Abstract
Background:
In some time-to-event analyses, it is unclear whether loss to follow-up should be treated as a censoring event or a competing event. Such ambiguity is particularly common in HIV research that uses routinely collected clinical data to report the timing of key milestones along the HIV care continuum. In this setting, loss to follow-up may be viewed as a censoring event, under the assumption that patients who are “lost” from a study clinic immediately enroll in care elsewhere, or a competing event, under the assumption that people “lost” are out of care all together.
Methods:
We illustrate an approach to address this ambiguity when estimating the 2-year risk of antiretroviral treatment initiation among 19,506 people living with HIV who enrolled in the IeDEA Central Africa cohort between 2006 and 2017, along with published estimates from tracing studies in Africa. We also assessed the finite sample properties of the proposed approach using simulation experiments.
Results:
The estimated 2-year risk of treatment initiation was 69% if patients were censored at loss to follow-up or 59% if losses to follow-up were treated as competing events. Using the proposed approach, we estimated that the 2-year risk of ART initiation was 62% (95% confidence interval: 61, 62). The proposed approach had little bias and appropriate confidence interval coverage under scenarios examined in the simulation experiments.
Conclusions:
The proposed approach relaxes the assumptions inherent in treating loss to follow-up as a censoring or competing event in clinical HIV cohort studies.
Keywords: Survival analysis, misclassification, HIV, antiretroviral therapy
Introduction
Early HIV diagnosis and linkage to care, rapid antiretroviral therapy (ART) initiation, and sustained viral suppression improve survival among people living with HIV and prevent onward transmission to their HIV-uninfected partners 1,2. Along the entire HIV care and treatment continuum, routinely collected clinical data (such as data from electronic health records) offer the potential to estimate the timing and persistence of key milestones, including initiation of ART and viral suppression. However, clinical data from routine service delivery settings are plagued by loss to follow-up. In contrast to interval cohort studies, where loss to follow-up simply masks observation of the outcome of interest, loss to follow-up in clinical cohort studies is a mixture of true loss to clinical care and loss to observation in that cohort. 3
Loss to clinical care is important because it can itself affect important health outcomes. For example, a patient with newly diagnosed HIV who has been lost early from clinical care may have no opportunity to initiate ART for the period that he is out of care. On the other hand, loss to observation from a specific clinical cohort does not always imply being lost from clinical care altogether. Patients may be lost to follow-up at a specific facility yet remain in clinical care by transferring to another facility. When such “transfers out” are not captured by records maintained at the first facility, this phenomenon is known as a “silent transfer.” In this scenario, patients may continue to experience outcomes along a continuum of care after being lost to follow-up in a specific clinical cohort 4. Such silent transfers are common, particularly in settings with decentralized health care systems and mobile populations, and may occur prior to treatment initiation.5 When monitoring the timing of treatment initiation using records from the first facility alone, silent transfers can lead to erroneous conclusions regarding the probability of treatment initiation over time.
The disposition of patients lost to follow-up in clinical cohorts has important implications for data analysis. For example, when estimating the cumulative incidence of ART initiation after entry into care, some patients are likely to become lost to follow-up at a study site before initiating ART. Traditional analyses of these data would proceed in one of two ways: 1) censor patients at loss to follow-up, which would yield a valid estimate of the cumulative incidence of ART initiation under the assumption that patients who are lost to follow-up at this facility initiate ART (presumably at a different facility) with the same probability at each time point as participants remaining in the study 6; or 2) treat loss to follow-up as a competing event that precludes ART initiation, which yields a valid estimate under the assumption that patients who are lost to follow-up from this facility are permanently lost to clinical care and do not initiate ART before the end of the study period.
Neither assumption is likely to hold for all patients. It is likely that some patients who are lost to follow-up at a specific facility are re-engaged in care elsewhere while others are truly out of care or have died. If these outcomes were known, patients who were lost to follow-up at a study site but engaged in care elsewhere would be censored, and death and complete disengagement from care would be treated as competing events. However, the disposition of patients lost to follow-up is usually unknown. Tracing studies have been conducted to understand what happens to these patients7,8. These studies provide estimates of the proportions of patients classified as lost to follow-up who have engaged in care elsewhere or died that can be used to adjust our analyses to account for the ambiguity in patient outcomes.
Here, we present an approach to use information from tracing studies or expert knowledge to account for what happens after loss to follow-up when estimating the cumulative incidence of key milestones along the HIV care continuum. We illustrate this approach to estimate cumulative incidence of ART initiation over time among eligible patients in the International epidemiology Databases to Evaluate AIDS (IeDEA) Central Africa cohort, and we evaluate the finite sample properties of the proposed approach using simulation experiments.
