Abstract
Implantable motor neuroprostheses can restore functionality to individuals with neurological disabilities by electrically activating paralyzed muscles in coordinated patterns. The typical design of neuroprosthetic systems relies on a single multi-use device, but this limits the number of stimulus and sensor channels that can be practically implemented. To address this limitation, a modular neuroprosthesis, the “Networked Neuroprothesis” (NNP), was developed. The NNP system is the first fully implanted modular neuroprosthesis that includes implantation of all power, signal processing, biopotential signal recording, and stimulating components. This paper describes the design of stimulation and recording modules, bench testing to verify stimulus outputs and appropriate filtering and recording, and validation that the components function properly while implemented in persons with spinal cord injury. The results of system testing demonstrated that the NNP was functional and capable of generating stimulus pulses and recording myoelectric, temperature, and accelerometer signals. Based on the successful design, manufacturing, and testing of the NNP System, multiple clinical applications are anticipated.
Keywords: electrical stimulation, implant, multi-function, neuroprosthesis, paralysis, spinal cord injury
I. INTRODUCTION
IMPLANTABLE motor neuroprosthesis systems have been used to restore functional capabilities for people with disabilities, such as spinal cord injury (SCI), stroke, cerebral palsy (CP), and multiple sclerosis (MS) [1–6]. Neuroprostheses restore movement by electrically activating paralyzed muscles in coordinated patterns to generate controlled movements that were lost. These systems have primarily focused on a single function such as hand grasp, cough assistance, or postural control and effectively improved function and quality of life [6–8].
While single function systems have been beneficial, paralysis often impacts multiple facets of life that people want restored [9]. Implanting a separate system for each function is impractical as each device requires its own interface and space in the body. Heterogeneity across and within pathologies also precludes a single approach that is applicable to everyone. For example, people with paralysis after complete SCI are impaired based on the level of injury while people with incomplete injuries have even greater heterogeneity within the population. Similarly, stroke survivors’ varied extent of hemiparesis is dependent on the size and location of the lesion. Therefore, a modular system that can serve as a platform technology and can be scaled and adapted based on an individual’s needs facilitates improving quality of life for the greatest number of people.
Our team has developed a distributed scalable modular system [10] that can be customized to address the multiple needs of people with paralysis by restoring lost function. The Networked Neuroprosthesis (NNP) system design was based on our analysis of anticipated clinical applications and our experience deploying neuroprosthetic systems for motor control [5, 6, 8, 11–16], but the concept is applicable to any implanted system of actuators and sensors where sensors must be placed in disparate locations in the body [5]. The NNP system uses a wired multipoint topology and consists of a central “power module” (PM) with daisy chained “remote modules” to provide function through sensing and neuromodulation. The power module supplies power to the remote modules and manages communication both within the system and between the system and external interfaces. The combination of remote modules incorporated into the system is determined based on the target application(s) and severity of the individual’s need. For example, Figure 1 shows a potential NNP configuration for trunk and hand function after SCI.
Fig. 1:
Potential NNP multi-function configuration for hand and trunk function. (PM – power module, PG4 – pulse generator module, BP2 – biopotential recording module)
The NNP System can operate untethered from any external input or control, but can also interface wirelessly with external components for additional features. Two external components have been developed: 1) a “Control Tower” (CT) and 2) a smartphone interface. The CT serves as the clinical programming link, the user’s system status interface, and as the user’s Charging is accomplished via an inductive transcutaneous link with the PM. The PM contains a rechargeable power supply for the entire NNP implanted system (three 200mAh 3.6V Li-ion batteries, Quallion LLC, QL0200I-A). A coil powered by the CT is applied to the skin over the location of the PM to charge the device. Thermistors inside and outside the body monitor tissue temperature for burn prevention. An external thermistor measures the temperature of the coil/skin interface to prevent overheating the exterior components while a thermistor adhered to the inside of the PM’s titanium capsule measures the PM temperature inside the body. The CT can be connected to a computer via USB for programming. The smartphone interface can monitor and control system status and settings via wireless communication with the PM over the MedRadio-band using a small radio dongle connected using the phone’s USB port.
The focus of this manuscript is the design and testing of the remote modules. The PM and charging characteristics of the fully implanted system are described in detail in [10]. Briefly, the PM enclosure is 68mm × 47mm × 14mm and is intended to be implanted in the chest or abdomen. The PM uses a 32-bit ARM-7 microprocessor (NXP Semiconductor LPC2129) which runs a real-time operating system (RTOS; Micrium μC/OS-III). The PM includes the same accelerometer and temperature sensor as the remote modules but has no independent stimulation or biopotential sensing capabilities. The PM performs DC to AC power conversion for transmission to all remote modules which are the primary interfaces with the nervous system. Implementation of an NNP System requires a module capable of stimulation to generate functional movement and/or a sensing module for measuring physiologic signals that could be used as command signals to detect intent and subsequently translated into appropriate assistance via communication through the PM. To achieve these features, we have developed a four-channel pulse generator module (PG4) and a two-channel biopotential recording module (BP2). This manuscript describes the design, benchtop testing, and first-in-human functionality of the first generation remote modules capable of stimulation (PG4) and biopotential recording (BP2). The presented modules create a framework for design and implementation of modules that will further enhance the capabilities and possible applications for the NNP System.
II. METHODS
A. Design Principles for Remote Modules
The design decisions for the remote modules in this distributed approach were guided by the goals of clinical implementation and translation. The choices were intended to make devices available to people with clinical needs as quickly as possible. Given this goal, the modules consist entirely of off-the-shelf electronic components and common power conditioning, current sensing, and processing electronics were utilized for both the PG4 and BP2 modules. While an application-specific integrated circuit (ASIC) may be smaller and improve power consumption, it would significantly increase development time and cost while potentially creating a rate limiting step by requiring production of that ASIC. Utilizing the same core components similarly simplifies the development time for both hardware and programming to create a first generation system for sensing and stimulation.
A common package design (capsule and header) is utilized for all remote modules, which facilitates the development process by reducing replication of required testing and simplifying clinical implementation. The package design allows longer or shorter length capsules to be built, accommodating future changes in the physical size of the PCB, without changing the feedthrough and header configurations or manufacturing procedures. The maximum dimensions of the common remote module package were constrained so that it could be implanted in the forearm of an average adult.
B. Remote Module Design
Module specific design specifications for the PG4 and BP2 modules are summarized in Tables 1 and 2 respectively while common specifications are listed in Table 3. The specifications were determined based on the requirements for implanted muscle-based stimulation from our prior work with people with paralysis [3, 4, 6, 8, 16–21].
