Abstract
Background:
Bleeding complications and acute limb ischemia (ALI) are devastating vascular complications in patients with STEMI. Cardiogenic shock (CS) can further increase this risk due to multi-organ failure. In the contemporary era, percutaneous mechanical circulatory support (pMCS) is commonly used for management of CS. We hypothesized that vascular complications may be an important determinant of clinical outcomes for CS due to STEMI (CS-STEMI).
Objective:
We evaluated 10-year national trends, resource utilization and outcomes of bleeding complications, and ALI in CS-STEMI.
Methods:
We performed a retrospective cohort study of CS-STEMI patients from a large US national database (National Inpatient Sample) between 2005–2014. Events were then divided into 4 different groups: No MCS, with IABP, PVAD (percutaneous ventricular assist device includes Impella or Tandem Heart) or extracorporeal membrane oxygenation (ECMO).
Results:
Bleeding complications and ALI were observed in 31,389 (18.2%) and 1,628 (0.9%) out of 172,491 admissions with CS-STEMI respectively. Between 2005–2014 overall trends increased for ALI, however, the number of bleeding events decreased. ALI was associated with increased in-hospital mortality in comparison to those without any ALI. However, bleeding complications were not associated with increased in-hospital mortality. Compared to patients without complications, both bleeding and ALI were associated with increased length of stay and hospitalization costs.
Conclusion
Bleeding and ALI are common complications associated with CS-STEMI in the contemporary era. Both complications are associated with increased hospital costs and length of stay. These findings highlight the need to develop algorithms focused on vascular safety in CS-STEMI.
Keywords: Cardiogenic Shock, STEMI, Bleeding Complications, Acute Limb Ischemia, Mechanical Circulatory Support Devices
Introduction:
Cardiogenic shock (CS) occurs in approximately 8–10% of all ST-segment elevation myocardial infarction (STEMI) patients, with reported mortality rates of around 40–50% (1–3). CS-STEMI remains a leading cause of death despite increased adoption of early revascularization strategies (2,4–6). STEMI patients are at higher risk of vascular events such as acute limb ischemia (ALI) and bleeding complications due to aggressive use of anticoagulation and antiplatelets for management of coronary thrombosis. Unlike STEMI alone, where radial access may be sufficient for coronary revascularization, femoral access is more common in CS-STEMI because of the potential need for percutaneous mechanical circulatory support (pMCS).
In the contemporary era, pMCS devices include intra-aortic balloon counterpulsation (IABP), percutaneous ventricular assist devices (PVAD: Impella or Tandem Heart) and veno-arterial extracorporeal membrane oxygenation (ECMO). These devices provide temporary support during high risk coronary intervention or for more prolonged support as a bridge to decision, recovery, durable ventricular assist device, or orthotopic heart transplant strategy. Since many of the temporary MCS especially Impella, Tandem Heart and ECMO require the use of large bore catheters delivered via the femoral route, vascular complications are common and may be a major determinant of outcomes in patients with CS-STEMI(7–9).
Limited data exploring the incidence and clinical outcomes of such vascular complications in patients with CS-STEMI exist. We hypothesized that both ALI and bleeding complications are common among patients with CS-STEMI. To explore this hypothesis, we employed the National Inpatient Sample (NIS) database to determine trends over time, incidence, and clinical outcomes associated with both bleeding complications and ALI in patients with CS-STEMI.
Methods:
Data source and study population
Data was obtained from the Nationwide Inpatient Sample (NIS) database which is maintained by the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project. This nationally representative database is the largest all-payer inpatient care database in the United States containing discharge data from a 20% stratified sample of different hospitals in various regions. It includes weights to allow researchers to generate national estimates from raw counts. Encounter-level information of hospital stays compiled in a uniform format with privacy protection of individual patients are included. Each year, over 7 million hospital stays are sampled nationwide, which, when weighted, generalizes to more than 35 million hospitalizations annually (10). Our study population includes all patients ≥18 years of age from 2005 to 2014 using International Classification of Diseases, 9th Edition (ICD-9) diagnosis codes for STEMI and CS (Supplementary table 1). Validation studies for CS for the ICD-9-CM code 785.51 have shown a specificity of 99.3%, a sensitivity of 59.8%, a positive predictive value of 78.8%, and a negative predictive value of 98.1% (11). Bleeding complication was defined using the previously validated study by Redfors et al as composite outcome of patients requiring blood transfusion, developing hematoma/hemorrhage and/or undergoing surgical or endovascular intervention to manage the bleeding (12). ALI patients were identified as those who experienced lower extremity embolism or thrombosis or those who underwent limb salvage interventions like surgical/endovascular repair or amputation during their hospitalization similar to the one used in previous studies using previously validated ICD-9 codes (13). Complete definitions and ICD-9 codes used for identification of MCS device or complications are mentioned in Supplementary table 1 (14,15).
Records with missing outcome were excluded from the analysis. This study was exempt from review by the institutional review board because NIS is a publically available national database.
Baseline variables and comparison groups.
Baseline variable included demographics (age, sex, race, median household income) and comorbid conditions (cardiac arrhythmias, hypertension, diabetes, chronic liver disease, obesity, dyslipidemia, chronic pulmonary disease, congestive heart failure, deficiency anemia, coagulopathy, chronic kidney disease peripheral vascular disease, alcohol abuse) were also reported. Revascularization strategies i.e. percutaneous coronary interventions, coronary artery bypass and/or fibrinolysis during the current admission were also identified. Hospital characteristics (bed size, location/teaching status, region of the hospital and primary payer) and insurance status were also reported. The ICD-9-CM and Clinical Classification Software codes were used to identify patient comorbidities. Elixhauser score was calculated based on the number of comorbidities (Supplementary table 2).
The overall cohort was grouped into 4 subgroups based on MCS device utilization: a) No MCS, b) only IABP, c) only PVAD (Impella or Tandem heart) and d) only ECMO device. Each analysis was stratified into these groups.
Study Outcomes and definitions
We evaluated the temporal trends of ALI and bleeding complications for each pMCS category from 2005–2014. Our primary outcome was to compare in-hospital mortality among patients with and without each of the complications (bleeding complications and ALI). Secondary outcomes included cost of hospitalization and length of stay (LOS). We also looked for total cost of hospitalization and average LOS as our secondary outcomes. We analyzed the outcomes of in-hospital mortality, average LOS and hospital cost among patients with no MCS, IABP, PVAD and ECMO group for patients with and without bleeding complications and ALI.
