Abstract
Background
Tisagenlecleucel, an anti-CD19 chimeric antigen receptor T cell therapy, has demonstrated efficacy in children and young adults with relapsed/refractory B cell acute lymphoblastic leukemia (B-ALL) in two multicenter phase 2 trials (ClinicalTrials.gov, NCT02435849 (ELIANA) and NCT02228096 (ENSIGN)), leading to commercialization of tisagenlecleucel for the treatment of patients up to age 25 years with B-ALL that is refractory or in second or greater relapse.
Methods
A pooled analysis of 137 patients from these trials (ELIANA: n=79; ENSIGN: n=58) was performed to provide a comprehensive safety profile for tisagenlecleucel.
Results
Grade 3/4 tisagenlecleucel-related adverse events (AEs) were reported in 77% of patients. Specific AEs of interest that occurred ≤8 weeks postinfusion included cytokine-release syndrome (CRS; 79% (grade 4: 22%)), infections (42%; grade 3/4: 19%), prolonged (not resolved by day 28) cytopenias (40%; grade 3/4: 34%), neurologic events (36%; grade 3: 10%; no grade 4 events), and tumor lysis syndrome (4%; all grade 3). Treatment for CRS included tocilizumab (40%) and corticosteroids (23%). The frequency of neurologic events increased with CRS severity (p<0.001). Median time to resolution of grade 3/4 cytopenias to grade ≤2 was 2.0 (95% CI 1.87 to 2.23) months for neutropenia, 2.4 (95% CI 1.97 to 3.68) months for lymphopenia, 2.0 (95% CI 1.87 to 2.27) months for leukopenia, 1.9 (95% CI 1.74 to 2.10) months for thrombocytopenia, and 1.0 (95% CI 0.95 to 1.87) month for anemia. All patients who achieved complete remission (CR)/CR with incomplete hematologic recovery experienced B cell aplasia; however, as nearly all responders also received immunoglobulin replacement, few grade 3/4 infections occurred >1 year postinfusion.
Conclusions
This pooled analysis provides a detailed safety profile for tisagenlecleucel during the course of clinical trials, and AE management guidance, with a longer follow-up duration compared with previous reports.
Keywords: pediatrics, receptors, chimeric antigen, hematologic neoplasms
Background
Tisagenlecleucel, an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, is effective for the treatment of children and young adults with relapsed or refractory B cell acute lymphoblastic leukemia (B-ALL) and adults with relapsed or refractory diffuse large B cell lymphoma (DLBCL).1–6 Clinical efficacy has also been demonstrated for other CAR T cell products for various B cell malignancies.7–17 CD19 CAR T cell products have been associated with clinically significant adverse events (AEs), particularly cytokine-release syndrome (CRS) and neurologic events.18 19 The magnitude and timing of these AEs vary depending on the CAR T cell construct, disease, and patient characteristics.18
A single-center phase 1/2a trial (NCT01626495)3 20 of tisagenlecleucel in 59 children and young adults with relapsed or refractory B-ALL demonstrated a 1 month complete remission (CR) rate of 93% and long-term disease control without additional therapy. CRS of any grade occurred in 88% of patients. Severe CRS (graded according to the University of Pennsylvania grading scale)21 22 occurred in 27% and was manageable with supportive measures and administration of the anti-interleukin 6 receptor antibody tocilizumab, often in combination with steroids and occasionally other immunosuppressive agents.20 Based on these single-center results, two phase 2 multicenter trials of tisagenlecleucel in children and young adults with relapsed or refractory B-ALL were initiated. ENSIGN (ClinicalTrials.gov, NCT02228096), conducted in the USA, was the first multicenter CAR T cell therapy trial and the first to use centrally manufactured cell product.23 24 ELIANA (NCT02435849) was the first global CAR T cell therapy trial and also used centrally manufactured cell product.4 25 CRS occurred in 81% (grade 3–4: 33%) and 77% (grade 3–4: 48%) of patients in ENSIGN and ELIANA, respectively, with no deaths attributable to CRS in either study. Neurologic events occurred in 33% and 13% of patients, respectively.24 25 The objective of this pooled safety analysis of ELIANA and ENSIGN is to provide a comprehensive, long-term evaluation of the tisagenlecleucel safety profile and AE management in a larger group of 137 children and young adults with relapsed or refractory B-ALL, particularly for lower frequency events that may have had limited characterization in earlier analyses.
Methods
Study design
ELIANA and ENSIGN were phase 2, single-arm, multicenter trials of tisagenlecleucel in children and young adults with relapsed or refractory B-ALL. The trials had similar designs, eligibility criteria, and dosing, permitting pooling of data for characterization of tisagenlecleucel safety.
Eligibility criteria included age ≥3 years at screening and ≤21 years at initial diagnosis, relapsed/refractory B-ALL (second or greater bone marrow relapse, or any bone marrow relapse after allogeneic hematopoietic stem cell transplant (HSCT) and ≥6 months from HSCT at time of infusion, or refractory disease defined as not achieving CR after two cycles of standard chemotherapy after initial diagnosis or one cycle of standard chemotherapy after relapse), and ≥5% lymphoblasts in bone marrow at screening. Patients with active central nervous system (CNS) leukemia involvement defined as CNS-3 were excluded from ELIANA. In ENSIGN, patients with active CNS-3 (except for CNS parenchymal/ocular disease, cranial nerve involvement, or significant leptomeningeal disease) were eligible if reduced to CNS-1/2 and there was documented evidence of disease stabilization for ≥3 months preinfusion.
The study designs comprised screening; pretreatment (leukapheresis, tisagenlecleucel product preparation, bridging chemotherapy per physician discretion, and protocol-defined lymphodepleting chemotherapy); tisagenlecleucel infusion; and primary, secondary, and survival follow-up.
Leukapheresis material was collected from patients, cryopreserved within 24 hours, and shipped to the central manufacturing facilities. Tisagenlecleucel was manufactured ex vivo using autologous T cells (described previously).26 27 On final product release testing, cryopreserved tisagenlecleucel cells were shipped to clinical sites for infusion. In both studies, the protocol-specified dose range was 0.2×106‒5.0×106 CAR-positive viable T cells/kg for patients with ≤50 kg body weight, and 0.1×108‒2.5×108 CAR-positive viable T cells for patients >50 kg.4 28 Multidisciplinary teams at clinical trial sites completed extensive training and education on leukapheresis, tisagenlecleucel-specific product infusion, patient logistics, and AE management.
Safety assessment
The safety population included all patients enrolled in ELIANA or ENSIGN who received tisagenlecleucel infusion. AEs that started or worsened after informed consent were recorded and assessed throughout the study until initiation of new therapy. After new therapy was initiated, AE collection was focused on the monitoring of delayed AEs per health authority guidance for AEs/serious AEs (ie, severe AEs potentially related to tisagenlecleucel, new incidence or exacerbation of pre-existing neurological disorders, rheumatologic or other autoimmune disorders, etc). Safety assessment and collection was the same for both trials and is continuing for up to 15 years postinfusion.
