e-Registry. Journal of Pain Research, 12, 1577–1604. 10.2147/jpr.S192174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urits I, Gress K, Charipova K, Habib K, Lee D, Lee C, … Viswanath O (2020). Use of cannabidiol (CBD) for the treatment of chronic pain. Best Practice & Research: Clinical Anaesthesiology, 34(3), 463–477. [DOI] [PubMed] [Google Scholar]
- van de Donk T, Niesters M, Kowal MA, Olofsen E, Dahan A, & van Velzen M (2019). An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain, 160(4), 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDolah HJ, Bauer BA, & Mauck KF (2019, September). Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin Proc, 94(9), 1840–1851. 10.1016/j.mayocp.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Velayudhan L, McGoohan KL, & Bhattacharyya S (2021). Evaluation of THC-Related Neuropsychiatric Symptoms Among Adults Aged 50 Years and Older: A Systematic Review and Metaregression Analysis. JAMA network open, 4(2), e2035913. 10.1001/jamanetworkopen.2020.35913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas MB, LeBaron VT, Gilson AM (2018). The use of cannabis in response to the opioid crisis: a review of the literature. Nursing Outlook, 66(1), 56–65. Doi: 10.1016/j.outlook.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Wade DT, Robson P, House H, Makela P, & Aram J (2003). A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clinical Rehabilitation, 17(1), 21–29. [DOI] [PubMed] [Google Scholar]
- Wan BA, Diaz P, Blake A, Chan S, Wolt A, Zaki P, … O’Hearn S (2017). Efficacy of different varieties of medical cannabis in relieving symptoms. Journal of Pain Management, 10(4), 375–383. [Google Scholar]
- Wong SSC, Chan WS, Cheung CW (2020). Analgesic effects of cannabinoids for chronic non-cancer pain: a systematic review and meta-analysis with meta-regression. Journal of Neuroimmune Pharmacology, 15,801–829. [DOI] [PubMed] [Google Scholar]
- Zuardi A, Crippa J, Dursun S, Morais S, Vilela J, Sanches R, & Hallak J (2010). Cannabidiol was ineffective for manic episode of bipolar affective disorder. Journal of Psychopharmacology, 24(1), 135–137. 10.1177/0269881108096521 [DOI] [PubMed] [Google Scholar]


