Abstract
Effective treatments for chronic pain without abuse liability are urgently needed. One in 5 adults suffer chronic pain and half of these patients report inefficient treatment. Mu opioid receptor agonists (MOP), including oxycodone, tramadol and morphine, are often prescribed to treat chronic pain, however, use of drugs targeting MOP can lead to drug dependency, tolerance and overdose deaths. Kappa opioid receptor (KOP) agonists have antinociceptive effects without abuse potential; however, they have not been utilised clinically due to dysphoria and sedation. We hypothesise that mixed opioid receptor agonists targeting the KOP and delta opioid receptor (DOP) would have a wider therapeutic index, with the rewarding effects of DOP negating the negative effects of KOP. MP1104, an analogue of 3-Iodobenzoyl naltrexamine, is a novel mixed opioid receptor agonist with potent antinociceptive effects mediated via KOP and DOP in mice without rewarding or aversive effects. In this study, we show MP1104 has potent, long-acting antinociceptive effects in the warm-water tail-withdrawal assay in male and female mice and rats; and is longer acting than morphine. In the paclitaxel-induced neuropathic pain model in mice, MP1104 reduced both mechanical and cold allodynia and unlike morphine, did not produce tolerance when administered daily for 23 days. Moreover, MP1104 did not induce sedative effects in the open-field locomotor activity test, respiratory depression in mice using whole-body plethysmography, or have cross-tolerance with morphine. This data supports the therapeutic development of mixed opioid receptor agonists, particularly mixed KOP/DOP agonists, as non-addictive pain medications with reduced tolerance.
Keywords: Mixed opioid receptor agonist, Neuropathic pain, Paclitaxel, Respiratory depression, Tolerance, Tail-withdrawal
1. Introduction
Chronic pain has become a worldwide issue, with global rates ranging from 11 to 50% of the adult population (Henderson et al., 2013; Kennedy et al., 2014; Fayaz et al., 2016). The high abuse potential of opioids prescribed for chronic pain also contributes to the global opioid epidemic, with 21–29% of patients abusing, and a further 8–12% addicted to prescription opioid medications (Vowles et al., 2015; Hedegaard et al., 2018). Despite vast efforts, opioid overdose deaths are continuing to increase due to the induction of respiratory depression (Okie, 2010; Berterame et al., 2016; Rudd et al., 2016), highlighting the urgent need for more effective, safer, non-addictive pain medications.
The main opioid receptors are mu (MOP), delta (DOP), kappa (KOP) and nociceptin opioid receptors (Stevens, 2009). Each opioid receptor subpopulation has a unique expression pattern and endogenous ligands that contribute to a unique analgesic effect, side-effects, and abilities to modulate different aspects of pain (Mayer and Saper, 2000). MOP analgesics such as morphine, oxycodone, and tramadol, are the most utilised pharmacological treatments for pain (Houmes et al., 1992; Lichtor et al., 1999; Caldwell et al., 2002). Unfortunately, on-target side-effects including tolerance, dependence, addiction, withdrawal, respiratory depression, hyperalgesia, and constipation, severely limit their clinical utility (Shook et al., 1990; Chu et al., 2006; Compton and Volkow, 2006; Goodman et al., 2007).
KOP agonists are a promising alternative, as they are non-rewarding, and have low abuse potential (Kivell and Prisinzano, 2010; Ueno et al., 2013). KOP agonists are effective in peripheral (Rivière, 2004 ), visceral (Binder et al., 2001) and inflammatory (Bileviciute-Ljungar et al., 2005) pre-clinical models of pain. DOP agonists also have antinociceptive (Brainin-Mattos et al., 2006; Gaveriaux-Ruff et al., 2008; Codd et al., 2009; Jones et al., 2009), anti-depressant (Broom et al., 2002; Naidu et al., 2007) and anxiolytic effects (Saitoh et al., 2004; Perrine et al., 2006; Vergura et al., 2008). In addition, it has been shown that administration of a KOP agonist and a DOP agonist can have a synergistic antinociceptive response (Miaskowski et al., 1990). Unfortunately, both KOP and DOP agonists have some unwanted side-effects that limit their clinical use. KOP activation causes dysphoria, anhedonia, aversion, depression and anxiety (Pfeiffer et al., 1986; Mello and Negus, 2000; Land et al., 2008), activation of DOP induces seizures (Bilsky et al., 1995; Jutkiewicz et al., 2005), and DOP agonists have abuse potential (Shippenberg et al., 2009; Pradhan et al., 2011; Mori et al., 2015). However, studies have shown that mixed opioid receptor agonists may be a viable strategy to generate a more desirable drug profile (Balboni et al., 2002; Váradi et al., 2016; Anand and Montgomery, 2018; Majumdar and Devi, 2018). We hypothesise that a mixed opioid receptor agonist targeting the KOP and DOP could have a wider therapeutic index, with the rewarding and positive effects of DOP opposing the aversive effects of KOP.
In this study, we explored the antinociceptive potential of the mixed opioid receptor agonist, 17-cyclopropylmethyl-3-hydroxy-4,5α-epoxy-7,8-en-6-β-[(3′-iodo)benzamido]-morphinan (MP1104), an analogue of 3-iodobenzoyl naltrexamine, a potent analgesic belonging to the 6β-amidoepoxymorphinan group of opioids (Váradi et al., 2015a, Váradi et al., 2015b). MP1104 has a higher affinity for KOP than MOP and DOP (Váradi et al., 2015a ). In the mouse tail-withdrawal assay, MP1104 produced 15-fold greater antinociceptive effects than morphine, without inducing seizures or rewarding effects (Váradi et al., 2015a). In addition, MP1104 did not induce aversion, anxiety, sedation or depressive-like effects in rats at doses that block the rewarding effects of cocaine (Atigari et al., 2019). More recently, MP1104 was found to attenuate pain-like behaviours in phases I and II of the formalin assay in both male and female mice (Ulker et al., 2020).
In the current study, we sought to determine whether the dual activation of KOP and DOP by MP1104 was effective in assays of nociceptive and chemotherapy-induced neuropathic pain. In addition, we tested for sedative, tolerance, withdrawal, and respiratory side-effects.
2. Methods
2.1. Animals
Male (n = 164) and female (n = 18) adult (8+ weeks) C57BL/6J mice (20–30 g), and adult male Sprague-Dawley rats (n = 55; 250–400 g) were housed within the vivarium at the School of Biological Sciences, Victoria University of Wellington, New Zealand. Mice were housed in temperature (19–20 °C) and humidity (55%) controlled rooms with lights maintained on a 12 h light/dark cycle (lights on at 07:00). All experimental procedures were approved and conducted in accordance with the guidelines of the Victoria University Animal Ethics Committee (approval numbers 22334, 21,480, and 25,751) and the New Zealand Animal Welfare Act, 1999. For the tolerance and withdrawal experiments, male CD1 mice (n = 100; 20–32 g) were obtained from Charles River Laboratories. The mice were maintained on a 12 h light/dark cycle and were housed in groups of five until testing. These animal studies were pre-approved by the Institutional Animal Care and Use Committees of the Memorial Sloan Kettering Cancer Center, in accordance with the 2002 National Institutes of Health Guide for the Care and Use of Laboratory Animals. For all animal procedures at both facilities, food and water were available ad libitum except during experimental procedures. All animals were handled by the experimenter for 2–3 days prior to testing to habituate the animals to handling stress, and all tests were performed by an experimenter blinded to the treatment groups.
2.2. Drugs
MP1104 and naltrindole (>98% purity) (Váradi et al., 2015a) were provided by Dr Susruta Majumdar (Center of Clinical Pharmacology, St. Louis College of Pharmacy and Washington University School of Medicine, USA). MP1104, DOP antagonist naltrindole, MOP antagonist β-Funaltrexamine (β-FNA) (Tocris Bioscience), and morphine were dissolved in a vehicle of dimethyl sulfoxide (DMSO), tween 80 (Sigma-Aldrich) and physiological saline in a ratio 2:1:7. The KOP antagonist nor-binaltorphimine (nor-BNI) (Tocris Bioscience) was diluted in physiological saline. The pre-treatment time was 15 min for naltrindole (15 mg/kg), whereas β-FNA (10 mg/kg) and nor-BNI (10 mg/kg) were injected 24 h prior to testing via subcutaneous (s.c.) injection. All drugs and solutions were stored in the dark at 4 °C. For the tolerance and withdrawal experiments, the naloxone, morphine pellets and placebo pellets were provided by the National Institute on Drug Abuse. Paclitaxel (Paclitaxel Ebewe; Sandoz Pty Ltd., NSW, Australia) was commercially available in concentrated liquid form (6 mg/mL). Paclitaxel was diluted to 0.4 mg/mL to give a mixture of absolute ethanol, Kolliphor EL (Sigma-Aldrich), and 0.9% saline in 1:1:18 ratio.
2.3. Warm-water tail-withdrawal assay
The warm-water tail-withdrawal assay measures latency to withdraw the tail following thermal stimulation and is mediated via spinal cord reflexes (Irwin et al., 1951). Warm-water tail-withdrawal assays were conducted as previously described (Thorn et al., 2011; Paton et al., 2017). Mice were restrained in plexiglass restrainers with an internal diameter of 24 mm, while rats were restrained by hand. Tail-withdrawal latencies were measured by immersing a third of the distal portion of the tail in a water-bath containing 50 ± 0.5 °C (mice) or 55 °C (rats) water. The time taken for withdrawal responses was recorded, with a 10 s (mice) or 15 s (rats) cut-off latency to avoid tissue damage.
To evaluate the duration of action of MP1104, a tail-withdrawal time-course was performed. Mice and rats were administered via intraperitoneal (i.p.) injection either vehicle, MP1104 (0.3 or 0.6 mg/kg) or morphine (10 mg/kg) and tail-withdrawal latencies measured to 480 min. The percent maximum possible effect (%MPE) of antinociception was calculated by applying the formula:
To measure whether these effects were mediated via KOP, MOP or DOP, animals were pre-treated with an antagonist for KOP (nor-BNI, 10 mg/kg, s.c., 24 h pre-treatment), MOP (β-FNA, 10 mg/kg, s.c., 24 h pre-treatment), or DOP (naltrindole, 15 mg/kg, s.c., 15 min pre-treatment).
Dose-response effects for tail-withdrawal were evaluated using a within-subject experimental design as described previously (Bohn et al., 2000; Paton et al., 2017). Animals were given s.c. injections (5 μL/g) of MP1104 at increasing concentrations and tail-withdrawal latency measured 1 h following administration of each cumulative dose. The potency (ED50) and efficacy (Emax) were calculated using non-linear four-parameter regression analysis (variable slope dose-response curve) using GraphPad Prism software version 7 (GraphPad, La Jolla, CA).
2.4. Chemotherapy-induced neuropathic pain
To induce chemotherapy-induced neuropathic pain (CINP), male C57BL/6J mice were administered paclitaxel (4 mg/kg, i.p.) four times on alternate days to give a cumulative dose of 16 mg/kg. This dosing regime is known to produce paclitaxel-induced neuropathic pain in mice (Deng et al., 2015; Paton et al., 2017). Control mice were given vehicle consisting of a 1:1:18 ratio of absolute ethanol, kolliphor EL, and 0.9% saline, respectively, and housed in separate cages to paclitaxel-treated mice. To measure the progress of paclitaxel-induced effects, mice were placed in chambers on an elevated mesh grid with holes of approximately 1 mm and allowed to habituate to the apparatus for 20 min. Each hind paw was measured twice for both mechanical and cold allodynia and the average value recorded. On days when paclitaxel was to be administered, mechanical and cold allodynia measurements were taken prior to the administration of paclitaxel.