AEs were assessed per the Common Terminology Criteria for Adverse Events, V.4.03,29 except for CRS (graded according to the University of Pennsylvania grading scale),21 22 and graft-versus-host disease (GVHD; graded using protocol-defined criteria).30 31 Serious AEs were defined per protocol (online supplemental file). Certain AEs (CRS, febrile neutropenia, prolonged cytopenias based on hematologic laboratory parameters, infections, non-infectious neurologic events, and tumor lysis syndrome) occurring ≤8 weeks postinfusion were considered to be of interest based on experience from ongoing tisagenlecleucel clinical studies.23 25 CRS, neurologic events, and neurologic episodes were defined per protocol (online supplemental file), and CRS was managed according to a protocol-specified algorithm (online supplemental table 1). Neurologic events were managed by the treating physician; the studies did not include a recommendation for corticosteroids. Prolonged cytopenias are defined as grade 3/4 cytopenias that are not resolved to grade ≤2 by day 28 postinfusion. Cytopenias occurring before tisagenlecleucel infusion were considered to be persistent if all reported laboratory results prior to infusion indicated grade 3/4 severity, with the last planned assessment occurring prior to the administration of lymphodepleting chemotherapy.
jitc-2020-002287supp001.pdf (1.2MB, pdf)
Statistical analysis
Duration of events was analyzed using the Kaplan-Meier method with unresolved events censored using the data cut-off date, death date, or discontinuation date, whichever came first. Nominal p values were calculated using χ2 tests for post hoc subgroup analyses, without adjusting for multiplicity. Time to resolution of grade 3/4 cytopenias to grade ≤2 were analyzed via the Kaplan-Meier method with unresolved events censored at last assessment, and 95% CIs were calculated using the log–log transformation within PROC LIFETEST (SAS V.9.3). Time from onset of remission to B cell recovery (≥1% CD19-positive cells in viable white cell count or ≥3% among lymphocytes in peripheral blood) was analyzed by the Kaplan-Meier method with continuing B cell aplasia censored at last assessment.
Results
Patients
Patient disposition and dosing in ELIANA (data cut-off: April 13, 2018) and ENSIGN (data cut-off: October 6, 2017) are described in table 1; demographics and baseline characteristics are shown in table 2. This analysis included 137 patients who received tisagenlecleucel infusion at a median of 43 days after enrollment and with a median follow-up of 24 months (table 1).
Table 1.
Disposition and dosing of infused patients
| Disposition | Patients |
| Received tisagenlecleucel infusion, n* | 137 |
| In ELIANA, n | 79 |
| In ENSIGN, n | 58 |
| Median time from enrollment to infusion, days (range) | 43 (24–133) |
| Median follow-up duration from infusion, months (range)† | 24 (0.1–36.5) |
| Median dose, CAR-positive viable T cells (range) | 1.0×108 (0.03×108–2.6×108) |
| In patients >50 kg (n=46), cells (range) | 1.7×108 (0.1×108–2.5×108) |
| In patients ≤50 kg (n=91), cells/kg body weight (range) | 3.3×106 (0.2×106–5.4×106) |
| Death following tisagenlecleucel infusion, n/N (%) | 44/137 (32) |
| ≤30 days postinfusion | 4 (3) |
| Leukemia progression | 2 (1) |
| AE‡ | 2 (1) |
| >30 days postinfusion | 40 (29) |
| Leukemia progression | 33 (24) |
| Other, prior to any further anticancer therapy§ | 2 (1) |
| Other, following treatment with additional anticancer therapy¶ | 5 (4) |
*Screening for ELIANA began April 8, 2015 at 25 sites in 11 countries across four continents.4 Screening for ENSIGN began August 14, 2014 at 13 sites in the USA.28
†Data cut-off dates were April 13, 2018 for ELIANA and October 6, 2017 for ENSIGN.
‡Due to cerebral hemorrhage possibly related to tisagenlecleucel treatment in the setting of coagulopathy on day 15, and embolic infectious stroke (mucormycosis) on day 25.
§Both were due to infections possibly related to tisagenlecleucel treatment: systemic candidiasis associated with prolonged pancytopenia on day 62, and HHV-6-positive encephalitis associated with a history of prolonged neutropenia and lymphopenia on day 53.
¶Due to pneumonia on day 506, veno-occlusive disease following HSCT on day 359, other complications from HSCT on day 461, acute respiratory failure on day 125, and unknown reason on day 464.
AE, adverse event; CAR, chimeric antigen receptor; HHV-6, human herpesvirus 6; HSCT, hematopoietic stem cell transplant.
Table 2.
Patient demographics and baseline clinical characteristics
| Characteristic, median (range) | All infused patients (n=137) |
| Age at screening, years | 12 (3–25) |
| Age category (years), n (%) | |
| 3–9 | 51 (37) |
| ≥10–<18 | 63 (46) |
| ≥18 | 23 (17) |
| Age at initial diagnosis, years | 7 (0–21) |
| Age category (years), n (%) | |
| 0–9 | 87 (64) |
| ≥10 | 50 (36) |
| Male sex, n (%) | 72 (53) |
| Disease status, n (%) | |
| Primary refractory | 11 (8) |
| Relapsed disease | 126 (92) |
| Prior HSCT, n (%) | 74 (54) |
| No. of previous lines of therapies | 3 (1–9) |
| Time from initial diagnosis to first relapse, months* | 32 (1–108) |
| Category, n (%) | (n=123) |
| <18 months | 30 (24) |
| 18–36 months | 42 (34) |
| >36 months | 51 (41) |
| Time from most recent relapse to infusion, months | 3 (1–14) |
| Blast count in bone marrow at enrollment, % | 73 (5.0–98.5) |
| CNS status classification at enrollment, n (%) | |
| CNS-1 | 118 (86) |
| CNS-2 | 17 (12) |
| CNS-3† | 1 (1) |
| Unknown | 1 (1) |
| Non-CNS extramedullary disease, n (%) | 16 (12) |
| Lymphodepleting chemotherapy, n (%)‡ | |
| Fludarabine and cyclophosphamide | 129 (94) |
| Cytarabine and etoposide | 3 (2) |
| None | 6 (4) |
Data are median (range) unless otherwise specified.
*Relapsed patients only.
†One patient had CNS-1 status at initial screening, CNS-3 status at time of enrollment, and CNS-1 status before tisagenlecleucel infusion.
‡One patient received both fludarabine/cyclophosphamide and cytarabine/etoposide.
CNS, central nervous system; HSCT, hematopoietic stem cell transplant.