Table 1:
PG4 design specifications
| Parameter | Specifications |
|---|---|
| Number of output channels | 4 channels |
| Waveform | Charge-balanced biphasic |
| Pulse shape | Rectangular cathodic phase, interphase delay, followed by passive recovery anodic phase |
| Current or voltage regulation | Current |
| Maximum nominal output voltage | 29V |
| Maximum output current | 20mA |
| Frequency Range | 1–50Hz |
| Pulse duration range | 1–255μs |
| Charge balancing method | Capacitor |
| Maximum net charge at 500 Ω | 5.1μC/phase |
| Leakage current at 500 Ω | ≤0.1μA |
Table 2:
BP2 design specifications
| Parameter | Specifications |
|---|---|
| Number of channels | 2 |
| Signal processing and feature extraction | Software based |
| Stimulus rejection | Software based |
| Gain range | 150 to 7500 (18 steps logarithmic) |
| AC coupling (High-pass filter) | 10Hz |
| Overload recovery time | <10ms |
| Modes of operation | Raw or Processed EMG signal |
| Anti-aliasing filter | 500 or 1000 Hz |
| Sampling rate | 1 or 2kHz |
| ADC resolution | 8 bits |
Table 3:
Common remote module design specifications
| Parameter | Specifications |
|---|---|
| Method of isolation | Capacitive and Silicon |
| Size | ≤ 55mm × 15mm × 5mm |
| DC Supply voltage | 3.3V |
| Network connections | 4 |
| CPU Clock | Up to 8MHz |
| Temperature sensor sampling rate and resolution | 0.1Hz and 0.625°C |
| Accelerometer sampling rate and resolution | Up to 40Hz and 6 bits, ±2g range |
| Power and communication sending and receiving | Along NNP network |
Four channels of stimulation in a module enables targeting muscles required for a hand system with one module placed in the forearm and two modules placed in the upper arm. The number of outputs was also maximized based on the number of connections that could be achieved with the enclosure and header size. The specified stimulation parameters (maximum of 20mA and 255μs) are commonly implemented for muscle-based electrodes and are capable of recruiting enough motor units to generate functional movement, even in large muscles [16]. Furthermore, the maximum charge per phase is 5.1μC; applied with an intramuscular electrode with surface area of 14.5mm2[22], charge density is 0.352μC/phase/mm2 which results in a k value of 0.254 which is well below the values associated with tissue injury for an electrode of this size [23]. Twelve Hz is the minimum stimulus frequency necessary for generating tetany with an implanted electrode while lower stimulus frequencies generate sub-tetanic contractions. Higher frequencies increase muscle output force, but also induce fatigue more quickly. Implementing a biphasic charge-balanced waveform prevents charge build up that could damage the tissues or the implanted electrode [24, 25]. Current-controlled stimulation is implemented because neural structures respond to charge; current controlled pulses are insensitive to changes in load which can be caused by aging or tissue changes resulting from body position or muscle contraction [24]. The pulse shape is defined based on prior successful neuroprosthesis designs [15, 24]. A maximum output voltage of 29V is based on electrode impedance and the necessary voltage required to produce the maximum current. Charge balancing is implemented through a capacitor to prevent charge buildup between pulses and prevent damage from DC currents. Leakage current limits are based on the relevant standard, ISO14708–2:2005(E).16.2.
Two channels of recording represent the minimum number of channels necessary for a hand grasp system as one set of electrodes produces logical commands, such as transitioning between grasp patterns while the other grades the extent of muscle activation [26]. Recording modules must be capable of amplifying the relatively small myoelectric signals so that features can be extracted to use as proportional command signals. Implementing signal processing in software facilitates flexibility for incorporating new approaches in future implementations. Gain range was selected based on EMG magnitude and experience from prior generation implants [13, 17] since EMG signals can cover a range of a few thousand microvolts but tend to be smaller in people with paralysis. While EMG frequency content typically ranges from 6 to 500Hz, the majority of the power is between 20 and 150Hz. High pass filtering at 10Hz removes motion artifact while sampling at 1 or 2kHz captures target frequency content. Antialiasing filter cutoffs were determined based on the sampling rates. Additionally, the recording module must be capable of removing stimulus artifacts to prevent contamination of the command signal during simultaneous stimulation of nearby muscles. 8-bit ADC resolution was selected as a compromise between maximizing information content while minimizing memory and power usage during command signal generation.
Isolation methods were selected to prevent DC currents that would cause corrosion of components. The CPU clock was chosen to allow faster data processing if necessary in the future. The choice was a compromise between processing speed and power consumption. For both temperature and acceleration sensors, dynamics and ranges of human motion and body temperature were used to determine sampling frequency and resolution. Four network connections in each module facilitates branching to multiple terminal modules, maximizing configurability and future expansion. If modules were limited to two network connections, this would prohibit branching in multiple directions and would require that components be daisy chained together, significantly increasing the amount of cabling and complicating lead routing.
The PG4 and BP2 were designed to meet these input requirements for the NNP system. The modules include the capacity for electrical stimulation via muscle- and nerve-based electrodes, myoelectric signal sensing, 3-axis acceleration sensing, and temperature sensing. Each module contains local processing capabilities to minimize the need for communication between modules, and each module can be programmed through its network connection to the PM in conjunction with a transcutaneous wireless link in the PM. Each remote module has four network connections, allowing one remote module, connected to the network, to branch to three additional remote modules, greatly simplifying installation in the body and maximizing architectural flexibility. A set of network cables (NC2) with two-conductor leads connect the modules together and distribute power and provide for bi-directional data communication. Figure 2 shows a PG4 and BP2 module with associated network cabling and electrode leads connected. Figure 3 shows the circuitry and how it fits within the enclosure.
Fig. 2:
Images of remote modules with attached cabling. a) PG4 module, b) BP2 module. Red cables are plugged into stimulus output ports, green cables are plugged into recording input ports, and blue cables are plugged into network ports. Capped network connections are unused.
Fig. 3:
Remote module circuit. a) PG4 flex-circuit unfolded, b) illustration of folding process, and c) folded module in titanium enclosure.
Both modules include the same power conditioning, current sensing, and processing electronics. A pair of capacitors isolates and couples network AC power from the remote module’s tissue interface. This electrically isolated the battery and network from the tissues while the remote modules interface with the tissue through the electrodes. The titanium enclosure serves as the return electrode for stimulation (PG4) and as the reference electrode for biopotential recording (BP2). A full wave rectifier converts the network’s AC signal to a DC signal while a buck converter generates the isolated 3.3V DC power source for the microcontroller and all circuitry that are part of the tissue interface. Additional bipolar DC power supplies are generated via separate rectifiers to power the non-isolated circuitry that sends and receives communication along the bus. Bidirectional communication is managed between the remote modules and the power module by having remote modules decode pulsewidth from the network waveform and then encode bits to send back via a 0 or 1mA current. Each module is also equipped with a ZXCT1010 for current sensing as part of diagnostic testing within individual modules and across the system. A AT90CAN128 microcontroller manages the relevant stimulation or recording on the respective remote modules and also measures inertial motion with a 3-axis KXTE9–2050 accelerometer and temperature with a TMP112AIDRLT temperature sensor. A CAN-based bootloader enables the device firmware to be updated in vivo over the wireless MedRadio-band (402–405 MHz) link via the power module.
1). Pulse Generator Module (PG4)
The four channel pulse generator module (PG4) is a remote module utilized for electrical stimulation of nerve and/or muscle tissue (Figure 4). The PG4 can deliver stimulation to four independent monopolar electrodes, using the metal case of the PG4 as the common return electrode.