METHODS
Motivating example
The parameter of interest in the motivating example is the risk 9, or cumulative incidence, of ART initiation over 2 years since entry into HIV care among patients who enrolled in care at one of 15 clinical care sites in Central Africa. Moreover, we compare the timing of ART initiation between participants enrolling in three calendar time periods roughly corresponding to three treatment eras: 1) 2006 – 2009 (when WHO guidelines recommended treatment initiation when CD4<200); 2) 2010 – 2015 (when WHO guidelines recommended treatment initiation when CD4<350); 3) 2016 – 2017 (when WHO guidelines recommended immediate treatment). During the first 2 years in care, many patients become lost to follow-up at the study sites before they have the opportunity to start ART, which may impede our ability to estimate the probability of ART initiation. We applied the proposed approach to account for the fact that it was unclear if these patients should be censored, under the assumption that they enrolled in care elsewhere, or if these losses to follow-up should be treated as competing events, under the assumption that these patients were permanently out of care or had died.
Study sample
The Central Africa IeDEA cohort has been described in detail elsewhere 10,11. Briefly, the Central Africa IeDEA cohort is a multi-country collaboration that compiles clinical data from patients receiving HIV care and treatment at participating health facilities in the Central African region. We included 19,506 participants living with HIV who 1) had a first documented clinic visit in a participating facility in Rwanda, Democratic Republic of Congo, or Burundi between 1 January 2006 and 31 December 2017; 2) had at least one follow-up clinic visit or laboratory test in the 6 months after enrollment (to restrict to those truly enrolled in care); and 3) were not known to have started ART prior to their first visit at a participating facility.
Outcome definitions
Patients were followed from entry into HIV care at a study site until documented ART initiation, death, transfer to another facility, loss to follow-up, or administrative censoring at 2 years after enrollment or on 31 December 2017. ART initiation was defined as documentation of having been prescribed a regimen of three or more antiretroviral drugs, and patients were considered to have died if there was a documented death in the clinic database; information on death was collected by clinic staff. Patients were considered lost to follow-up on their last visit date prior to a 6 month-gap in documented clinic visits or laboratory records 12–14 and were not allowed to re-enter the cohort for this analysis.
Approach
In settings with no loss to follow-up, one may estimate the risk of ART initiation using the Aalen–Johansen estimator 15. The Aalen–Johansen estimator is used rather than the Kaplan–Meier estimator to account for the unavoidable competing event of death and allow loss to clinical care to be treated as a competing event, when appropriate. When loss to follow-up is present, the analyst has traditionally been faced with 2 options: 1) to censor patients at loss to follow-up under an assumption that the hazard of starting ART at time t is identical for patients lost to follow-up and those under observation; or 2) to treat loss to follow-up as a competing event under the assumption that patients have zero probability of initiating ART after loss to follow-up.
In time-to-event analyses, the decision to treat a specific endpoint as a censoring event or a competing event can have a dramatic impact on results. In particular, when calculating risk using the Kaplan–Meier or Aalen–Johansen estimators 15, censored individuals’ person–mass is redistributed onto persons who continue under follow-up, such that events that occur after a participant is censored carry more ‘weight’ than events before that participant was censored, reflecting the implicit assumption that censored participants experienced the same hazard of the event of interest as participants remaining under observation 16. This assumption is often labeled “no informative censoring” 17,18. In contrast, when a participant experiences a competing event, his unobserved event is not redistributed to future event times, under the implicit assumption that a competing event precludes the event of interest from occurring 19. Accordingly, if any participants are lost to follow-up, censoring them at loss to follow-up will yield a higher risk of an outcome of interest than treating loss to follow-up as a competing event 20.
We first estimate the risk of ART initiation under the two standard approaches to illustrate a likely best case scenario for ART initiation, in which patients are censored at loss to follow-up under the assumption that they are in care elsewhere and have the same probability of ART initiation as people remaining in the study, and worst case scenario, in which we treat loss to follow-up as a competing event that precludes ART initiation.
Next, we accounted for loss to follow-up using tracing weights according to the following algorithm implemented in a dataset with one record per person:
For participants not lost to follow-up (i.e., those who had documented ART initiation, death, or transfer, or were administratively censored prior to [or without ever] meeting the definition of loss to follow-up or experiencing any of the above events): Assign a weight of 1.
- For participants lost to follow-up:
- Create a second, duplicate, record.
- Censor the first record at loss to follow-up and assign a weight of ρi, where ρi is the probability that the patient is enrolled in care elsewhere.
- Treat the second record as though the patient experienced a competing event at the time of loss to follow-up and assign a weight of 1-ρi.
Apply desired estimator in the expanded and weighted data.
Under each approach (i.e., censoring at loss to follow-up, treating loss to follow-up as a competing event, and accounting for loss to follow-up using the proposed approach), we report the risk functions for ART initiation, the risk of ART initiation at 2 years after care enrollment, and subdistribution hazard ratios comparing ART initiation over the three calendar time periods. Risks were estimated by applying a weighted Aalen–Johansen estimator in the extended dataset outlined above, and hazard ratios were estimated using a weighted subdistribution Cox proportional hazards model21 in the extended dataset. Details on the statistical approach can be found in eAppendix 1, sample SAS and R code to implement the approach can be found in eAppendix 2, and an R package to implement the proposed approach can be found at https://github.com/edwardsjk/mccc.