Overall safety
Most patients (77%) experienced grade 3/4 AEs suspected to be related to tisagenlecleucel. The most common grade 3/4 tisagenlecleucel-related AEs were CRS (42%) and febrile neutropenia (28%). A complete listing of common (>20%) AEs suspected to be related to tisagenlecleucel is provided in online supplemental table 2. Certain AEs, such as grade 4 CRS, grade 3/4 neurologic events, and grade 3/4 infections, are of specific interest in this patient population (table 3). These complications were common in the first 8 weeks from infusion: grade 4 CRS developed in 22% of patients, grade 3/4 neurologic events occurred in 10% of patients, and grade 3/4 infections occurred in 19% of patients. There was no convincing evidence that the likelihood of these events depended on patient age, sex, or if the patient had previously undergone allogeneic HSCT (table 4). There were three patients who died due to AEs that were possibly related to tisagenlecleucel (cerebral hemorrhage in the setting of coagulopathy on day 15, systemic candidiasis associated with prolonged pancytopenia on day 62, and human herpesvirus-6 (HHV-6) encephalitis associated with a history of prolonged neutropenia and lymphopenia on day 53). An additional 35 (26%) patients died from leukemia progression and 5 (4%) patients died after treatment with additional anticancer therapy (table 1).
Table 3.
Specific AEs of interest occurring within 8 weeks after tisagenlecleucel infusion in >1 patient
| Specific AEs of interest, n (%) | All infused patients (n=137) | ||
| Any grade | Grade 3 | Grade 4 | |
| CRS* | 108 (79) | 27 (20) | 30 (22) |
| Neurologic events† | 50 (36)‡ | 14 (10)§ | 0 |
| Confusion | 13 (9) | 0 | 0 |
| Encephalopathy | 12 (9) | 6 (4) | 0 |
| Delirium | 11 (8) | 3 (2) | 0 |
| Agitation | 7 (5) | 0 | 0 |
| Tremor | 7 (5) | 0 | 0 |
| Somnolence | 6 (4) | 2 (1) | 0 |
| Hallucination | 5 (4) | 0 | 0 |
| Irritability | 5 (4) | 0 | 0 |
| Seizure | 5 (4) | 2 (1) | 0 |
| Mental status changes | 4 (3) | 1 (1) | 0 |
| Cognitive disorder | 3 (2) | 1 (1) | 0 |
| Dysarthria | 3 (2) | 1 (1) | 0 |
| Lethargy | 3 (2) | 0 | 0 |
| Muscular weakness | 3 (2) | 1 (1) | 0 |
| Depressed level of consciousness | 2 (1) | 1 (1) | 0 |
| Dysphagia | 2 (1) | 2 (1) | 0 |
| Febrile neutropenia¶ | 46 (34) | 44 (32) | 2 (1) |
| Prolonged cytopenias** | 55 (40) | 21 (15) | 25 (18) |
| Prolonged white cell count decrease | 23 (17) | 7 (5) | 11 (8) |
| Prolonged neutrophil count decrease | 15 (11) | 2 (1) | 11 (8) |
| Prolonged platelet count decrease | 15 (11) | 4 (3) | 8 (6) |
| Prolonged thrombocytopenia | 11 (8) | 3 (2) | 7 (5) |
| Prolonged anemia | 9 (7) | 5 (4) | 0 |
| Prolonged lymphocyte count decrease | 9 (7) | 3 (2) | 3 (2) |
| Prolonged neutropenia | 7 (5) | 2 (1) | 4 (3) |
| Prolonged febrile neutropenia¶ | 4 (3) | 4 (3) | 0 |
| Prolonged lymphopenia | 2 (1) | 2 (1) | 0 |
| Prolonged pancytopenia | 2 (1) | 2 (1) | 0 |
| Infections¶ | 58 (42) | 22 (16) | 4 (3) |
| Viral infectious disorders | 19 (14) | 5 (4) | 1 (1) |
| Rhinovirus infection | 5 (4) | 0 | 0 |
| Oral herpes | 2 (1) | 1 (1) | 0 |
| Herpes simplex | 2 (1) | 1 (1) | 0 |
| Human herpesvirus 6 infection | 2 (1) | 1 (1) | 0 |
| Encephalitis viral | 2 (1) | 1 (1) | 1 (1) |
| Gastroenteritis norovirus | 2 (1) | 0 | 0 |
| Bacterial infectious disorders | 24 (18) | 13 (9) | 0 |
| Staphylococcal infection | 7 (5) | 3 (2) | 0 |
| Staphylococcal bacteremia | 3 (2) | 3 (2) | 0 |
| Clostridium difficile infection | 5 (4) | 3 (2) | 0 |
| Clostridium difficile colitis | 4 (3) | 1 (1) | 0 |
| Fungal infectious disorders | 8 (6) | 2 (1) | 1 (1) |
| Candida infection | 3 (2) | 0 | 1 (1) |
| Oral candidiasis | 2 (1) | 0 | 0 |
| Infections-pathogen unspecified | 29 (21) | 8 (6) | 2 (1) |
| Conjunctivitis | 5 (4) | 0 | 0 |
| Pneumonia | 3 (2) | 2 (1) | 0 |
| Oral infection | 2 (1) | 0 | 0 |
| Gastroenteritis | 2 (1) | 1 (1) | 0 |
| Nail infection | 2 (1) | 0 | 0 |
| Tumor lysis syndrome | 5 (4) | 5 (4) | 0 |
*Graded according to the University of Pennsylvania grading scale.21 22
†Neurologic events is a group term for events under the standard Medical Dictionary for Regulatory Activities queries for non-infectious encephalopathy and delirium; headache is not included in the definition. The specific events listed are those that occurred in >1 patient.
‡Twelve grade 1/2 neurologic events in eight patients were unresolved at time of death (n=6) or data cut-off (n=2). Median duration was 23 (range, 1‒62) days. Five events were assessed as related to tisagenlecleucel (confusion, encephalopathy, dysarthria, tremor, and agitation).
§Two grade 3 neurologic events in two patients were unresolved at time of death: muscular weakness (8 days) and dysarthria (8 days); neither was assessed as related to tisagenlecleucel.
¶Compared with febrile neutropenia reported as an AE, grade 3/4 neutropenia with fever ≥38.3°C occurred in 63% of patients within 8 weeks after infusion.
**Prolonged cytopenias are defined as grade 3/4 cytopenias that are not resolved to grade ≤2 by day 28 postinfusion. The specific events listed are those that occurred in >1 patient.
AE, adverse event; CRS, cytokine-release syndrome.
Table 4.
Specific AEs of interest by patient subgroups
| Subgroup | Patients, n | AEs within 8 weeks after tisagenlecleucel infusion, N (%) | ||
| Grade 4 CRS* | Grade 3/4 neurologic events | Grade 3/4 infections | ||
| All infused | 137 | 30 (22) | 14 (10) | 26 (19) |
| Age, years | ||||
| 3–9 | 51 | 11 (22) | 6 (12) | 11 (22) |
| ≥10–<18 | 63 | 13 (21) | 5 (8) | 8 (13) |
| ≥18 | 23 | 6 (26) | 3 (13) | 7 (30) |
| Sex | ||||
| Male | 72 | 16 (22) | 5 (7) | 11 (15) |
| Female | 65 | 14 (22) | 9 (14) | 15 (23) |
| Prior HSCT | ||||
| Yes | 74 | 13 (18) | 5 (7) | 14 (19) |
| No | 63 | 17 (27) | 9 (14) | 12 (19) |
*Graded according to the University of Pennsylvania grading scale.21 22 Data for patients with grade 4 CRS only are shown to better characterize the most severe events.