2.4.1. Mechanical allodynia
Mechanical allodynia was measured using von Frey filaments (20-piece set; #58011, Stoelting, IL, USA). Von Frey filament numbers 2 to 9 were used to measure the sensitivity to mechanical stimuli, beginning with filament 5, and using a simplified up-down method (Bonin et al., 2014). Briefly, a positive response to filament application resulted in the use of the next lower filament in the subsequent test, but if no response was observed, the next higher filament was used. The process continued until five filaments were used. The paw withdrawal threshold was calculated using the outcome of the fifth filament. A value of 0.5 filament intervals was added or subtracted to the result if the response to the fifth filament was negative or positive, respectively (Bonin et al., 2014).
2.4.2. Cold allodynia
Cold allodynia was measured using a drop of acetone administered to the plantar surface of the hind paw as described previously (Deng et al., 2015; Paton et al., 2017). The total response time of relevant behaviours, including licking, shaking, elevating or biting the paw, was measured.
2.4.3. Dose-response procedure in mice with established chemotherapy-induced neuropathic pain
The dose-response experiments were carried out on day 15 following the initial induction of CINP. Mice were administered cumulative doses of MP1104, morphine or equivalent volumes of vehicle, with a 1 h interval between each cumulative dose. The doses were administered via s.c. injection to allow for a sustained rate of absorption. Non-linear regression analysis was used to calculate ED50 and Emax and four-parameter variable slope with least-squares ordinary fit used to fit the data curve in GraphPad Prism software version 7.
2.4.4. Evaluation of chronic MP1104 administration on chemotherapy-induced neuropathic pain
The effects of repeated MP1104 administration on mechanical and cold allodynia were evaluated in mice with established paclitaxel-induced neuropathic pain. This experimental design allowed evaluation of tolerance following chronic administration of MP1104 or morphine. On day 15, CINP mice were allocated into treatment groups so that mechanical allodynia scores were equal. Mice were administered daily injections of either MP1104 (1.2 mg/kg, i.p.), morphine (10 mg/kg, i.p.) or vehicle for 23 days (days 16–38). On even-numbered days, mechanical and cold allodynia behaviours were measured 1 h following injection.
2.5. Open-field locomotor activity test
The open-field arena was a 45 × 45 cm chamber that was virtually separated into two zones, comprising a 5 cm width border zone and a middle zone. The animals were placed in the center of the chamber and allowed to move freely for 15 min, while their horizontal ambulatory activity was tracked with the aid of a video-tracking system using SMART 3.0 software. Treatment groups included vehicle, morphine (10 mg/kg, i.p.) and MP1104 (0.6 and 1.2 mg/kg, i.p.).
2.6. Tolerance and withdrawal studies
For the initial withdrawal experiment, male CD1 mice were administered twice-daily injections with either morphine (5 mg/kg, s.c.) or MP1104 (1 mg/kg, s.c.) for 5 days. Naloxone (1 mg/kg, s.c.) was administered to precipitate withdrawal, and animals were evaluated for the number of jumps over 15 min (Ling et al., 1984; Gistrak et al., 1989; Majumdar et al., 2011; Grinnell et al., 2014). Similarly, in the second withdrawal experiment, in morphine (75 mg free-base) or placebo pelleted animals, after 3 days withdrawal was precipitated with either naloxone (1 mg/kg, s.c.) or MP1104 (0.66 mg/kg, s.c.).
Cross-tolerance studies were also performed in morphine (75 mg free-base) or placebo pelleted mice. Antinociceptive tolerance was assessed by performing a dose-response radiant heat tail-flick assay using an Ugo Basile model 37,360 instrument as previously described (Váradi et al., 2015a, 2015b). The intensity was set to achieve a baseline between 2 and 3 s. Baseline latencies were determined before experimental treatments for all mice. Morphine or MP1104 were administered via s.c. injection with 30 min between subsequent doses. Tail flick antinociception was assessed with a maximal 10 s latency to minimize damage to the tail. Data were analysed as percent maximal effect, % MPE, and was calculated according to the formula:
2.7. Whole-body plethysmography in unrestrained awake mice
Mice were acclimatised to the room for 30 min and habituated to the respiratory chamber for 10 min every day for 2 weeks. The respiratory measurements were taken as previously described (Lim et al., 2014). In brief, volume changes were calibrated by injecting known amounts of air into the chamber, the pressure deflection of this injection was used to calibrate the pressure transducer and was recorded for later analysis (Colman and Miller, 2001; Lewanowitsch et al., 2006). On the experimental day, mice were placed into the respiratory chamber for 10 min with 3 baselines taken at 5, 7, and 9 min. The animal was then removed from the chamber and injected within a 2 min window. The animal was then returned to the chamber and recordings taken at 5, 10, 15, 20, 30, 40, 50, and 60 min post-administration. Data were analysed using the LabChart Software v8 (Dunedin, NZ) using previously described macro analysis (Lim et al., 2014). Tidal volume and minute volume were then calculated using equations (1)–(3) and presented as a percent of the baseline measurements:
| (1) |
Where;
VT = tidal volumePT = pressure deflection due to tidal volumePK = pressure deflection due to each μL injectionVK = volume of each calibration injectionTCORE
= core temperature of each mouse
PB = barometric pressurePC = water vapor pressure at chamber temperature relative humidity in chamber
PCORE = pressure at body temperature
(water vapor pressure at body temperature × 1)
| (2) |
| (3) |
2.8. Statistical analysis
One-way and two-way interaction analysis was carried out with GraphPad Prism version 7. Data from repeated measures experiments were analysed using two-way repeated-measures ANOVA followed by Bonferroni or Turkey’s multiple comparison tests and were compared to vehicle-treated control animals. To evaluate dose-response effects, the data were transformed to logarithmic values (base 10), and a non-linear regression was performed using a four-parameter variable slope with least-squares ordinary fit to determine ED50 and Emax values from the dose-response curve. For mechanical allodynia, the top constraint was set to no more than 9.5 and for cold allodynia, the bottom constraint was set at no less than 0. No constraints were set for tail-withdrawal analyses. Data sets were tested prior to statistical analysis for normality using the D’Agostino and Pearson omnibus normality test. For data sets with p < 0.05 in the normality test were analysed with the Mann-Whitney non-parametric test.
The three-way interaction analysis was carried out with IBM SPSS Statistics (version 26) using a three-way mixed ANOVA, with treatment and sex as the between-subjects variables, and time as the within-subjects variable. Normality was assessed using the Shapiro-Wilk test, the homogeneity of variances was assessed with Levene’s test, and the sphericity was tested using Mauchly’s test. If the assumption of sphericity was violated, the Greenhouse-Geisser correction was applied. The Bonferroni correction was applied for the pairwise comparisons of simple main effects.
3. Results
3.1. MP1104profile
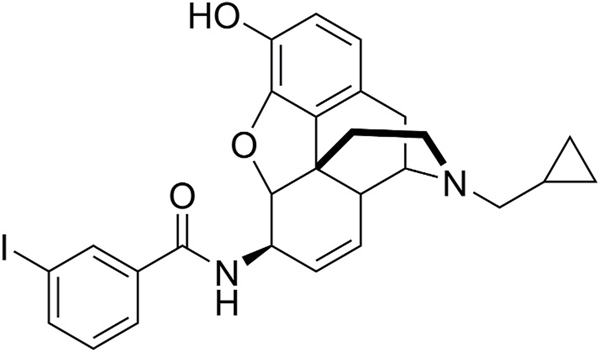
MP1104 is an analogue of 3-iodobenzoyl naltrexamine, a potent analgesic belonging to the 6β-amidoepoxymorphinan group of opioids (Fig. 1). MP1104 is a novel mixed opioid agonist that has high in vitro binding affinity and activity at the KOP, MOP and DOP (Váradi et al., 2015a).
Fig. 1.
The chemical structure of MP1104.
3.2. Onset and duration of action of MP1104 in the warm-water tail-withdrawal assay
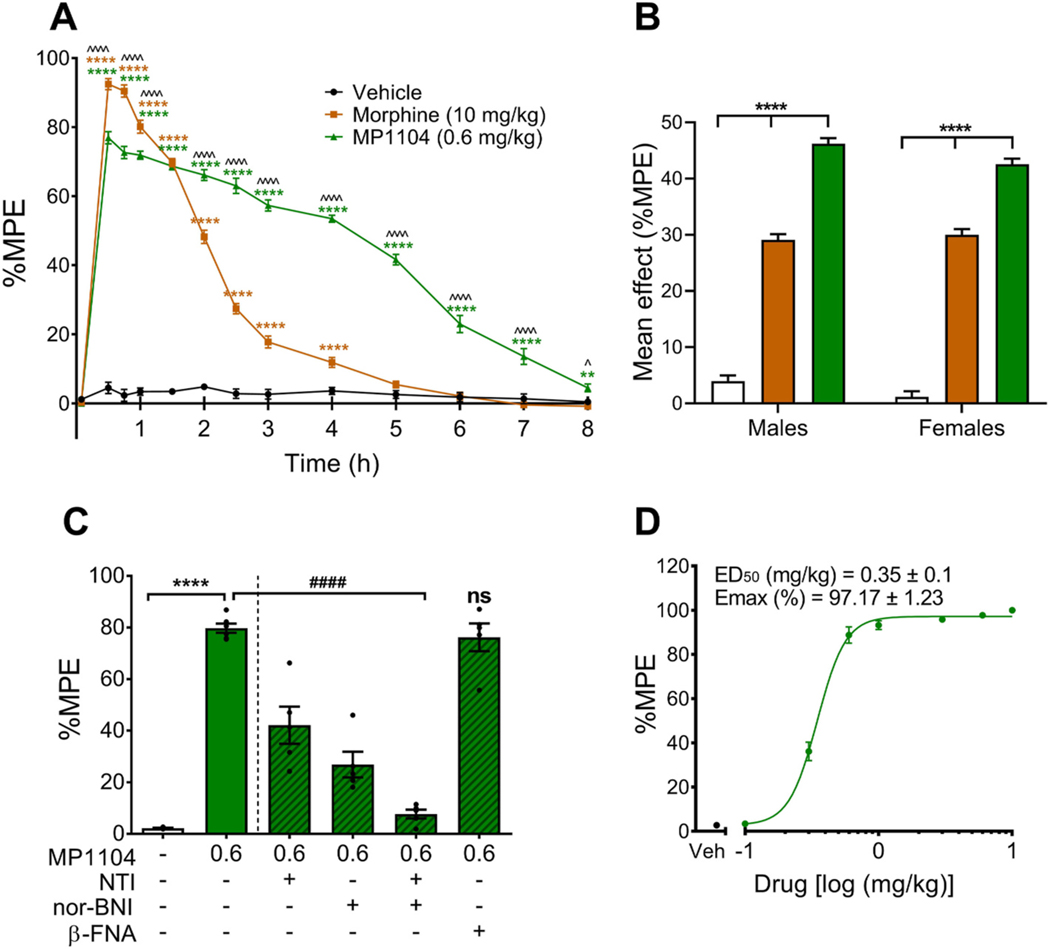
The warm-water tail-withdrawal assay was used to assess the onset and duration of action of MP1104. To investigate any sex differences in the antinociceptive effects of MP1104, both male and female mice were evaluated using the warm-water tail-withdrawal assay (Fig. 2A and B). The mean baseline latencies were not significantly different between the sexes, with baseline latencies of 2.0 ± 0.1 s for males and 1.9 ± 0.1 s for females. There was no three-way interaction of treatment, sex and time (F(14.4,216.7) = 0.907, p = 0.555), however, there were significant two-way interactions between treatment and time (Fig. 2A, p < 0.0005) and between treatment and sex (Fig. 2B, p = 0.027). MP1104 (0.6 mg/kg) produced antinociceptive effects starting at 30 min, and lasting up to 8 h, whilst morphine (10 mg/kg) produced effects up to 4 h (Fig. 2A). Between 30 and 60 min, morphine had more potent antinociceptive effects than MP1104, however, between 2 and 8 h, MP1104 produced significantly greater antinociceptive effects than morphine (Fig. 2A). The mean effects showed that MP1104 (0.6 mg/kg) had more potent antinociceptive effects than morphine (10 mg/kg) and vehicle within both the male and female mice (Fig. 2B).
