Abstract
Purpose
The field of quality-of-life (QOL) research would benefit from learning about and integrating systems science approaches that model how social forces interact dynamically with health and affect the course of chronic illnesses. Our purpose is to describe the systems science mindset and to illustrate the utility of a system dynamics approach to promoting QOL research in chronic disease, using diabetes as an example.
Methods
We build a series of causal loop diagrams incrementally, introducing new variables and their dynamic relationships at each stage.
Results
These causal loop diagrams demonstrate how a common set of relationships among these variables can generate different disease and QOL trajectories for people with diabetes and also lead to a consideration of non-clinical (psychosocial and behavioral) factors that can have implications for program design and policy formulation.
Conclusions
The policy implications of the causal loop diagrams are discussed, and empirical next steps to validate the diagrams and quantify the relationships are described.
Keywords: Health disparities, Quality of life, Psychosocial, Health, System science, Cognitive reserve, Policy
Over the past two decades, the field of quality-of-life (QOL) research has evolved and matured, evidencing growth in methodological sophistication and application. In addition to its long-standing application to outcome research, QOL tools and methods are increasingly used in comparative effectiveness research [1] and health policy decision-making [2]. Additionally, research on the utility of QOL tools in clinical practice supports their use for improving provider-patient communication [3]. Finally, recent developments in formalizing the use of patient- reported outcomes at the United States Federal Drug Administration have led to QOL tools being a critical source of information for federal approval of new pharmacotherapies and medical devices [4].
In parallel with the growth in the field of QOL research, there have been substantial advances in theoretical models for understanding how social forces influence health. Comprehensive models of care, such as the original chronic care model [5–7], focus on evidence-based health care system changes to meet the needs of chronically ill patients. While such models can be useful for linking health system, clinical, and patient components that affect disease management outcomes [8], they are typically applied somewhat narrowly to focus on clinical concepts in a unidirectional manner; that is, the factors are all “inputs,” and are not conceptualized or considered in a dynamic manner, such as how they interact and impact each other over time. It is important to go beyond a static, clinical view and look at other factors in the patient’s life that can and do affect the disease trajectory [8, 9].
In response to this desire for a more dynamic understanding and modeling of how social forces impact health [10], the interdisciplinary field of systems science research has developed. This field adds value to more traditional research methods by aiding in the development and testing of integrated models that examine how these factors interact with each other and with health status. For example, some of this research has examined the dynamic mechanisms by which income, education, neighborhood quality, employment stability, and other socioeconomic factors influence health [11]. Other research has revealed changes in access to important resources may have direct or indirect effects on health outcomes [12, 13]. Social network analysis, agent- based modeling, and system dynamics modeling are three such systems science approaches that show great promise for health intervention research [14].
Systems science approaches utilize qualitative, nonlinear thinking to model relationships between a host of factors that influence health [15]. The approaches can reveal what factors lead to worse-than-expected health outcomes, (i.e., health disparities) as well as what factors lead to better-than-expected health outcomes (i.e., resilience). The resulting model is then substantiated by extant research and becomes the basis for simulation modeling and exploration of alternative strategies that yield likely points of intervention to make social change [15, 16].
We believe that the field of QOL research would benefit from learning about and integrating such a systems science approach. Our purpose is thus to describe the systems science zeitgeist and to illustrate its utility for QOL research and health system reform. Using diabetes as an example, we will demonstrate the application of systems methods to QOL research. To do this, we will build a causal loop diagram incrementally, introducing new variables and their dynamic relationships at each stage. These causal loop diagrams demonstrate how the relationships can lead to different disease and QOL trajectories for people with diabetes. This approach builds on documented relationships that other researchers have validated. It synthesizes and integrates the literature into a more dynamic model to fuel hypothesis testing in future empirical research. In addition to illustrating how systems science thinking leads to a consideration of non-traditional factors, we will also discuss the policy implications of the causal relationships that are diagramed. Table 1 provides a glossary of terms central to system dynamics, a systems science approach that we are using as an example.
Table 1.
