Abstract
An accessory liver lobe is a congenital anomaly of hepatic tissue most commonly due to embryonic heteroplasia. Rarely, accessory liver lobes can undergo torsion and present as an acute surgical emergency. Although common in certain animals, there are only a few reported cases of accessory lobe torsion in humans. We report a multi-modality radiographic diagnosis of an acute torsion and subsequent infarct of an accessory liver lobe following minor trauma in a 29-year old male patient.
Keywords: Accessory liver lobe, Torsion, Liver, Computed tomography, Magnetic resonance imaging, Ultrasound
Introduction
An accessory liver lobe (ALL) is an uncommon congenital anomaly of hepatic tissue secondary to embryonic heteroplasia and may either be sessile or pedunculated [1]. The majority of cases of ALL are usually asymptomatic and are incidentally detected during surgery. One serious complication that can arise, particularly in the pedunculated subtype is liver torsion. This can result in abdominal pain and impaired liver function [2]. Although it can frequently be seen in animals including dogs and rabbits, this complication is rarely seen in humans [3,4]. Interestingly, in the small subset of cases reported, a few have shown an association with a history of omphalocele repair [5].
We report an acute torsion and subsequent infarction of an accessory lobe following minor trauma in a 29-year old male patient with a history of omphalocele repair. This case report highlights the role of a multi-modality imaging approach to diagnosis and pre-operative assessment of accessory hepatic lobe torsion.
Case report
A 29-year old male with DiGeorge syndrome presented to his local emergency department with worsening abdominal pain, nausea and emesis one week after lifting a heavy wagon which resulted in a fall that knocked him to the ground. Past surgical history is significant for repaired omphalocele.
On admission, the patient's vital signs were within normal limits. His physical exam was significant for 3/6 holosystolic murmur, left upper quadrant tenderness with palpation, abdominal fullness and mild guarding. Also noted was a midline surgical scar superior to the umbilicus. Laboratory studies revealed HGB = 12.6 g/dl (normal = 13-17 g/dl), HCT = 37.2% (normal = 39-48), INR = 1.2 (normal 0.9-1.1), WBC = 13.38 K/uL (normal = 4.0-10.4 K/uL), PLT = 119 K/uL (normal 150-350 K/uL), ALT =999 unit/L (normal = 0-41 unit/L), AST =275 unit/L (normal = 0-40 unit/L), total bilirubin = 2.3 mg/dL (normal = 0.0-1.2 mg/dL), direct bilirubin = 0.2 mg/dL (normal = 0.0-0.3 mg/dL), and lipase = 12 unit/L (normal = 13-60 unit/L).
An abdominal ultrasound from an outside institution demonstrated a heterogeneously hypoechoic, avascular mass in the left upper abdominal quadrant [Fig. 1, A-C]. At that time, the differential diagnosis included a perihepatic hematoma given the patient's recent history of trauma, however an abdominal mass could not be excluded. Given the indeterminate appearance of the mass, contrast-enhanced CT of the abdomen and pelvis was recommended to further characterize it.
Fig. 1.
A-C – (A) Grayscale ultrasound image of the left abdomen demonstrates a heterogenous, predominantly hypoechoic mass. (B) Color-flow Doppler image demonstrates no color flow within the mass. (C) Grayscale ultrasound image demonstrates the right hepatic lobe for comparison.
The subsequent contrast enhanced CT demonstrated a large heterogeneous collection in the left abdomen, just inferior to left hepatic lobe concerning for development of a perihepatic hematoma [Fig. 2, A-B].
Fig. 2.
A-B – (A) Contrast-enhanced, axial and sagittal CT images demonstrate a large, low-attenuation mass in the left abdomen.
A CT angiogram was performed for further evaluation of the vasculature and to assess for additional sites of bleeding [Fig. 3, A-D]. The CT angiogram revealed a large non-enhancing mass in the central aspect of the abdomen grossly similar in appearance to the prior CT. There was no active extravasation of contrast and the smooth contour of the collection was not suggestive of an infiltrative process such as a recent hemorrhage. The axial images suggested the presence of non-enhancing vascular structures, which was supported by the coronal images [Fig. 4, A-C] suggesting the possibility of a torsed hepatic lobe.
Fig. 3.
A-D – (A) Contrast-enhanced, axial CTA image in arterial phase demonstrates the torsed accessory liver lobe compared to the normal right hepatic lobe (B). (C) Contrast-enhanced, axial CTA image in venous phase demonstrates the torsed accessory liver lobe compared to the normal right hepatic lobe (D). Notice the dilated, fluid-filled loops of small bowel in keeping with reactive small bowel ileus (arrows).
Fig. 4.
A-C – (A) Contrast-enhanced, coronal CTA image in delayed venous phase demonstrates a mixed-attenuation mass in the anterior abdomen (thin arrow). (B) Contrast-enhanced, coronal CTA image in arterial phase demonstrates the hepatic artery extending inferiorly toward the mass (thin arrow). (C) Contrast enhanced, coronal CTA image in delayed venous phase demonstrates portal and hepatic venous structures in the normal liver extending toward and away from the mass, respectively. The mass shows tubular structures, likely hepatic vein (thin arrow) and portal vein (thick arrow), which are better demonstrated due to the lower attenuation of the torsed ALL.
