Abstract
Introduction
Methicillin-susceptible Staphylococcus aureus (MSSA) bacteraemia is a frequent condition, with high mortality rates. There is a growing interest in identifying new therapeutic regimens able to reduce therapeutic failure and mortality observed with the standard of care of beta-lactam monotherapy. In vitro and small-scale studies have found synergy between cloxacillin and fosfomycin against S. aureus. Our aim is to test the hypothesis that cloxacillin plus fosfomycin achieves higher treatment success than cloxacillin alone in patients with MSSA bacteraemia.
Methods
We will perform a superiority, randomised, open-label, phase IV–III, two-armed parallel group (1:1) clinical trial at 20 Spanish tertiary hospitals. Adults (≥18 years) with isolation of MSSA from at least one blood culture ≤72 hours before inclusion with evidence of infection, will be randomly allocated to receive either cloxacillin 2 g/4-hour intravenous plus fosfomycin 3 g/6-hour intravenous or cloxacillin 2 g/4-hour intravenous alone for 7 days. After the first week, sequential treatment and total duration of antibiotic therapy will be determined according to clinical criteria by the attending physician.
Primary endpoints: (1) Treatment success at day 7, a composite endpoint comprising all the following criteria: patient alive, stable or with improved quick-Sequential Organ Failure Assessment score, afebrile and with negative blood cultures for MSSA at day 7. (2) Treatment success at test of cure (TOC) visit: patient alive and no isolation of MSSA in blood culture or at another sterile site from day 8 until TOC (12 weeks after randomisation).
We assume a rate of treatment success of 74% in the cloxacillin group. Accepting alpha risk of 0.05 and beta risk of 0.2 in a two-sided test, 183 subjects will be required in each of the control and experimental groups to obtain statistically significant difference of 12% (considered clinically significant).
Ethics and dissemination
Ethical approval has been obtained from the Ethics Committee of Bellvitge University Hospital (AC069/18) and from the Spanish Medicines and Healthcare Product Regulatory Agency (AEMPS, AC069/18), and is valid for all participating centres under existing Spanish legislation. The results will be presented at international meetings and will be made available to patients and funders.
Trial registration number
The protocol has been approved by AEMPS with the Trial Registration Number EudraCT 2018-001207-37. ClinicalTrials.gov Identifier: NCT03959345; Pre-results.
Keywords: infectious diseases, microbiology, clinical trials
Strengths and limitations of this study.
The primary endpoints are strong composite outcomes that will assess mortality, clinical and microbiological failure at 7 and 90 days after randomisation.
The multicentre nature of the study supports the generalisability of the results.
A blinded adjudication committee will evaluate the key study endpoints and mitigate the observer bias inherent in the open-label design.
Given the increased risk of sodium overload, patients with cardiac failure and hepatic cirrhosis will be excluded.
Introduction
Staphylococcus aureus is one of the most common causes of bacteraemia and endocarditis in industrialised countries, and has particularly high hospitalisation and mortality rates (and associated costs).1 2 Healthcare exposure and the increasing use of invasive devices have contributed to the high burden of the disease.3
Mortality rates at 90 days due to methicillin-susceptible Staphylococcus aureus (MSSA) bacteraemia range between 20% and 30%.4 5 Mortality has been linked to factors such as age, comorbidities, source of infection, pathogen virulence elements and optimisation of antibiotic treatment.6 Complicated S. aureus bacteraemia is common, and is an indicator of poor prognosis.7 Indeed, every continued day of bacteraemia has been associated with a higher risk of mortality.8 9
Although MSSA bacteraemia is a common and life-threatening infection, it is still unclear whether combination therapy can reduce duration of bacteraemia or reduce mortality compared with the current standard of care (monotherapy beta-lactams). For over 50 years, the standard treatment of MSSA bacteraemia has been antistaphylococcal penicillin monotherapy.10 Today, there is a growing interest in identifying new therapeutic regimens able to reduce the rate of therapeutic failure and improve the outcomes obtained with the standard of care.