Statistical analysis
Weighted data was used for all analyses. We compared the in-hospital mortality, length of stay, and cost of hospitalization among the patients with and without bleeding complications or ALI using unadjusted and adjusted logistic regression analyses among each device group. The multivariate hierarchical logistic regression models adjusted for age, sex, race, congestive heart failure, liver disease, dyslipidemia, alcohol abuse, anemia peripheral vascular disease, pulmonary circulation disorder, chronic pulmonary disease, coagulopathy, renal failure diabetes, hypertension, PCI or CABG or fibrinolytic therapy in current admission, cardiac arrest, and vasopressor use.
We also performed multivariable comparison for per number of documented transfusions by logistic regression analysis for inpatient mortality with each event of transfusion in comparison to those with no transfusion (Supplementary table 6)
Baseline demographics, comorbidities and hospital characteristics between those with and without bleeding complications or ALI in patients with CS-STEMI were compared using the Pearson χ2 test for categorical variables and one-way ANOVA for continuous variables to identify significant univariate associations. Categorical and continuous variables were reported as percentages and mean ± standard error (SE), respectively. Temporal trends of events of bleeding complications and ALI were reported as absolute values for each calendar year and compared using 1-way ANOVA. Mean LOS and total cost of hospitalization were reported and compared using univariate analysis by 1-way ANOVA. All data extraction and analyses were performed using SPSS (Version 25.0 Armonk, NY). Two-sided p value <0.05 was used for statistical significance.
Results:
Baseline Characteristics
Between 2005–2014, 172,491 admissions with a primary diagnosis of CS-STEMI were registered, of which 18.2% (31,389/172,491) developed bleeding complications and 0.9% (1,628/172,491) had ALI. Baseline characteristics of the patients with and without bleeding complications or ALI are provided in Table 1. Supplementary Table 3 and 4 shows the baseline characteristics with and without bleeding complications or ALI for patients with No MCS, with IABP, PVAD and ECMO.
Table 1:
Baseline characteristics of CS-STEMI patients with and without bleeding complications, acute limb ischemia and stroke.
| CS with STEMI | Total Bleeding Complications | Acute Limb Ischemia | ||||
|---|---|---|---|---|---|---|
| Yes | No | P-value | Yes | No | P-value | |
| No. of observations(weighted) | 31389 | 141102 | 1628 | 170863 | ||
| Age (in years) (Mean± SE) | 67.6 (±0.07) | 66.6 (±0.03) | <0.001 | 63.3 (±0.2) | 66.8 (±0.03) | <0.001 |
| Female | 41.8% | 35.2% | <0.001 | 42.0% | 36.3% | <0.001 |
| Race | <0.001 | <0.001 | ||||
| White | 74.4% | 78.6% | 78.1% | 77.8% | ||
| Black | 7.1% | 6.5% | 7.1% | 6.6% | ||
| Hispanic | 9.0% | 7.3% | 8.2% | 7.6% | ||
| Asian or Pacific Islander | 4.1% | 2.8% | 0.7% | 3.1% | ||
| Native American | 0.5% | 0.6% | 0% | 0.6% | ||
| Others | 4.8% | 4.2% | 6.0% | 4.3% | ||
| Chronic Medical Conditions (%) | ||||||
| Cardiac arrhythmia | 49.8% | 46.9% | <0.001 | 48.5% | 47..4% | 0.39 |
| Cardiac arrest | 20.0% | 20.9% | <0.001 | 24.3% | 20.7% | <0.001 |
| Hypertension | 55.3% | 51.1% | <0.001 | 43.4% | 52.0% | <0.001 |
| Chronic liver disease | 1.6% | 1.3% | <0.001 | 1.8% | 1.3% | 0.06 |
| Diabetes | 32.8% | 29.2% | <0.001 | 27.0% | 29.8% | 0.01 |
| Obesity | 10.7% | 9.5% | <0.001 | 9.3% | 9.7% | 0.62 |
| Dyslipidemia | 37.0% | 39.8% | <0.001 | 27.9% | 39.4% | <0.001 |
| Chronic pulmonary disease | 22.1% | 20.4% | <0.001 | 23.4% | 20.7% | 0.008 |
| Pulmonary Circulation disorder | 0.4% | 0.2% | <0.001 | 1.3% | 0.2% | <0.001 |
| Congestive Heart Failure | 4.8% | 2.3% | <0.001 | 6.0% | 2.7% | <0.001 |
| Anemia | 33.1% | 15.4% | <0.001 | 17.5% | 18.6% | 0.3 |
| Coagulopathy | 24.1% | 10.7% | <0.001 | 23.2% | 13.1% | <0.001 |
| Chronic Kidney Disease | 18.4% | 14.1% | <0.001 | 12.7% | 14.9% | 0.01 |
| Peripheral Vascular Disease | 13.0% | 9.5% | <0.001 | 73.0% | 9.5% | <0.001 |
| Alcohol abuse | 4.4% | 4.1% | 0.003 | 3.6% | 4.1% | 0.30 |
| h/o MI | 6.1% | 6.7% | <0.001 | 4.7% | 6.6% | 0.003 |
| h/o PCI | 7.7% | 7.9% | 0.5 | 6.5% | 7.9% | 0.055 |
| h/o CABG | 2.1% | 2.8% | <0.001 | 1.7% | 2.7% | 0.01 |
| Atrial fibrillation | 26% | 21.6% | <0.001 | 22.5% | 22.4% | 0.9 |
| h/o CVA | 4.6% | 4.5% | 0.6 | 4.1% | 4.5% | 0.5 |
| Elixhauser Comorbidities | <0.001 | <0.001 | ||||
| 0 | 5.4% | 10.9% | 3.1% | 10.0% | ||
| 1–3 | 55.1% | 65.2% | 52.6% | 63.5% | ||
| ≥4 | 39.5% | 23.9% | 44.3% | 26.5% | ||
| Revascularization Methods | ||||||
| CABG | 31.5% | 11.6% | <0.001 | 19.8% | 15.1% | <0.001 |
| PCI | 54.4% | 67.4% | <0.001 | 61.9% | 65.1% | <0.001 |
| Fibrinolytic therapy | 2.3% | 1.9% | <0.001 | 4.9% | 2.0% | <0.001 |
| Vasopressor use | 7.8% | 6.1% | <0.001 | 9.0% | 6.4% | <0.001 |
| Expected primary payer | <0.001 | <0.001 | ||||
| Medicare | 56.6% | 53.1% | 44.8% | 53.8% | ||
| Medicaid | 7.7% | 7.4% | 10.3% | 7.4% | ||
| Private | 26.8% | 28.9% | 34.6% | 28.4% | ||
| Others | 2.5% | 3.1% | 1.8% | 3.0% | ||
| Hospital bed size | <0.001 | 0.06 | ||||
| Small | 6.5% | 8.3% | 6.5% | 8.0% | ||
| Medium | 20.9% | 22.3% | 21.4% | 22.1% | ||
| Large | 72.5% | 68.3% | 72.1% | 69.9% | ||
| Hospital teaching status | <0.001 | <0.001 | ||||
| Rural | 4.4% | 6.9% | 3.3% | 6.4% | ||
| Urban, Non-teaching | 37.9% | 39.1% | 32.7% | 38.9% | ||
| Urban, teaching | 57.7% | 54.1% | 63.9% | 54.6% | ||
Bleeding Complications
Clinical characteristics of patients with CS-STEMI with and without bleeding complications are detailed in Table 1. Briefly patients with bleeding complications were more likely to be female and had high prevalence of comorbidities of: diabetes, hypertension, coagulopathy, anemia or chronic kidney disease and had a higher composite number of comorbidities as suggestive by an Elixhauser score ≥4 (39.5% vs 23.9%; with vs without bleeding complication, p<0.001).