Fig. 2.
Effect of MP1104 in the warm-water tail-withdrawal assay in C57BL/6J mice. (A) MP1104 showed significant antinociceptive effects in male and female mice that lasted up to 8 h. Three-way mixed ANOVA followed by Bonferroni post-tests (n = 12). (B) The mean effect analysis showed that MP1104 exhibited significantly increased antinociceptive effects compared to morphine and vehicle-controls in male (M) and female (F) mice (n = 6). (C) Percent maximal antinociceptive effect (%MPE) measured at 30 min in male mice showed that nor-BNI (10 mg/kg, s.c.) and naltrindole (NTI, 15 mg/kg, s.c.) administered individually and in combination significantly reduced the effects of MP1104 (0.6 mg/kg). On the other hand, MOP antagonist, β-FNA (10 mg/kg, s.c.), had no significant (ns) effect on MP1104 tail-withdrawal latencies. One-way ANOVA with Bonferroni post-tests (n = 5–6). (D) Cumulative dose-response effects of MP1104 administered to mice with %MPE calculated as a percent based on the pre-treatment baseline latencies. Animals were given s.c. injections of MP1104 at increasing concentrations and tail-withdrawal latency measured 1 h following administration. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ^p < 0.05, ^^^^p < 0.0001 for MP1104 compared to morphine. ####p < 0.0001 for addition of antagonist compared to MP1104. Values expressed as mean ± SEM.
Antinociceptive effects were evaluated in the presence of selective MOP (β-FNA, 10 mg/kg, s.c.), KOP (nor-BNI, 10 mg/kg, s.c.) and DOP (naltrindole, 15 mg/kg, s.c.) antagonists (Fig. 2C). The %MPE was calculated for each treatment group at 30 min, the time-point that showed peak significant antinociceptive effects (Fig. 2C). MP1104 at 0.6 mg/kg (p < 0.0001) dose showed significant antinociceptive effects compared to vehicle-controls (Fig. 2C). Pre-treatment with the MOP selective antagonist β-FNA did not alter antinociceptive effects, whereas pre-treatment with the selective DOP (naltrindole) (p < 0.0001) or KOP (nor-BNI) (p < 0.0001) antagonists attenuated the antinociceptive effects of MP1104 (0.6 mg/kg). When both nor-BNI and naltrindole were co-administered, MP1104 showed no significant antinociceptive effects, confirming the lack of MOP involvement in MP1104 induced antinociception, consistent with previous findings in mice (Váradi et al., 2015a).
Both mice and rats were used in this study to correlate our finding to previous reports evaluating side-effects and therapeutic effects of MP1104 utilising these same species (Váradi et al., 2015a; Atigari et al., 2019; Ulker et al., 2020). In the warm-water tail-withdrawal assay, MP1104 (0.6 mg/kg) showed a long duration of action of 8 h in both mice (Fig. 2A) and rats (Fig. S1). In addition, the antinociceptive effect in rats was attenuated following pre-treatment with KOP and DOP antagonists (Fig. S1). MP1104 was also found to be potent and efficacious in the cumulative dose tail-withdrawal assay in mice (ED50 = 0.35 ± 0.1 mg/kg) (Fig. 2D) and rats (ED50 = 0.37 ± 0.1 mg/kg) (Fig. S1).
3.3. Cumulative dose-response effects of MP1104 on mechanical and cold allodynia
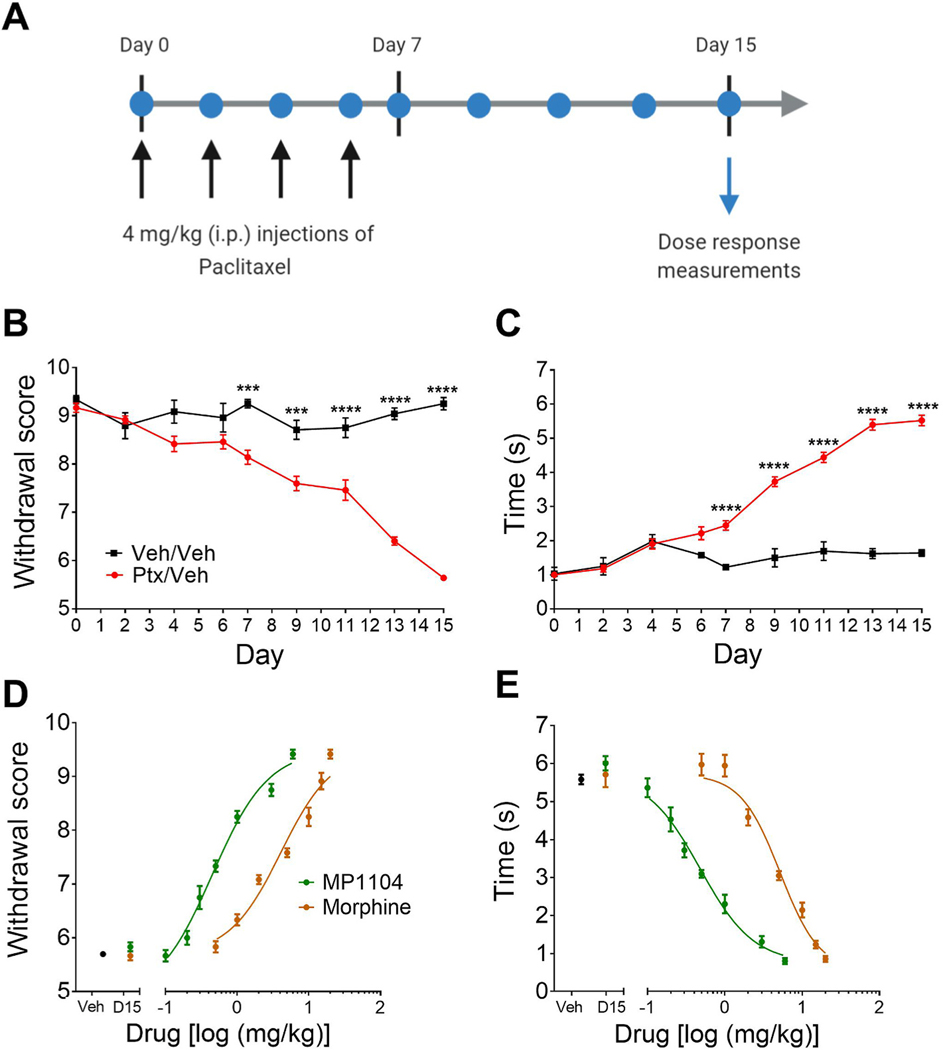
Paclitaxel, when administered as indicated in Fig. 3A, induced both mechanical (Fig. 3B) and cold (Fig. 3C) allodynia in mice. Maximal mechanical and cold allodynia was established at day 15 as described previously (Paton et al., 2017). Paclitaxel-induced mechanical allodynia on days 7–15 is shown as a reduction in withdrawal score measured by von Frey filaments (Fig. 3B) and an increase in response time to a cold acetone stimulus (Fig. 3C). MP1104 showed significant attenuation of both mechanical (Fig. 3D) and cold (Fig. 3E) allodynia. MP1104 showed a 9-fold increase in potency (ED50 = 0.449 mg/kg) in tests of mechanical allodynia compared to morphine (ED50 = 4.07 mg/kg) (p = 0.0386, Student’s t-test) (Fig. 3D). MP1104 was found to have 10-fold higher potency (ED50 = 0.479 mg/kg) compared to morphine (ED50 = 5.179 mg/kg) (p = 0.0371, Student’s t-test) in measures of cold allodynia (Fig. 3E).
Fig. 3.
Dose-response effects of MP1104 on paclitaxel-induced neuropathic pain in male C57BL/6J mice. (A) Experimental design. Mice were administered 4 mg/kg i.p. paclitaxel on days 0, 2, 4 and 6. Paclitaxel administration led to significant (B) mechanical and (C) cold allodynia. On day 15, MP1104 attenuated both (D) mechanical and (E) cold allodynia in mice with established paclitaxel-induced neuropathic pain. Veh indicates paclitaxel-treated mice injected with the vehicle; D15 indicates the day 15 baseline values prior to the dose-response experiment. Ptx, paclitaxel; Veh, vehicle. Two-way repeated-measures ANOVA with Bonferroni post-tests. ***p < 0.001, ****p < 0.0001. Values expressed as mean ± SEM, n = 6.