Strategies combining cloxacillin with aminoglycosides have not shown any significant improvement in patients’ outcomes, and have been associated with a higher risk of nephrotoxicity.11 A randomised multicentre study conducted in the UK, which included around 1000 patients and compared the efficacy of the rifampicin combination with the standard treatment for S. aureus bacteraemia, did not show a reduction in early or late mortality for the combined therapy compared with monotherapy.12 Nor did two recent studies comparing a beta-lactam and daptomycin (DAP) combination with beta-lactams in monotherapy to treat MSSA bacteraemia show any differences in mortality between groups.4 13
Among the combinations that might improve the outcome of patients with MSSA bacteraemia, cloxacillin plus fosfomycin is an appealing strategy. Fosfomycin is a bactericidal antibiotic which inhibits synthesis of N-acetylmuramic acid, a precursor of bacterial wall peptidoglycan, and is highly active against most strains of S. aureus.14 Cross-resistance with other antibiotic groups is very uncommon. Nevertheless, because of the risk of the development of resistance when administered as monotherapy, fosfomycin must be administered in combination with another antibiotic.15 In vitro and small-scale studies have demonstrated a synergistic effect of cloxacillin plus fosfomycin against S. aureus,16 and several different beta-lactam combinations have been successfully used in difficult-to-treat methicillin-resistant S. aureus (MRSA) infections.17 18
In a recent multicentre trial, we showed that DAP plus fosfomycin in MRSA bacteraemia achieved better outcomes in a subgroup of younger severely ill patients and faster clearance of bacteraemia than DAP alone.19 To date, however, no other randomised studies evaluating the efficacy of cloxacillin plus fosfomycin for treating MSSA bacteraemia have been published or registered in the ClinicalTrials.gov database.
We hypothesise that combining cloxacillin plus fosfomycin during the initial 7 days of treatment achieves better outcomes than cloxacillin alone in patients with MSSA bacteraemia. The primary objective of the study is to determine and compare mortality, clinical and microbiological failure at 7 and 90 days after randomisation by allocated treatment.
Methods and analysis
Study design and setting
We will perform a multicentre, superiority, randomised, open-label, phase IV–III, two-armed parallel group (1:1) clinical trial. Patients will be recruited from 20 tertiary hospitals in Spain (a list of study sites is available in the online supplemental material). The trial has been registered in the EudraCT and ClinicalTrials databases. The protocol follows the Standard Protocol Items: Recommendations for Interventional Trials initiatives, and the results will be presented in accordance with the Consolidated Standards of Reporting Trials statement.20 21
bmjopen-2021-051208supp001.pdf (214.6KB, pdf)
Study population
Inclusion criteria
Subjects aged ≥18 years.
At least one blood culture positive for MSSA ≤72 hours before inclusion, with evidence of active infection.
Written informed consent from the participant or the legal representative (LR).
Exclusion criteria
Severe clinical status with expected death <24 hour.
Severe hepatic cirrhosis (Child-Pugh C).
Moderate-to-severe cardiac chronic failure (NYHA (New York Heath Association class III–IV).
Prosthetic endocarditis.
History of significant allergy to β-lactams or fosfomycin (defined as previous type 1 hypersensitivity reaction to any β-lactams or fosfomycin, or history of serious non-type 1 hypersensitivity reaction to any penicillin or fosfomycin).
Known S. aureus fosfomycin non-susceptibility.
Polymicrobial bacteraemia with more than one micro-organism in blood cultures.
A positive pregnancy test or pregnancy or lactation at the time of inclusion.
Myasthenia gravis.
Participation in another clinical trial.
Previous participation in the present clinical trial.
Social problems, cognitive or psychiatric impairment which might be expected to affect adherence to the protocol.
Acute SARS-CoV-2 infection.
Intervention
Patients will be randomly assigned to receive intravenous cloxacillin 2 g every 4 hours plus fosfomycin 3 g every 6 hours, or to receive cloxacillin 2 g every 4 hours intravenously for the duration of 7 days. If creatinine clearance is <30 mL/min, cloxacillin will be administered at dose of 2 g every 6 hours. The fosfomycin dose will be adjusted according to creatinine clearance, as explained in table 1.
Table 1.