Temporal trend and overall events of bleeding complications in patients with CS-STEMI
Figure 1 shows the overall temporal trend of bleeding complications from 2005 to 2014 among patients with CS-STEMI. We observed that there was decrease in the overall bleeding complications in patients from 17.3% in 2005 to 14.1% in 2014. Similar decrease of trends was seen in patients requiring blood transfusions (16.1% from 2005 to 14.1% in 2014) and with events of hematoma (4.5% from 2005 to 3.4% in 2014). However, we observed slight increase in the events of surgical/endovascular intervention from 1.50% in 2005 to 2.19% in 2014. In all the groups (those with no MCS, IABP, PVAD or ECMO) the most common bleeding complications was those requiring blood transfusions.
Figure 1:
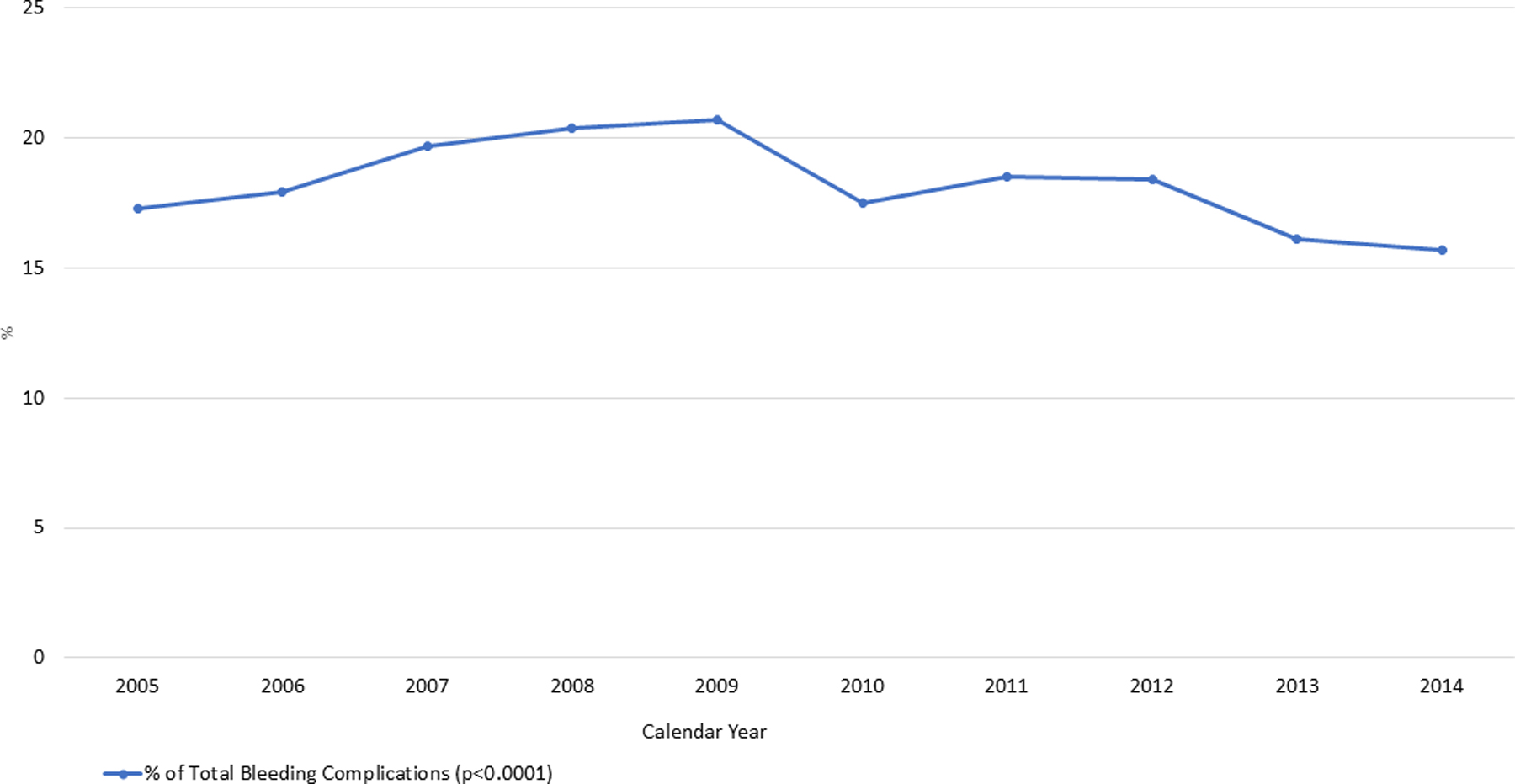
Trend of Incidence of Bleeding complications in Cardiogenic Shock with STEMI from 2005–2014
Overall events of bleeding complications in patients with no MCS was 15.9% (12,869/81,020), IABP was 19.4% (16,877/86,795), PVAD was 29.9% (622/2079) and ECMO was 54.2% (240/444). After adjustment for demographics and comorbidities, adjusted risk of having an event of bleeding complications in comparison to no MCS with IABP, PVAD and ECMO is shown in supplementary table 5.