AE, adverse event; CRS, cytokine-release syndrome; HSCT, hematopoietic stem cell transplant.
Cytokine-release syndrome
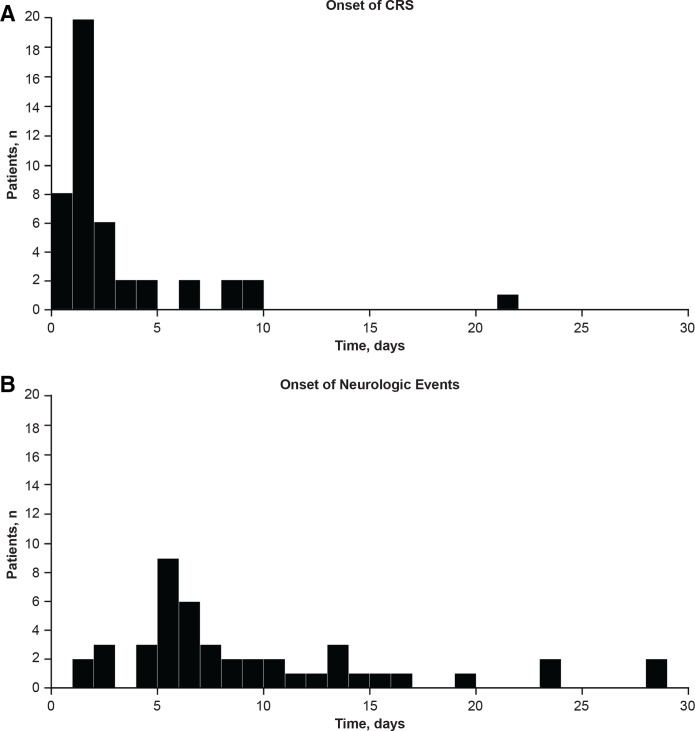
CRS occurred in 108 (79%) patients (table 3), with all but three (3%) of cases occurring within 14 days of infusion. The median time to CRS onset was 3 (range: 1–22) days and to resolution was 8 (range: 1–36) days. The median time to resolution of grade 3/4 CRS was 10 (range: 4–36) days (table 5). CRS recurred in four patients 2–5 days after the resolution of the first episode and at the same grade (grades 2–3) as the first episodes. CRS was managed using a protocol-specified algorithm (online supplemental table 1). Anti-cytokine therapy, including tocilizumab, was administered in 44 (41%) patients. Of the 43 patients who received tocilizumab, 42 (98%) had grade 3–4 CRS and 1 patient had grade 2 CRS. In the patients who received tocilizumab, the median onset of CRS was 2 (range: 1–22) days, median time to administration of tocilizumab from onset of CRS was 5 (range: 1–19) days, and the median duration of CRS was 11 (range: 5–33) days. There were no deaths attributed to CRS.
Table 5.
CRS characterization
| Characteristic, median (range) | Patients with CRS (N=108) |
| Time to onset, days | 3 (1–22) |
| Time from onset of CRS to grade 4 CRS, days | 4.5 (1–18) |
| Duration, days | |
| Any grade CRS | 8 (1–36) |
| Grade 3/4 CRS | 10 (4–36) |
| Fever ≥38.6°C, n (%) | 103 (95) |
| Time to onset, days | 3 (1–22) |
| Duration, days | 6 (1–36) |
| ICU admission, n (%) | 58 (54) |
| Time to ICU admission, days | 6 (1–24) |
| Duration of ICU stay, days | 7.5 (1–66)* |
| Anticytokine therapy, n (%)† | 44 (41) |
| Tocilizumab | 43 (40) |
| 1 dose | 23 (21) |
| 2 doses | 14 (13) |
| 3 doses | 6 (6) |
| Corticosteroids | 25 (23) |
| Siltuximab | 5 (5) |
| Other | 7 (6) |
| Hypotension that required intervention, n (%) | 60 (56) |
| High-dose vasopressors, n (%)‡ | 33 (31) |
| Oxygen supplementation, n (%) | 54 (50) |
| Intubation, n (%) | 18 (17) |
| Duration of intubation, days | 8 (4–26) |
| Dialysis, n (%)§ | 12 (11) |
| Duration of dialysis, days | 13.5 (2–61) |
| Fibrinogen <1.0 g/L, n (%) | 9 (8) |
| Outcome, n (%) | |
| Recovered/resolved | 106 (98)¶ |
| Not recovered/resolved | 2 (2)** |
Only the first CRS episode is summarized for each patient. Data are median (range) unless otherwise specified.
*Excluding outliers (ie, one patient with an ICU stay of 66 days), median (range) is 7 (1–34) days.
†Administered per the protocol-specific CRS management algorithm (online supplemental table 1). 24 of the 25 patients who received corticosteroids also received tocilizumab. All five patients who received siltuximab also received tocilizumab and corticosteroids. Of the seven patients who received ‘other’ anticytokine therapy, all experienced grade 4 CRS and received etanercept in addition to tocilizumab and corticosteroids, two also received siltuximab, and one also received infliximab.
‡High-dose vasopressors (defined in the University of Pennsylvania grading scale21 22) included vasopressin, norepinephrine, dopamine, phenylephrine, and epinephrine.
§Patients were dialyzed during CRS to manage fluid overload and/or acute kidney injury with only five patients having grade 3/4 creatinine elevation.
¶In one patient, CRS resolved, but the patient died due to HHV-6 encephalitis.
**Both patients died due to leukemia progression with CRS ongoing.
CRS, cytokine-release syndrome; HHV-6, human herpesvirus-6; ICU, intensive care unit.
Grade 4 CRS occurred in 27% (25/94) of patients who had ≥50% bone marrow blasts at the time of enrollment and in 12% (5/43) of patients with <50% blasts (p=0.081), but these results need to be interpreted cautiously as tumor burden at the time of tisagenlecleucel infusion was not assessed and patients received bridging treatment between enrollment and infusion. Hypotension that required intervention developed in 56% of patients with CRS and high dose vasopressors were used in 31%. Fifty per cent of patients received oxygen supplementation and 17% were intubated. Hypofibrinogenemia was seen in 8% (9/108) of patients with CRS; hypofibrinogenemia occurred more frequently and was most severe in patients with grade 4 CRS (online supplemental table 3).
Neurologic events
Within 8 weeks after infusion, 50 patients (36%) experienced 55 neurologic episodes, defined as overlapping or successive neurologic events, at a median of 8 (range: 2‒53) days from infusion. Common neurologic toxicities during this period are listed in table 3. The median time to resolution was 6 days for episodes of any grade and 10.5 days for grade 3/4 episodes. Most (91% (50/55)) of these episodes occurred in patients who also developed CRS but not necessarily at the same time. Three patients developed neurologic symptoms up to 2 days prior to CRS, 35 patients developed neurologic symptoms during CRS (median time to onset, 7 (range: 2‒29) days postinfusion), and 9 patients developed neurologic symptoms after resolution of CRS. The post-CRS symptoms developed at a median of 5 (range: 1‒39) days after CRS resolution. The time of onset of CRS and neurologic events in patients who experienced both events are shown in figure 1.