A Glossary of Terms
| Concept | Definition |
|---|---|
| Systems thinking | A nonlinear way of thinking about social problems that stresses chains of reciprocal causal relationships among the variables |
| Causal loop diagram | A visual representation of a problem that specifies what processes are hypothesized to give rise to problematic behavior in a system |
| Balancing loop | A loop in which all the variables act to counteract change, i.e., bringing the system back to status quo |
| Reinforcing loop | A loop in which all the variables amplify the directional change by prompting either growth or decay |
| Endogenous factors | Individual and societal characteristics that are dynamic and changeable, and which interact to influence disease trajectories |
| Exogenous factors | Static, unchanging background characteristics that create the environment in which a person is dealing with the disease (e.g., ethnicity, cultural norms, country of origin, gender) |
| Passive cognitive reserve | Brain reserve based on past enrichment activities, such as educational and occupational achievement, childhood exposure to cultural, artistic, musical, and other enrichment |
| Active cognitive reserve | Current enrichment activities that maintain brain capacity, such as current practices of exercise, cultural, artistic, musical, spiritual/religious, and other leisure pursuits that enrich brain function and cognitive flexibility |
Applications of system dynamics to health problems
System dynamics modeling has been shown to promote deep understanding of complex human and organizational problems and has been demonstrated to be useful for informing the development and implementation of effective policies in many domains [17–19]. The hallmark of the system dynamics approach is the study of feedback mechanisms, or cybernetics, which can be used to explain how things change over time [20–23]. Health services and clinical care have been a major focus for system dynamics studies since the 1970s [24]. Applications to public health and health services research have typically studied population-level problems, although organizational studies and models of individual level biologic or medical phenomena have been conducted. For example, system dynamics has been used to examine health care quality improvement [25–31]; patient flow and wait time optimization [32–37]; clinical workforce demand [38–40]; community-based systems of care [41–44]; urgent care facility utilization [45–47]; patient-level clinical assessment [48–50]; epidemiology and disease surveillance [51–55]; global health care management [56–58]; behavioral health interventions in tobacco [55, 59–62], substance abuse [63–66], and mental health [67, 68]; as well as in managing chronic illness [44, 69, 70].
In addition, a system dynamics model called Health-Bound was developed for the Centers for Disease Control and Prevention (CDC) to help people understand the critical relationship between “upstream” efforts to prevent illness and the demand for the relationships between a community’s access to care, health status, and socioeconomic level [10]. A later generation of the model is being used as part of a project called ReThink Health to help communities better understand the potential benefits of combining preventive efforts with measures to improve the delivery of health care.
Background on diabetes
Diabetes is a debilitating chronic illness that is considered an epidemic [71], affecting over six percent of the world’s population [72, 73]. The disease disproportionately affects Hispanics and non-Hispanic blacks, with incidence estimated to be 66 and 77 % higher than the non-Hispanic white national population, respectively [74]. More prevalent in older [71] and urban-dwelling [72], diabetes is often accompanied by other comorbid conditions, such as heart disease, kidney disease, obesity, and depression [74–77]. Diabetes is an important area of concern for healthcare professionals, both because of the global burden of disease and because its course is exacerbated by non-adherence to medical regimens [78–80]. As is the case with most chronic diseases, living with diabetes is a process of daily decision-making and participation in self-care [75, 81–83]. The current standard of care of diabetes involves not only medical monitoring and treatment, but also patient education and psychosocial support to enable patient participation [75, 84]. However, patient participation in care and adherence to treatment regimens depend very much on what else is going on in a patient’s life (e.g., employment, social relationships, family support) which, in turn, is affected by the clinical and psychological impacts of the disease. System dynamics and the causal diagrams developed in the next section enable us to capture the closed loop nature of the interactions between these aspects of a patient’s life and the subsequent progression of their illness.
Building the causal loop diagram
Diabetes management focuses on the patients’ participation in their care [78, 85–89]. This participation is operationalized not only as adherence to pharmacotherapy regimens, but also to dietary and lifestyle practices that are known to prevent the progression of disease [78, 80, 86]. When clinical health status creates symptoms, the patient becomes more aware and alarmed of their diagnosis [90] and thus is propelled to participate more effectively in medical care [86, 91]. Figure 1 shows a causal feedback loop through associated symptoms and complications that affect perceived need for care and cause the patient to participate more effectively. This loop is referred to as a balancing loop (denoted by a “B”), because it has a buffering effect that trends toward a position of stability or homeostasis over time [92]. In this sense, balancing loops are goal directed and serve to resist movement in a particular direction (downward or upward) that might otherwise be a consequence of the disease.
Fig. 1.