Clinical Outcomes of patients with and without bleeding complications
Among all patients with CS-STEMI, in-hospital mortality was 27.4% in patients who experienced bleeding complications and 30.8% in patients who did not (p<0.001). After adjusting for demographic factors, medical history, and additional intervention (as listed in Methods), the adjusted odds of in-hospital morality among patients with bleeding complications were lower compared to those without bleeding complications (aOR: 0.79; CI: 0.77–0.81) (Table 2). Bleeding complications were also associated with lower in-hospital mortality in the no MCS, IABP, and PVAD groups. Among the ECMO cohort, 62.1% of those without bleeding complications died compared to a mortality rate of 50.6% in those with bleeding complications but was not statistically significant after adjusting for underlying comorbidities (Table 2). However, bleeding complications were associated with a significantly higher LOS (15 days vs 10 days; with vs without bleeding complication, p<0.001) and cost of hospitalization ($210,258 vs 139,409; with vs without bleeding complication, p<0.001) (Table 2).
Table 2:
Inpatient mortality, cost and LOS in Bleeding complications
| Total Bleeding complications | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| In-Hospital mortality | Length of Stay (in days)** | Cost of Hospitalization (in dollars) | |||||||||
| With bleeding complications No./Total (%) | Without bleeding complications No./Total (%) | p-value | Adjusted Odds Ratio* | p-Value | With bleeding complications | Without bleeding complications | p-value | With bleeding complications | Without bleeding complications | p-value | |
| Cardiogenic shock with STEMI | 8616/31388 (27.4%) | 43432/14110 2 (30.8%) | <0.001 | 0.79 (0.77–0.81) | <0.001 | 15 | 10 | <0.001 | 210,258 | 139,409 | <0.001 |
| CS with STEMI without MCS | 3810/12869 (29.4%) | 23652/68151 (34.7%) | <0.001 | 0.72 (0.69–0.75) | <0.001 | 14 | 8 | <0.001 | 166,063 | 95,576 | <0.001 |
| CS with STEMI and IABP | 4106/16877 (24.3%) | 18268/69918 (26.1%) | <0.001 | 0.82 (0.79–0.86) | <0.001 | 14 | 11 | <0.001 | 155,176 | 220,107 | <0.001 |
| CS with STEMI and PVAD | 201/603 (32.3%) | 653/1457 (44.8%) | <0.001 | 0.66 (0.52–0.83) | <0.001 | 18 | 13. | <0.001 | 403,242 | 265,020 | <0.001 |
| CS with STEMI and ECMO | 122/241 (50.6%) | 126/203 (62.1%) | 0.01 | 0.67 (0.37–1.22) | 0.19 | 27 | 24 | 0.23 | 523,168 | 437,824 | 0.36 |
Adjusted for age, sex, race, smoking status, dyslipidemia, alcohol abuse, congestive heart failure, liver disease, peripheral vascular disease, deficiency anemia, chronic pulmonary disease, coagulopathy, renal failure diabetes, hypertension, cardiac arrest and vasopressor use.
included all patients that survived the index admission.
We also observed that receiving 2 transfusions (aOR:1.43, 95% CI: 1.19 – 1.71, p<0.001) or ≥3 transfusions (aOR:1.41, 95% CI: 1.11 – 1.77, p = 0.004) was associated with increased in-hospital mortality as compared to those not requiring any transfusions (Supplementary table 6).
Acute Limb Ischemia:
Patients with ALI were younger (63.3 vs 66.8; with vs without ALI, p<0.001), female and had higher prevalence of following comorbidities: congestive heart failure, coagulopathy and peripheral vascular disease. They also had higher Elixhauser score ≥4 (44.3% vs 26.5%; with vs without ALI, p<0.001) (Table 1). The overall incidence of ALI increased from 0.8% in 2005 to 1.4% in 2014 (p=0.02) as shown in Figure 2. Overall events of ALI in patients with no MCS was 0.8% (631/81,019), IABP was 0.9% (805/86,795), PVAD was 3.6% (74/2079) and ECMO was 7.7% (34/444). After adjustment for demographics and comorbidities, adjusted risk of events of ALI in comparison to no MCS with IABP, PVAD and ECMO is shown in supplementary table 5.
Figure 2:
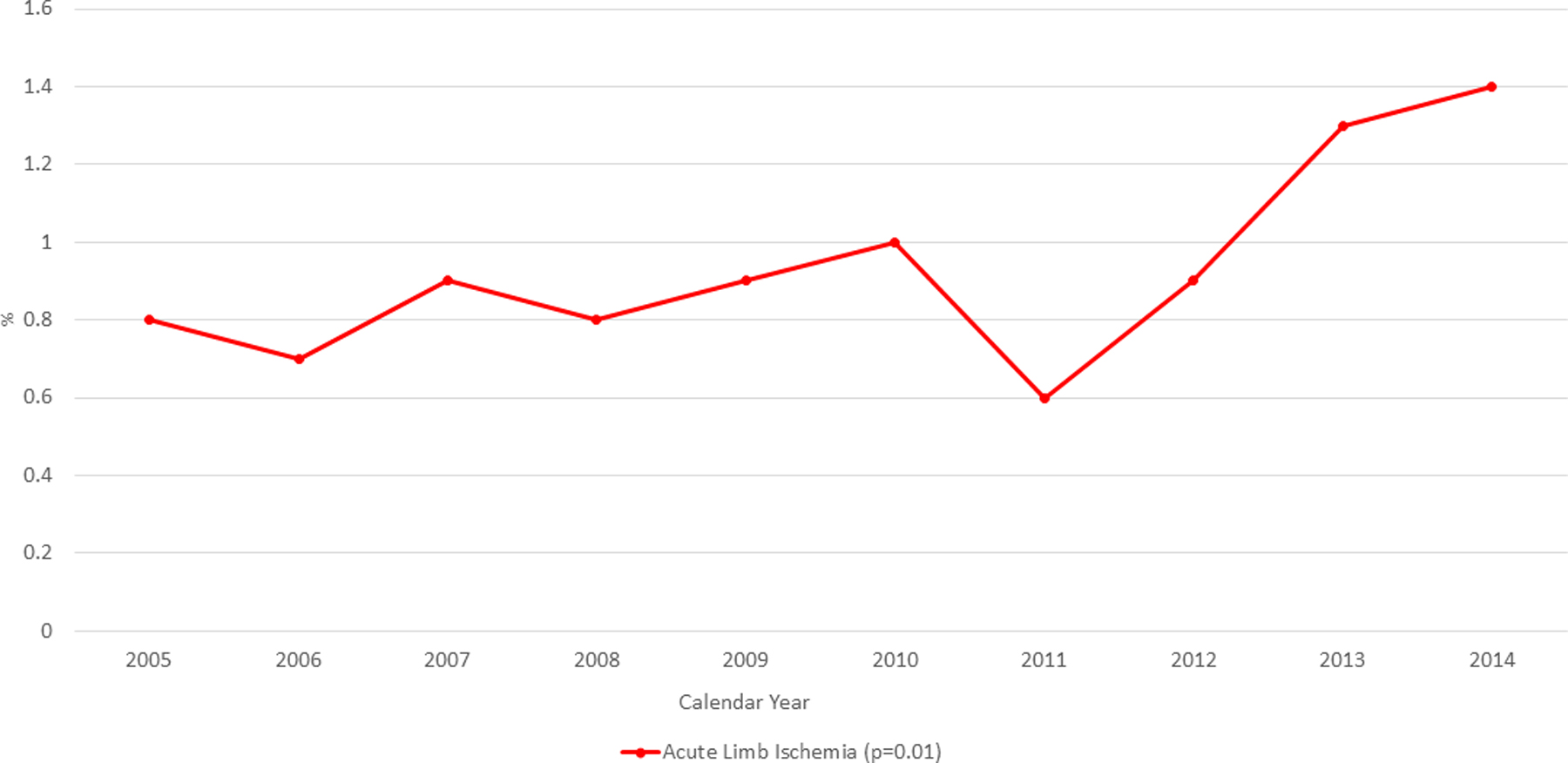