Fosfomycin dosage adjusted to renal function
| Creatinine clearance (mL/min) | Fosfomycin dosage |
| >40 | 3 g every 6 hours |
| 20–40 | 3 g every 12 hours |
| 10–20 | 3 g every 24 hours |
| <10 | 3 g every 48 hours |
| Haemodialysis | 3 g after haemodialysis |
| Continuous renal replacement therapy | 3 g every 24 hours |
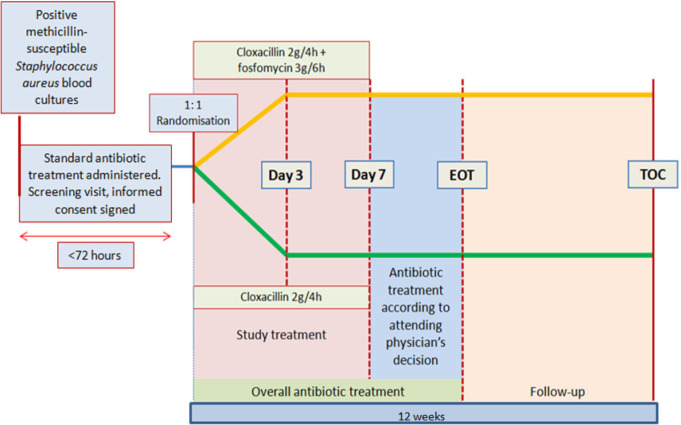
This treatment will be administered during the first 7 days after randomisation. After the first week, the choice of antibiotic strategy and the duration of overall antibiotic treatment will be determined according to clinical criteria by the attending physician, based on current guidelines. Uncomplicated bacteraemia (no evidence of complicated bacteraemia) will be treated for 10–14 days, and complicated bacteraemia (defined as infection with haematogenous seeding, progression of infection beyond the primary focus, persistent bacteraemia, skin alterations suggestive of acute systemic infection, presence of non-catheter device, haemodialysis) for 4–6 weeks at least, depending on the source of the infection and other clinical considerations.22 23 Removal of a focus of infection as soon as possible and performance of echocardiogram will be prioritised. The assessment schedule is summarised in table 2. A schematic diagram of study design is shown in figure 1.
Table 2.
The SAFO evaluation schedule
| Visit day | Screening | 0 | 3 | 7 | EOT | Unscheduled visit* | TOC | |
| All patients | ||||||||
| Eligibility criteria | X | |||||||
| Pregnancy test† | X | |||||||
| Informed consent | X | |||||||
| Randomisation | X | |||||||
| Clinical evaluation | X | X | X | X | X | X‡ | ||
| Quick SOFA score | X | X | ||||||
| Blood cultures | X | X | X | X | X | X‡ | ||
| Blood count and biochemical analysis§ | X | X | X | X | X‡ | |||
| Adverse events record | X | X | X | X | X | |||
| Concomitant medication | X | X | X | X | ||||
| Subgroup of patients with pharmacokinetic/pharmacodinamic (PK/PD) subanalysis | ||||||||
| Lithium heparin blood sample (2×5 mL) | X | |||||||
*Unscheduled visit will be performed only in case of clinical infectious symptoms and signs.
†Pregnancy test will be performed only in woman of childbearing age.
‡In absence of infective symptoms, clinical assessment may be made by phone call; blood culture and blood analysis will not be necessary.
§Complete blood count, biochemical analysis (C reactive protein, creatinine, urea, creatinine clearance, AST, ALT, INR, bilirubin, sodium, potassium, calcium, acid–base analysis) and coagulation test (prothrombin test/INR).
ALT, alanine aminotransferase; AST, aspartate amino transferase; EOT, end of treatment; INR, International Normalized Ratio; SOFA, Sequential Organ Failure Assessment; TOC, test of cure.
Figure 1.
Study design. EOT, end of treatment; TOC, test of cure.
Outcomes
Efficacy will be analysed by intention to treat in all randomised patients, using a hierarchical testing procedure in the following order: treatment success at day 7 followed by treatment success at TOC visit. Furthermore, a per-protocol analysis will also be performed.
Primary endpoints
Treatment success at day 7 from randomisation is a composite outcome defined by all the following criteria met after randomisation:
Patient alive at day 7.
Clinical improvement measured by stable or improved quick Sequential Organ Failure Assessment (SOFA) score (compared with baseline) at day 7.
Patient afebrile at day 7.
Negative MSSA blood cultures at day 7.
Treatment success at TOC visit, defined by presence of all of the following:
Patient alive at TOC.
No isolation of MSSA in blood culture and/or at another sterile site from day 8 until the TOC visit (12 weeks after randomisation). In case of patients with a prolonged course of antibiotic treatment (more than 10 weeks), the TOC visit will be performed 2 weeks after the end of treatment (EOT).