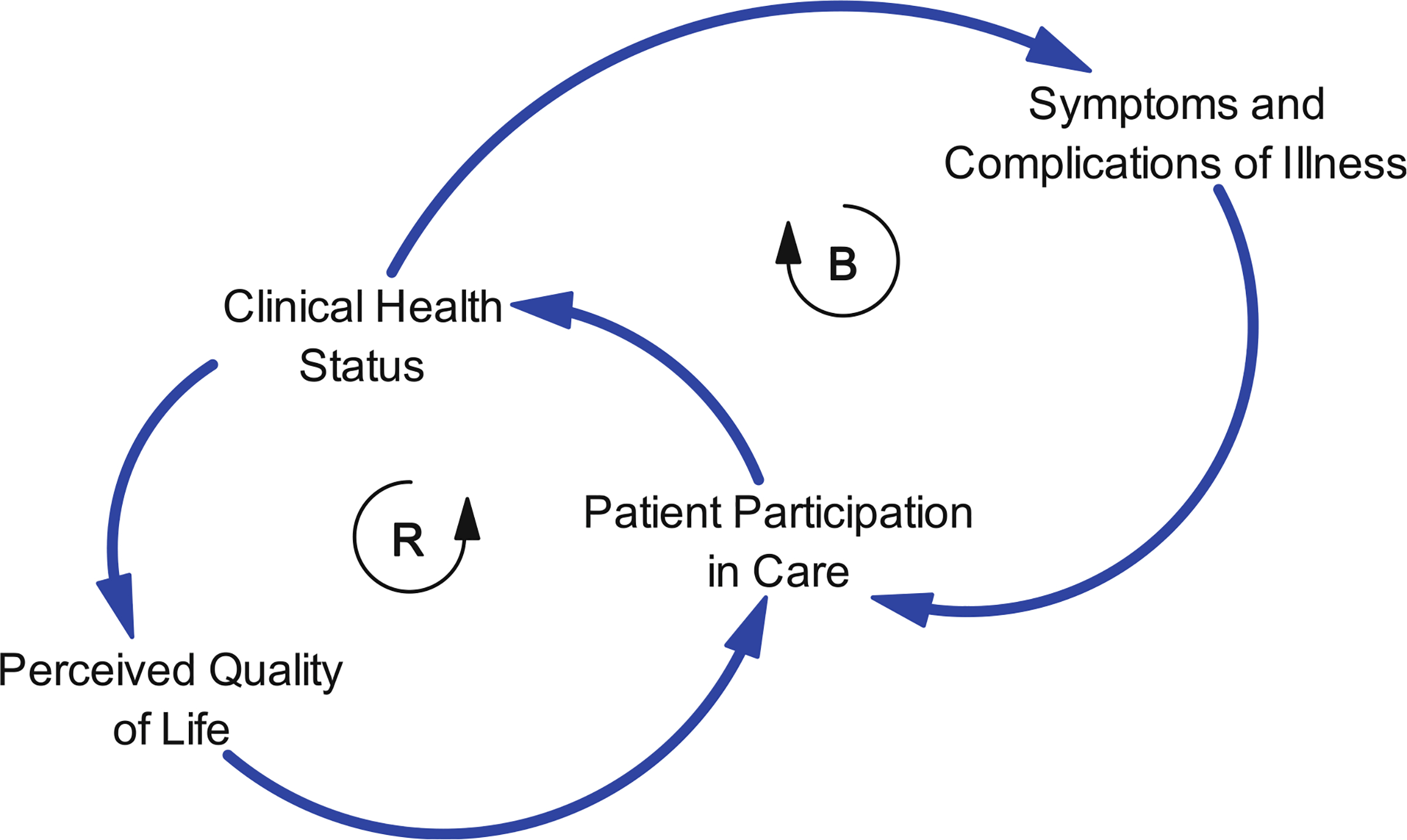
A simple depiction of factors influencing patient participation in care, including a balancing loop as patients respond to diabetes symptoms and complications and a reinforcing loop that can produce decline or improvement based on patient responses to perceived QOL
However, as shown in Fig. 1, there is more going on that affects the patient’s participation. Clinical health status may affect one’s perceived QOL which affects participation in care and, in turn, one’s clinical health status [84, 93–95]. Declining QOL, as perceived by the patient, may reduce the patient’s motivation to participate in care and accelerate the decline in both clinical health status and perceived QOL. This forms another type of causal loop, a reinforcing or positive feedback loop, with relationships that drive behavior in a particular direction rather than resisting changes as the balancing loop does. Reinforcing loops are denoted with an “R” and can generate upward and downward trends in the variables that define the loop [92]. Thus, improvements in perceived QOL can lead to improved patient participation in care and clinical health status. This will be an important concept as we project the various trajectories that might be implied by these causal relationships.
The next several Figures embellish upon these fundamental dynamics of diabetes and chronic illness in general. Added variables at each stage are shown in bold italics, and new feedback loops added are shown as thick lines.
As shown in Fig. 2, perceived QOL is going to be affected not only by clinical health status, but also by the patient’s own standard for QOL, which reflects his/her past experience, or history [84, 93]. In this figure, we have included two primary socioeconomic factors—the ability to afford healthcare and the ability to work (i.e., earn a living)—that feed into a number of important abilities, such as the ability to live in safe and pleasant housing, and the ability to purchase health-enhancing food, such as fresh produce and whole-grain bread.