Trend of incidence of Acute Limb ischemia in patient with Cardiogenic Shock and STEMI from 2005–2014
Overall, ALI in patients with CS-STEMI had higher in-hospital mortality in compared to those without ALI (35.9% vs 30.1%; aOR: 1.21, 95% CI: 1.09–1.36) (Table 3). ALI patients who underwent surgical and/or endovascular intervention were noted to have lower in-hospital mortality in compare to those without any intervention with ALI (Supplementary Table 7). Patients with ALI in comparison to those without were observed to have higher LOS (19 days vs 11 days; ALI vs without ALI, p<0.001) and cost of hospitalization ($261,168 vs $144,218; ALI vs without ALI; p<0.001) among patient with CS-STEMI (Table 3).
Table 3:
In-hospital mortality, cost and LOS in Acute limb ischemia
| Acute Limb Ischemia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| In-Hospital mortality | Length of Stay (in days) ** | Cost of Hospitalization (in dollars) | |||||||||
| With acute limb ischemia No./Total (%) | Without acute limb ischemia No./Total (%) | p-value | Adjusted Odds Ratio* | P-value | With acute limb ischemia No./Total (%) | Without acute limb ischemia No./Total (%) | p-value | With acute limb ischemia No./Total (%) | Without acute limb ischemia No./Total (%) | p-value | |
| Cardiogenic Shock with STMI | 585/1628 (35.9%) | 51464/17086 3 (30.1%) | <0.001 | 1.21 (1.09–1.36) | <0.001 | 19 | 11 | <0.001 | 261,168 | 144,218 | <0.001 |
| CS with STEMI without MCS | 264/631 (41.8%) | 27198/80388 (33.8%) | <0.001 | 1.49 (1.25–1.77) | <0.001 | 19 | 9 | <0.001 | 197,195 | 105,985 | <0.001 |
| CS with STEMI and IABP | 232/805 (28.8%) | 22143/85990 (25.8%) | 0.04 | 0.96 (0.81–1.13) | 0.63 | 18 | 11 | <0.001 | 253,738 | 166,908 | <0.001 |
| CS with STEMI and PVAD | 25/74 (33.8%) | 828/2004 (41.3%) | 0.19 | 0.30 (0.16–0.56) | <0.001 | 15 | 15 | 0.92 | 292,310 | 307,405 | 0.16 |
| CS with STEMI and ECMO | 19/33 (57.6%) | 229/411 (55.7%) | 0.83 | 1.25 (0.48–3.24) | 0.64 | 30 | 26 | 0.32 | 619,830 | 498,420 | 0.08 |
Adjusted for age, sex, race, smoking status, dyslipidemia, alcohol abuse, congestive heart failure, liver disease, peripheral vascular disease, deficiency anemia, chronic pulmonary disease, coagulopathy, renal failure diabetes, hypertension, cardiac arrest and vasopressor use.
included all patients that survived the index admission.
Discussion:
We now report one of the largest analyses focused on vascular complications in the setting of CS-STEMI. Specifically, we identified that while rates of bleeding complications remain high, overall trends show a reduction in bleeding events over time across all CS-STEMI patients from 2005–2014. We further identified that bleeding events are not associated with increased in-hospital mortality in CS-STEMI patients. We observed that the incidence of ALI in CS-STEMI patients increased between 2005 and 2014 and is associated with higher in-hospital mortality. Both bleeding complications and ALI were associated with longer LOS and higher hospitalization costs. Percutaneous MCS use is associated with increased rates of vascular complications, length of stay and hospitalization costs.
Despite significant improvement in the medical management and development of new technologies, in-hospital mortality in patients managed with CS-STEMI remains high (16). Over the past decade, multiple studies have been reported using various pMCS in these patients for ventricular support and to improve hemodynamics(8,17–19). Many of these devices require large bore catheters which can increase vascular complications. When analyzing major trials comparing pMCS, most of these studies were underpowered to detect significant differences in complications such as bleeding or ALI (20). Using a large US national database, we observed a decrease in the bleeding complications and significant increase in the incidence of ALI between 2005 and 2014.