Using external information about ρi
The true probability that a patient is engaged in care at another clinical site, ρi, is unknown and must be estimated. While, ideally, one would conduct a tracing study in the population of interest by ascertaining the status of a random sample of patients lost to follow-up at the study sites, often additional data collection at a study site is not feasible. In this setting, one may inform the proposed approach using information from tracing studies conducted in similar settings. For example, tracing studies were conducted among patients in ART programs lost to follow-up at types of health facilities similar to those in our study in East Africa, western Africa, and southern Africa. These studies are summarized in a systematic review by Zurcher et al. 22 and several other recent publications 23,24. A subset of these studies reported both the number of patients successfully traced and the number found to be enrolled in care elsewhere (i.e., undocumented, or silent, transfers). This proportion ranged from 2% to 54%. Because ART programs were rapidly expanding during the study time period, calendar year was likely an important predictor of whether a patient lost from a facility included in the study enrolled in care elsewhere. To account for this trend in the proportion of silent transfers, we modeled this proportion as a function of calendar year when patient tracing occurred and used the results to predict the proportion of patients lost from study sites who had silently transferred at the time that each patient in the main study (i.e., our dataset) was lost to follow-up. We let this predicted proportion stand in for the probability that a specific patient lost to follow-up was actually in care elsewhere, .
Specifically, we gathered published estimates into a dataset along with the year of data collection for each estimate. Then, we fit the logistic regression model
where ps was the proportion of patients who appeared lost in study s who were later found to be in care, and Zswas the year that study s was conducted (or the study midpoint). In the logistic model, each study s was weighted by the total number of patients successfully traced.
We next used estimates of to predict for hypothetical studies conducted at the time that each patient in the study was lost to follow-up. Under the assumption that individuals lost to follow-up in a given year in the study were exchangeable with participants in the tracing studies conducted in those years, was estimated as the predicted value
where Xi was the year in which patient i became lost from the study and . Because year was included in the models, all patients lost in the same year had the same estimated probability of being in care elsewhere.
In eAppendix 1, we show how ρi could be informed by prior knowledge rather than estimated from external data. We also conducted a sensitivity analysis (shown in eAppendix 4) in which we varied ρi for all participants from 0.02 to 0.54, to reflect results from tracing studies with the lowest and highest proportion of patients found to be in care elsewhere.
Incorporating uncertainty about ρi
Uncertainty in the final point estimates should reflect both sampling error in the main study and uncertainty about ρi. Uncertainty about ρi is represented in this analysis by the estimated standard errors around the predicted proportions in care. Standard errors for the risks and subdistribution hazard ratios were estimated as the standard deviations of the point estimates from 1000 bootstrap samples of the study data. To propagate uncertainty about ρi through the analysis, we set the probability that an individual was in care in each bootstrap sample k as , where was a random draw from the normal distribution
Simulations
We explored the finite sample properties of the proposed approach using a series of simulation experiments. Simulation experiments and results are described in eAppendix 3.
All analyses were conducted in R 3.6.0 and SAS 9.4.
This study was approved by Institutional Review Boards at the Albert Einstein College of Medicine and the University of North Carolina at Chapel Hill.
RESULTS
Example
Of the 19,506 participants, 63% were female and the majority (52%) were between the ages of 21 and 40 (Table 1). The rate of participant enrollment was similar across the three calendar time periods, resulting in similar numbers of participants enrolled in each of the first two periods and slightly under half that number enrolled in the third period. Of participants who became lost to follow-up before starting ART, over half entered care during the first period. In the second period, a greater proportion of new patients were, by definition, eligible for ART at the time of enrollment. By the third period, when guidelines stipulated early treatment, very few patients were lost prior to initiating ART. Participant characteristics by calendar period are described in eAppendix 5.
Table 1.
Demographic and clinical characteristics of 19,506 patients who entered HIV care between 1 January 2006 and 31 December 2017 at 15 Central Africa IeDEA clinical sites and were followed for ART initiation up to 2 years.