Fig. 4:
Block diagram of PG4
The PG4 stimulus pulses have a constant-current cathodic phase followed by a passive-recharge anodic phase to achieve charge balance. Stimulation parameters meet the specifications in Table 2: pulse duration 0–255μs, 1μs resolution; pulse amplitude 0–20mA, 100μA resolution; and stimulus period 20–1000ms, resolution 1ms. These parameters are sufficient for all muscle-based and nerve-based electrodes that we have utilized in motor neuroprostheses [18]. The PG4 also has circuitry to detect if the compliance voltage is insufficient to generate the requested stimulation current through the electrode and tissue load. This allows for the clinical programmer to reduce the compliance voltage if the necessary currents and/or impedances across all channels are low. Future software revisions could allow for automated pulse-by-pulse compliance voltage adjustment to save further power. Compliance detection can also be used to diagnose open circuits (e.g., a broken or unplugged lead) during or after surgical implantation.
The connections within the output stage of the PG4 are shown in Figure 5. The components specific to the PG4 to generate stimulus parameters are a boost converter (LT3464ETS8) which is controlled by a digital to analog converter (LTC2630CSC6) to set the compliance voltage for the current source from the unregulated input voltage (4.5–9.0V). An analog switch (MAX313LCUE) and a series of logic gates and MOSFETs direct the current to the target channel. A 10-bit voltage output DAC (MAX5355) is used to control the gate voltage of a MOSFET within a feedback circuit that maintains the current output. Input from a current sensing resistor which is in series with the stimulation output automatically increases or decreases the MOSFET gate voltage thereby implementing a digitally controlled current source. Although the hardware can support a range of 0–22mA in 22uA steps, only 8-bit resolution with a 20mA maximum was implemented in software as indicated above. The gate voltage is monitored by an analog comparator in the microcontroller and triggers an out-of-regulation indication within the pulse generation firmware when a maximum voltage is exceeded. Switch timing is regulated by timers on the microcontroller that control the logic gates. Passive recharge after each stimulus pulse is managed by the same switch along with DC blocking ceramic capacitors. The current PG4 firmware utilizes 35/128KB available flash, 3.7/4KB available RAM, and runs at 1MHz.
Fig. 5:
Stimulus circuitry with the elements and path shown for a single channel. Text with lines on the left hand side indicate the function and transmitted information to/from the microcontroller. ‘Compliance detect’ is an input to the microcontroller while the remaining signals are outputs to control pulse generation circuitry
2). Biopotential Recording Module (BP2)
Myoelectric signals (MES) from muscles under voluntary control are recorded by the biopotential recording module (BP2) (Figure 6). The BP2 can record MES from two sites and process the signals independently for different control needs. MES processing in the BP2 includes digital blanking to remove stimulus artifacts (realized in firmware), large selectable gain range (150–7500) and software-based signal processing, as shown in Table 2.
Fig. 6:
Block diagram of BP2
The hardware-based signal processing includes a low-pass filter implemented with an X2Y capacitor to remove high frequency common mode and differential signals. Next, an instrumentation amplifier (LT1168ACS8) amplifies the signals with a gain of 150. Then a 10Hz highpass filter removes DC offset and motion artifact. All filters are first order. A second stage amplifier applies a programmable gain from 1 to 50 on a logarithmic scale (18 steps). A 500Hz antialiasing filter is applied and the signals are sampled at up to 2kHz by the microcontroller. The microcontroller is programmed to calculate the following time-domain features for use as command signals for stimulation: Mean Absolute Value, Mean Value, Zero Crossings, Slope Sign Changes, and Waveform Length [27]. Alternative features could be extracted by modifying the firmware. The current BP2 firmware utilizes 35/128KB available flash, 3.3/4KB available RAM, and runs at 4MHz.
C. Remote Module Communication
The NNP network is both a power and communication network for all implanted components. The NNP System uses a packet-oriented protocol, an extension of the ISO 11898 Controller-Area-Network (CAN) serial communications protocol. The microcontroller features an integrated CAN controller for data transfer within the network. Key features of the CAN protocol that are critical to the NNP network are the inherent priority assigned to each message without interrupts and rapid yet extensive error checking for each message. This allows highly reliable message traffic to be delivered at rates in excess of that required for the NNP network. The “data consistency” of the CAN protocol guarantees that all information is visible and acknowledged by all modules connected along a network segment. Additionally, there is no bus time wasted on message handshaking and it is possible to achieve 100% of the bus utilization time if necessary.
The hardware layer of the network utilizes an in-house design to achieve an alternating current communication signal to minimize risk to the tissue in the event that a network cable is damaged. To accommodate this requirement while using two conductors, power is transmitted in charge-balanced pulses. Modulation of the width of pulses by the PM allows communication to the remote modules (described below). Oscilloscope traces of the network physical layer are shown in Figure 7.
Fig. 7:
Traces showing the network physical layer running at 9.5V shown at different resolutions. a) Five pairs of pulses comprising a single CAN bit. b) Superimposed view of a single dominant and recessive network cycle. The data were recorded separately from the top plot but demonstrate a zoomed-in representation of the type of data in the top plot. c) Round trip delay of a remote module CAN controller transmitting a bit and receiving the same bit back from the network.
To obtain the necessary synchronization in the CAN protocol, multiple network cycles are used to generate a single CAN bit. To transmit a bit, the PM drives the pulse widths of the network signal: a dominant CAN bit results in narrow pulses while a recessive bit results in wide pulses. The remote module network circuitry decodes the width as less than or greater than 500ns (1 or 0) and triggers a flipflop on a change between widths. The output of the flipflop directly drives the CAN controller’s RX line. For the remote module CAN controller to send a dominant CAN bit, the TX line drives the current “talkback”, which is decoded by the PM during a 200ns “quiet” window (Figure 7b) and encoded back onto the network as several narrow (dominant) pulses. The roundtrip delay of a remote module transmitting a bit and receiving the same bit back from the network varies by a “half network cycle” (1μs) (Figure 7b) plus a minimum delay of ~700ns that comes from propagation delays in network circuitry (primarily the 500ns delay line). The roundtrip delay is a critical design criterion for determining the maximum CAN bitrate, because CAN arbitration requires that the transmitted and received bit is “simultaneous” within a small fraction of the total bit time (dependent on CAN “bittime” parameters). While the underlying network circuitry can theoretically support CAN bitrates higher than 100kbit/s—and the bitrate with alternate communication protocols could be 500kbit/s maintaining the full network cycle for DC balance, or 1Mbit/s using “half network cycles”—the 100kbit/s rate is sufficient to meet the intended clinical needs. Further, it is well matched with the 100kbit/s radio protocol.
Power is transferred during the pulses in the waveform resulting in transfer at a 60% duty cycle during recessive pulses (wide), and 40% during dominant pulses (narrow).
D. Electrodes Interfacing Between Remote Modules and Human Tissue
The remote modules are designed to employ previous generations of electrodes which have been used successfully in humans for the past two decades [28]. The stimulating modules use two styles of muscle-based stimulating electrodes, the “epimysial” [14] and “intramuscular” [22] electrodes. The PG4 is also capable of monopolar stimulation through nerve cuff electrodes [29]. Recording electrodes include bipolar versions of the intramuscular and epimysial electrode [13, 26, 30]. These electrodes previously received FDA approval for use [8]; their use has enabled faster translation into human subject testing. For implementation with the NNP, the recording/stimulating end and lead are identical to prior versions but the two conductor connector was designed specifically for the NNP System. We have also developed an intra-cable adaptor that will allow us to connect our previous pin-and-spring-style connector [31] to our new NNP design. This enables us to update past research participants with our new NNP technology, if they should so choose.