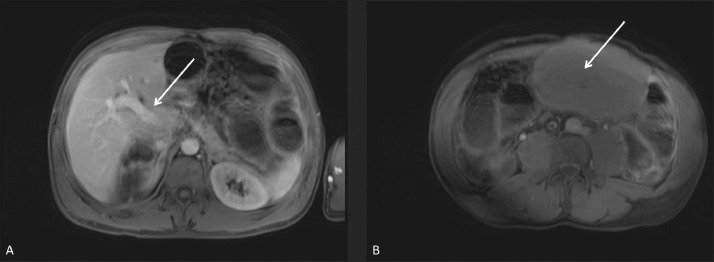
After further discussion between radiology and surgical teams, including pediatric surgery, the decision was made to obtain an enhanced MRI of the abdomen to help characterize the mass [Fig. 5, A-C], as well as rule-out other findings in this patient with known congenital defects. The MRI demonstrated a non-enhancing mass in the upper abdomen, which communicated with the undersurface of the normal appearing left hepatic lobe via a thin stalk. After review of the prior CTs [Fig. 6, A-B] and MRI [Fig. 7, A-B], it was determined that this stalk contained a vascular pedicle, which appeared twisted upon itself. This confirmed the suspicion of torsion of an accessory liver lobe.
Fig. 5.
A-C – (A) T2-weighted, coronal MRI image shows well-delineated, low-signal, branching hepatic and portal veins in the edematous torsed ALL. (A-C) T2-weighted, coronal MRI images show the torsed ALL with tapering vessels extending into the thin pedicle (arrows).
Fig. 6.
A-D – (A-D) Contrast-enhanced, axial CT image shows the thin, torsed pedicle of the ALL (arrows).
Fig. 7.
A-B – (A-B) Contrast-enhanced, T1-weighted, axial MRI images show normal enhancement of the portal vein (arrow) compared to (B) no enhancement in the torsed ALL (arrow)..
The patient subsequently underwent an exploratory laparotomy 3 days after his initial visit to the outside emergency department. Pre-operatively, the patient's hemoglobin and hematocrit remained stable from admission. His total and direct bilirubin peaked at 2.4 mg/dL and 0.5 mg/dL, respectively, on the day before surgery, while his remaining LFTs continued to trend towards normal.
At the time of surgery, a torsed stalk was visualized connecting a normal appearing left liver lobe to an ischemic appearing accessory liver segment associated with segment 3. The pedicle was detorsed 720 degrees and the ischemic accessory lobe was excised. Histopathology revealed infarcted liver with abundant parenchymal hemorrhage and a superficial cap of acute inflammation.
By post-operative day #2, the patient's LFTs had normalized except for the ALT which remained elevated at 340 unit/L. Over the remainder of the patient's hospitalization, the patient's remaining laboratory studies normalized. The patient continued to improve and was discharged home in stable condition eight days after admission.
Discussion
Accessory hepatic lobes are a rare congenital malformation with only a few case reports in the literature describing their presentation [6]. Their incidence in some reports is less than 1% of the population, however that number is likely underestimated given the majority are asymptomatic [7]. The most common and well known location of an accessory liver lobe is an elongated inferior right liver lobe, better known as a Riedel lobe.
In our patient, the accessory liver lobe originated from the left hepatic lobe, and may have been originally viewed as a pedunculated “beaver tail liver” [8]. Unfortunately no prior imaging was available to assess the now excised lobe in its native orientation.
In the majority of the population, the liver has several ligamentous attachments to the abdominal wall and diaphragm. This includes the coronary ligament, triangular ligament, and the falciform ligament. Given the patient's history of prior omphalocele repair, it is unknown if the entire triangular ligament was attached to the left liver lobe and the accessory lobe. Within the limited number of published cases of accessory liver lobe torsion, there are several which occurred in patients with a history of omphalocele repair [9,10]. The lack of a ligamentous attachment, either surgical or congenital, was likely a contributing factor, and a history of omphalocele repair should raise the suspicion of hepatic torsion.
The imaging diagnosis can be variable depending on the degree of torsion. As the previously vascularized lobe infarcts, some of its imaging characteristics lose their similarity to the normal appearing adjacent liver. The ultrasound findings of a liver infarct are represented as hypoechoic, nonvascular regions on conventional and Doppler sonography [11]. Similarly, the “mass” seen in our patient was originally described as a heterogeneously hypoechoic (relative to normal liver parenchyma) and echogenic masslike structure without flow on color Doppler which lead to the initial diagnosis of a hematoma.
The initial CT demonstrated a heterogeneous collection extending from hepatic segment 3. The study showed subtle hyperdensities traversing the mass, which did not enhance. Given complete torsion and lack of blood flow to the accessory lobe, these hyperdensities were not immediately recognized as vessels, but were initially thought to represent mixed-age blood products.