Treatment failure is defined by the presence of one of the following conditions: all-cause mortality at TOC, withdrawal from the study due to adverse events related to the treatment, requirement of an additional MSSA-active antibiotic until day 7, and lack of clinical improvement at day 7.
Secondary endpoints
Clinical
All-cause mortality at day 7, EOT and TOC visit.
Persistent bacteraemia (at least one positive blood culture) at day 3 and persistent bacteraemia at day 7 after randomisation.
Microbiological relapse, defined by at least one positive blood culture for MSSA at least 72 hours after a preceding negative culture.
Microbiological treatment failure, defined by a positive sterile site culture for MSSA at least 14 days after randomisation.
Number of patients with persistent and relapsing bacteraemia.
Number of patients with complicated bacteraemia, defined as persistent bacteraemia, endocarditis or metastatic emboli, presence of prosthetic devices.
Length of intensive care unit stay.
Duration of intravenous antibiotic treatment.
We will perform exploratory subgroup analyses for patients at high risk (those with metastatic infection, unknown focus of bacteraemia, endocarditis and pneumonia) for both primary outcomes. On participants with persistent bacteraemia subgroup analysis will be focused on treatment success at TOC.
Microbiological
In vitro cloxacillin plus fosfomycin combination synergy (see online supplemental material).
Emergence of fosfomycin-resistant strains during therapy in the combination treatment arm.
Operon agr functionality and its relationship with minimum inhibitory concentration (MIC) changes to vancomycin (VAN) and DAP and with biofilm production.
VAN and DAP MIC as markers of complications during bacteraemia. Isolates with rising VAN MICs are associated with thicker cell walls and dysfunctional agr profiles. These profiles are involved in quorum sensing, activation of S. aureus toxins and other virulence factors, leading to more resistant but less virulent strains.
Whole genome sequencing and its changes in patients with treatment failure.
Pharmacological
Patients recruited at the coordinating centre (Bellvitge University Hospital) will be included in a pharmacological substudy, after obtaining additional signed informed consent. The variables assessed will be:
Minimum and maximum concentration in steady state of fosfomycin and cloxacillin, and pharmacokinetic variability of these concentrations.
Associations between pharmacokinetic parameters and efficacy.
Safety
Safety of cloxacillin plus fosfomycin as compared with cloxacillin alone (see online supplemental material).
Follow-up and data collection
During the first week of treatment, all patients will be assessed at days 1, 3 and 7 by a member of the investigating team, and followed up daily by an infectious diseases specialist. Scheduled visits are reported in table 2. A follow-up visit will be arranged for all participants at EOT (48 hours after the last dose of antibiotic treatment) and at TOC. At this last visit, a structured telephone interview will be performed to assess outcomes.
All data will be recorded on a secure web application used for building and managing online databases (REDCap). Authorised staff will be free to examine the records for quality assurance and audit purposes.
Endpoint assessment
The primary endpoints will be assessed by a committee comprising three independent senior infectious disease specialists with extensive experience in S. aureus bacteraemia and endocarditis. This committee will be blinded to treatment allocation and to patient identification. Committee members will receive a data extract containing patients’ demographical data, comorbidities, source of infection, quick SOFA score at baseline and day 7, date and results of blood and sterile cultures between randomisation and TOC, as well as date of death if applicable.
Statistical analysis plan
Sample size
Prior data indicate a success rate in the cloxacillin alone group of 74%.4 To achieve a success rate in the experimental group of 86% (ie, an absolute difference of 12%, considered as clinically significant), we will need 183 experimental subjects and 183 control subjects to reject the null hypothesis of an equal success rate with a probability of 80%. The probability of type I error associated with this test is 5%, and a dropout rate of 5% has been anticipated.
Allocation
Participants will be block randomised to receive monotherapy or combination using an internet-based, concealed computer-generated random allocation sequence. Random blocks will be of size 4 or 6. The randomised sequence allocation will be stored in the Biostatistics Unit at Biomedical Research Institute of Bellvitge (IDIBELL) and will not be available to any member of the research team.