E. Experimental Design for Device Verification and Validation
Each module type was evaluated on the benchtop and in a human user with paralysis to demonstrate the expected functionality.
1). Benchtop Verification
PG4:
Output voltage was measured on an oscilloscope (THS3024 Tektronix, Beaverton, OR) across a resistor to verify the desired waveform was achieved for varied pulse amplitude, pulsewidth, and impedance values. Pulse amplitude was varied from 4 to 20mA in 4mA increments with a fixed pulsewidth of 250us across a 500 Ω resistor. Pulsewidth was tested from 50us to 250us in 50us increments at 10mA across a 500 Ω resistor. Lastly, pulsewidth and pulse amplitude were fixed at 250us and 10mA for testing across 250, 500, and 1000 Ω resistors. Stimulator output was also tested with an electrode and anode in saline and a 10ohm resistor in series with the electrode. Voltage was measured across the resistor on a battery powered oscilloscope to confirm that the prescribed stimulator current was appropriately maintained.
To verify that charge balancing is effective without undesired charge buildup, the PG4 was tested based on the cardiac standard of 0.1μA DC leakage current per ISO14708–2:2005(E).16.2. Stimulation was applied through a low pass filter with a 10s time constant to facilitate buildup of any unbalanced charge. The four channels on a PG4 stimulated with maximum pulse parameters (20mA, 255us) and the minimum intrapulse interval (1ms between channels) at 12.5Hz for 60s. Leakage current was determined based on the outcome of this test.
BP2:
A differential function generator (Model 220 MediCal Instruments, Columbus, OH) with variable frequency and amplitude sine waves provided inputs to be recorded by a BP2. A gain of 1500 was implemented in all benchtop tests with sampling at 1000Hz. With a fixed 1mV peak to peak input, input frequencies were implemented at 1Hz, 2Hz, 5Hz, 10Hz, 20Hz, 50Hz, 100Hz, 200Hz, and 400Hz to verify effective filtering. With a fixed frequency of 100Hz, peak to peak amplitudes were input at 0mV, 0.1mV, 0.2mV, 0.5mV, 1.0mV, and 2.0mV to verify effective recording across amplitudes. These ranges represent potential inputs that would be experienced physiologically.
Power Consumption (both modules):
A benchtop system was implemented to measure the contribution of each remote module to total system power consumption as a function of the remote module state and the network voltage. Preliminary testing indicated additional remote modules in the same state and at the same network voltage added linearly to total power consumption and that increasing network voltage increases system power. We tested each remote module state at 5.5, 6.5, 7.5, 8.5 and 9.5V. The network voltage can vary between 4.6 and 9.5V; however, lower voltages cannot generate the stimulus parameters necessary for clinical implementation. Higher network voltage enables supporting more remote modules within a system, higher instantaneous loading demands by each remote module, and tolerance to more series resistance in the network cables. The bench system did not use or simulate resistive and capacitive network cables which vary based on surgical installations. Measurements were completed using current sensing incorporated on each battery cell of the PM. A 5s running average in combination with each batteries’ voltage measurement provided a total system power measurement that reflected the actual power drawn from the batteries and includes the inefficiencies in DC/AC/DC conversions and powering network circuitry. To obtain the contribution for each remote module, we subtracted the baseline power measurement with only the PM on—with the network energized without any remote modules attached. We measured the total power separately for a BP2 and PG4 in the following states: 1) Inactive, 2) Idle, 3) Active with Minimal Stimulation, 4) Active with Maximum Stimulation, and 5) Active with Recording. “Inactive” is the lowest power state, where the microcontroller is essentially off and cannot respond to network commands—the network must be power cycled to return the module to an online, idle state. The “Idle” state allows the module to be interrogated and commanded over the network but does not support active functions of recording or stimulating. During the “Active with Minimum Stimulation” state with the PG4, power was measured with the stimulator active while maintaining full compliance voltage and controlling switch circuitry but not generating any pulses (effectively 0μs, 0mA, 20Hz). Stimulus parameters during the “Active with Maximum Stimulation” were 255μs, 20mA, and 20Hz on all four channels, 1ms apart. Stimulation was applied through 500Ω loads on all four channels. During the Active with Recording condition, the BP2 the analog amplifier circuitry was powered and the microcontroller clock ran at 4MHz to support EMG feature calculation.
2). Human User Validation
The NNP system has been surgically implanted in persons with SCI as part of an ongoing clinical trial implementing the NNP as a hand and trunk system. As part of the system, a BP2 module was implanted inferior to the clavicle to measure from wrist extensor muscles which were under voluntary control. Stimulation modules were implanted in the forearm, upper arm, and abdomen with electrodes generating hand opening and closing and trunk support [8, 21]. Data have been collected from the remote modules of multiple users during the clinical trial process. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. The registry number is NCT02329652.
PG4:
Kinematic responses were visually inspected and photographed to verify that appropriate hand grasp postures could be generated. Stimulation was applied to muscles to generate a variety of hand patterns, including a pinch grasp and a power grasp pattern [17].
BP2:
Recordings were completed under several conditions with gains ranging from 195 to 1500. In the first case, EMG was recorded while the participant was instructed to contract without stimulation to demonstrate a myoelectric signal without concurrent stimulation artifact. Next, the participant relaxed while stimulation was applied to an adjacent muscle and then a muscle significantly further away in the body. In the second case, the participant was instructed to contract during ongoing simultaneous stimulation to demonstrate a command signal can be recorded while stimulation is applied. EMG was recorded from brachioradialis. Stimulation was applied to extensor carpiulnaris (ECU) at the maximum levels used during hand function since it is the closest muscle with a stimulating electrode. The ECU tendon was transferred to the extensor carpi radialis longus tendon in this instance. Digital blanking was applied to remove stimulus artifact and M-waves; waveform length feature was extracted from the remaining 16ms of myoelectric signal during the interpulse interval.
Implanted physiological sensors across modules:
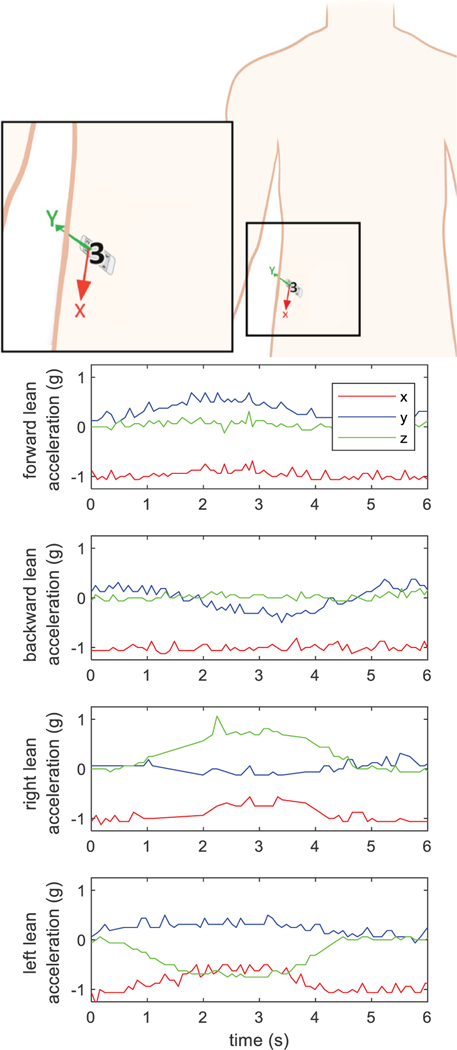
Accelerometer signals were measured during trunk tilt. The participant started in an upright posture, leaned in one of the cardinal directions (anterior, posterior, left, and right), and then returned to the upright posture. The 3-axis signals were recorded at 10Hz to demonstrate signal acquisition in an implant user. Temperature signals were measured at each remote module at a single time point to demonstrate feasibility of recording throughout the body.