3.4. Effect of chronic administration of MP1104 on CINP-induced mechanical and cold allodynia
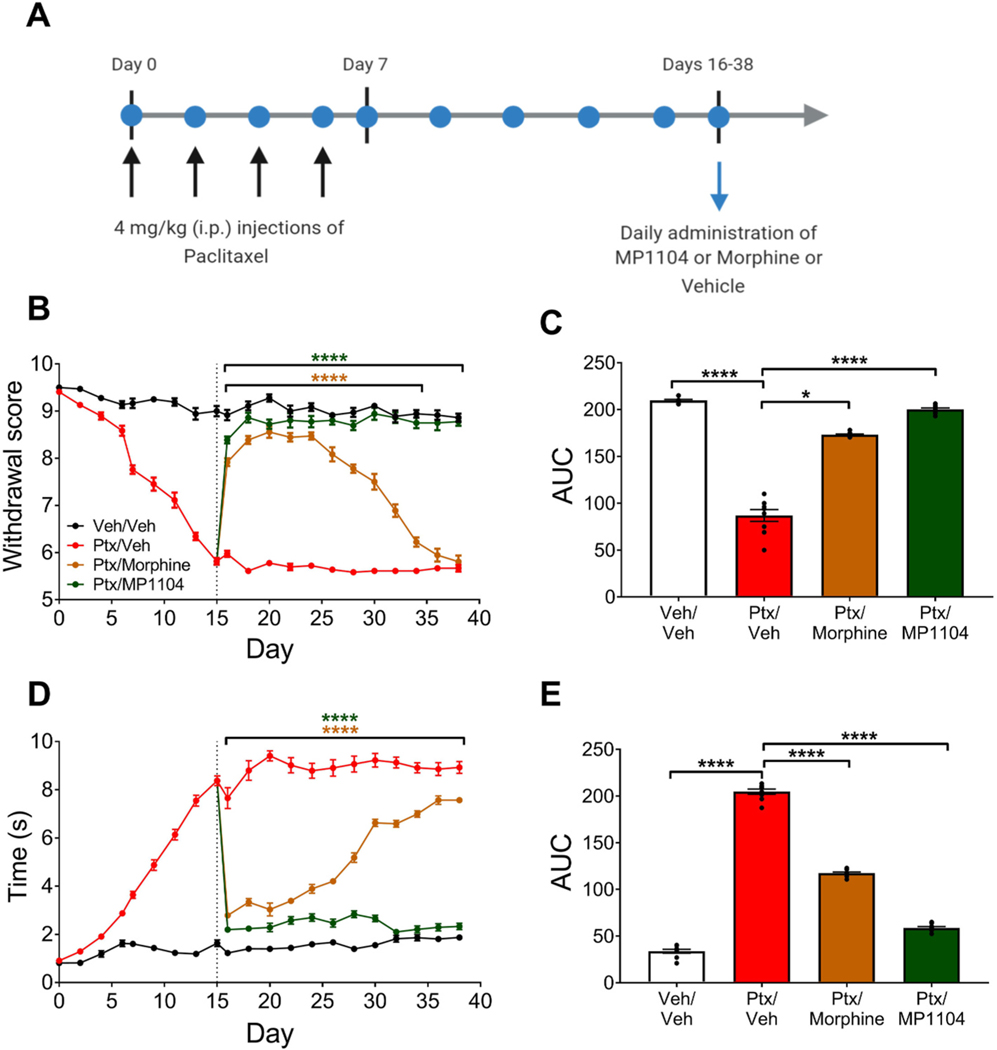
Mice were administered MP1104 (1.2 mg/kg), morphine (10 mg/kg) or vehicle daily beginning on day 16 following the initial induction of CINP (Fig. 4A). Two-way repeated-measures ANOVA revealed a significant interaction between the treatment and time for the mechanical allodynia measurements (F(33,352) = 29.1, p < 0.0001). Administration of MP1104 attenuated paclitaxel-induced mechanical allodynia for the entire experiment (days 16–38; p < 0.0001) (Fig. 4B). In contrast, morphine treatment showed significant antinociceptive effects that only lasted until day 34 (days 16–34; p <0.0001) (Fig. 4B). To understand the main effect of treatment, the area under the curve was calculated from days 16–38. MP1104 (p < 0.0001) and morphine (p < 0.05) reduced mechanical thresholds compared to paclitaxel/vehicle-treated mice (Fig. 4C).
Fig. 4.
Chronic administration of MP1104 and morphine in chemotherapy-treated C57BL/6J mice. (A) Experimental design for chronic treatment of MP1104 or morphine in mice with established paclitaxel-induced neuropathic pain. MP1104 (1.2 mg/kg) and morphine (10 mg/kg) daily i.p. injections reduced paclitaxel-induced (B–C) mechanical and (D–E) cold allodynia. MP1104 completely restored mechanical threshold and cold allodynia to non-diseased levels. The area under the curve (AUC) showed morphine and MP1104 reduced (C) mechanical and (E) cold allodynia compared to the vehicle/paclitaxel group. Two-way ANOVA with Bonferroni post-tests; vehicle/vehicle, MP1104 and morphine *p < 0.05, ****p < 0.0001 compared to the vehicle/paclitaxel control treatment group. Values expressed as mean ± SEM, n = 9. Ptx, paclitaxel; Veh, vehicle.
Cold allodynia was also assessed using the acetone test. There was significant interaction between treatment and time (F(33,352) = 23.98, p < 0.0001; two-way ANOVA with Bonferroni post-tests). MP1104 and morphine reduced paclitaxel-induced cold allodynia on all days assessed (days 16–38; p < 0.0001) (Fig. 4D). Moreover, MP1104 treatment reduced the cold response times back to the same level as non-diseased control levels (vehicle/vehicle treatment group (p > 0.999)) (Fig. 4D). The area under the curve analysis showed a significant effect of treatment (F(3,24) = 19.29, p < 0.0001), and Bonferroni post-tests showed that MP1104 and morphine reduced the paclitaxel-induced cold allodynia effects (p < 0.0001; Fig. 4E).
3.5. Open-field locomotor activity test
The open-field activity test was used to evaluate the locomotor effects of MP1104 in adult male C57BL/6J mice (Fig. 5A). The mean total distance travelled by mice treated with 0.6 and 1.2 mg/kg of MP1104 (5998 ± 722.4 cm and 6544 ± 499.6 cm, respectively) were not significantly different from the vehicle-treated control mice (6475 ± 704.2 cm; Fig. 5B). However, mice treated with morphine (10 mg/kg) had significantly (p < 0.01) increased locomotor activity (16,166 ± 1491 cm) compared to the vehicle controls (Fig. 5B). Morphine-treated mice showed increased activity in both the middle zone and border (Fig. 5C). However, neither morphine nor MP1104-treated mice showed a significant difference in the amount of time spent in the middle or border zone compared to the vehicle controls (Fig. 5D).
Fig. 5.
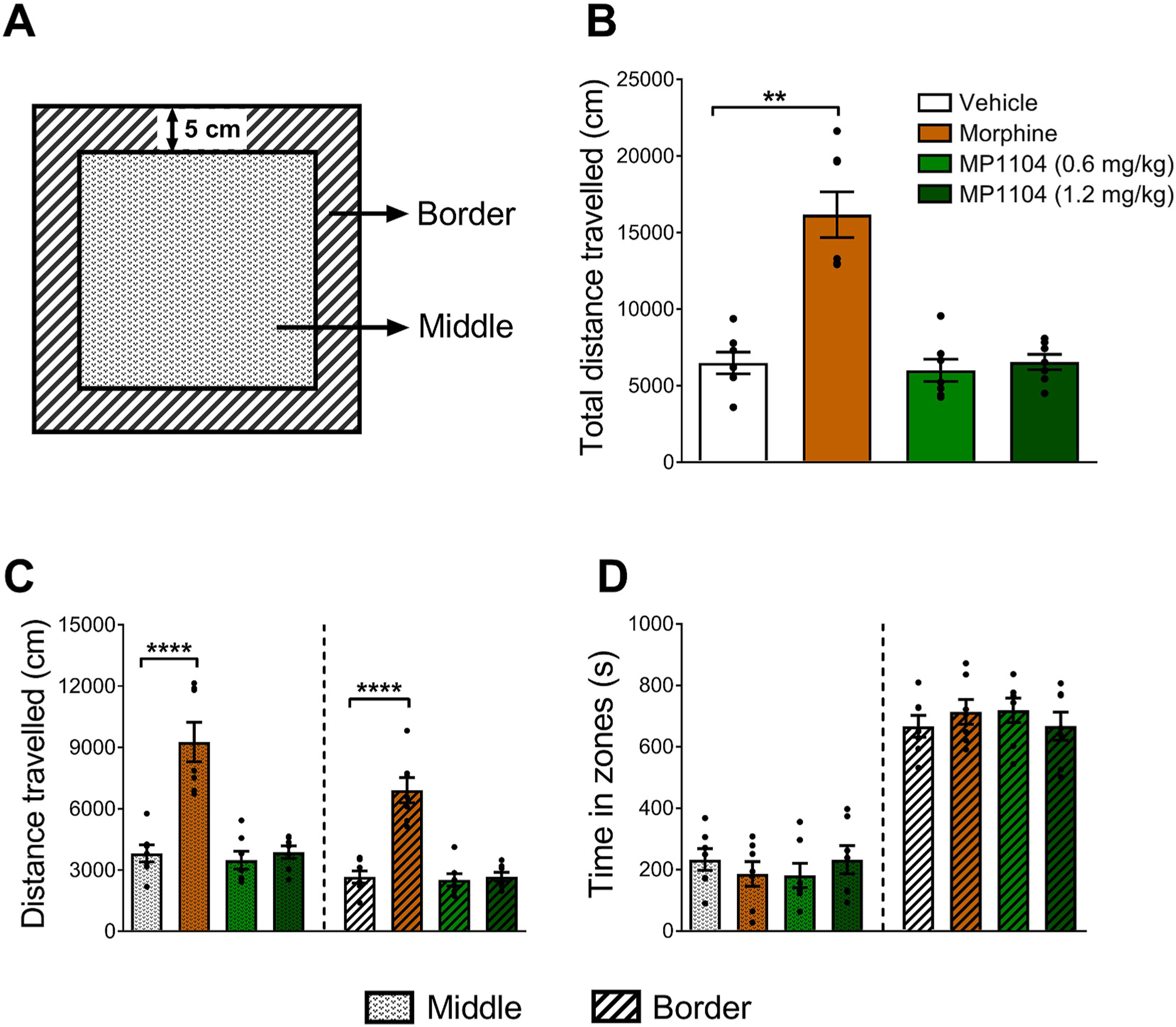
The locomotor effects of MP1104 were evaluated using the open-field activity test in male C57BL/6J mice. (A) Graphical representation of the open-field locomotor activity chamber divided into 2 zones, the outer border and the middle zone. (B) The total distance travelled and (C) the distance travelled in the middle vs border zone showed that the locomotor effects of MP1104 (0.6 and 1.2 mg/kg) were not significantly different to vehicle controls, whereas, morphine (10 mg/kg) significantly increased locomotor activity throughout both zones. (D) The time spent in the middle vs border zones showed no differences between any of the treatment groups. One-way ANOVA (B) and two-way (C–D) ANOVA with Bonferroni post-tests; **p < 0.01, ****p < 0.0001 compared to vehicle. Values expressed as mean ± SEM, n = 7.