| Characteristics | Overall (N = 19,506) | Patients lost to follow-up (Nlost = 4387) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Male sex | 7257 | 37 | 1645 | 38 |
| Country of health facility | ||||
| Burundi | 4387 | 22 | 997 | 23 |
| Democratic Republic of Congo | 3268 | 17 | 293 | 7 |
| Rwanda | 11,851 | 61 | 3097 | 71 |
| Age at enrollment (in years) | ||||
| 10 or under | 1442 | 7 | 294 | 7 |
| 11 – 20 | 1196 | 6 | 283 | 5 |
| 21 – 30 | 5717 | 29 | 1485 | 34 |
| 31 – 40 | 6429 | 33 | 1380 | 31 |
| 41 – 50 | 3336 | 17 | 696 | 16 |
| 51+ | 1386 | 7 | 249 | 6 |
| Calendar year of enrollment | ||||
| 2006 – 2009 | 7929 | 41 | 2765 | 63 |
| 2010 – 2014 | 8325 | 43 | 1478 | 34 |
| 2015 – 2017 | 3252 | 17 | 144 | 3 |
ART: antiretroviral therapy
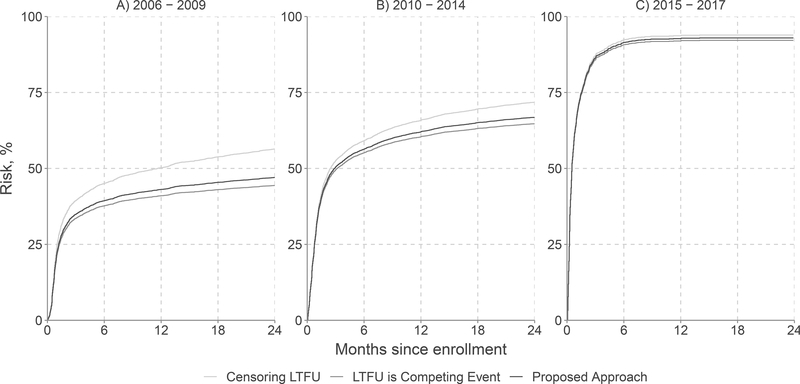
Overall, 63% of patients (n=12,253) started ART and 23% (n=4387) were lost to follow-up within 2 years after entry into care (Table 2). The proportion lost to follow-up prior to ART initiation was highest for enrollees from 2006 – 2009 (35%) and dropped to 4% by enrollees in the 2015 – 2017 period. Under an approach that censored participants at loss to follow-up, the overall 2-year risk of ART initiation was 69% (95% CI: 67.8, 69.3), ranging from 56% in the earliest calendar time period to 94% in the most recent period (Table 3). If losses to follow-up were treated as competing events, the overall 2-year risk of ART initiation was 59% (95% CI: 58.6, 60.0), ranging from 44% in the earliest period to 92% in the most recent period. Figure 1 presents the entire 2-year risk functions estimated using all three approaches in each period.
Table 2.
Outcomes at 2 years since entry into care among 19,506 patients who entered HIV care at one of 15 Central Africa IeDEA sites between 1 January 2006 and 31 December 2017
| Outcome | Overall n = 19,506 |
Patients entering care between 2006 – 2009 n = 7929 |
Patients entering care between 2010 – 2014 n = 8325 |
Patients entering care between 2015 – 2017 n = 3252 |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Started ART | 12,253 | 63 | 3942 | 50 | 5438 | 65 | 2873 | 88 |
| Recorded deaths a | 192 | 1.0 | 79 | 1.0 | 98 | 1.2 | 15 | 0.51 |
| Documented transfer to another facility | 88 | 0.54 | 50 | 0.63 | 34 | 0.43 | 4 | 0.12 |
| Lost to follow-up prior to ART | 4387 | 23 | 2765 | 35 | 1478 | 18 | 144 | 4.4 |
| Administratively censored | 2586 | 13 | 1093 | 14 | 1277 | 15 | 216 | 6.6 |
The reported number of deaths is known to underestimated, as there is likely misclassification between loss to follow-up prior to ART and death.
Table 3.
Risk of ART initiation by 2 years, and subdistribution hazard ratios comparing ART initiation between calendar time periods, among 19,506 patients who entered HIV care at one of 15 Central Africa IeDEA sites between 1 January 2006 and 31 December 2017
| Approach | 2-year risk, % | 95% CI | Subdistribution hazard ratio | 95% CI |
|---|---|---|---|---|
| Censoring at loss to follow-up | ||||
| Overall | 68 | 68, 69 | ||
| 2006 – 2009 | 56 | 55, 58 | 1 | |
| 2010 – 2014 | 72 | 71, 73 | 1.4 | 1.4, 1.5 |
| 2015 – 2017 | 94 | 93, 95 | 4.1 | 3.9, 4.4 |
| Treating loss to follow-up as a competing event | ||||
| Overall | 59 | 59, 60.0 | ||
| 2006 – 2009 | 44 | 43, 45 | 1 | |
| 2010 – 2014 | 65 | 64, 66 | 1.8 | 1.6, 1.7 |
| 2015 – 2017 | 92 | 91, 93 | 4.8 | 4.5, 5.1 |
| Accounting for loss to follow-up using the proposed approach | ||||
| Overall | 62 | 61, 62 | ||
| 2006 – 2009 | 47 | 46, 48 | 1 | |
| 2010 – 2014 | 67 | 66, 68 | 1.6 | 1.5, 1.7 |
| 2015 – 2017 | 93 | 92, 94 | 4.8 | 4.5, 5.1 |
CI: Confidence interval
Figure 1.