The CTA was subsequently obtained to assess for persistent hemorrhage. There was no active extravasation of contrast and the smooth contour of the collection was not suggestive of an infiltrative process such as acute hemorrhage. The axial images suggested the presence of non-enhancing vascular structures, which was supported by the coronal images. In addition, the left hepatic artery and the left portal vein from the unaffected liver were demonstrated extending toward the mass on the arterial phase of imaging, and a left hepatic vein branch was demonstrated extending away from the mass on the venous phase of imaging. These additional finding resulted in the addition of a torsed accessory liver lobe to the differential diagnosis.
MRI has the advantage over CT with its contrast resolution based on tissue characteristics rather than tissue densities. Because of this, the non-enhancing vasculature extending from the normal liver to the pedicle and into the accessory lobe could be more readily appreciated. The T2-signal was increased in the torsed accessory lobe indicating edema, which is consistent with an acute torsion. A chronic torsion would appear as cystic changes as the infarcted tissue atrophied. Through several different imaging modalities, the challenges of diagnosing such a rare entity are clearly demonstrated.
Conclusion
Accessory liver lobes are a rare clinical entity that are likely underrepresented in the population given their asymptomatic nature. A rare but important presentation of this entity is the accessory liver lobe that undergoes torsion. This usually occurs with the pedunculated subtype. Within the literature of documented cases of accessory hepatic lobe torsion, several have occurred in the setting of prior omphalocele repair. With the imaging studies available for review, we demonstrate that with complete torsion, ultrasound may be the least helpful modality for the diagnosis of this entity. Contrast enhanced CT, particularly a CTA, was more helpful in the diagnostic process, but the MRI allowed us to best delineate the anatomy and therefore the underlying pathology. However, CT and CTA likely remain the first line imaging studies for the evaluation of accessory liver lobe torsion. CT imaging is readily available and rapid, which increases the likelihood of a timely diagnosis and increased chances of salvaging the ischemic segment or lobe. Going forward, the history of abdominal wall surgery, acute pain, and trauma in the setting of an accessory liver lobe should raise the suspicion for torsion, and potentially allow for rapid transition to surgery and salvage of the torsed segment.
Patient consent statement
Patient consent has not been obtained.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Wang C, Cheng L, Zhang Z, Xie T, Ding H, Deng Q. Accessory lobes of the liver: a report of 3 cases and review of the literature. Intractable Rare Dis Res. 2012;1(2):86–91. doi: 10.5582/irdr.2012.v1.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glenisson M, Salloum C, Lim C, Lacaze L, Malek A, Enriquez A. Accessory liver lobes: anatomical description and clinical implications. J Visc Surg. 2014;151(6):451–455. doi: 10.1016/j.jviscsurg.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Scheck MG. Liver lobe torsion in a dog. Can Vet J. 2007;48(4):423–425. [PMC free article] [PubMed] [Google Scholar]
- 4.Graham J, Basseches J. Liver lobe torsion in pet rabbits: clinical consequences, diagnosis, and treatment. Vet Clin North Am Exot Anim Pract. 2014;17(2):195–202. doi: 10.1016/j.cvex.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Koumanidou C, Nasi E, Koutrouveli E, Theophanopoulou M, Hilari S, Kakavakis K. Torsion of an accessory hepatic lobe in a child: ultrasound, computed tomographic, and magnetic resonance imaging findings. Pediatr Surg Int. 1998;13(7):526–527. doi: 10.1007/s003830050391. [DOI] [PubMed] [Google Scholar]
- 6.Hundal RS, Ali J, Korsten MA, Khan AM. Torsion and infarction of an accessory liver lobe. Z Gastroenterol. 2006;44(12):1223–1226. doi: 10.1055/s-2006-926847. [DOI] [PubMed] [Google Scholar]
- 7.Jambhekar K, Pandey T, Kaushik C, Shah HR. Intermittent torsion of accessory hepatic lobe: An unusual cause of recurrent right upper quadrant pain. Indian J Radiol Imaging. 2010;20(2):135–137. doi: 10.4103/0971-3026.63046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang H, Han J, Ridley WE, Ridley LJ. Beaver tail liver: Anatomic variant. J Med Imaging Radiat Oncol. 2018;62(Suppl 1):57. doi: 10.1111/1754-9485.05_12784. [DOI] [PubMed] [Google Scholar]
- 9.Umehara M, Sugai M, Kudo D, Hakamada K, Sasaki M, Munakata H. Torsion of an accessory lobe of the liver in a child: report of a case. Surg Today. 2009;39(1):80–82. doi: 10.1007/s00595-008-3772-0. [DOI] [PubMed] [Google Scholar]
- 10.Elmasalme F, Aljudaibi A, Matbouly S, Hejazi N, Zuberi MS. Torsion of an accessory lobe of the liver in an infant. J Pediatr Surg. 1995;30(9):1348–1350. doi: 10.1016/0022-3468(95)90502-2. [DOI] [PubMed] [Google Scholar]
- 11.Park AH, Tran TA, Neychev V. Accessory liver lobe attached to the wall of the gallbladder. Cureus. 2019;11(11):e6113. doi: 10.7759/cureus.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]