3.6. Tolerance and withdrawal studies
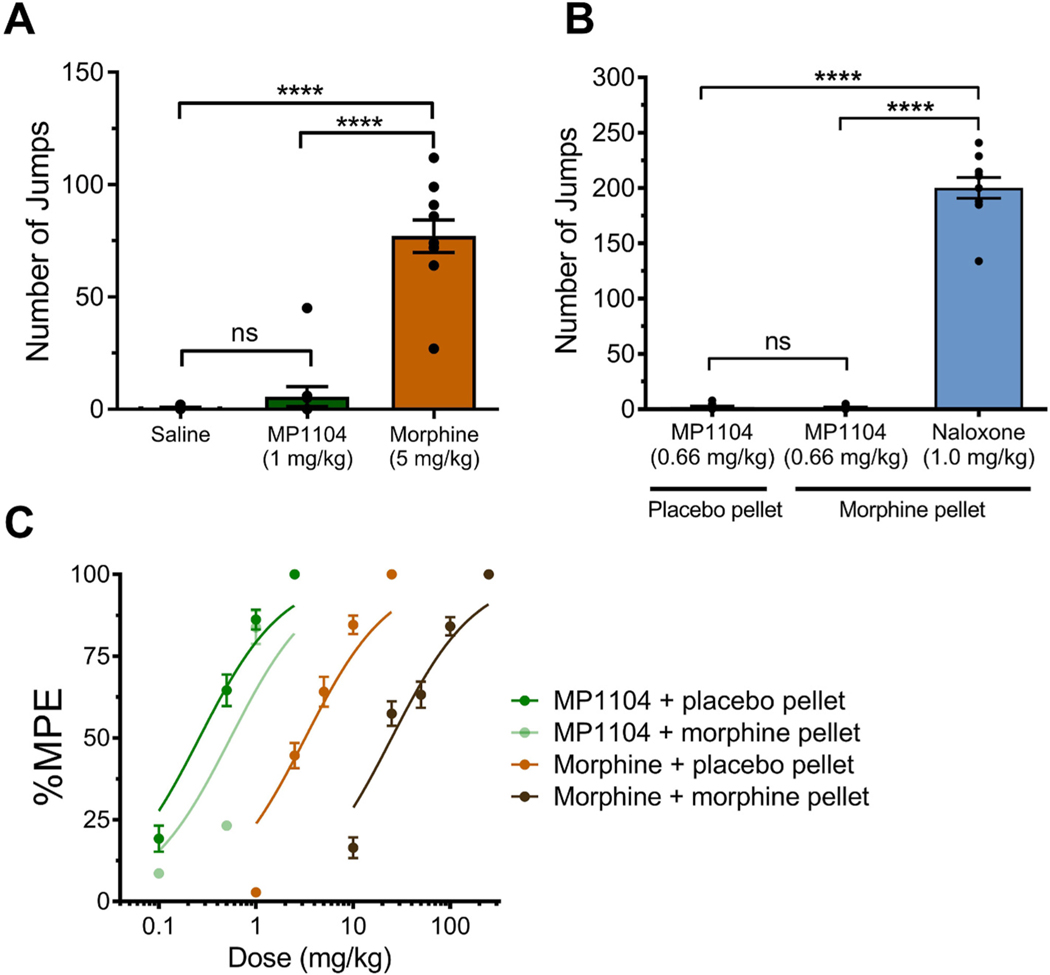
Chronic administration of traditional mu-opioids leads to physical dependence. We have previously shown that daily administration of morphine (5 mg/kg, s.c.) twice a day led to antinociceptive tolerance in mice to morphine by day 5 (Váradi et al., 2016). These morphine-treated mice show naloxone precipitated withdrawal syndrome. The same model was used with MP1104 and mice were treated twice a day at 1 mg/kg s.c. for 5 days and then challenged with naloxone (F(2,27) = 75.93, p < 0.0001). Contrary to morphine treated group (p < 0.0001 compared to saline control), the MP1104 treated mice showed no signs of naloxone induced withdrawal (p >0.9999 compared to saline control; Fig. 6A).
Fig. 6.
Cross-tolerance and withdrawal effects of MP1104 in male CD1 mice. (A) Mice chronically treated with morphine (5 mg/kg twice daily) showed robust naloxone-precipitated withdrawal, while mice chronically treated with MP1104 (1 mg/kg twice daily) did not. (B) MP1104 (0.66 mg/kg) did not precipitate withdrawal in mice chronically exposed to a morphine pellet. One-way ANOVA with Bonferroni multiple comparisons test. (C) Antinociceptive dose-response effects in mice chronically exposed to morphine or placebo pellet. There was a 7-fold rightward shift in the dose-response effect of morphine in morphine-pelleted mice, whereas MP1104 did not show cross-tolerance with morphine. Values expressed as mean ± SEM, n = 10. ****p < 0.0001, ns = non-significant.
We implanted one pellet of morphine (75 mg free-base) which results in slow release of morphine and leads to marked morphine tolerance and dependence in mice (Cicero and Meyer, 1973; Yoburn et al., 1985; Majumdar et al., 2011; Grinnell et al., 2014). Naloxone demonstrated withdrawal in morphine pelleted animals (measured by involuntary jumping in mice) indicating that the morphine-pelleted mice were physically dependent (F(2,27) = 443.5, p < 0.0001). MP1104, on the other hand, failed to demonstrate any evidence of withdrawal in the morphine-pelleted animals (p > 0.9999 for comparison of MP1104 administration to mice with morphine pellet vs mice with placebo pellet; Fig. 6B).
Cross-tolerance with MOP agonists is often used to demonstrate a common mechanism of action. A cumulative dose-response was carried out with morphine and MP1104 in the morphine-pelleted animals. Morphine showed a 7-fold decrease in tail withdrawal antinociception (p < 0.0001), with the ED50 increasing from 3.2 to 22.4 mg/kg s.c. (Fig. 6C). However, the morphine-pelleted animals retained antinociceptive efficacy to MP1104, and the shift in the antinociceptive dose-response curve was only 2-fold (Fig. 6C).
3.7. Opioid-induced respiratory depression studies
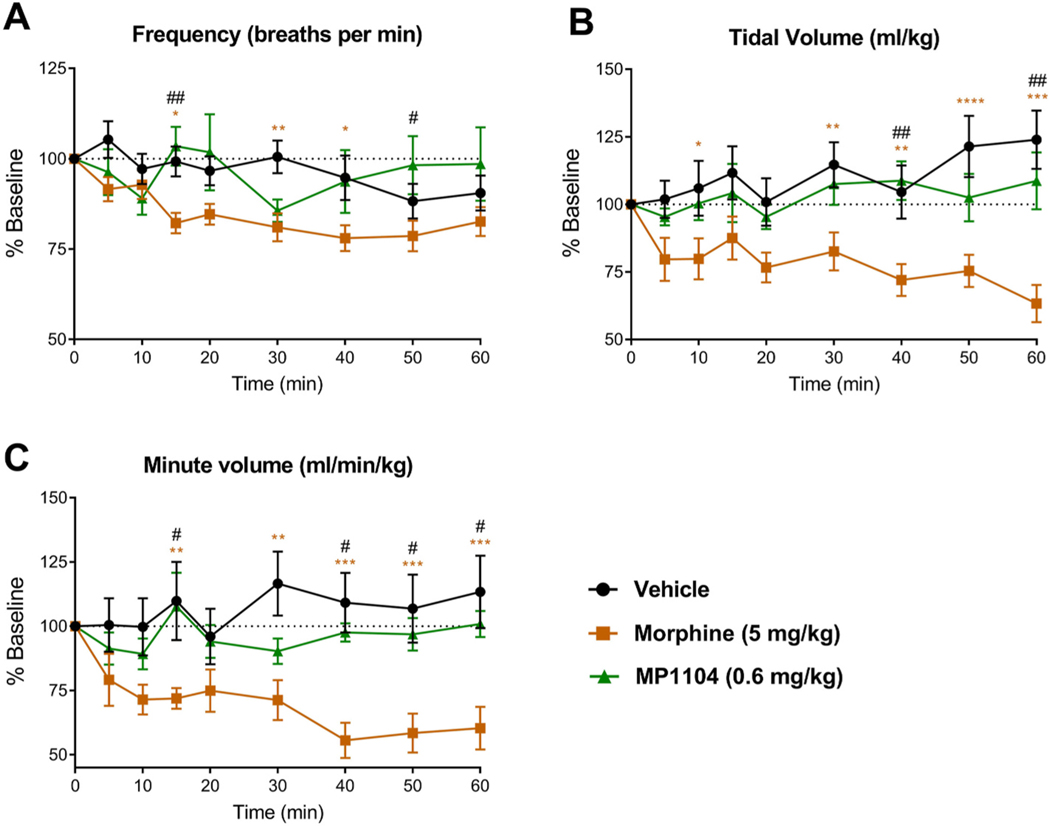
To assess the ability of MP1104 (0.6 mg/kg, s.c) to induce respiratory depression we utilised whole-body plethysmography in unrestrained, awake, male C57BL/6J mice. The effects on frequency (F(16, 136) = 2.525, p = 0.002), tidal volume (F(16, 136) = 2.322, p = 0.0047), and minute volume (F(16, 136) = 2.162, p = 0.009) were compared to vehicle-treated controls. Bonferroni post-tests revealed MP1104 did not decrease respiratory frequency compared to vehicle (p > 0.05), whereas, morphine decreased respiratory frequency at the 5, 15 and 30–60 min time points (p <0.05) (Fig. 7A). Similarly, for tidal volume, MP1104 had no effect compared to vehicle (p > 0.05), whereas morphine decreased the tidal volume at the 10 and 30–60 min time points (p < 0.05) (Fig. 7B). Morphine decreased minute volume at 15, 30–60 min (p < 0.001), whilst there was no decrease in MP1104 treated mice over the 60 min period (p > 0.9999) (Fig. 7C). The analysis of other respiratory parameters showed an increase in exhalation time and total cycle time at peak effect with morphine and MP1104 (Fig. S2). Only morphine increased the inspiration time (Fig. S2).
Fig. 7.
The respiratory effects of MP1104 were assessed in whole-body plethysmography in awake, unrestrained male C57BL/6J mice. MP1104 (0.6 mg/kg) had no effect on (A) respiratory frequency, (B) tidal volume or (C) minute volume. However, morphine (5 mg/kg) significantly decreased all three parameters. Two-way repeated-measures ANOVA with Bonferroni post-tests; *p < 0.05, **p < 0.01, ***p < 0.001 compared to vehicle; #p < 0.05, ##p < 0.01 for MP1104 compared to morphine. Values expressed as mean ± SEM, n = 6–7.
4. Discussion
MP1104 is an analogue of 3-iodobenzoyl naltrexamine (Fig. 1), with previous studies revealing potent antinociceptive actions in C57BL/6J (ED50 = 0.22 mg/kg, s.c.) and CD1 mice (ED50 = 0.25 mg/kg, s.c.) in tail-withdrawal assays (Váradi et al., 2015a); and in male and female ICR mice (0.05–1.0 mg/kg, s.c.) in the formalin assay (Ulker et al., 2020). In the current study, we examined whether the dual KOP/DOP agonist actions of MP1104 would be effective at modulating pain and investigated the side-effect profile in mice.
In C57BL/6J mice, the onset of action of MP1104 was 30 min (Fig. 2A), consistent with previous studies in CD1 male mice (Váradi et al., 2015a). However, in rats, the onset of action was at 45 min and the time of peak effect was between 2 and 3 h (Fig. S1). This is suggestive of different pharmacokinetic effects, which may be due to a difference in metabolism of MP1104 in mice and rats. There is evidence to support that opioid drug metabolism varies in different animals, including rodents and humans (Sawa and Oka, 1976; Oguri et al., 1990; Chandrasekaran et al., 2010). Moreover, Chandrasekaran et al. (2010) showed that MOP antagonist, methlynaltrexone metabolised to a higher extent in mice compared to rats, dogs and humans.
The mean effect analysis of the warm-water tail withdrawal assay showed that MP1104 was more potent than morphine in both male and female mice (Fig. 2B). To evaluate the potency and efficacy of MP1104 at attenuating CINP, we evaluated the dose-response effects. MP1104 was 9-times more potent than morphine in attenuating mechanical allodynia (Fig. 3D) and 10-times more potent in attenuating cold allodynia (Fig. 3E). It is important to note that we used a 1 h interval between subsequent doses in this cumulative dosing paradigm, which may have allowed for morphine to be cleared at a faster rate than MP1104, due to the peak effect of morphine occurring at 30 min (Fig. 2A). However, since MP1104 is up to 10-times more potent than morphine, it is not likely that this confounding factor explains this entire increase in potency. In fact, using our warm-water tail withdrawal dose-response paradigm with a 30 min interval, we have previously shown morphine to have an ED50 of 5.3 mg/kg (Crowley et al., 2016). By comparing the results for MP1104 in the present paper (ED50 = 0.35 mg/kg; Fig. 2D), this makes MP1104 15-times more potent than morphine, reflecting the increase in potency seen in the paclitaxel-induced neuropathic pain model.