Risk of antiretroviral therapy initiation over 2 years since entry into care among 19,506 patients who entered HIV care at one of 15 Central Africa IeDEA sites between 1 January 2006 and 31 December 2017 after censoring patients lost to follow-up (LTFU; light grey curves), treating loss to follow-up as a competing event (dark grey curves), and accounting for loss to follow-up using the proposed approach (black curves)
Applying the individual values of , the estimated overall 2-year risk of ART initiation was 62% (95% CI: 61.0, 62.3), ranging from 47% in the earliest period to 93% in the most recent period. Under all approaches, subdistribution hazard ratios illustrated the acceleration in ART initiation in later periods. Subdistribution hazard ratios were similar under all approaches considered. Details regarding values of may be found in eAppendix 2.
In the simulation experiments, handling loss to follow-up using the proposed tracing weights yielded results with smaller bias and root mean squared error than treating loss to follow-up as a censoring or competing event. Moreover, the proposed approach yielded near 95% CI coverage in most scenarios while approaches that treated loss to follow-up as a censoring or competing event produced coverage that was below the nominal level in all scenarios examined. Poor confidence interval coverage among the standard approaches was due primarily to bias; standard errors were similar between the approaches, though the proposed approach yielded standard errors that were slightly larger than the standard approaches, on average.
DISCUSSION
Weighting participants who were lost to follow-up from clinical HIV programs using the proposed approach leverages information from published tracing studies and allows investigators to relax the dubious assumptions inherent in treating loss to follow-up as a censoring event or a competing event. In all calendar time periods examined, applying the tracing weights yielded an estimated probability of ART initiation above that estimated by treating loss to follow-up as a competing event, but below that estimated by censoring patients at loss to follow-up. Simulation experiments confirmed that the proposed approach yields results with little bias and appropriate CI coverage in settings similar to the example.
The proposed approach offers a formal method for incorporating knowledge about the probability of being in care after loss to follow-up into the estimated risk and its confidence interval. This knowledge was encoded in results from tracing studies of people lost to HIV care at specific study sites in Africa. While tracing studies have been used to reduce bias in mortality estimates 25,26 and estimates of retention in care 24,27, they have not been widely used to estimate the risk of other outcomes along the continuum of HIV care. Unlike approaches that use external validation data to account for misclassification of the outcome of interest (e.g., 28,29), here, we propose using these external validation data to account for “misclassification” between a competing event and a censoring event.
In this example, the high proportion of patients experiencing the outcome (here, ART initiation) in each calendar period prior to loss to follow-up limited the difference between results obtained using the proposed approach and other candidate approaches (e.g., treating loss to follow-up as a censoring or competing event). However, as demonstrated in the simulation experiments, when investigating outcomes with substantial loss to follow-up prior to the outcome of interest, the choice of how to handle losses to follow-up will have a more profound impact on the results.
A primary assumption underlying the proposed approach is that the probability of being engaged in care, given that a participant appears to be lost, is the same between the main study sample and the data provided in the tracing studies. This assumption may be relaxed to be conditional on a set of measured variables by including these variables in the model for . In our example, because the final analysis was stratified by calendar time, we included calendar year in the model for to allow to vary by person based on her date of loss to follow-up. In general, the model for should include any variable later stratified upon or used as an exposure, in addition to any factors that are likely to affect the probability of silent transfer and whose distribution varies between the tracing data and the main study data 30. However, the richness of the model for is limited by the coarseness of the tracing data; in many settings tracing data are available only in aggregate form without individual-level covariates; for example, published tracing data are likely to include only calendar year and geographic setting rather than rich data on participant characteristics. When possible, tracing studies should be designed to collect the individual- and facility-level covariates believed to be associated with the probability of silent transfer to allow quantitative transportability of the findings to other settings. However, in settings where a variable thought to both predict and differ between the main study and tracing data is not included in the tracing data, one could include that variable in the model for and place an informative prior distribution on the regression coefficient corresponding to that variable using Markov Chain Monte Carlo or data augmentation. 31 Alternatively, one could conduct a sensitivity analysis under presumed values of within strata of covariates measured in the main study.
The standard approaches ignored the uncertainty in the probability of silent transfer, either treating as fixed at 0 (i.e., treating loss to follow-up as a competing event) or as fixed at 1 (i.e., censoring at loss to follow-up). In the proposed approach, uncertainty in the estimation of is propagated through to the final CIs around the estimated risk using the two-stage bootstrap approach defined above, resulting in wider CIs than the standard approaches, in general. However, in the example described in this paper, the widths of the CIs obtained using the proposed approach were not substantially wider than CIs obtained other approaches considered, likely due to the small proportion of patients lost to follow-up prior to ART and the large numbers of participants included in the tracing studies (resulting in low variability around ). In eAppendix 3, we illustrated that standard errors estimated under the proposed approach increased as the size of the tracing study decreased, resulting in wider confidence intervals.