Figure 1.
Time of onset of CRS and neurologic events among patients who experienced both events. CRS, cytokine-release syndrome.
Neurologic events occurring ≤8 weeks postinfusion correlated with CRS severity (p<0.001; online supplemental figure 1 and table 4). These neurologic events occurred in 57% (13/23) of patients with a history of preinfusion neurologic events (eg, central nervous system hemorrhage, cerebrovascular accident, and non-infectious encephalopathy/delirium in medical history) versus 32% (37/114) of patients without such disease history (p=0.051). However, neither a prior diagnosis of CNS leukemia (yes: 37% vs no: 37%) nor prior history of CNS radiotherapy (yes: 31% vs no: 42%) correlated with frequency of neurologic events (although it should be noted that patients with CNS-3 leukemia were excluded). Neurologic events occurring >8 weeks postinfusion were rare (occurring in 4.3% of 116 patients >8 weeks to 1 year postinfusion and in 1.7% of 59 patients >1 year postinfusion). Neurologic events were managed with supportive care and anticonvulsants (online supplemental table 5).
Prolonged grade 3/4 cytopenias
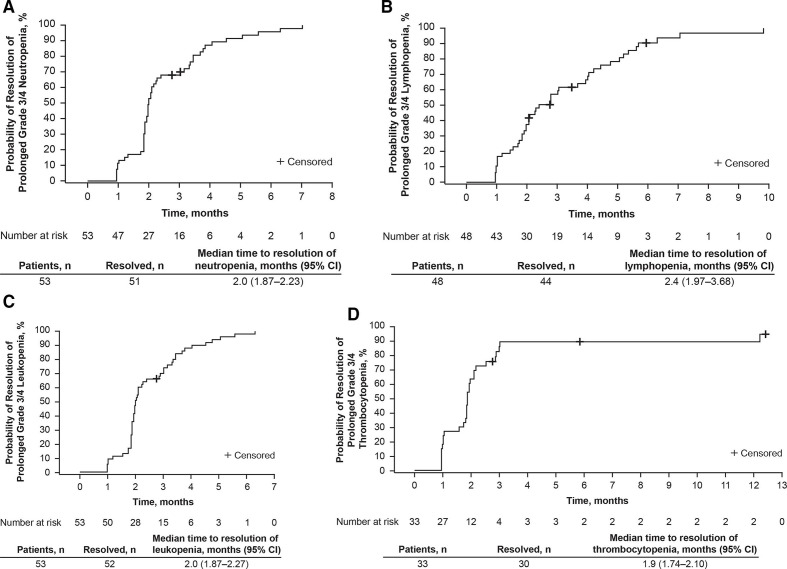
All patients experienced cytopenias postinfusion. Median time to resolution of grade 3/4 cytopenia to grade ≤2 was 2.0 (95% CI 1.87 to 2.23) months for neutropenia, 2.4 (95% CI 1.97 to 3.68) months for lymphopenia, 2.0 (95% CI 1.87 to 2.27) months for leukopenia, 1.9 (95% CI 1.74 to 2.10) months for thrombocytopenia, and 1.0 (95% CI 0.95 to 1.87) month for anemia (figure 2). Prolonged thrombocytopenia was more common in patients with thrombocytopenia before infusion compared with those who had normal platelet counts at the time of infusion (11/14 (79%) vs 47/123 (38%); p=0.009). Prolonged neutropenia was also more common in patients who were neutropenic prior to infusion compared with patients who were not (24/31 (77%) vs 57/106 (54%); p=0.03).
Figure 2.
Kaplan-Meier analysis of time to resolution of prolonged grade 3/4 (A) neutropenia,* (B) lymphopenia,† (C) leukopenia,‡ and (D) thrombocytopenia§ to grade 2 or better in patients with response (CR/CRi) and indicated cytopenia at day 28 after tisagenlecleucel infusion. *CTCAE grading, neutrophils/mm3: grade 1: <LLN to 1500; grade 2: 1000–<1500; grade 3: 500–<1000; grade 4: <500. †CTCAE grading, lymphocytes/mm3: grade 1: <LLN to 800; grade 2: 500–<800; grade 3: 200–<500; grade 4: <200. ‡CTCAE grading, WBC/mm3: grade 1: <LLN to 3000; grade 2: 2000–<3000; grade 3: 1000–<2000; grade 4: <1000. §CTCAE grading, platelets/mm3: grade 1: <LLN to 75,000; grade 2: 50,000–<75,000; grade 3: 25,000–<50,000; grade 4: <25,000. CR, complete remission; CRi, complete remission with incomplete hematologic recovery; CTCAE, Common Terminology Criteria for Adverse Events; LLN, lower limit of normal; NE, not estimable; WBC, white blood cell.
B cell aplasia was seen in all patients with CR/CR with incomplete hematologic recovery (CRi) and was managed with immunoglobulin replacement therapy per institutional guidelines. An estimated 66% of evaluable patients in ongoing response at both 12 and 24 months continued to have B cell aplasia (online supplemental figure 2). Since immunoglobulin replacement was often initiated prior to observation of hypogammaglobulinemia in patients with B cell aplasia, the rate of hypogammaglobulinemia (57/237 patients (42%)) is lower than that of B cell aplasia.
Infections
Infections developed in 49/137 (36%) patients ≤4 weeks postinfusion (grade 3/4, 14%) and in 18/126 (14%) patients 4‒8 weeks postinfusion (grade 3/4, 8%; table 3). Twenty-eight of 81 (35%) patients with prolonged grade 3/4 neutropenia developed grade 3/4 infections after day 28. Three patients in ELIANA had fatal infections (systemic candidiasis on day 62 in the setting of prolonged grade 3/4 pancytopenia, HHV-6 encephalitis on day 53 in the setting of prolonged grade 3/4 neutropenia and lymphopenia, and lower respiratory tract infection on day 506 following treatment with additional anticancer therapy). Among those with ≥1-year follow-up, 7/59 patients (12%) reported grade 3/4 infections ≥1 year postinfusion in the absence of leukemia relapse, all of which resolved. These include respiratory infections (upper respiratory tract infection, respiratory syncytial viral bronchiolitis, and pneumonia), otitis media, bacterial meningitis (pneumococcal), herpes zoster, sepsis, and Campylobacter and Clostridium difficile infection. All patients were receiving immunoglobulin. The case of bacterial meningitis occurred in a patient on regular immunoglobulin replacement and was caused by a pneumococcal serotype not covered by the 23 polyvalent vaccine.