Following chronic administration, MP1104 attenuated mechanical allodynia for the entire experimental period, however, morphine only reduced mechanical allodynia up to day 34 (Fig. 4B). Studies have shown that selective MOP agonists attenuate paclitaxel-induced neuropathic pain in male Sprague-Dawley (Mori et al., 2014) and Wistar rats (Pascual et al., 2010), however, the current study showed that morphine rapidly shows tolerance. This highlights that MP1104, unlike morphine, does not show tolerance in this model, however, the mechanisms through which MP1104 exerts its antinociceptive actions in CINP needs to be fully evaluated.
Few compounds possessing dual KOP/DOP activity have been evaluated in thermal nociceptive models of pain (Daniels et al., 2005; Tang et al., 2010). However, recent studies have evaluated the effects of selective KOP agonists in neuropathic pain (Paton et al., 2017; Coffeen et al., 2018). One study showed that lappaconitine (diterpenoid alkaloid) exhibited antinociceptive effects mediated by dynorphin A in the spinal cord without tolerance (Sun et al., 2018). Both pharmacological and genetic data highlight DOP agonists as promising alternatives to MOP analgesics in the treatment of chronic pain (Zhang et al., 2006; Bie and Pan, 2007; Cahill et al., 2007; Kabli and Cahill, 2007); and DOP agonists are more potent than MOP and KOP agonists in neuropathic pain (Mika et al., 2001; Obara et al., 2009; Nozaki et al., 2012). DOP knockout male and female C57BL/6 mice showed enhanced development of mechanical and thermal allodynia following sciatic nerve ligation (Nadal et al., 2006). These results reveal the involvement of DOP in neuropathic pain and suggest a potential therapeutic use for DOP agonists. Selective DOP agonists are known to cause seizures (Bilsky et al., 1995; Jutkiewicz et al., 2005), however, MP1104 did not cause seizures at 30-times the antinociceptive dose (Váradi et al., 2015a), indicating it is likely to have a broad therapeutic window.
Studies in mice (Fantegrossi et al., 2005; Paris et al., 2011), rats (Gallantine and Meert, 2008; Wang et al., 2009) and rhesus monkeys (Butelman and Kreek, 2001; Butelman et al., 2009) have shown that KOP agonists have sedative side-effects. This can be a confounding factor when using pain-stimulated behavioural models, as the sedative effects can lead to ‘false positive’ outcomes (Negus, 2019). Therefore, we evaluated the locomotor effects of MP1104 in the open-field activity test. MP1104 (0.6–1.2 mg/kg) treated mice did not have differences in locomotor activity compared to vehicle-treated mice, whereas, morphine (10 mg/kg) treated mice showed hyper-locomotor activity (Fig. 5B and C). This indicates that the behaviours observed in the pain models were due to antinociceptive effects of MP1104, rather than sedative effects. Further examination of the time spent in each zone of the chamber showed there was no difference between vehicle and the treatment groups (Fig. 5D). Time spent in the outer border of open-field locomotor chambers is routinely used as a measure of anxiety in rodents (Prut and Belzung, 2003). This data showing that MP1104 does not induce anxiogenic-like behaviours supports previous studies performed in rats using the elevated plus maze (Atigari et al., 2019).
The effects of MP1104 were also evaluated in a series of withdrawal studies. Morphine, but not MP1104 exposed mice showed robust naloxone precipitated withdrawal (Fig. 6A). Moreover, MP1104 did not precipitate withdrawal in morphine tolerant mice (Fig. 6B). The dependence studies in morphine-pelleted mice also suggest that MP1104 has a mechanism of action independent of MOP. Unlike morphine, MP1104 doesn’t show physical dependence and retains antinociceptive efficacy in morphine-dependent mice (Fig. 6B). This confirms that the in vivo actions of MP1104 are not mediated by MOP, as MOP partial agonists would show naloxone precipitated withdrawal in a morphine-dependent animal, and antinociceptive effects of MP1104 would be blocked by β-FNA (Fig. 2C).
We then investigated the induction of respiratory depression by MP1104. We found that morphine significantly reduced respiratory frequency (Fig. 7A), tidal volume (Fig. 7B), and minute volume (Fig. 7C), while MP1104 had no significant effects. As previously discussed, the behavioural effects of MP1104 are thought to be dependent on KOP and DOP activation, not the MOP. Prototypical agonists of both KOP and DOP induce limited respiratory effects (Castillo et al., 1986; Codd et al., 2009). In addition, KOP and DOP agonists have been shown to rescue the respiratory depressive effects of MOP agonists when co-administered (Dosaka-Akita et al., 1993; Su et al., 1998; Vankova et al., 1996). Furthermore, the MOP/DOP mixed agonist, DPI-125, was shown to have reduced respiratory depression, with DOP activation believed to be ‘dampening’ the MOP mediated respiratory depression (Gengo et al., 2003; Yi et al., 2017). Further investigation is required to understand the combination of receptors activated by MP1104 to produce no respiratory side-effects.
Interestingly, although MP1104 has high affinity towards MOP, KOP and DOP in vitro, there is a lack of MOP in vivo activity. This is consistent with previous studies evaluating antinociception and reward (Váradi et al., 2015a; Atigari et al., 2019; Ulker et al., 2020). MP1104 may produce a metabolite that lacks MOP action in vivo but retains KOP and DOP action or that metabolism may cause the hydrolysis of the amide bond that would result in the formation of a MOP antagonist such as naltrexamine, however, there is no evidence of this and further studies are required to fully evaluate the differences between in vitro and in vivo effects. Nevertheless, studies have shown difference in opioid receptor binding profiles (Pasternak et al., 1987; Ulens et al., 2001) and difference in the antinociceptive effects in rodents (Abbott and Palmour, 1988) by the metabolites of morphine, morphine-6-O-glucuronide (M6G) and morphine-3-O-glucuronide (M3G). In cloned human MOP, KOP, DOP, compared with morphine, M6G exhibited higher potency at the MOP, lower potency at the KOP, and similar potency at the DOP, while a 1000-fold non-selective reduction in potency via opioid receptors was observed with M3G (Ulens et al., 2001). Moreover, M3G failed to antagonise the effects of morphine at the MOP unlike naloxone (Ulens et al., 2001). There is also a possibility that MP1104 acts on KOP/DOP heterodimers. KOP/DOP heterodimers are believed to be tissue-specific and previous studies have shown a DOP/KOP heterodimer-specific agonist exhibited spinal-mediated antinociception (Waldhoer et al., 2005; Jacobs et al., 2019). Therefore, we cannot rule out the possibility that MP1104 may act at DOP/KOP heterodimers. However, a previous study using double and triple opioid receptor knock out mice failed to find evidence of KOP/DOP heterodimers in vivo (Yoo et al., 2014).
5. Conclusion
MP1104 is long-acting, potent and efficacious at modulating nociceptive pain, in a KOP- and DOP-dependent manner. In the CINP model, MP1104 was highly potent at attenuating both mechanical and cold allodynia. In addition, MP1104 did not show sedation, anxiogenic effects, naloxone-precipitated withdrawal, cross-tolerance with morphine, or respiratory depression. We have previously shown that MP1104 produced no seizure activity (Váradi et al., 2015a ), depression, aversion, anxiety or rewarding effects (Atigari et al., 2019). Therefore, we conclude that MP1104 has significant improvements over morphine in the duration of action, potency and side-effects, highlighting the potential therapeutic utility of mixed KOP/DOP agonists for development of safer, more effective analgesics. This is significant in the context of the current opioid epidemic and the dearth of clinical options available for treating chronic pain.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Craig Doney and Neville Higgison (Victoria University of Wellington) for their technical assistance setting up the plethysmography equipment and Stacey Parbhu (Victoria University of Wellington) for animal husbandry.
Funding
This work was supported by Research for Life, the Wellington Medical Research Foundation (2017); NIDA [grant numbers DA045884, DA046487]; NIAAA [grant number AA026949]; the Office of the Assistant Secretary of Defense for Health Affairs through the Peer-Reviewed Medical Research Program [grant number W81XWH-17-1-0256]; and start-up funds from the Center for Clinical Pharmacology, St. Louis College of Pharmacy and Washington University. This research was funded in part through the NIH/NCI Cancer Center Support Grant [grant number P30 CA008748]. D.V. Atigari received a doctoral scholarship from Victoria University of Wellington. The funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations:
- β-FNA
β-Funaltrexamine
- CINP
chemotherapy-induced neuropathic pain
- DMSO
dimethyl sulfoxide
- DOP
delta opioid receptor
- i.p.
intraperitoneal
- KOP
kappa opioid receptor
- MOP
mu opioid receptor
- MP1104
17-Cyclopropylmethyl-3-hydroxy-4,5α-epoxy-7,8-en-6-β-[(3′-iodo)benzamido]-morphinan
- nor-BNI
nor-binaltorphimine
- s.c.
subcutaneous
Footnotes
Declaration of competing interest
S. Majumdar is co-founder of Sparian BioSciences. All other authors report no conflicts of interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2020.108445.