Estimates of are subject to systematic error in addition to random error. In particular, the tracing studies used to estimate likely suffer from selection bias due to logistical challenges in tracing all participants lost from a specific facility. Methods for addressing systematic error when using estimates from tracing studies is an important area of future research. Simple approaches that could be employed when using the proposed methods include reweighting the relative importance of various tracing studies according to perceived study quality, or calculating bounds 32 on the proportion of silent transfers from tracing studies as part of a sensitivity analysis.
In our example, we presented a descriptive analysis comparing the cumulative incidence of ART initiation in selected Central Africa facilities among three calendar time periods roughly corresponding to eras with different treatment guidelines. Had we wished to estimate a causal effect in a setting with confounding, or generalize or transport results to a different target population, we could have combined the proposed approach with inverse probability of treatment 33 or sampling 30,34 weights.
While we focused on an example specific to research in clinical cohorts of people in care for HIV, the proposed approach is relevant beyond HIV. For example, this approach could be used to account for the ambiguity brought about by loss to follow-up from datasets produced by pharmaceutical claims, substance use treatment facilities, school records, employment records, or antenatal care records, among others.
Formal approaches to incorporate external sources of knowledge to address bias in epidemiologic studies are critical to improving inference. With increasing access to clinical data for epidemiologic research, handling inevitable losses to follow-up will be a growing challenge faced by analysts that must be considered carefully in order to minimize bias. The tracing weights proposed here add a weapon to the epidemiologist’s arsenal available for combatting systematic bias in estimates of outcome incidence and associations by integrating external information.
Supplementary Material
Acknowledgements
Funding sources:
Research reported in this publication was supported by the National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), the National Heart, Lung, and Blood Institute (NHLBI), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Fogarty International Center (FIC), the National Library of Medicine (NLM), and the Office of the Director (OD) under Award Number U01AI096299 (Central Africa-IeDEA). This work was also funded by NIAID under award K01AI125087 and Fogarty International Center under award K01TW010272.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Data access: Central Africa IeDEA has a concept approval process by which interested parties can apply for data access. Details are available at https://www.iedea.org/regions/central-africa/. Example code is available in the online supplementary material and an R package to implement the proposed approach is available at the first author’s github page: https://github.com/edwardsjk/mccc.
Site investigators and cohorts:
Nimbona Pélagie, Association Nationale de Soutien aux Séropositifs et Malade du Sida (ANSS), Burundi; Patrick Gateretse, Jeanine Munezero, Valentin Nitereka, Théodore Niyongabo, Christelle Twizere, Centre National de Reference en Matière de VIH/SIDA, Burundi; Hélène Bukuru, Thierry Nahimana, Centre Hospitalo-Universitaire de Kamenge (CHUK), Burundi; Elysée Baransaka, Patrice Barasukana, Eugene Kabanda, Martin Manirakiza, François Ndikumwenayo, CHUK/Burundi National University, Burundi; Jérémie Biziragusenyuka, Ange Marie Michelline Munezero, Hospital Prince Régent Charles (HPRC), Burundi; Tabeyang Mbuh, Kinge Thompson Njie, Edmond Tchassem, Kien-Atsu Tsi, Bamenda Hospital, Cameroon; Rogers Ajeh, Mark Benwi, Marc Lionel Ngamani, Victorine Nkome, Grace Toutou, Clinical Research Education and Consultancy (CRENC), Cameroon; Anastase Dzudie, CRENC and Douala General Hospital, Cameroon; Akindeh Mbuh, CRENC and University of Yaoundé, Cameroon; Djenabou Amadou, Amadou Dodo Balkissou, Eric Ngassam, Eric Walter Pefura Yone, Jamot Hospital, Cameroon; Alice Ndelle Ewanoge, Norbert Fuhngwa, Ernestine Kendowo, Chris Moki, Denis Nsame Nforniwe, Limbe Regional Hospital, Cameroon; Catherine Akele, Faustin Kitetele, Patricia Lelo, Martine Tabala, Kalembelembe Pediatric Hospital, Democratic Republic of Congo; Emile Wemakoy Okitolonda, Cherubin Ekembe, Kinshasa School of Public Health, Democratic Republic of Congo; Merlin Diafouka, Martin Herbas Ekat, Dominique Mahambou Nsonde, CTA Brazzaville, Republic of Congo; Adolphe Mafou, CTA Pointe-Noire, Republic of Congo; Nicole Ayinkamiye, Jules Igirimbabazi, Bethsaida Health Center, Rwanda; Emmanuel Ndamijimana, Providance Uwineza, Busanza Health Center, Rwanda; Emmanuel Habarurema, Marie Luise Nyiraneza, Gahanga Health Center, Rwanda; Dorothee Mukamusana, Liliane Tuyisenge, Gikondo Health Center, Rwanda; Catherine Kankindi, Christian Shyaka, Kabuga Health Center, Rwanda; Marie Grace Ingabire, Bonheur Uwakijijwe, Kicukiro Health Center, Rwanda; Jules Ndumuhire, Marie Goretti Nyirabahutu, Masaka Health Center, Rwanda; Yvette Ndoli, Oliver Uwamahoro, Nyarugunga Health Center, Rwanda; Ribakare Muhayimpundu, Sabin Nsanzimana, Eric Remera, Esperance Umurarungu, Rwanda Biomedical Center, Rwanda; Lydia Busingye, Alex M Butera, Josephine Gasana, Thierry Habiryayo, Charles Ingabire, Jules Kabahizi, Jean Chrysostome Kagimbana, Faustin Kanyabwisha, Gallican Kubwimana, Benjamin Muhoza, Athanase Munyaneza, Gad Murenzi, Francoise Musabyimana, Francine Mwiza, Gallican Nshogoza Rwibasira, Jean d’Amour Sinayobye, Patrick Tuyisenge, Rwanda Military Hospital, Rwanda; Chantal Benekigeri, Jacqueline Musaninyange, WE-ACTx Health Center, Rwanda.