Other events
Cardiac toxicities primarily developed during the first 8 weeks of the study (43/137, 31%; grade 3/4, 7%) and were rare thereafter (7/116 (6%) from 8 weeks to 1 year postinfusion; none thereafter). The most common cardiac toxicity was tachycardia (26%; online supplemental table 6); cardiac events of interest included arrhythmia, cardiac dysfunction, valvular dysfunction, and pericardial effusion. Importantly, cardiac events were transient and resolved in all survivors with the exception of one patient who had ongoing left ventricular dysfunction at the time of last contact.
Acute kidney injury developed in 32/137 (23%) patients (grade 3/4, 14%) and was largely transient. Twelve patients required dialysis during CRS (table 5). Reversible grade 3/4 liver function abnormalities including increased levels of aspartate aminotransferase (16%; all had CRS), alanine aminotransferase (14%; all had CRS), and total bilirubin (9%; 11/12 had CRS) were reported.
Among the 74 patients with prior HSCT, GVHD was uncommon following tisagenlecleucel infusion. Two cases (3%) were reported, including a case of grade 1 skin GVHD on day 26, and a case of grade 2 gastrointestinal GVHD on day 73, both of which resolved.
No replication-competent lentivirus was detected in any patient postinfusion. Secondary malignancies were reported in three patients, one with an underlying TP53 germline mutation who later developed glioblastoma multiforme and two with myelodysplastic syndrome (one of which was transient and completely resolved within 3.5 months). No cases of secondary malignancy related to insertional mutagenesis were observed.
Discussion
This pooled safety analysis from two phase 2 multicenter studies in patients with relapsed or refractory B-ALL is the longest follow-up of the largest dataset in this patient population and provides a robust investigation of the tolerability and safety profile for tisagenlecleucel occurring during these trials and their management strategies. Given the evolution in grading of CRS and management of CAR T therapy-related AEs since the time of these studies, these data represent a historical reference point for future research in this patient population and help guide the management of complications for patients receiving treatment outside of clinical trials. In these analyses, we identified risk factors for common AEs following tisagenlecleucel infusion. In particular, patients with high tumor burden at enrollment had higher rates of severe CRS than patients with low tumor burden; patients with severe CRS were more likely to experience neurologic events compared with patients without CRS; and patients with preinfusion thrombocytopenia or neutropenia were more likely to experience prolonged cytopenias postinfusion. It should be noted that the nominal p values provided in this analysis were not adjusted for multiplicity and should be interpreted with caution. Toxicities typically occurred ≤8 weeks postinfusion, decreased thereafter and, with the exception of B cell aplasia, were usually transient. No replication-competent lentivirus was detected in any patients and no cases of secondary malignancy related to insertional mutagenesis were observed. Death ≤30 days postinfusion occurred in 3% of patients, half of which were attributed to leukemia progression.
This study used the University of Pennsylvania (Penn) CRS grading scale.21 22 Other scales, such as the CRS Revised Grading System developed by Lee et al32 or the ASTCT CRS Consensus Grading scale33 are also in use and a recent analysis of adult patients with relapsed or refractory DLBCL who experienced CRS following tisagenlecleucel infusion34 demonstrated that the Penn scale graded CRS higher than the Lee or ASTCT scales. Higher tumor burden has been demonstrated as a risk factor for more severe CRS symptoms regardless of the grading scale used.35 36 To safely employ a therapy associated with known severe, but reversible, CRS in multiple centers worldwide, we developed a CRS treatment algorithm (online supplemental table 1) and a specialized site training program, with ultimately no deaths attributable to CRS. Interleukin 6 receptor blockade with tocilizumab along with corticosteroids and appropriate supportive care resulted in rapid improvement of symptoms in most patients, similar to prior trials.3 37 38
Many individual AEs are associated with CRS, such as fever, febrile neutropenia, tachycardia, and coagulopathy, and may be under-reported due to collection under the syndrome ‘CRS’ as opposed to being reported separately, a potential limitation of this analysis.
Neurologic events were common, strongly associated with CRS, occurred mostly during CRS or shortly after its resolution, and their frequency and severity increased with higher grade CRS. Although we did not find evidence that a prior history of CNS leukemia was a risk for neurologic events, it should be noted that patients with active CNS leukemia at screening were excluded. Based on prior clinical trial experience with tisagenlecleucel and observations during the current trials, we developed clinical guidelines to support the management of neurotoxicity associated with tisagenlecleucel. We recommend a thorough baseline neurologic evaluation prior to treatment with tisagenlecleucel, particularly in patients with a history of pre-existing CNS disorder or leukemia. Prophylactic anticonvulsants (eg, levetiracetam) may be considered in patients with history of seizure, prior neurotoxicity, or history of focal CNS symptoms or lesions, as these may increase the risk of neurotoxicity39 and create a focus for seizure activity. As subclinical seizures can contribute to encephalopathy, we recommend continuous electroencephalogram be considered in patients with depressed level of consciousness. At the time these studies were conducted, corticosteroids were not recommended except in cases of severe CRS refractory to tocilizumab due to the concern for potential cytolytic effects on CAR T cells. Since these studies were completed, corticosteroids, siltuximab, and anakinra have been reported to aid the management of neurologic events associated with other CAR T cell treatments.19 40 41
Cardiac events, including arrhythmia, cardiac dysfunction, valvular dysfunction, and pericardial effusion, mostly occurred ≤8 weeks postinfusion (especially during CRS) and resolved in a large proportion of patients; there were no such treatment-related events >8 weeks postinfusion. The mechanisms behind cardiovascular toxicity associated with CAR T cell therapies are not well understood, although they may be a consequence of CRS.42 43 Data from retrospective analyses show that these events occur early and are associated with CRS,43 consistent with our results. A recent analysis of patients who survived ≥1 year following CAR T cell infusion did not describe any cardiovascular toxicities occurring or continuing ≥90 days after infusion.44
Prolonged cytopenias can occur with chemoimmunotherapies and CAR T cell therapies.44–46 Our pooled analyses demonstrated that pre-existing cytopenia is a risk factor for prolonged cytopenias, most infections occurred during the prolonged but ultimately transient neutropenia and lymphopenia that developed in most patients, and that infections were rarely fatal.
B cell aplasia, which serves as a surrogate for CD19-directed CAR T cell function, occurred in all responding patients from antigen-specific, on-target, off-tumor effect on CD19-positive B cells and was prolonged in many cases in this analysis and in the previous analysis from ELIANA.4 In this population of children and young adults with B-ALL, immunoglobulin replacement was used in nearly all patients with CR/CRi. As a result, a high rate of hypogammaglobulinemia was not observed. Taken together with the low rate of grade 3/4 infections occurring >1 year postinfusion, these data suggest that the risk of life-threatening infections in this population can be managed with intravenous immunoglobulin replacement and appropriate supportive care therapy. Data from a phase 1/2 clinical trial of CD19 CAR T cells in patients with relapsed or refractory ALL, non-Hodgkin lymphoma, or chronic lymphocytic leukemia also demonstrated that the majority of late infections were mild.44 Of note, evaluation of longer term outcomes related to prolonged B cell aplasia is limited by the current length of follow-up in these studies. Long-term safety events are being monitored for up to 15 years postinfusion, as required by health authorities.