Bleeding is a common complication in STEMI due to increase usage of thrombolytics, anticoagulation and antiplatelets, and is one of the important clinical outcomes in acute coronary syndrome (21–24). The overall risk of major bleeding in STEMI patients ranges between 2–10% (25–30). In our study we observed that the incidence of bleeding complications was 18.2%. This increased risk of bleeding in CS-STEMI patients may be due to multiorgan failure, a hyper-inflammatory milieu, increased utilization of femoral access, and higher usage of pMCS which requires both prolonged use of systemic anticoagulation and large bore catheters (8,9,31). Collectively, these findings suggest that pMCS devices are associated with higher risk of vascular complications. More focus on managing vascular complications is required in CS-STEMI, especially when pMCS devices are used. In the STEMI-DTU Pilot trial, vascular complications associated with Impella were rare and occurred during device removal, not device implantation (32). This observation led to the development of techniques specifically designed to mitigate vascular complications during Impella removal such as the double Perclose post-closure method (33). Our findings suggest that a renewed focus and collaboration with vascular surgeons and peripheral interventional specialists must occur to improve outcomes in CS-STEMI.
Our study population included only patients with CS which may have resulted in an overall higher number of bleeding events when compared to prior studies. PCI was the most common revascularization strategy among patients with bleeding complications. However, there were higher CABG and fibrinolytic therapy performed among patients with bleeding complication in comparison to those without bleeding. We observed that all cause in-hospital mortality did not increase in group with bleeding complications in comparison to those without. ExTRACT-TIMI 25 trial showed that non-intracranial hemorrhage major or minor bleeding were not independently associated with increased short- or long-term mortality. However, development of CS during the index hospitalization was associated with increased mortality (27). Since our study includes all patients with CS regardless of bleeding complications, overall in-hospital mortality remained high suggesting that many of these patients are critically sick. Furthermore, many of our cases received blood transfusions or underwent surgical or endovascular intervention to control bleeding event which may be associated with improved mortality. Previous studies have demonstrated mixed mortality outcomes in acute myocardial infarction patients with bleeding requiring blood transfusions (24,34). In our study we observed that having more than 2 transfusions during index hospitalization was associated with significantly higher adjusted in-hospital mortality, which is consistent with the harmful effect of having severe bleeding and increased blood transfusions in any clinical settings (12,35–37). One of the study with 78,794 Medicare patients hospitalized with acute myocardial infarction, showed reduced in-hospital mortality associated with transfusion use (24). Transfusions are also common after ventricular assist device implantation (38). Short-term MCS similarly may be associated with an increase in blood transfusions due to hemolysis, hemodilution when using extracorporeal circuits, and expected bleeding at the time of device implantation (i.e. surgical cutdowns). Furthermore, transfusions increase oxygen carrying capacity of the blood, which when combined with increased systemic perfusion driven by a short-term MCS may improve clinical outcomes for CS-STEMI patients. In addition, with increasing experience in utilization of MCS devices have led to an early identification of complication requiring blood transfusion and better techniques to control this bleeding.”
ALI is another dreaded vascular complication in CS-STEMI that can be potentially due to poor hemodynamic state, increase use of vasopressors, and large bore cannulas for pMCS (3,39–41). In addition, many of the STEMI patients may have history of previous peripheral artery disease which predisposes the risk of developing ALI (40,42,43). In our study ALI was observed in 0.9% of patients with CS-STEMI. Similar to previous studies we noted that the risk of ALI is increased with the use of devices requiring larger size canula such as PVAD or ECMO (8,42,44). About 50% of the ALI hospitalizations in our study underwent intervention with or without pMCS. Similar results were seen by Abaunza et al in patients with Impella in which 7 out of 12 underwent invasive interventions with ALI (45). Overall mortality was better in patients who underwent interventions in comparison to those with medical management. We observed similar findings with overall in-hospital mortality was lower in patients who underwent interventions for ALI (Supplementary Table 7). ALI was associated with overall higher in-hospital mortality in patients with CS-STEMI in comparison to those without ALI. However, among all the sub-groups patients with PVAD did not show higher in-hospital mortality with ALI. Abaunza et al showed comparable findings as vascular complications did not affect 30-day mortality in their study. The authors suggest that these findings were observed in very sick patient population and may not be linked to the lower-risk population (45).
Our analysis identified that patients with ALI had higher vasopressor use and prevalence of peripheral vascular disease. Several strategies have been proposed to improve limb perfusion such as antegrade distal perfusion catheter which can be considered in patients with previous history of peripheral vascular disease (8). In addition, our study showed that presence of ALI in patients with CS-STEMI was accompanied with higher cost of hospitalization and longer hospital stay with or without pMCS.
All pMCS are associated with cannulation related complications such as vessel perforation, arterial dissection and distal ischemia. Among all devices used for CS-STEMI, IABP have the lowest risk of ALI or bleeding complications. This can be due to use of smaller size catheter 7.5–8F and they do not require high anticoagulation dose and may not require any if used with 1:1 counterpulsation. IABP can result in ALI due to wrong insertion into side branches such as profunda or superficial femoral artery as neither of them are large enough to accommodate insertion of the device without causing obstruction to the arterial flow(8). However, incidence of vascular complications such as ALI or bleeding complications are much higher in patients with PVAD due to increase anticoagulation and from increased systemic hemolysis. Both impella and tandem heart require larger arterial cannula which significantly increases risk of distal limb ischemia in comparison to IABP (19). Similarly, ECMO requires large bore catheters for both arterial and venous which can significantly increase these risks (46). In addition to continuous anticoagulation use with high ACT goals, increase destruction of platelets can also increase the risk of bleeding in patients with ECMO. Tight monitoring and close titration of ACT goals for anticoagulation is essential in patients at risk of bleeding complications for patients with any pMCS device. Performing a routine peripheral angiography before cannula selection and early recognition of those at risk of developing ALI can play a key role in decreasing events limb ischemia in these patients. Furthermore, keeping a low threshold for using percutaneous downstream perfusion canula in high risk patients can help reducing events of distal ischemia(19,47).
Limitations
Our study has several limitations that need to be acknowledged. Like previous large discharge databases, the NIS follows a retrospective cross-sectional study design and one of the main limitations in this study is the inability to draw causality among the various pMCS devices and their risk for vascular complications as time to event is limited. However, we reported the incidence of bleeding complications and ALI among all groups (without any device, with IABP, PVAD or ECMO). Also, being a retrospective database, patient related information and data may be missing or incomplete. We were unable to use BARC, CRUSADE or TIMI classifications for bleeding due to lack of laboratory values in NIS database, however, we used previously validated methods to define our events of bleeding complications (12). Moreover, the NIS does not provide objective vitals such as blood pressure and heart rate nor echocardiographic findings which are essential markers in assessing management of CS. The multivariate hierarchical logistic regression model was adjusted for all the variables listed in baseline characteristics as they can be possible confounders. However, there can still be variation in the hemodynamic profile of patients with different pMCS devices which was not provided in this database. Finally, as with many projects that use the national database, the use of a registry database to calculate the impact of vascular complications on mechanical support with pVADs is at risk of coding errors (48). These limitations are counterbalanced by a larger sample size and absence of reporting bias which usually results from selective publications from specialized centres. Nevertheless, our study answered a lot of questions that could not be collected from larger clinical trials and single center observational studies due to insufficient power. Furthermore, the ICD-CM codes used for bleeding, complications and ALI have been well validated in previous studies (12,13).