References
- Abbott FV, Palmour RM, 1988. Morphine-6-glucuronide: analgesic effects and receptor binding profile in rats. Life Sci. 43, 1685–1695. 10.1016/0024-3205(88)90479-1. [DOI] [PubMed] [Google Scholar]
- Anand JP, Montgomery D, 2018. Multifunctional opioid ligands. In: Jutkiewicz E. (Ed.), Delta Opioid Receptor Pharmacology and Therapeutic Applications, Handbook of Experimental Pharmacology, vol. 247. Springer, Cham, pp. 21–51. [DOI] [PubMed] [Google Scholar]
- Atigari DV, Uprety R, Pasternak GW, Majumdar S, Kivell BM, 2019. MP1104, a mixed kappa-delta opioid receptor agonist has anti-cocaine properties with reduced side-effects in rats. Neuropharmacology 150, 217–228. 10.1016/j.neuropharm.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni G, Guerrini R, Salvadori S, Bianchi C, Rizzi D, Bryant SD, Lazarus LH, 2002. Evaluation of the Dmt−tic pharmacophore: conversion of a potent δ-opioid receptor antagonist into a potent δ agonist and ligands with mixed properties. J. Med. Chem 45, 713–720. 10.1021/jm010449i. [DOI] [PubMed] [Google Scholar]
- Berterame S, Erthal J, Thomas J, Fellner S, Vosse B, Clare P, Hao W, Johnson DT, Mohar A, Pavadia J, Samak AK, Sipp W, Sumyai V, Suryawati S, Toufiq J, Yans R, Mattick RP, 2016. Use of and barriers to access to opioid analgesics: a worldwide, regional, and national study. Lancet 387, 1644–1656. 10.1016/S0140-6736(16)00161-6. [DOI] [PubMed] [Google Scholar]
- Bie B, Pan ZZ, 2007. Trafficking of central opioid receptors and descending pain inhibition. Mol. Pain 3, 37. 10.1186/1744-8069-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bileviciute-Ljungar I, Saxne T, Spetea M, 2005. Anti-inflammatory effects of contralateral administration of the κ-opioid agonist U-50,488 H in rats with unilaterally induced adjuvant arthritis. Rheumatology 45, 295–302. 10.1093/rheumatology/kei156. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F, 1995. SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J. Pharmacol. Exp. Therapeut 273, 359–366. [PubMed] [Google Scholar]
- Binder W, Machelska H, Mousa S, Schmitt T, Rivière PJ, Junien JL, Stein C, Schäfer M, 2001. Analgesic and antiinflammatory effects of two novel κ-opioid peptides. Anesthesiology 94, 1034–1044. 10.1097/00000542-200106000-00018. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG, 2000. μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature 408, 720–723. 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Bories C, De Koninck Y, 2014. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol. Pain 10, 26. 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainin-Mattos J, Smith ND, Malkmus S, Rew Y, Goodman M, Taulane J, Yaksh TL, 2006. Cancer-related bone pain is attenuated by a systemically available δ-opioid receptor agonist. Pain 122, 174–181. 10.1016/j.pain.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Rice KC, Traynor JR, Woods JH, 2002. Behavioral effects of delta-opioid receptor agonists: potential antidepressants? Jpn. J. Pharmacol 90, 1–6. 10.1254/jjp.90.1. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Kreek MJ, 2001. κ-Opioid receptor agonist-induced prolactin release in primates is blocked by dopamine D2-like receptor agonists. Eur. J. Pharmacol 423, 243–249. 10.1016/s0014-2999(01)01121-9. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Prisinzano TE, Deng H, Rus S, Kreek MJ, 2009. Unconditioned behavioral effects of the powerful κ-opioid hallucinogen salvinorin A in nonhuman primates: fast onset and entry into cerebrospinal fluid. J. Pharmacol. Exp. Therapeut 328, 588–597. 10.1124/jpet.108.145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A, 2007. Trafficking of δ-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol. Sci 28, 23–31. 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Caldwell JR, Rapoport RJ, Davis JC, Offenberg HL, Marker HW, Roth SH, Yuan W, Babul N, Lynch PM, 2002. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: results from a randomized, placebo-controlled, double-blind trial and an open-label extension trial. J. Pain Symptom Manag 23, 278–291. 10.1016/S0885-3924(02)00383-4. [DOI] [PubMed] [Google Scholar]
- Castillo R, Kissin I, Bradley EL, 1986. Selective kappa opioid agonist for spinal analgesia without the risk of respiratory depression. Anesth. Analg 65, 350–354. [PubMed] [Google Scholar]
- Chandrasekaran A, Tong Z, Li H, Erve JC, DeMaio W, Goljer I, McConnell O, Rotshteyn Y, Hultin T, Talaat R, Scatina J, 2010. Metabolism of intravenous methylnaltrexone in mice, rats, dogs, and humans. Drug Metab. Dispos 38, 606–616. 10.1124/dmd.109.031179. [DOI] [PubMed] [Google Scholar]
- Chu LF, Clark DJ, Angst MS, 2006. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J. Pain 7, 43–48. 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Meyer ER, 1973. Morphine pellet implantation in rats: quantitative assessment of tolerance and dependence. J. Pharmacol. Exp. Therapeut 184, 404–408. [PubMed] [Google Scholar]
- Codd EE, Carson JR, Colburn RW, Stone DJ, Van Besien CR, Zhang SP, Wade PR, Gallantine EL, Meert TF, Molino L, Pullan S, Razler CM, Dax SL, Flores CM, 2009. JNJ-20788560 [9-(8-azabicyclo[3.2.1]oct-3-ylidene)-9H-xanthene-3-carboxylic acid diethylamide], a selective delta opioid receptor agonist, is a potent and efficacious antihyperalgesic agent that does not produce respiratory depression, pharmacologic tolerance, or physical dependence. J. Pharmacol. Exp. Therapeut 329, 241–251. 10.1124/jpet.108.146969. [DOI] [PubMed] [Google Scholar]
- Coffeen U, Canseco-Alba A, Simón-Arceo K, Almanza A, Mercado F, León-Olea M, Pellicer F, 2018. Salvinorin A reduces neuropathic nociception in the insular cortex of the rat. Eur. J. Pain 22, 311–318. 10.1002/ejp.1120. [DOI] [PubMed] [Google Scholar]
- Colman AS, Miller JH, 2001. Modulation of breathing by mu1 and mu2 opioid receptor stimulation in neonatal and adult rats. Respir. Physiol 127, 157–172. 10.1016/s0034-5687(01)00240-7. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND, 2006. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 81, 103–107. 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Crowley RS, Riley AP, Sherwood AM, Groer CE, Shivaperumal N, Biscaia M, Paton K, Schneider S, Provasi D, Kivell BM, Filizola M, Prisinzano TE, 2016. Synthetic studies of neoclerodane diterpenes from salvia divinorum: identification of a potent and centrally acting mu opioid analgesic with reduced abuse liability. J. Med. Chem 59 (24), 11027–11038. 10.1021/acs.jmedchem.6b01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS, 2005. A bivalent ligand (KDAN-18) containing δ-antagonist and κ-agonist pharmacophores bridges δ2 and κ1 opioid receptor phenotypes. J. Med. Chem 48, 1713–1716. 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG, 2015. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1–dependent withdrawal. Biol. Psychiatr 77, 475–487. 10.1016/j.biopsych.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosaka-Akita K, Tortella FC, Holaday JW, Long JB, 1993. The kappa opioid agonist U-50,488H antagonizes respiratory effects of mu opioid receptor agonists in conscious rats. J. Pharmacol. Exp. Therapeut 264, 631–637. 10.21236/ada263043. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Kugle KM, Valdes LJ III, Koreeda M, Woods JH, 2005. Kappa-opioid receptor-mediated effects of the plant-derived hallucinogen, salvinorin A, on inverted screen performance in the mouse. Behav. Pharmacol 16, 627–633. 10.1097/00008877-200512000-00005. [DOI] [PubMed] [Google Scholar]
- Fayaz A, Croft P, Langford R, Donaldson L, Jones G, 2016. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. Br. Med. J. Open 6, e010364. 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF, 2008. Antinociceptive and adverse effects of μ-and κ-opioid receptor agonists: a comparison of morphine and U50488-H. Basic Clin. Pharmacol. Toxicol 103, 419–427. 10.1111/j.1742-7843.2008.00306.x. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL, 2008. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur. J. Neurosci 27, 2558–2567. 10.1111/j.1460-9568.2008.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengo PJ, Pettit HO, O’Neill SJ, Su YF, McNutt R, Chang KJ, 2003. DPI-3290 [(+)-3-((alpha-R)-alpha-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-hydroxybenzyl)-N-(3-fluorophenyl)-N-methylbenzamide]. II. A mixed opioid agonist with potent antinociceptive activity and limited effects on respiratory function. J. Pharmacol. Exp. Therapeut 307, 1227–1233. 10.1124/jpet.103.054429. [DOI] [PubMed] [Google Scholar]
- Gistrak MA, Paul D, Hahn E, Pasternak G, 1989. Pharmacological actions of a novel mixed opiate agonist/antagonist: naloxone benzoylhydrazone. J. Pharmacol. Exp. Therapeut 251, 469–476. [PubMed] [Google Scholar]
- Goodman AJ, Le Bourdonnec B, Dolle RE, 2007. Mu opioid receptor antagonists: recent developments. ChemMedChem 2, 1552–1570. 10.1002/cmdc.200700143. [DOI] [PubMed] [Google Scholar]
- Grinnell SG, Majumdar S, Narayan A, Le Rouzic V, Ansonoff M, Pintar JE, Pasternak GW, 2014. Pharmacologic characterization in the rat of a potent analgesic lacking respiratory depression, IBNtxA. J. Pharmacol. Exp. Ther 350, 710–718. 10.1124/jpet.114.213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Miniño AM, Warner M, 2018. Drug Overdose Deaths in the United States, 1999–2017. NCHS Data Brief, No 329. National Centre for Health Statistics, Hyattsville, MD. [Google Scholar]
- Henderson JV, Harrison CM, Britt HC, Bayram CF, Miller GC, 2013. Prevalence, causes, severity, impact, and management of chronic pain in Australian general practice patients. Pain Med. 14, 1346–1361. 10.1111/pme.12195. [DOI] [PubMed] [Google Scholar]
- Houmes RJ, Voets MA, Verkaaik A, Erdmann W, Lachmann B, 1992. Efficacy and safety of tramadol versus morphine for moderate and severe postoperative pain with special regard to respiratory depression. Anesth. Analg 74, 510–514. 10.1213/00000539-199204000-00007. [DOI] [PubMed] [Google Scholar]
- Irwin S, Bennett DR, Hendershot LC, Seevers M, Houde RW, 1951. The effects of morphine, methadone and meperidine on some reflex responses of spinal animals to nociceptive stimulation. J. Pharmacol. Exp. Therapeut 101, 132–143. [PubMed] [Google Scholar]
- Jacobs BA, Pando MM, Jennings EM, Jamshidi RJ, Zamora JC, Chavera TS, Clarke WP, Berg KA, 2019. Signaling characteristics and functional regulation of delta opioid-kappa opioid receptor (DOP-KOP) heteromers in peripheral sensory neurons. Neuropharmacology 151, 208–218. 10.1016/j.neuropharm.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Griffin AM, Gawell L, Lavoie R, Delorme D, Roberts E, Brown W, Walpole C, Xiao W, Boulet J, Labarre M, Coupal M, Butterworth J, St-Onge S, Hodzic L, Salois D, 2009. N,N-Diethyl-4-[(3-hydroxyphenyl)(piperidin-4-yl)amino] benzamide derivatives: the development of diaryl amino piperidines as potent delta opioid receptor agonists with in vivo anti-nociceptive activity in rodent models. Bioorg. Med. Chem. Lett 19, 5994–5998. 10.1016/j.bmcl.2009.09.072. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Traynor JR, Woods JH, 2005. Separation of the convulsions and antidepressant-like effects produced by the delta-opioid agonist SNC80 in rats. Psychopharmacology 182, 588–596. 10.1007/s00213-005-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Cahill CM, 2007. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain 127, 84–93. 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S, 2014. Prevalence of persistent pain in the US adult population: new data from the 2010 national health interview survey. J. Pain 15, 979–984. 10.1016/j.jpain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Kivell B, Prisinzano TE, 2010. Kappa opioids and the modulation of pain. Psychopharmacology 210, 109–119. 10.1007/s00213-010-1819-6. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C, 2008. The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. J. Neurosci 28, 407–414. 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewanowitsch T, Miller JH, Irvine RJ, 2006. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Life Sci. 78, 682–688. 10.1016/j.lfs.2005.05.062. [DOI] [PubMed] [Google Scholar]