The severity of hypofibrinogenemia occurring in patients with CRS in our analysis correlated with CRS severity, consistent with other studies of CD19-targeted CAR T cell therapy.35 47 48 Clinical guidelines were developed for the use of fibrinogen replacement to treat tisagenlecleucel-associated coagulopathy with hypofibrinogenemia.49
Our pooled analysis also allowed for the evaluation of rare events such as GVHD, which occurred in only two patients in these studies. Nevertheless, we recommend avoiding leukapheresis for CAR T cell manufacture in patients with active GVHD. Furthermore, the current recommendation is to stop systemic GVHD therapies for ≥14 days prior to leukapheresis, to confirm no grade 2–4 acute or extensive chronic GVHD occurred after leukapheresis, and to ensure that ≥12 weeks had elapsed from HSCT at the time of leukapheresis.
Overall, results of this large pooled safety analysis from two multicenter studies of tisagenlecleucel establish a comprehensive safety profile in children and young adults with B-ALL in the context of the clinical trials performed prior to regulatory approvals,3 including characterization of rare and long-term events. This analysis, the longest follow-up of the largest dataset in this patient population, provides guidance to clinicians who care for these patients. Although the AEs of tisagenlecleucel can be severe, they are manageable and do resolve. Site training and adherence to a defined CRS treatment algorithm are of key importance, as are standard supportive measures for infections, coagulopathy, and cytopenias. Long-term follow-up shows that late events are rare. Further understanding of the pathogenesis of CAR T related AEs and data from real-world analyses will guide refinement of treatment or prevention in the future.
Acknowledgments
We would like to thank all the patients and their families and caregivers for participating in these studies, as well as the investigators and site personnel. Additionally, we would like to thank Abhijit Agarwal of Novartis Pharmaceuticals Corporation (East Hanover, New Jersey, USA) for ongoing support and review of the manuscript. Medical writing assistance was provided by Beena John, PhD, and Rozena Varghese, PharmD, CMPP, of ICON plc (North Wales, Pennsylvania, USA).
Footnotes
Twitter: @gruppsteve
Deceased: PAW is deceased.
Contributors: All authors substantially contributed to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and the drafting of the work or revising it critically for important intellectual content; and provided final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: These studies and writing assistance were funded by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. Representatives of the sponsor participated in study design, collection, data interpretation, and development/submission of the manuscript.
Competing interests: JEL has received research and/or clinical trial support from Incyte, Kamada, Mesoblast, and Biogen and has participated in consulting, study steering committees, or scientific/clinical advisory boards for Novartis, bluebird bio, Incyte, Ironwood, Mesoblast, Omeros, Oncoimmune, Talaris, and X4 Pharmaceuticals. SAG has received research and/or clinical trial support from Novartis, Servier, and Kite and has participated in consulting, study steering committees, or scientific/clinical advisory boards for Novartis, Cellectis, Adaptimmune, Eureka, TCR2, Juno, GlaxoSmithKline, Vertex, Cure Genetics, Humanigen, and Roche. MAP has participated in steering committees for the ENSIGN and ELIANA trials for Novartis, advisory boards, and educational activities for Novartis. ACD is a current employee of bluebird bio; bluebird bio has had no support or oversight of this research or manuscript. SR has received clinical trial support from Novartis, Servier, and Celgene and has participated in consulting, study steering committees, or scientific/clinical advisory boards for Novartis, Servier, Celgene, Cellectis, Kite/Bristol-Myers Squibb, JazzPharma, and Amgen. GDM has received payment and honoraria as a consultant to Novartis Pharma and for serving on the speaker’s bureau for Kymriah. KJA has participated in a speaker bureau and received travel accommodations and expenses from Novartis. MRV has participated in advisory boards for Novartis, Fate Therapeutics, and B-Mogen and has stock options from Fate Therapeutics and B-Mogen. JB has participated in study steering committees, advisory boards, and educational activities for Novartis, and advisory boards for Kite and Janssen. TWL has consulted for Novartis, Cellectis, Loxo Oncology, Eli Lilly, and Bayer; has received research funding from Novartis, Pfizer, and Bayer; and is currently affiliated with the Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA and the Division of Oncology, Center for Childhood Cancer Research and Cancer Immunotherapy Program, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA. HB has participated in consulting for Novartis and Jazz Pharmaceuticals and his department has received financial reimbursement for participation in tisagenlecleucel trials for Novartis. AB has received research and/or clinical trial support from Novartis, Servier, and Kite and participated in consulting, study steering committees, or scientific/clinical advisory boards for Novartis, Servier, Celgene, Jazz Pharma, AstraZeneca, Janssen, and Amgen. MWB has participated in advisory boards for Novartis and Thunder Biotech. BDM has received a travel grant from Jazz Pharma; her department has received financial reimbursement and compensation for participation in CTL019 trials for Novartis, and consulting for Novartis. MQ has received support for service on clinical advisory boards for Novartis and Bristol-Myers Squibb. SMD has received research support from Alexion Pharmaceuticals and served in a consulting role for Novartis. CLP has received support for service on clinical advisory board for Novartis.TAD has no conflicts to declare. PB has received institutional research grants from medac, Riemser, and Neovii and institutional compensation for advisory activity and speakers bureau from Miltenyi, Amgen, Novartis, Servier, medac, and Riemser. KS has no conflicts to declare. PAW was an employee of Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. RM has no conflicts to declare. LY is an employee of Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. ML is an employee of Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. LKE is an employee of Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. CHJ has received research support from Novartis and Tmunity Therapeutics. SLM has received clinical trial support from Novartis and has served in a consulting role, on advisory boards, or on study steering committees for Novartis, Kite, and Wugen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: The authors note with sadness the passing of their colleague Patricia A Wood on October 25, 2019.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided is anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Both studies were approved by institutional review boards at each participating institution and regulatory agencies. Patients and/or their guardians provided written informed consent. Patients/the public did not participate in study design or dissemination. Details of these approvals have been published elsewhere.