Conclusion
Using a large national database, our study showed that the vascular events such as bleeding complications and ALI are common among patients with CS-STEMI. Bleeding complications did not increase the risk of in-hospital mortality; however, ALI are associated with higher in-hospital mortality. Both the complications were associated with significantly higher hospital stay and healthcare costs in patients with CS-STEMI. Considering significant growth in the increase of pMCS in recent era better strategies are needed to avoid these complications, including antegrade perfusion catheters, precision coagulation monitoring, early detection and better clinical management once a complication is diagnosed.
Supplementary Material
Supplementary Table 1: ICD-9 Diagnosis and Procedural Codes
Supplementary Table 2: List of Elixhauser comorbidities.
Supplementary Table 3: Baseline characteristics of patients with and without bleeding complications for patients with No MCS, IABP, PVAD or ECMO.
Supplementary Table 4: Baseline characteristics of patients with and without acute limb ischemia for patients with No MCS, IABP, PVAD or ECMO.
Supplementary Table 5: Adjusted risk of having an event of bleeding complications and ALI among patients with IABP, PVAD or ECMO in comparison to those with no MCS.
Supplementary Table 6: Adjusted adverse event risk by number of transfusions in patients with CS-STEMI and bleeding complications.
Supplementary Table 7: In-hospital mortality with and without surgical/endovascular intervention in patients with ALI.
Acknowledgments
Disclosure of funding: Funding from NIH 1R01HL139785–01 to NKK
Abbreviations:
- CS
Cardiogenic Shock
- STEMI
ST segment elevation myocardial infarction
- CS-STEMI
Patients with STEMI and cardiogenic shock
- pMCS
Percutaneous mechanical circulatory support
- IABP
Intra aortic balloon pump
- PVAD
Percutaneous ventricular assist device includes Impella or Tandem Heart
- ECMO
Extracorporeal membrane oxygenation
- ALI
acute limb ischemia
- LOS
Length of stay
Footnotes
Conflict of interest: NKK: Consulting/Speaker Honoraria and Research Grants from Abbott, Abiomed, Boston Scientific, LivaNova, Medtronic, Maquet, preCARDIA, and MD Start.
References:
- 1.Babaev A, Frederick PD, Pasta DJ et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294:448–54. [DOI] [PubMed] [Google Scholar]
- 2.Jeger RV, Radovanovic D, Hunziker PR et al. Ten-year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med 2008;149:618–26. [DOI] [PubMed] [Google Scholar]
- 3.Lo N, Magnus Ohman E. Mechanical Circulatory Support in ST-Elevation Myocardial Infarction. In: Watson TJ, Ong PJL, Tcheng JE, editors. Primary Angioplasty: A Practical Guide. Singapore, 2018:253–273. [PubMed] [Google Scholar]
- 4.Backhaus T, Fach A, Schmucker J et al. Management and predictors of outcome in unselected patients with cardiogenic shock complicating acute ST-segment elevation myocardial infarction: results from the Bremen STEMI Registry. Clin Res Cardiol 2018;107:371–379. [DOI] [PubMed] [Google Scholar]
- 5.Rathod KS, Koganti S, Iqbal MB et al. Contemporary trends in cardiogenic shock: Incidence, intra-aortic balloon pump utilisation and outcomes from the London Heart Attack Group. Eur Heart J Acute Cardiovasc Care 2018;7:16–27. [DOI] [PubMed] [Google Scholar]
- 6.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J 2019. [DOI] [PubMed]
- 7.Cheng R, Hachamovitch R, Kittleson M et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610–6. [DOI] [PubMed] [Google Scholar]
- 8.Rihal CS, Naidu SS, Givertz MM et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care (Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). J Card Fail 2015;21:499–518. [DOI] [PubMed] [Google Scholar]
- 9.Thiele H, Jobs A, Ouweneel DM et al. Percutaneous short-term active mechanical support devices in cardiogenic shock: a systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 2017;38:3523–3531. [DOI] [PubMed] [Google Scholar]
- 10.Qian G, Wu C, Chen YD, Tu CC, Wang JW, Qian YA. Predictive factors of cardiac rupture in patients with ST-elevation myocardial infarction. J Zhejiang Univ Sci B 2014;15:1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert L, Blais C, Hamel D et al. Evaluation of care and surveillance of cardiovascular disease: can we trust medico-administrative hospital data? Can J Cardiol 2012;28:162–8. [DOI] [PubMed] [Google Scholar]
- 12.Redfors B, Watson BM, McAndrew T et al. Mortality, Length of Stay, and Cost Implications of Procedural Bleeding After Percutaneous Interventions Using Large-Bore Catheters. JAMA Cardiol 2017;2:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baril DT, Ghosh K, Rosen AB. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. J Vasc Surg 2014;60:669–77 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ando T, Akintoye E, Uemura T et al. Palliative care referral in ST-segment elevation myocardial infarction complicated with cardiogenic shock in the United States. Heart Lung 2020;49:25–29. [DOI] [PubMed] [Google Scholar]
- 15.Pahuja M, Adegbala O, Mishra T et al. Trends in the Incidence of In-Hospital Mortality, Cardiogenic Shock, and Utilization of Mechanical Circulatory Support Devices in Myocarditis (Analysis of National Inpatient Sample Data, 2005–2014). J Card Fail 2019;25:457–467. [DOI] [PubMed] [Google Scholar]
- 16.Wayangankar SA, Bangalore S, McCoy LA et al. Temporal Trends and Outcomes of Patients Undergoing Percutaneous Coronary Interventions for Cardiogenic Shock in the Setting of Acute Myocardial Infarction: A Report From the CathPCI Registry. JACC Cardiovasc Interv 2016;9:341–351. [DOI] [PubMed] [Google Scholar]
- 17.Kar B, Gregoric ID, Basra SS, Idelchik GM, Loyalka P. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol 2011;57:688–96. [DOI] [PubMed] [Google Scholar]
- 18.Sjauw KD, Engstrom AE, Vis MM et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J 2009;30:459–68. [DOI] [PubMed] [Google Scholar]
- 19.Thiele H, Sick P, Boudriot E et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26:1276–83. [DOI] [PubMed] [Google Scholar]