Coordinating and Data Centers:
Adebola Adedimeji, Kathryn Anastos, Madeline Dilorenzo, Lynn Murchison, Jonathan Ross, Marcel Yotebieng, Albert Einstein College of Medicine, USA; Diane Addison, Ellen Brazier, Heidi Jones, Elizabeth Kelvin, Sarah Kulkarni, Denis Nash, Matthew Romo, Olga Tymejczyk, Institute for Implementation Science in Population Health, Graduate School of Public Health and Health Policy, City University of New York (CUNY), USA; Batya Elul, Columbia University, USA; Xiatao Cai, Allan Dong, Don Hoover, Hae-Young Kim, Chunshan Li, Qiuhu Shi, Data Solutions, USA; Robert Agler, Kathryn Lancaster, The Ohio State University, USA; Mark Kuniholm, University at Albany, State University of New York, USA; Andrew Edmonds, Angela Parcesepe, Jess Edwards, University of North Carolina at Chapel Hill, USA; Olivia Keiser, University of Geneva; Stephany Duda; Vanderbilt University School of Medicine, USA; April Kimmel, Virginia Commonwealth University School of Medicine, USA.
References
- 1.The INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. New England Journal of Medicine. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau B, Gange SJ, Moore RD. Interval and clinical cohort studies: epidemiological issues. AIDS Res Hum Retroviruses. 2007;23(6):769–776. doi: 10.1089/aid.2006.0171 [DOI] [PubMed] [Google Scholar]
- 4.Hallett TB, Eaton JW. A side door into care cascade for HIV-infected patients? Journal of acquired immune deficiency syndromes (1999). 2013;63 Suppl 2:S228–32. doi: 10.1097/QAI.0b013e318298721b [DOI] [PubMed] [Google Scholar]
- 5.Hickey MD, Omollo D, Salmen CR, et al. Movement between facilities for HIV care among a mobile population in Kenya: transfer, loss to follow-up, and reengagement. AIDS Care. 2016;28(11):1386–1393. doi: 10.1080/09540121.2016.1179253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. Springer; 2nd edition; 2005. [Google Scholar]
- 7.Chammartin F, Zürcher K, Keiser O, et al. Outcomes of Patients Lost to Follow-up in African Antiretroviral Therapy Programs: Individual Patient Data Meta-analysis. Clinical Infectious Diseases. 2018;67(11):1643–1652. doi: 10.1093/cid/ciy347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes CB, Sikazwe I, Sikombe K, et al. Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: Findings from a multistage sampling-based survey. PLOS Medicine. 2018;15(1):e1002489. doi: 10.1371/journal.pmed.1002489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole SR, Hudgens MG, Brookhart MA, Westreich D. Risk. American Journal of Epidemiology. 2015;181(4):246–250. doi: 10.1093/aje/kwv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross J, Edmonds A, Hoover DR, et al. Association between pregnancy at enrollment into HIV care and loss to care among women in the Democratic Republic of Congo, 2006–2013. Atanga PN, ed. PLOS ONE. 2018;13(4):e0195231. doi: 10.1371/journal.pone.0195231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adedimeji A, Edmonds A, Hoover D, et al. Characteristics of HIV-Infected Children at Enrollment into Care and at Antiretroviral Therapy Initiation in Central Africa. Andrei G, ed. PLOS ONE. 2017;12(1):e0169871. doi: 10.1371/journal.pone.0169871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesko CR, Edwards JK, Cole SR, Moore RD, Lau B. When to Censor? American Journal of Epidemiology. 2018;187(3). doi: 10.1093/aje/kwx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi BH, Yiannoutsos CT, Westfall AO, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS medicine. 2011;8(10):e1001111–e1001111. doi: 10.1371/journal.pmed.1001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesko CR, Edwards JK, Moore RD, Lau B. Censoring for Loss to Follow-up in Time-to-event Analyses of Composite Outcomes or in the Presence of Competing Risks. Epidemiology. 2019;30(6):817. doi: 10.1097/EDE.0000000000001073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aalen OO, Johansen S. An empirical transition matrix for non-homogeneous markov chains based on censored observations. Scandinavian Journal of Statistics. 1978;5(3):141–150. [Google Scholar]