Data analysis
The main analysis will be performed for the intention-to-treat population, which will include all randomised patients included in the study with a primary outcome assessment. If no statistical significance is detected by day 7 in the hierarchy, then no further hypothesis testing will be performed. The analysis will be repeated in the per-protocol population. All patients who receive at least one dose of treatment will be included in the safety analysis.
The χ2 test will be used to test the binary endpoints of the success rate. The relative risk for success rate will be calculated, accompanied by 95% CIs. Absolute risk difference and 95% CI will also be reported. The time-to-event outcomes, including the time of response, and overall survival will be estimated using the Kaplan-Meier method.24 To account for competing risks, cause-specific cox regression models will be used, and event cause cumulative incidence functions will be plotted.25 All analyses and data management will be performed with R software, V.4.0.4 or superior.26
Monitoring
Monitoring plans
The data monitoring board will ensure the correct progress of the study in terms of safety, and also the sample size assumptions.
Harms—Data Safety and Monitoring Board (DSMB)
An independent DSMB will review safety data and provide advice about the continuation, modification and/or termination of the study, as well as adherence to the protocol, recruitment, outcomes and additional data related to participants’ safety. The DSMB will be composed by specialists in pharmacology, biostatistics and infectious diseases. The review by the DSMB will be performed when half of the sample size will be reached.
Adverse events reporting and quantification
An adverse event will be defined as any injury related to medical management occurring during the patient’s participation in the study, even if it is not related to the study medication.
An adverse drug event will be defined as any medication-related adverse event occurring during the patient’s participation in the clinical trial.
An adverse drug reaction will be defined as any ‘adverse drug event’ occurring when the medication is used as directed and at the usual dosage.
Serious adverse event or reaction will be defined as an event or reaction that:
Results in death.
Is life-threatening.
Causes persistent or significant disability.
Causes a congenital anomaly/birth defect.
Requires inpatient hospitalisation or prolongation of existing hospitalisation (not related to basal diseases).
Adverse drug events of particular interest for the study
Hypokalaemia and hypocalcaemia: blood analysis will be performed every 2–3 days during the first week to permit potassium and calcium control. Furthermore, administration of potassium supplement will be recommended from the first day of treatment to avoid this complication.
Sodium overload: since both fosfomycin and cloxacillin carry a high sodium load, daily physical examination and administration of a low dose of a diuretic such as furosemide will be recommended to avoid hypertension, oedema and acute cardiac failure.
Reporting
Any adverse events occurring during the patient’s participation in the clinical trial will be recorded on the clinical chart by the principal investigator (PI) at each scheduled visit. The PI will record its possible relationship to the study drug.
The electronic case report form should record only the following: serious adverse drug events; adverse events (of any degree) related to the study medication, in the opinion of the PI; adverse events (of any degree) leading to modification of the dosage of the study drug or its interruption/early discontinuation; adverse events of particular interest for the study.
The sponsor will be notified of all serious adverse events within 24 hours of their occurrence.
Trial status
The SAFO trial opened its first recruitment site on 31 May 2019. The first patient was enrolled on 1 July 2019. Follow-up is expected to be completed by May 2022.
Declaration
Ethics
The trial will be conducted in accordance with the principles of the most recent Declaration of Helsinki (agreed by the 64th World Medical Association General Assembly in 2013), the Good Clinical Practice guidelines and the current local legislation.
The study was authorised by the Spanish Medicines and Healthcare Products Regulatory Agency (AEMPS, 18-0905) and by the Bellvitge University Hospital Ethics committee (AC069/18).
The PI or collaborator at each site will provide patients with the information sheet, and he/she will explain the nature of the study and the objectives and clarify any doubts. Written informed consent will be obtained from all patients or from their LRs if they lack capacity, before enrollment (online supplemental file). Patients (or their LRs) are free to withdraw from the trial at any time; this will be explicitly stated on the patient’s information sheet.
Patients’ personal and clinical information will be managed in accordance with European Regulation 2016/679 and Spanish legislation. The trial protocol was approved by the research ethics committee on 28 March 2019 and by the AEMPS on 8 April 2019. The informed consent form and information sheet were approved by the research ethics committee on 28 March 2019. The emendation regarding ‘acude SARS-CoV-2 infection’ as exclusion criteria was approved by the research ethics committee and by the AEMPS on 29 November 2020.