- 20.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014;64:1407–15. [DOI] [PubMed] [Google Scholar]
- 21.Bundhun PK, Janoo G, Chen MH. Bleeding events associated with fibrinolytic therapy and primary percutaneous coronary intervention in patients with STEMI: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox KA, Carruthers K, Steg PG et al. Has the frequency of bleeding changed over time for patients presenting with an acute coronary syndrome? The global registry of acute coronary events. Eur Heart J 2010;31:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steg PG, Huber K, Andreotti F et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J 2011;32:1854–64. [DOI] [PubMed] [Google Scholar]
- 24.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med 2001;345:1230–6. [DOI] [PubMed] [Google Scholar]
- 25.Boden H, Velders MA, van der Hoeven BL, Cannegieter SC, Schalij MJ. In-hospital major bleeding and its clinical relevance in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol 2013;112:1533–9. [DOI] [PubMed] [Google Scholar]
- 26.Cohen M Predictors of bleeding risk and long-term mortality in patients with acute coronary syndromes. Curr Med Res Opin 2005;21:439–45. [DOI] [PubMed] [Google Scholar]
- 27.Giugliano RP, Giraldez RR, Morrow DA et al. Relations between bleeding and outcomes in patients with ST-elevation myocardial infarction in the ExTRACT-TIMI 25 trial. Eur Heart J 2010;31:2103–10. [DOI] [PubMed] [Google Scholar]
- 28.Kikkert WJ, Zwinderman AH, Vis MM et al. Timing of mortality after severe bleeding and recurrent myocardial infarction in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 2013;6:391–8. [DOI] [PubMed] [Google Scholar]
- 29.O’Gara PT, Kushner FG, Ascheim DD et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:485–510. [DOI] [PubMed] [Google Scholar]
- 30.Numasawa Y, Kohsaka S, Ueda I et al. Incidence and predictors of bleeding complications after percutaneous coronary intervention. J Cardiol 2017;69:272–279. [DOI] [PubMed] [Google Scholar]
- 31.Miller PE, Solomon MA, McAreavey D. Advanced Percutaneous Mechanical Circulatory Support Devices for Cardiogenic Shock. Crit Care Med 2017;45:1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapur NK, Alkhouli MA, DeMartini TJ et al. Unloading the Left Ventricle Before Reperfusion in Patients With Anterior ST-Segment-Elevation Myocardial Infarction. Circulation 2019;139:337–346. [DOI] [PubMed] [Google Scholar]
- 33.Kapur NK, Hirst C, Zisa D. Advances in Vascular Post-Closure With Impella. Cardiovasc Revasc Med 2019;20:94–95. [DOI] [PubMed] [Google Scholar]
- 34.Rao SV, Jollis JG, Harrington RA et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004;292:1555–62. [DOI] [PubMed] [Google Scholar]
- 35.Corwin HL, Gettinger A, Pearl RG et al. The CRIT Study: Anemia and blood transfusion in the critically ill--current clinical practice in the United States. Crit Care Med 2004;32:39–52. [DOI] [PubMed] [Google Scholar]
- 36.Gili S, D’Ascenzo F, Lococo MF et al. Impact of blood transfusion on in-hospital myocardial infarctions according to patterns of acute coronary syndrome: Insights from the BleeMACS registry. Int J Cardiol 2016;221:364–70. [DOI] [PubMed] [Google Scholar]
- 37.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544–52. [DOI] [PubMed] [Google Scholar]
- 38.Slaughter MS, Rogers JG, Milano CA et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241–51. [DOI] [PubMed] [Google Scholar]
- 39.Kovacic JC, Nguyen HT, Karajgikar R, Sharma SK, Kini AS. The Impella Recover 2.5 and TandemHeart ventricular assist devices are safe and associated with equivalent clinical outcomes in patients undergoing high-risk percutaneous coronary intervention. Catheter Cardiovasc Interv 2013;82:E28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjauw KD, Konorza T, Erbel R et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol 2009;54:2430–4. [DOI] [PubMed] [Google Scholar]
- 41.Thiele H, Zeymer U, Neumann FJ et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287–96. [DOI] [PubMed] [Google Scholar]
- 42.O’Neill WW, Schreiber T, Wohns DH et al. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol 2014;27:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saw J, Bhatt DL, Moliterno DJ et al. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol 2006;48:1567–72. [DOI] [PubMed] [Google Scholar]
- 44.Hatch J, Baklanov D. Percutaneous Hemodynamic Support in PCI. Curr Treat Options Cardiovasc Med 2014;16:293. [DOI] [PubMed] [Google Scholar]
- 45.Abaunza M, Kabbani LS, Nypaver T et al. Incidence and prognosis of vascular complications after percutaneous placement of left ventricular assist device. J Vasc Surg 2015;62:417–23. [DOI] [PubMed] [Google Scholar]
- 46.Sklar MC, Sy E, Lequier L, Fan E, Kanji HD. Anticoagulation Practices during Venovenous Extracorporeal Membrane Oxygenation for Respiratory Failure. A Systematic Review. Ann Am Thorac Soc 2016;13:2242–2250. [DOI] [PubMed] [Google Scholar]
- 47.Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation 2001;104:2917–22. [DOI] [PubMed] [Google Scholar]
- 48.Sarrazin MS, Rosenthal GE. Finding pure and simple truths with administrative data. JAMA 2012;307:1433–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: ICD-9 Diagnosis and Procedural Codes
Supplementary Table 2: List of Elixhauser comorbidities.
Supplementary Table 3: Baseline characteristics of patients with and without bleeding complications for patients with No MCS, IABP, PVAD or ECMO.
Supplementary Table 4: Baseline characteristics of patients with and without acute limb ischemia for patients with No MCS, IABP, PVAD or ECMO.
Supplementary Table 5: Adjusted risk of having an event of bleeding complications and ALI among patients with IABP, PVAD or ECMO in comparison to those with no MCS.
Supplementary Table 6: Adjusted adverse event risk by number of transfusions in patients with CS-STEMI and bleeding complications.
Supplementary Table 7: In-hospital mortality with and without surgical/endovascular intervention in patients with ALI.