Power Consumption:
Power consumption was measured from the entire system to verify that values in human use were comparable to benchtop testing.
III. RESULTS
Figure 2 shows the final devices. This design was tested on the benchtop and in individuals with SCI.
A. Benchtop Verification:
First we tested the stimulus output of the PG4 while it was controlled and powered by the power module. Figure 8 shows the stimulus output measured across 250, 500, and 1000Ω resistors. The desired pulse width and interphase interval are maintained independent of pulse amplitude (Figure 8a). The system also outputs the desired pulsewidth (Figure 8b). These responses are consistent across varied impedance values with scaled amplitudes suggesting viable responses when implanted in the body (Figure 8c). During testing in the saline bath, the cathodic pulse had a comparable rectangular shape to the traces shown in Figure 8 and the expected amplitude based on the requested current. Although charge between phases is not shown here, the variable recharge phases demonstrate the charge balanced nature of the response. The DC balancing test resulted in a leakage current that was less than the threshold of 0.1μA.
Fig. 8:
Benchtop stimulus responses from the PG4 across a resistor: a) PW = 250μs, PA = 4, 8, 12, 16, 20mA, R=500 Ω, b) PW = 50, 100, 150, 200, 250μs, PA = 10mA, R=500 Ω c) PW=250μs, PA = 10, 5, 2.5mA, R=250, 500, 1000 Ω
To evaluate the recording capabilities of the BP2, sine waves of varying input frequencies were recorded to demonstrate acquisition within the typical range of 10–500Hz for EMG signals. Figure 9 shows recordings from the BP2 at varying frequencies. Figure 9a shows the frequency response relative to the theoretical frequency response for the analog circuits modeled in LTSpice. Variability is within component tolerances. The recordings demonstrate effective rejection of signals with frequency content less than 10Hz to remove motion artifact. Within the 20–150Hz range, which represents the majority of power in the EMG spectrum [32], the signal is recorded with sufficient sampling and without significant attenuation. This signal acquisition demonstrates the potential for recording command signals. Figure 9b shows consistently matching recordings for varied input amplitudes at 100Hz. The recorded signal has been referred to input voltages by dividing out the gain and accounting for the A/D resolution and reference voltage. A small amount of attenuation can be seen in the recording compared to the input signal but appears consistent across varying amplitudes. These signals demonstrate the ability to record inputs of varying intensity which is important for a proportional control signal.
Fig. 9:
Benchtop recording from a function generator with the BP2: a) Filter output relative to a 1mV P-P input at varied frequencies, Gain = 1500. Blue dots indicate recorded responses at a given frequency, the black line represents the modeled representation of the circuit, the yellow band shows the frequency range from 20–200Hz. b) Input: varied amplitudes at 100Hz, Gain = 1500.
Relative power consumption by the PG4 and BP2 are shown in Figure 10. A network voltage of 5.5V was unable to support maximum stimulation parameters. At 6.5V and higher values, full capabilities were supported. The remote modules consumed about 45mW in the Inactive mode alone with greater consumption at higher network voltages. Idle mode increased consumption by about 10mW. Power usage was consistent across network voltages during stimulation and accounted for 28–53% of power consumption in the PG4. Consumption during recording was consistent across network voltages, accounting for 31–35% of power.
Fig. 10:
Power consumption by the PG4 and BP2 Remote Module at varying network voltages (5.5V-9.5V) and activity types: 1) Inactive, 2) Idle, 3) Minimum Stimulation, 4) Maximum Stimulation, and 5) Active Recording.
B. Human Validation:
Functionality of the remote modules was assessed in users implanted with the NNP system. Remote modules were implanted in the trunk, upper arm, and forearm. Stimulating electrodes provided trunk control and hand grasp while recording from EMG electrodes controlled hand grasp. Data presented here were collected more than 10 months postimplant.
The PG4 modules generated hand postures that prior implanted systems have produced [13]. Figure 11a shows stimulation producing a pinch grasp while Figure 11b shows stimulation generating a power grasp.
Fig. 11:
Demonstration of grasp postures: a) pinch grasp, b) power grip
The BP2 module recorded myoelectric signals that could be converted into command signals. The 8-bit recording (−128 to 127) is shown in Figure 12 during a series of different test conditions. The signal from a volitional contraction without simultaneous stimulation is shown in Figure 12a. The participant started relaxed, contracted their wrist extensor, and then relaxed again. Figures 12b and 12c show the response recorded from the same muscle while the individual remains relaxed and stimulation is separately applied to a nearby muscle (finger extensor) and a further muscle (hip extensor) respectively. Volitional effort during simultaneous stimulation applied to a nearby muscle is shown in Figure 12d. Constant stimulation was applied while the participant contracted and then relaxed the muscle under volitional control. Digital blanking was applied to remove stimulus artifact with the resulting waveform shown in Figure 12e. A zoomed in version of frames (d) and (e) are shown in Figure 12f. The corresponding waveform length feature is shown in Figure 12g. Although more of the frame in Figure 12f could have been used to create each sample in Figure 12g, the presented processing demonstrates that extracting only 15ms per frame still generates an effective command signal. The signal shows a clear increase during contraction that could be converted into a proportional command signal. Additional processing, such as adaptive filtering, could be implemented during the conversion to a command signal [26].
Fig. 12:
EMG recordings in an implant recipient. a) voluntary effort recorded from ECRL (gain=195) without stimulation, b) stimulus artifact recorded from ECRL (gain=195) during stimulation applied to APL-EDC (tendon-transferred muscle) while user was relaxed (no voluntary effort), c) stimulus artifact recorded from ECRL (gain=195) during stimulation to gluteus maximus while user was relaxed, d) voluntary effort and artifact recorded from brachioradialis (gain=1500) while stimulation was applied to ECU, e) blanking applied to extract myoelectric command signal from (d) (14ms extracted per frame), f) smaller window of the signals from (d) (red) and (e) (blue), and g) waveform length extracted from blanked myoelectric signal in (e) and scaled to 0–255.
Each module is instrumented with a 3-axis accelerometer. Figure 13 shows the temporal acceleration dynamics for a PG4 in the lower-right abdomen while the user completed a series of movements from erect to each of the target directions: forward, backward, left, and right. The figures show a clear shift and then return to baseline about the axes matching the surgical notes from implantation.
Fig. 13:
Accelerometer data from a module in abdomen demonstrating measurement during trunk tilt. The Z-axis is directed medially and posteriorly (into the page).
Temperature recording was assessed with a single sample recorded from each remote module of the seven remote modules in an individual. The average temperature (standard deviation) was 35.1 (1.3) °C. The average temperature was slightly higher in the abdomen (36.1 °C) compared to modules in the shoulder (35.5 °C), upper arm (34.7 °C), and forearm (32.5 °C).