- Lichtor JL, Sevarino FB, Joshi GP, Busch MA, Nordbrock E, Ginsberg B, 1999. The relative potency of oral transmucosal fentanyl citrate compared with intravenous morphine in the treatment of moderate to severe postoperative pain. Anesth. Analg 89, 732–738. 10.1097/00000539-199909000-00038. [DOI] [PubMed] [Google Scholar]
- Lim R, Zavou MJ, Milton PL, Chan ST, Tan JL, Dickinson H, Murphy SV, Jenkin G, Wallace EM, 2014. Measuring respiratory function in mice using unrestrained whole-body plethysmography. JoVE 90, e51755. 10.3791/51755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling G, MacLeod JM, Lee S, Lockhart SH, Pasternak GW, 1984. Separation of morphine analgesia from physical dependence. Science 226, 462–464. 10.1126/science.6541807. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Devi LA, 2018. Strategy for making safer opioids bolstered. Nature 553, 286–288. 10.1038/d41586-018-00045-1. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan Y-X, Pasternak GW, 2011. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc. Natl. Acad. Sci. Unit. States Am 108, 19778–19783. 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E, Saper C, 2000. The Biological Basis for Mind Body Interactions. Elsevier, Amsterdam, pp. 245–253. [Google Scholar]
- Mello NK, Negus SS, 2000. Interactions between kappa opioid agonists and cocaine: preclinical studies. Ann. N. Y. Acad. Sci 909, 104–132. 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Taiwo YO, Levine JD, 1990. κ- and δ-opioid agonists synergize to produce potent analgesia. Brain Res. 509, 165–168. 10.1016/0006-8993(90)90327-8. [DOI] [PubMed] [Google Scholar]
- Mika J, Przewłocki R, Przewłocka B, 2001. The role of δ-opioid receptor subtypes in neuropathic pain. Eur. J. Pharmacol 415, 31–37. 10.1016/s0014-2999(01)00814-7. [DOI] [PubMed] [Google Scholar]
- Mori T, Itoh T, Yoshizawa K, Ise Y, Mizuo K, Saeki T, Komiya S, Masukawa D, Shibasaki M, Suzuki T, 2015. Involvement of mu- and delta-opioid receptor function in the rewarding effect of (+/−)-pentazocine. Addiction Biol. 20, 724–732. 10.1111/adb.12169. [DOI] [PubMed] [Google Scholar]
- Mori T, Kanbara T, Harumiya M, Iwase Y, Masumoto A, Komiya S, Nakamura A, Shibasaki M, Kanemasa T, Sakaguchi G, Suzuki T, 2014. Establishment of opioid-induced rewarding effects under oxaliplatin-and Paclitaxel-induced neuropathy in rats. J. Pharmacol. Sci 126, 47–55. 10.1254/jphs.14134fp. [DOI] [PubMed] [Google Scholar]
- Nadal X, Baños JE, Kieffer BL, Maldonado R, 2006. Neuropathic pain is enhanced in δ-opioid receptor knockout mice. Eur. J. Neurosci 23, 830–834. 10.1111/j.1460-9568.2006.04569.x. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Lichtman AH, Archer CC, May EL, Harris LS, Aceto MD, 2007. NIH 11082 produces anti-depressant-like activity in the mouse tail-suspension test through a delta-opioid receptor mechanism of action. Eur. J. Pharmacol 566, 132–136. 10.1016/j.ejphar.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, 2019. Core outcome measures in preclinical assessment of candidate analgesics. Pharmacol. Rev 71 (2), 225–266. 10.1124/pr.118.017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki C, Le Bourdonnec B, Reiss D, Windh RT, Little PJ, Dolle RE, Kieffer BL, Gavériaux-Ruff C, 2012. δ-Opioid mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J. Pharmacol. Exp. Therapeut 342, 799–807. 10.1124/jpet.111.188987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R, 2009. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain 141, 283–291. 10.1016/j.pain.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Oguri K, Hanioka N, Yoshimura H, 1990. Species differences in metabolism of codeine: urinary excretion of codeine glucuronide, morphine-3-glucuronide and morphine-6-glucuronide in mice, rats, Guinea pigs and rabbits. Xenobiotica 20, 683–688. 10.3109/00498259009046884. [DOI] [PubMed] [Google Scholar]
- Okie S, 2010. A flood of opioids, a rising tide of deaths. N. Engl. J. Med 363, 1981–1985. 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Reilley KJ, McLaughlin JP, 2011. Kappa opioid receptor-mediated disruption of novel object recognition: relevance for psychostimulant treatment. J. Addiction Res. Ther S4 10.4172/2155-6105.S4-007, 007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Burgos E, Martín MI, 2010. Antinociceptive effect of three common analgesic drugs on peripheral neuropathy induced by paclitaxel in rats. Pharmacol. Biochem. Behav 95, 331–337. 10.1016/j.pbb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Bodnar RJ, Clark JA, Inturrisi CE, 1987. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 41, 2845–2849. 10.1016/0024-3205(87)90431-0. [DOI] [PubMed] [Google Scholar]
- Paton KF, Kumar N, Crowley RS, Harper JL, Prisinzano TE, Kivell BM, 2017. The analgesic and anti-inflammatory effects of Salvinorin A analogue β-tetrahydropyran Salvinorin B in mice. Eur. J. Pain 21, 1039–1050. 10.1002/ejp.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM, 2006. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br. J. Pharmacol 147, 864–872. 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM, 1986. Psychotomimesis mediated by kappa opiate receptors. Science 233, 774–776. 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL, 2011. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol. Sci 32, 581–590. 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C, 2003. The open field as a paradigm to measure the effects of drug on anxiety-like behaviours: a review. Eur. J. Pharmacol 463, 3–33. 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rivière PJM, 2004. Peripheral kappa-opioid agonists for visceral pain. Br. J. Pharmacol 141, 1331–1334. 10.1038/sj.bjp.0705763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L, 2016. Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR Morb. Mortal. Wkly. Rep 65, 1445–1452. 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J, 2004. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J. Pharmacol. Sci 95, 374–380. 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Sawa A, Oka T, 1976. Effects of narcotic analgesics on serotonin metabolism in brain of rats and mice. Jpn. J. Pharmacol 26, 599–605. 10.1254/jjp.26.599. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Thompson AC, 2009. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol. Psychiatr 65, 169–174. 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook JE, Watkins WD, Camporesi EM, 1990. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am. Rev. Respir. Dis 142, 895–909. 10.1164/ajrccm/142.4.895. [DOI] [PubMed] [Google Scholar]
- Stevens CW, 2009. The evolution of vertebrate opioid receptors. Front. Biosci 14, 1247–1269. 10.2741/3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YF, McNutt RW, Chang KJ, 1998. Delta-opioid ligands reverse alfentanil-induced respiratory depression but not antinociception. J. Pharmacol. Exp. Therapeut 287, 815–823. [PubMed] [Google Scholar]
- Sun ML, Ao JP, Wang YR, Huang Q, Li TF, Li XY, Wang YX, 2018. Lappaconitine, a C18-diterpenoid alkaloid, exhibits antihypersensitivity in chronic pain through stimulation of spinal dynorphin A expression. Psychopharmacology 235, 2559–2571. 10.1007/s00213-018-4948-y. [DOI] [PubMed] [Google Scholar]
- Tang Y, Yang J, Lunzer MM, Powers MD, Portoghese PS, 2010. A κ opioid pharmacophore becomes a spinally selective κ-δ agonist when modified with a basic extender arm. ACS Med. Chem. Lett 2, 7–10. 10.1021/ml1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Zhang Y, Peng BW, Winter JC, Li JX, 2011. Effects of imidazoline I2 receptor ligands on morphine-and tramadol-induced antinociception in rats. Eur. J. Pharmacol 670, 435–440. 10.1016/j.ejphar.2011.09.173. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Mori A, Yanagita T, 2013. One year long-term study on abuse liability of nalfurafine in hemodialysis patients. Int. J. Clin. Pharm. Ther 51, 823–831. 10.5414/CP201852. [DOI] [PubMed] [Google Scholar]
- Ulens C, Baker L, Ratka A, Waumans D, Tytgat J, 2001. Morphine-6β-glucuronide and morphine-3-glucuronide, opioid receptor agonists with different potencies. Biochem. Pharmacol 62, 1273–1282. 10.1016/s0006-2952(01)00761-4. [DOI] [PubMed] [Google Scholar]
- Ulker E, Toma W, White A, Uprety R, Majumdar S, Damaj MI, 2020. The antinociceptive effects of a dual kappa-delta opioid receptor agonist in the mouse formalin test. Behav. Pharmacol 31, 174–178. 10.1097/FBP.0000000000000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankova ME, Weinger MB, Chen DY, Bronson JB, Motis V, Koob GF, 1996. Role of central mu, delta-1, and kappa-1 opioid receptors in opioid-induced muscle rigidity in the rat. Anesthesiology 85, 574–583. 10.1097/00000542-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Eans SO, Ganno ML, Subrath JJ, LeRouzic V, Hunkele A, Pasternak GW, McLaughlin JP, Majumdar S, 2015a. Synthesis and characterization of a dual kappa-delta opioid receptor agonist analgesic blocking cocaine reward behavior. ACS Chem. Neurosci 6, 1813–1824. 10.1021/acschemneuro.5b00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váradi A, Palmer TC, Haselton N, Afonin D, Subrath JJ, Le Rouzic V, Hunkele A, Pasternak GW, Marrone GF, Borics A, 2015b. Synthesis of carfentanil amide opioids using the ugi multicomponent reaction. ACS Chem. Neurosci 6, 1570–1577. 10.1021/acschemneuro.5b00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A, Pagirsky J, Eans SO, Medina JM, Xu J, Pan YX, Borics A, Pasternak GW, McLaughlin JP, Majumdar S, 2016. Mitragynine/Corynantheidine pseudoindoxyls as opioid analgesics with mu agonism and delta antagonism, which do not recruit β-Arrestin-2. J. Med. Chem 59, 8381–8397. 10.1021/acs.jmedchem.6b00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, Trapella C, Lazarus LH, Regoli D, Guerrini R, Salvadori S, Calo G, 2008. Anxiolytic- and antidepressant-like activities of H-Dmt-Tic-NH-CH(CH2-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides 29, 93–103. 10.1016/j.peptides.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN, 2015. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156, 569–576. 10.1097/01.j.pain.0000460357.01998.f1. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL, 2005. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. Unit. States Am 102, 9050–9055. 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Tao YM, Li FY, Wang YH, Xu XJ, Chen J, Cao YL, Chi ZQ, Neumeyer JL, Zhang A, Liu JG, 2009. Pharmacological characterization of ATPM [(−)-3-aminothiazolo[5,4-b]-N-cyclopropylmethylmorphinan hydrochloride], a novel mixed kappa-agonist and mu-agonist/-antagonist that attenuates morphine antinociceptive tolerance and heroin self-administration behavior. J. Pharmacol. Exp. Therapeut 329, 306–313. 10.1124/jpet.108.142802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SP, Kong QH, Li YL, Pan CL, Yu J, Cui BQ, Wang YF, Wang GL, Zhou PL, Wang LL, Gong ZH, Su RB, Shen YH, Yu G, Chang KJ, 2017. The opioid receptor triple agonist DPI-125 produces analgesia with less respiratory depression and reduced abuse liability. Acta Pharmacol. Sin 38, 977–989. 10.1038/aps.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoburn BC, Chen J, Huang T, Inturrisi CE, 1985. Pharmacokinetics and pharmacodynamics of subcutaneous morphine pellets in the rat. J. Pharmacol. Exp. Therapeut 235, 282–286. [PubMed] [Google Scholar]
- Yoo JH, Bailey A, Borsodi A, Tóth G, Matifas A, Kieffer BL, Kitchen I, 2014. Knockout subtraction autoradiography: a novel ex vivo method to detect heteromers finds sparse KOP receptor/DOP receptor heterodimerization in the brain. Eur. J. Pharmacol 731, 1–7. 10.1016/j.ejphar.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Guan JS, 2006. Role of delivery and trafficking of δ-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol. Sci 27, 324–329. 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.