- 16.Satten GA, Datta S. The Kaplan–Meier Estimator as an Inverse-Probability-of-Censoring Weighted Average. The American Statistician. 2001;55(3):207–210. doi: 10.1198/000313001317098185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ. Selection Bias Due to Loss to Follow Up in Cohort Studies. Epidemiology (Cambridge, Mass). 2016;27(1):91–97. doi: 10.1097/EDE.0000000000000409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards JK, Cole SR, Westreich D. All your data are always missing: Incorporating bias due to measurement error into the potential outcomes framework. International Journal of Epidemiology. 2015;44(4). doi: 10.1093/ije/dyu272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen PK, Geskus RB, Witte TD, Ã HP. Competing risks in epidemiology : possibilities and pitfalls. 2012;(January):861–870. doi: 10.1093/ije/dyr213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards JK, Hester LL, Gokhale M, Lesko CR. Methodologic Issues when Estimating Risks in Pharmacoepidemiology. Current Epidemiology Reports. 2016;3(4):285–296. doi: 10.1007/s40471-016-0089-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 22.Zürcher K, Mooser A, Anderegg N, et al. Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Tropical Medicine & International Health. 2017;22(4):375–387. doi: 10.1111/tmi.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Kop ML, Nagide PI, Thabane L, et al. Retention in clinic versus retention in care during the first year of HIV care in Nairobi, Kenya: a prospective cohort study. J Int AIDS Soc. 2018;21(11):e25196. doi: 10.1002/jia2.25196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyakato P, Kiragga AN, Kambugu A, Bradley J, Baisley K. Correction of estimates of retention in care among a cohort of HIV-positive patients in Uganda in the period before starting ART: a sampling-based approach. BMJ open. 2018;8(4):e017487–e017487. doi: 10.1136/bmjopen-2017-017487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger M, Spycher BD, Sidle J, et al. Correcting Mortality for Loss to Follow-Up: A Nomogram Applied to Antiretroviral Treatment Programmes in Sub-Saharan Africa. Bisson GP, ed. PLoS Medicine. 2011;8(1):e1000390–e1000390. doi: 10.1371/journal.pmed.1000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schomaker M, Gsponer T, Estill J, Fox M, Boulle A. Non-ignorable loss to follow-up : correcting mortality estimates based on additional outcome ascertainment. 2014;(July 2013). doi: 10.1002/sim.5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng EH, Odeny TA, Lyamuya R, et al. Retention in Care and Patient-Reported Reasons for Undocumented Transfer or Stopping Care Among HIV-Infected Patients on Antiretroviral Therapy in Eastern Africa: Application of a Sampling-Based Approach. Clinical Infectious Diseases. 2016;62(7):935–944. doi: 10.1093/cid/civ1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakoyannis G, Yiannoutsos CT. Impact of and Correction for Outcome Misclassification in Cumulative Incidence Estimation. 2015:1–15. doi: 10.1371/journal.pone.0137454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards JK, Cole SR, Moore RD, Mathews WC, Kitahata M, Eron JJ. Sensitivity analyses for misclassification of cause of death in the parametric G-formula. American Journal of Epidemiology. 2018;187(8):1808–1816. doi: 10.1093/aje/kwy028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westreich D, Edwards JK, Lesko CR, Stuart E, Cole SR. Transportability of Trial Results Using Inverse Odds of Sampling Weights. American Journal of Epidemiology. 2017;186(8). doi: 10.1093/aje/kwx164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenland S Relaxation penalties and priors for plausible modeling of nonidentified bias sources. Statistical Science. 2009;24(2):195–210. doi: 10.1214/09-STS291 [DOI] [Google Scholar]
- 32.Cole SR, Hudgens MG, Edwards JK, et al. Nonparametric Bounds for the Risk Function. Am J Epidemiol. 2019;188(4):632–636. doi: 10.1093/aje/kwz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology (Cambridge, Mass). 2003;14(6):680–686. doi: 10.1097/01.EDE.0000081989.82616.7d [DOI] [PubMed] [Google Scholar]
- 34.Cole SR, Stuart EA. Generalizing evidence from randomized clinical trials to target populations: The ACTG 320 trial. American journal of epidemiology. 2010;172(1):107–115. doi: 10.1093/aje/kwq084 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.