Data sharing plan and dissemination
Sharing of data generated by this project is an essential part of our proposed activities and will be carried out in several different ways. We would wish to make our results available both to the community of scientists interested in infectious diseases and the biology of S. aureus to avoid unintentional duplication of research.
The preliminary results will be presented at international and national infectious diseases conferences and will be published in peer-reviewed journals. The results will also be made available to patients, caregivers and funders through press and social media communications. A corporative Twitter account will be created to establish direct contact with the general public and other healthcare professionals. Any formal presentation or publication of data collected from this study will be considered as a joint publication by the participating investigators and will follow the recommendations of the International Committee of Medical Journal Editors.
Individual participant data that underlie the results, after deidentification (text, tables, figures and appendices) will be available immediately following publication and ending 5 years following article publication. Data will be shared with researchers who provide a methodologically sound proposal to achieve aims in the approved suggestions. Propositions should be directed to the corresponding author.
Patients and public involvement
Patients will not be involved in either the enrollment or the execution of the trial, or in the assessment of the interventions. However, before the beginning of the study, a number of patients with previous S. aureus bacteraemia were contacted by phone to obtain their feedback about the study.
Protocol amendments
No protocol modifications will become effective until approved by the relevant authorities and by the Drug Research Ethics Committee (CEIm). Exceptions will be made for any changes to protect patients from imminent harm and those concerning exclusively logistic or administrative aspects.
Supplementary Material
Acknowledgments
We thank the CERCA Programme/Generalitat de Catalunya for their institutional support. We would also like to thank the Biostatistics Unit and the Pharmacology department of the IDIBELL for technical help.
Footnotes
Twitter: @GaschOriol_Inf
Collaborators: The SAFO study group and the Spanish Network for Research in Infectious Diseases (REIPI)
Contributors: SG, GC, JMA, JC, DB, MAD, AP, SC and MP conceived and designed the study. SG, GC, MP and JC wrote and revised the manuscript. CT and NP designed and wrote statistical analysis plan. PH and SV critically reviewed the protocol. SG-Z, GC, RS-J, JMA, LM, JL-C, OG, AG-G, SI, GG-P, EC, LB-P, IO, AJ-S, LEL-C, GE, MA, MJG-P, FG, JRP, MLP-B, RMB, MTP-R, YM, MBL-Y and GH contributed to the aquisition of data. All authors have read and approved the final manuscript.
Funding: The SAFO trial is supported by a competitive grant awarded by the Fondo de Investigaciones Sanitarias at the Spanish government’s National Institute of Health Research, Instituto de Salud Carlos III (ISCIII), (FIS PI17/01116). This study was supported by Plan Nacional de I+D+i 2017–2021 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0005).
Competing interests: GH received a research grant from ERN (19PNJ145).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Bergin SP, Holland TL, Fowler VG. Bacteremia, sepsis, and infective endocarditis associated with Staphylococcus aureus. Curr Top Microbiol Immunol 2015;409:263–96. [DOI] [PubMed] [Google Scholar]
- 2. Stewardson AJ, Allignol A, Beyersmann J, et al. The health and economic burden of bloodstream infections caused by antimicrobial-susceptible and non-susceptible Enterobacteriaceae and Staphylococcus aureus in European hospitals, 2010 and 2011: a multicentre retrospective cohort study. Euro Surveill 2016;21:30319. 10.2807/1560-7917.ES.2016.21.33.30319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Souli M, Ruffin F, Choi S-H, et al. Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis 2019;69:1868–77. 10.1093/cid/ciz112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grillo S, Cuervo G, Carratalà J, et al. Impact of β-lactam and daptomycin combination therapy on clinical outcomes in methicillin-susceptible Staphylococcus aureus bacteremia: a propensity score-matched analysis. Clin Infect Dis 2019;69:1480–8. 10.1093/cid/ciz018 [DOI] [PubMed] [Google Scholar]