References
- 1.Kymriah (tisagenlecleucel) . Full prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2018. [Google Scholar]
- 2.Kymriah (tisagenlecleucel) . Summary of product characteristics. Dublin, Ireland: Novartis Europharm Limited; 2018. [Google Scholar]
- 3.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–54. 10.1056/NEJMoa1708566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56. 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 7.Locke FL, Ghobadi A, Jacobson CA, et al. Long-Term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019;20:31–42. 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2020;382:1331–42. 10.1056/NEJMoa1914347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah BD, Bishop MR, Oluwole OO, et al. End of phase I results of ZUMA-3, a phase 1/2 study of KTE-X19, anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in adult patients (pts) with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL). J Clin Oncol 2019;37:7006. 10.1200/JCO.2019.37.15_suppl.7006 [DOI] [Google Scholar]
- 10.Wayne A, Huynh V, Hijiya N. ZUMA-4 phase 1: KTE-x19, an anti-CD19 CAR T cell therapy, in children and adolescents with R/R B-ALL. Pediatric Blood and Cancer 2019;66:S23–4. [Google Scholar]
- 11.Jacobson CA, Chavez JC, Sehgal AR, et al. Interim analysis of zuma-5: a phase 2 study of axicabtagene ciloleucel (AXI-cel) in patients with relapsed/refractory indolent non-Hodgkin lymphoma. HemaSphere 2020;4:105. [Google Scholar]
- 12.Abramson J, Palomba ML, Gordon L. Safety and efficacy results from transcend NHL 001, a multicenter phase 1 study of lisocabtagene maraleucel (Liso-cel) in relapsed/refractory (R/R) large B-cell lymphoma (LBCL). Oncolo Res Treatment 2020;43:215. [Google Scholar]
- 13.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med 2019;380:1726–37. 10.1056/NEJMoa1817226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berdeja JG, Alsina M, Shah ND, et al. Updated results from an ongoing phase 1 clinical study of bb21217 anti-Bcma CAR T cell therapy. Blood 2019;134:927. 10.1182/blood-2019-126660 [DOI] [Google Scholar]
- 15.San Miguel J, Shah N, Oriol A, et al. Idecabtagene vicleucel (IDE-CEL; BB2121), a BCMA-targeted CAR T cell therapy, in patients with relapsed and refractory multiple myeloma: initial karmma results. HemaSphere 2020;4:61–2. [Google Scholar]
- 16.Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 2019;134:2361–8. 10.1182/blood.2019001641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol 2020;38:1938–50. 10.1200/JCO.19.03279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teachey DT, Bishop MR, Maloney DG, et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit 'ALL'. Nat Rev Clin Oncol 2018;15:218. 10.1038/nrclinonc.2018.19 [DOI] [PubMed] [Google Scholar]
- 19.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. 10.1038/nrclinonc.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. JCO 2016;34:3011. 10.1200/JCO.2016.34.15_suppl.3011 [DOI] [Google Scholar]
- 21.Porter DL, Hwang W-T, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139. 10.1126/scitranslmed.aac5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter D, Frey N, Wood PA, et al. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol 2018;11:35. 10.1186/s13045-018-0571-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maude SL, Pulsipher MA, Boyer MW, et al. Efficacy and safety of CTL019 in the first US phase II multicenter trial in pediatric relapsed/refractory acute lymphoblastic leukemia: results of an interim analysis. Blood 2016;128:2801. 10.1182/blood.V128.22.2801.2801 [DOI] [Google Scholar]
- 24.Maude SL, Grupp SA, Mody R, et al. An updated analysis of tisagenlecleucel in pediatric/ young adult patients with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) in a US multicenter clinical trial (ENSIGN). HemaSphere 2018;2:41. [Google Scholar]
- 25.Grupp SA, Maude SL, Rives S, et al. Updated analysis of the efficacy and safety of Tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (r/r) acute lymphoblastic leukemia. Blood 2018;132:895. 10.1182/blood-2018-99-112599 [DOI] [Google Scholar]
- 26.Tyagarajan S, Schmitt D, Acker C, et al. Autologous cryopreserved leukapheresis cellular material for chimeric antigen receptor-T cell manufacture. Cytotherapy 2019;21:1198–205. 10.1016/j.jcyt.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 27.Tyagarajan S, Spencer T, Smith J. Optimizing CAR-T cell manufacturing processes during pivotal clinical trials. Mol Ther Methods Clin Dev 2020;16:136–44. 10.1016/j.omtm.2019.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US National Institutes of Health . Study of efficacy and safety of CTL019 in pediatric ALL patients (NCT02228096). Available: https://clinicaltrials.gov/ct2/show/NCT02228096 [Accessed 29 Oct 2018].
- 29.US Department of Health and Human Services . Common terminology criteria for adverse events (CTCAE) version 4.03. Available: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf [Accessed 29 Oct 2018].
- 30.Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J Rare Dis 2007;2:35. 10.1186/1750-1172-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blood and Marrow Transplant Clinical Trials Network . Definitions of chronic GVHD. Available: https://web.emmes.com/study/bmt2/public/Definition/Definition_of_Chronic_GVHD.pdf [Accessed 29 Oct 2018].
- 32.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–95. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–38. 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuster SJ, Maziarz RT, Rusch ES, et al. Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. Blood Adv 2020;4:1432–9. 10.1182/bloodadvances.2019001304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay KA, Hanafi L-A, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017;130:2295–306. 10.1182/blood-2017-06-793141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tedesco VE, Mohan C. Biomarkers for predicting cytokine release syndrome following CD19-Targeted CAR T cell therapy. J Immunol 2021;206:1561–8. 10.4049/jimmunol.2001249 [DOI] [PubMed] [Google Scholar]
- 37.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–33. 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509–18. 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gofshteyn JS, Shaw PA, Teachey DT, et al. Neurotoxicity after CTL019 in a pediatric and young adult cohort. Ann Neurol 2018;84:537–46. 10.1002/ana.25315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Topp M, Van Meerten T, Houot R, et al. Earlier steroid use with axicabtagene ciloleucel (axi-cel) in patients with relapsed/refractory large B cell lymphoma. Blood 2019;134:243. 10.1182/blood-2019-126081 [DOI] [Google Scholar]
- 41.Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv 2020;4:3123–7. 10.1182/bloodadvances.2020002328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamal FA, Khaled SK. The cardiovascular complications of chimeric antigen receptor T cell therapy. Curr Hematol Malig Rep 2020;15:130–2. 10.1007/s11899-020-00567-4 [DOI] [PubMed] [Google Scholar]
- 43.Ghosh AK, Chen DH, Guha A. CAR T cell therapy–related cardiovascular outcomes and management: systemic disease or direct cardiotoxicity? JACC CardioOncol 2020;2:97–109. 10.1016/j.jaccao.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant 2020;26:26–33. 10.1016/j.bbmt.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill S, Carney D, Ritchie D, et al. The frequency, manifestations, and duration of prolonged cytopenias after first-line fludarabine combination chemotherapy. Ann Oncol 2010;21:331–4. 10.1093/annonc/mdp297 [DOI] [PubMed] [Google Scholar]
- 46.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321–30. 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang H, Liu L, Guo T, et al. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol 2019;98:1721–32. 10.1007/s00277-019-03685-z [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Qi K, Cheng H, et al. Coagulation disorders after chimeric antigen receptor T cell therapy: analysis of 100 patients with relapsed and refractory hematologic malignancies. Biol Blood Marrow Transplant 2020;26:865–75. 10.1016/j.bbmt.2019.11.027 [DOI] [PubMed] [Google Scholar]
- 49.Buechner J, Grupp SA, Hiramatsu H, et al. Practical guidelines for monitoring and management of coagulopathy following tisagenlecleucel CAR T-cell therapy. Blood Adv 2021;5:593–601. 10.1182/bloodadvances.2020002757 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-002287supp001.pdf (1.2MB, pdf)
Data Availability Statement
Data are available on reasonable request. Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided is anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. This trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.