Total power consumption in a system with a PM, six PG4s, and a BP2 averaged about 800mW. Accounting for the PM power consumption, seven remote modules consumed 550–675mW with a network voltage of 8.5V, dependent on the stimulation configuration and parameters. This usage enabled about 2–2.5 hours of active daily use between charges.
IV. DISCUSSION
The remote modules achieved the design features and specifications that were outlined in Tables 1, 2, and 3 both through benchtop testing and in testing with people with paralysis. The key innovation of the NNP concept is that it provides, within a single system, the capacity to implement various configurations of implanted technology, both for stimulation and recording sites, and for addressing multiple body functions by incorporating additional modules. The networked architecture allows the NNP to be applied equally well to modest disabilities using a few components or severe disabilities requiring many components.
The fully-implanted, rechargeable-battery-powered network, along with the design of the remote modules, reduces or eliminates the need for external components during functional use, resulting in systems that are easy for users to operate, robust, cosmetically acceptable, and applicable to a broad range of neurological indications.
This design significantly improves capabilities compared to prior neuroprosthetic systems utilized to provide motor function in people with paralysis from SCI and stroke (Table 4) [33–35]. The stimulation and recording parameters are comparable to previously implemented motor neuroprosthesis systems [14, 15]. As summarized in Table 4, commercial devices designed for spinal cord stimulation (SCS) or deep brain stimulation (DBS) are capable of longer pulse widths and higher stimulation frequencies [36–40]. These features may be necessary for neural targets in the brain and spinal cord, but are generally not required for peripheral nerve stimulation and myoelectric recording [13]. The significant distinguishing feature of the NNP approach is the system architecture and its relevance to clinical needs for people with paralysis. Commercially available systems are intended to target a focal region in the body (typically brain or spinal cord) and use a “star” topology [10]. While the star topology is appropriate for many applications, restoring motor function requires coordination between multiple muscles throughout the limbs and torso. Further, the ability to position sensors across joints increases opportunities for advanced control (accelerometer) or even health monitoring (temperature sensor). This distributed approach also facilitates system upgrades. As new modules are developed, they can be incorporated into the system, such as the development of a module for cortical recording [41]. Upgrades can be achieved by connecting to an open network port in the existing system and does not necessitate removing or replacing any components. In addition to the distributed approach facilitating clinical implementation, it increases the commercial viability of translation in order to impact the lives of people with paralysis. Individual populations of people with paralysis are considered too small to support marketing of multiple devices, with each device being optimized for a specific need. A platform ecosystem that allows individualization with a hardware set that can be customized based on clinical goals maximizes the likelihood of sustainable commercial translation [42].
Table 4:
PG4 and BP2 in context of existing motor neuroprostheses, commercial neurostimulators and devices in development.
| Device | Architecture Design |
Stimulus Outputs Leads Channels Sources |
Stimulus Parameters Pulsewidth range Amplitude range Frequency range |
Biopotential Inputs Leads Channels |
Additional sensing |
|---|---|---|---|---|---|
| Presented Device (NNP) | |||||
| PG4 (NNP) (per module) | Distributed | 4 Leads 1 Channel/Lead |
0–255us 0–20mA 1–50Hz |
N/A | 1 Inertial 1 Temperature |
| BP2 (NNP) (per module) | Distributed | N/A | N/A | 2 Leads 2 Bipolar channels |
1 Inertial 1 Temperature |
| Prior Motor Neuroprosthesis | |||||
| Implanted Stimulator Telemeter (CWRU) |
Star | 12 Leads 1 Channel/Lead | 0–255us 0–20mA 1–50Hz |
2 Bipolar channels | |
| Commercial Neurostimulators | |||||
| Wavewriter Alpha (Boston Scientific) | Star | 4 Leads 8 Channels/Lead |
20–1000us 0–25.5mA 2–1200Hz |
N/A | N/A |
| Percept PC (Medtronic) | Star | 2 Leads 8 Channels/Lead |
20–450us 0–25.5mA 2–250Hz |
1 Lead 6 Channels | N/A |
| Activa RC+S (Medtronic) | Star | 4 Leads 4 Channels/Lead |
60–450us 0–25.5mA 30–250Hz |
1 Lead 4 Channels 16 Contacts | 1 Inertial sensor in IPG |
| Senza Omnia (Nevro) | Star | 2 Leads 8 Channels/Lead |
20us-1ms 0–15mA 2–10,000Hz |
N/A | N/A |
| In Development | |||||
| Rozgic [44] | Star | 1 Lead 64 channels 8 drivers | Pulsewidth not available 0–5.1mA Frequency not available |
1 Lead 64 channels | N/A |
| SenseBack [45] | Star | 1 Lead 32 Bidirectional Channels |
Pulsewidth not available 5–315uA Frequency not available |
1 Lead 32 Bidirectional Channels |
N/A |
N/A – not applicable
While the modular design with off the shelf components facilitates creating an available system that can be scaled to a variety of user needs, there are also trade-offs and limitations. The system is not optimized for a single solution, which can increase overall system cost, power consumption, and size.
While each output channel in the PG4 could drive a single contact in a cuff, it does not support current steering with multiple active channels within a cuff electrode. The stimulation module acts as the anode instead of allowing alternate channels to serve as the anode. Implementation beyond motor tasks would also benefit from increased stimulus parameter options such as higher frequency ranges and higher pulsewidths. The present design was not optimized for power consumption, a contributing factor to the significant power consumption from the modules. It is worth noting that 4 channels of stimulation outputting the maximum parameters at 20Hz across a 500Ω resistor only accounts for 4mW of the 110mW consumed by the PG4 so there is significant room for improved power efficiency. The network voltage implemented in implant recipients will be a function of the number of remote modules, stimulus levels required by target functions, and cable lengths between modules. Efficiency of different module elements is greater at varying network voltages as well. Compliance voltage generation is more efficient at higher network voltages resulting in a more consistent contribution of stimulation. In contrast, elements that rely primarily on regulated 3.3V power are more efficient at lower network voltages as indicated in the shift in power consumption across inactive states.
The flexibility and modularity of this approach creates a wide array of possible combinations of stimulus parameters and recording configurations that make it unrealistic to sample the entire space. As a result, the system has only been tested within a very specific range even though the hardware and software could be configured for greater recording ranges and variable sampling rates.
The NNP system has potential for advanced stimulation control due to the network of sensors that can be incorporated into the system. It also presents new possibilities for health monitoring through the combination of on-board sensors and attached electrodes.
This novel architecture facilitates system expansion, technical upgrades, and functional enhancements. The NNP System is an enabling technology that provides a platform upon which clinical applications can be developed for a multitude of neurological disorders. Because of the modularity of the wired multi-point topology, the NNP System can easily accommodate new remote modules that are developed with future technological advances as they become available, such as cortical recording [41] and enhanced orientation sensing [43], thus ensuring a state-of-the-art system that maximizes the function provided in each clinical application. Thus we expect that, in the future, new modules will be created which can be used interchangeably with the existing system through the common network connection.
V. CONCLUSION
This study presented the effective design and implementation of remote modules for a networked neuroprosthesis system for neural modulation and biopotential recording. This system has potential to significantly improve quality of life for people with paralysis and could be implemented in people with SCI, MS, cerebral palsy, and stroke. The system could be configured for hand grasp, reach, trunk support, transfer assistance, bladder control, standing, and stepping. The design of additional modules could further expand target clinical applications for more advanced control of movement and additional autonomic functions.