- 5. Rieg S, Joost I, Weiß V, et al. Combination antimicrobial therapy in patients with Staphylococcus aureus bacteraemia-a post hoc analysis in 964 prospectively evaluated patients. Clin Microbiol Infect 2017;23:406.e1–406.e8. 10.1016/j.cmi.2016.08.026 [DOI] [PubMed] [Google Scholar]
- 6. van Hal SJ, Jensen SO, Vaska VL, et al. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012;25:362–86. 10.1128/CMR.05022-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gasch O, Camoez M, Dominguez MA, et al. Predictive factors for mortality in patients with methicillin-resistant Staphylococcus aureus bloodstream infection: impact on outcome of host, microorganism and therapy. Clin Microbiol Infect 2013;19:1049–57. 10.1111/1469-0691.12108 [DOI] [PubMed] [Google Scholar]
- 8. Minejima E, Mai N, Bui N, et al. Defining the breakpoint duration of Staphylococcus aureus bacteremia predictive of poor outcomes. Clin Infect Dis 2020;70:566–73. 10.1093/cid/ciz257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuehl R, Morata L, Boeing C, et al. Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis 2020;20:1409–17. 10.1016/S1473-3099(20)30447-3 [DOI] [PubMed] [Google Scholar]
- 10. Gudiol F, Aguado JM, Almirante B, et al. Executive summary of the diagnosis and treatment of bacteremia and endocarditis due to Staphylococcus aureus. A clinical guideline from the Spanish Society of clinical microbiology and infectious diseases (SEIMC). Enferm Infecc Microbiol Clin 2015;33:626–32. 10.1016/j.eimc.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 11. Cosgrove SE, Vigliani GA, Fowler VG, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009;48:713–21. 10.1086/597031 [DOI] [PubMed] [Google Scholar]
- 12. Thwaites GE, Scarborough M, Szubert A, et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (arrest): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:668–78. 10.1016/S0140-6736(17)32456-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng MP, Lawandi A, Butler-Laporte G, et al. Adjunctive daptomycin in the treatment of methicillin-susceptible Staphylococcus aureus bacteremia: a randomized, controlled trial. Clin Infect Dis 2021;72:e196–203. 10.1093/cid/ciaa1000 [DOI] [PubMed] [Google Scholar]
- 14. Popovic M, Steinort D, Pillai S, et al. Fosfomycin: an old, new friend Eur J Clin Microbiol Infect Dis 2010;29:127–42. 10.1007/s10096-009-0833-2 [DOI] [PubMed] [Google Scholar]
- 15. Drugeon HB, Courtieu AL. The role of culture media on the fosfomycin sensitivity of six Serratia strains and their resistant mutants. Chemotherapy 1982;28:345–50. 10.1159/000238102 [DOI] [PubMed] [Google Scholar]
- 16. Kastoris AC, Rafailidis PI, Vouloumanou EK, et al. Synergy of fosfomycin with other antibiotics for gram-positive and gram-negative bacteria. Eur J Clin Pharmacol 2010;66:359–68. 10.1007/s00228-010-0794-5 [DOI] [PubMed] [Google Scholar]
- 17. Grabein B, Graninger W, Rodríguez Baño J, et al. Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature. Clin Microbiol Infect 2017;23:363–72. 10.1016/j.cmi.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 18. del Río A, Gasch O, Moreno A, et al. Efficacy and safety of fosfomycin plus imipenem as rescue therapy for complicated bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: a multicenter clinical trial. Clin Infect Dis 2014;59:1105–12. 10.1093/cid/ciu580 [DOI] [PubMed] [Google Scholar]
- 19. Pujol M, Miró J-M, Shaw E, et al. Daptomycin plus fosfomycin versus daptomycin alone for methicillin-resistant staphylococcus bacteremia and endocarditis: a randomized clinical trial. Clin Infect Dis 2021;72:1517–25. 10.1093/cid/ciaa1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schulz KF, et al. Consort 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726. 10.7326/0003-4819-152-11-201006010-00232 [DOI] [PubMed] [Google Scholar]
- 22. Fowler VG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003;163:2066–72. 10.1001/archinte.163.17.2066 [DOI] [PubMed] [Google Scholar]
- 23. Fowler VG, Justice A, Moore C, et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005;40:695–703. 10.1086/427806 [DOI] [PubMed] [Google Scholar]
- 24. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 25. Wolkewitz M, Cooper BS, Bonten MJM, et al. Interpreting and comparing risks in the presence of competing events. BMJ 2014;349:g5060. 10.1136/bmj.g5060 [DOI] [PubMed] [Google Scholar]
- 26. R core team . R: a language and environment for statistical computing. Austria: R Found Stat Comput Vienna, 2016. http//wwwR-Project.org [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-051208supp001.pdf (214.6KB, pdf)