ACKNOWLEDGMENT
We thank Erika Woodrum for creating illustrations and taking photographs for the figures. We also thank Martha Liechty for creating figure illustrations. We thank Lisa Lombardo and Musa Audu for assisting in data collection for Figure 13.
This paper was submitted for review on 12/11/20. This work was funded by the following grants: NINDS UH2/3-NS-103863; NIBIB R01-EB-001740; NINDS U01-NS-069517; PVA Research Foundation Grant #2816; and Ohio Third Frontier Program IPP #13-316.
Contributor Information
Nathaniel S. Makowski, Department of Physical Medicine & Rehabilitation, MetroHealth Medical Center (MHMC) and Case Western Reserve University (CWRU), Cleveland, OH 44109 USA
Alexandru Campean, LSCVAMC and is currently with Synapse Biomedical Inc, Oberlin, OH 44074..
Joris M. Lambrecht, Department of Biomedical Engineering, CWRU.
James R. Buckett, Department of Biomedical Engineering, CWRU.
James D. Coburn, Department of Biomedical Engineering, CWRU.
Ronald L. Hart, Research Service, Louis Stokes Cleveland Veterans Affairs Medical Center, Cleveland, OH 44106.
Michael E. Miller, Motion Study Laboratory, LSCVAMC.
Fred W. Montague, Department of Biomedical Engineering, CWRU.
Tim Crish, CWRU and is currently with Synapse Biomedical Inc..
Michael J. Fu, Department of Electrical Engineering and Computer Science, CWRU.
Kevin L. Kilgore, Departments of Physical Medicine & Rehabilitation and Orthopaedics, MHMC and CWRU, the Research Service at the LSCVAMC, and the Department of Biomedical Engineering at CWRU.
P. Hunter Peckham, Department of Physical Medicine & Rehabilitation, MHMC and CWRU. He was with the Department of Biomedical Engineering, CWRU..
Brian Smith, Department of Biomedical Engineering, CWRU..
REFERENCES
- [1].Ho CH, Triolo RJ, Elias AL, Kilgore KL, DiMarco AF, Bogie K, Vette AH et al. , “Functional electrical stimulation and spinal cord injury,” Phys Med Rehabil Clin N Am, vol. 25, no. 3, pp. 631–54, ix, August 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kilgore KL, Kilgore KL, Ed. Introduction and fundamental requirements of neuroprostheses, 1st ed. (Implantable Neuroprostheses for Restoring Function). Cambridge, UK: Woodhead Publishing, 2015. [Google Scholar]
- [3].Knutson JS, Chae J, Hart RL, Keith MW, Hoyen HA, Harley MY, Hisel TZ et al. , “Implanted neuroprosthesis for assisting arm and hand function after stroke: A case study,” J Rehabil Res Dev, vol. 49, no. 10, pp. 1505–16, December 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Makowski NS, Kobetic R, Lombardo LM, Foglyano KM, Pinault G, Selkirk SM, and Triolo RJ, “Improving Walking with an Implanted Neuroprosthesis for Hip, Knee, and Ankle Control After Stroke,” Am J Phys Med Rehabil, vol. 95, no. 12, pp. 880–888, December 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Peckham PH and Kilgore KL, “Challenges and opportunities in restoring function after paralysis,” IEEE Trans Biomed Eng, vol. 60, no. 3, pp. 602–9, March 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Triolo RJ, Bailey SN, Foglyano KM, Kobetic R, Lombardo LM, Miller ME, and Pinault G, “Long-Term Performance and User Satisfaction With Implanted Neuroprostheses for Upright Mobility After Paraplegia: 2- to 14-Year Follow-Up,” Arch Phys Med Rehabil, vol. 99, no. 2, pp. 289–298, February 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].DiMarco AF, Kowalski KE, Hromyak DR, and Geertman RT, “Long-term follow-up of spinal cord stimulation to restore cough in subjects with spinal cord injury,” J Spinal Cord Med, vol. 37, no. 4, pp. 380–8, July 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Peckham PH, Keith MW, Kilgore KL, Grill JH, Wuolle KS, Thrope GB, Gorman P. et al. , “Efficacy of an implanted neuroprosthesis for restoring hand grasp in tetraplegia: a multicenter study,” Arch Phys Med Rehabil, vol. 82, no. 10, pp. 1380–8, October 2001. [DOI] [PubMed] [Google Scholar]
- [9].Anderson KD, “Targeting recovery: priorities of the spinal cord-injured population,” J Neurotrauma, vol. 21, no. 10, pp. 1371–83, October 2004. [DOI] [PubMed] [Google Scholar]
- [10].Kilgore KL, Smith B, Campean A, Hart RL, Lambrecht JM, Buckett JR, and Peckham PH, “Powering strategies for implanted multi-function neuroprostheses for spinal cord injury,” Healthc Technol Lett, vol. 7, no. 3, pp. 81–86, June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Keith MW, Peckham PH, Thrope GB, Stroh KC, Smith B, Buckett JR, Kilgore KL et al. , “Implantable functional neuromuscular stimulation in the tetraplegic hand,” J Hand Surg Am, vol. 14, no. 3, pp. 524–30, May 1989. [DOI] [PubMed] [Google Scholar]
- [12].Kilgore K, Sensors for motor neuroprostheses (Intelligent implantable sensor systems for medical applications). Cambridge, UK: Woodhead Publishing, 2013. [Google Scholar]
- [13].Kilgore KL, Hoyen HA, Bryden AM, Hart RL, Keith MW, and Peckham PH, “An implanted upper-extremity neuroprosthesis using myoelectric control,” The Journal of hand surgery, vol. 33, no. 4, pp. 539–50, April 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Smith B, Peckham PH, Keith MW, and Roscoe DD, “An externally powered, multichannel, implantable stimulator for versatile control of paralyzed muscle,” IEEE Trans Biomed Eng, vol. 34, no. 7, pp. 499–508, July 1987. [DOI] [PubMed] [Google Scholar]
- [15].Smith B, Tang Z, Johnson MW, Pourmehdi S, Gazdik MM, Buckett JR, and Peckham PH, “An externally powered, multichannel, implantable stimulator-telemeter for control of paralyzed muscle,” IEEE Trans Biomed Eng, vol. 45, no. 4, pp. 463–75, April 1998. [DOI] [PubMed] [Google Scholar]
- [16].Triolo RJ, Bailey SN, Miller ME, Rohde LM, Anderson JS, Davis JA Jr., Abbas JJ et al. , “Longitudinal performance of a surgically implanted neuroprosthesis for lower-extremity exercise, standing, and transfers after spinal cord injury,” Arch Phys Med Rehabil, vol. 93, no. 5, pp. 896–904, May 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hart RL, Bhadra N, Montague FW, Kilgore KL, and Peckham PH, “Design and testing of an advanced implantable neuroprosthesis with myoelectric control,” IEEE Trans Neural Syst Rehabil Eng, vol. 19, no. 1, pp. 45–53, February 2011. [DOI] [PubMed] [Google Scholar]
- [18].Kilgore KL, Anderson KD, and Peckham PH, “Neuroprosthesis for individuals with spinal cord injury,” Neurol Res, pp. 1–13, July 30 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kilgore KL, Peckham P, and Keith MW, “Twenty year experience with implanted neuroprostheses,” Conf Proc IEEE Eng Med Biol Soc, vol. 2009, pp. 7212–5, 2009. [DOI] [PubMed] [Google Scholar]
- [20].Memberg WD, Polasek KH, Hart RL, Bryden AM, Kilgore KL, Nemunaitis GA, Hoyen HA et al. , “Implanted neuroprosthesis for restoring arm and hand function in people with high level tetraplegia,” Arch Phys Med Rehabil, vol. 95, no. 6, pp. 1201–1211 e1, June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Triolo RJ, Bailey SN, Miller ME, Lombardo LM, and Audu ML, “Effects of stimulating hip and trunk muscles on seated stability, posture, and reach after spinal cord injury,” Arch Phys Med Rehabil, vol. 94, no. 9, pp. 1766–75, September 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Memberg WD, Peckham H, and Keith MW, “A surgically-implanted intramuscular electrode for an implantable neuromuscular stimulation system,” IEEE Trans Rehabil Eng, vol. 2, no. 2, pp. 80–91, 1994. [Google Scholar]
- [23].Shannon RV, “A model of safe levels for electrical stimulation,” IEEE Trans Biomed Eng, vol. 39, no. 4, pp. 424–6, April 1992. [DOI] [PubMed] [Google Scholar]
- [24].Mortimer JT and Bhadra N, “Fundamentals of Electrical Stimulation,” in Fundamentals and Mechanisms of Neuromodulation, vol. 3, Krames ES, Peckham PH, and Rezai AR, Eds. 2 ed. (Neuromodulation Comprehensive Textbook of Principles, Technologies, and Therapies, pp. 71–82. [Google Scholar]
- [25].Mortimer JT, Kaufman D, and Roessman U, “Intramuscular electrical stimulation: tissue damage,” Ann Biomed Eng, vol. 8, no. 3, pp. 235–44, 1980. [DOI] [PubMed] [Google Scholar]
- [26].Hart RL, Kilgore KL, and Peckham PH, “A comparison between control methods for implanted FES hand-grasp systems,” IEEE Trans Rehabil Eng, vol. 6, no. 2, pp. 208–18, June 1998. [DOI] [PubMed] [Google Scholar]
- [27].Hudgins B, Parker P, and Scott RN, “A new strategy for multifunction myoelectric control,” IEEE Trans Biomed Eng, vol. 40, no. 1, pp. 82–94, January 1993. [DOI] [PubMed] [Google Scholar]
- [28].Kilgore KL, Peckham PH, Keith MW, Montague FW, Hart RL, Gazdik MM, Bryden AM et al. , “Durability of implanted electrodes and leads in an upper-limb neuroprosthesis,” J Rehabil Res Dev, vol. 40, no. 6, pp. 457–68, Nov-Dec 2003. [DOI] [PubMed] [Google Scholar]
- [29].Polasek KH, Hoyen HA, Keith MW, Kirsch RF, and Tyler DJ, “Stimulation stability and selectivity of chronically implanted multicontact nerve cuff electrodes in the human upper extremity,” IEEE Trans Neural Syst Rehabil Eng, vol. 17, no. 5, pp. 428–37, October 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Memberg WD, Stage TG, and Kirsch RF, “A fully implanted intramuscular bipolar myoelectric signal recording electrode,” Neuromodulation, vol. 17, no. 8, pp. 794–9; discussion 799, December 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Letechipia JE, Peckham PH, Gazdik M, and Smith B, “In-line lead connector for use with implanted neuroprosthesis,” IEEE Trans Biomed Eng, vol. 38, no. 7, pp. 707–9, July 1991. [DOI] [PubMed] [Google Scholar]
- [32].De Luca CJ, Gilmore LD, Kuznetsov M, and Roy SH, “Filtering the surface EMG signal: Movement artifact and baseline noise contamination,” J Biomech, vol. 43, no. 8, pp. 1573–9, May 28 2010. [DOI] [PubMed] [Google Scholar]
- [33].Audu M, Kobetic R, Selkirk S, and Triolo RJ, “Lower-Extremity Motor System Neuroprostheses,” in Neuromodulation, vol. 3, Peckham PH and Kilgore KL, Eds. 2nd ed. San Diego, CA: Academic Press, 2018. [Google Scholar]
- [34].P. H KKL Peckham, “Stimulation for Return of Upper-Extremity Function,” in Neuromodulation, vol. 3, Peckham PH and Kilgore KL, Eds. 2nd ed. San Diego, CA: Academic Press, 2018. [Google Scholar]
- [35].Knutson JS, Wilson RD, Makowski NS, and Chae J, “Functional Electrical Stimulation for Return of Function After Stroke,” in Neuromodulation, vol. 3, Peckham PH and Kilgore KL, Eds. 2nd ed. San Diego, CA: Academic Press, 2018. [Google Scholar]
- [36].Bourget D, Bink H, Stanslaski S, Linde D, Arnett C, Adamski T, and Denison T, “An implantable, rechargeable neuromodulation research tool using a distributed interface and algorithm architecture,” in 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpelier, 2015, pp. 61–65. [Google Scholar]
- [37].Medtronic, “Percept PC Neurostimulator with BrainSense Technology,” ed, 2020, pp. 8–9. [Google Scholar]
- [38].Medtronic, “Activa RC 37612 Multi-program rechargeable neurostimulator,” ed, 2020, pp. 8–9. [Google Scholar]
- [39].Nevro, “Physician Implant Manual - Senza, Senza II, Senza Omnia,” ed, 2020, p. 18. [Google Scholar]
- [40].Scientific B, “WaveWriter Alpha and WaveWriter Alpha Prime Implantable Pulse Generators,” ed, 2020, p. 3. [Google Scholar]
- [41].Bullard AJ, Nason SR, Irwin ZT, Nu CS, Smith B, Campean A, Peckham PH et al. , “Design and testing of a 96-channel neural interface module for the Networked Neuroprosthesis system,” Bioelectron Med, vol. 5, p. 3, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Borton DA, Dawes HE, Worrell GA, Starr PA, and Denison TJ, “Developing Collaborative Platforms to Advance Neurotechnology and Its Translation,” Neuron, vol. 108, no. 2, pp. 286–301, October 28 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lambrecht JM and Kirsch RF, “Miniature low-power inertial sensors: promising technology for implantable motion capture systems,” IEEE Trans Neural Syst Rehabil Eng, vol. 22, no. 6, pp. 1138–47, November 2014. [DOI] [PubMed] [Google Scholar]
- [44].Rozgic D, Hokhikyan V, Jiang W, Akita I, Basir-Kazeruni S, Chandrakumar H, and Markovic D, “A 0.338 cm(3), Artifact-Free, 64-Contact Neuromodulation Platform for Simultaneous Stimulation and Sensing,” IEEE Trans Biomed Circuits Syst, vol. 13, no. 1, pp. 38–55, February 2019. [DOI] [PubMed] [Google Scholar]
- [45].Williams I, Brunton E, Rapeaux A, Liu Y, Luan S, Nazarpour K, and Constandinou T, “SenseBack - An Implantable System for Bidirectional Neural Interfacing,” IEEE Trans Biomed Circuits Syst, vol. PP, September 10 2020. [DOI] [PubMed] [Google Scholar]