Fig. 2.
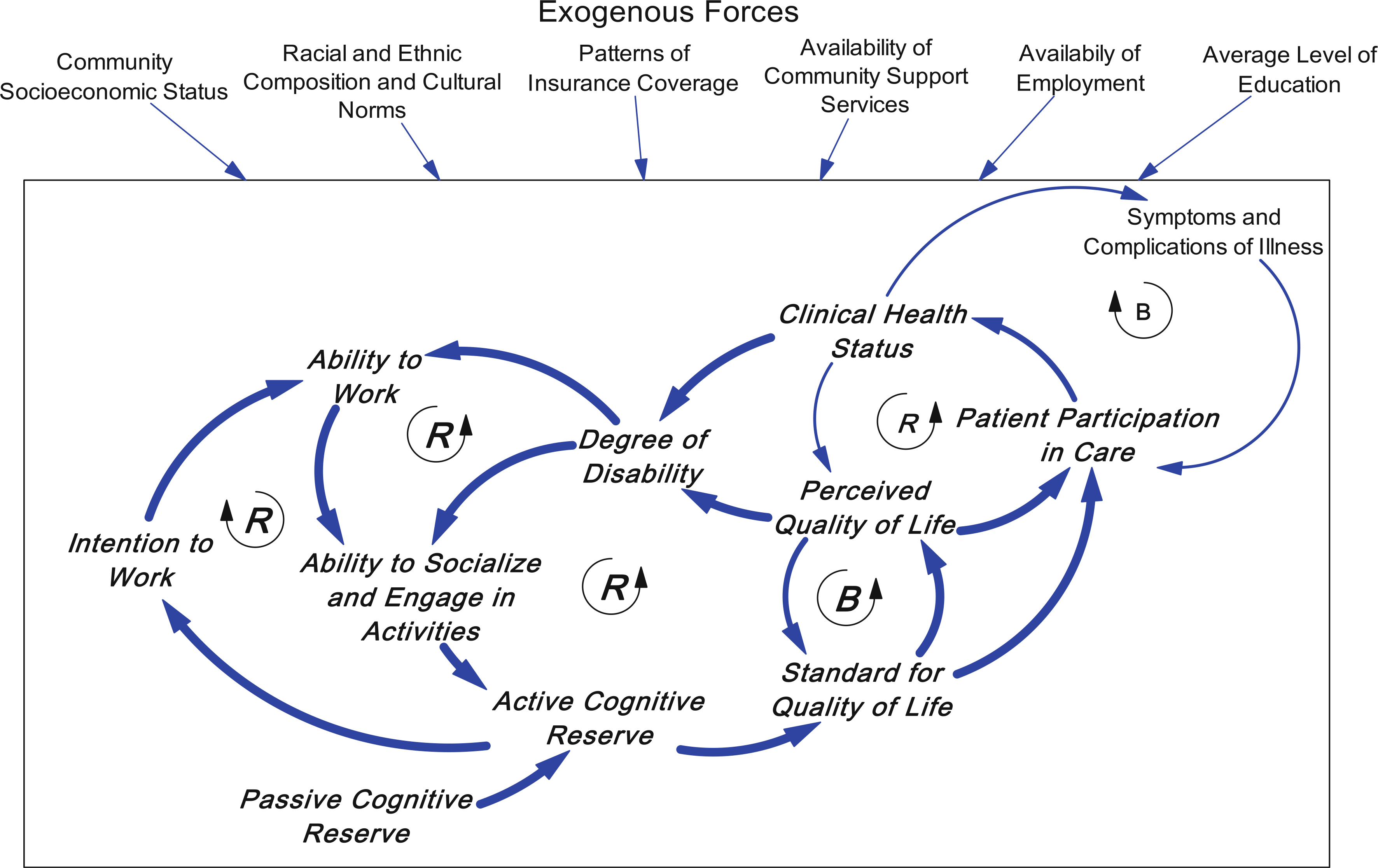
Exogenous variables are shown impinging on the box, together with other community characteristics, and constitute the environment in which individual behavior occurs. Exogenous variables can affect individual behavior (e.g., average level of education or availability of employment can affect an individual’s ability to work); but are only minimally affected by changes in any one individual’s status. Two new reinforcing loops show that not being able to work or engage in social activities, reduce one’s active cognitive reserve and lower one’s standard for QOL, which leads to deterioration in one’s perceived QOL. The degree of disability is affected both by one’s clinical health status and also by one’s perceived QOL, which can be disabling as well. There is also a balancing loop in which the patient’s standard for quality of life can help to stabilize the perceived QOL. An additional reinforcing loop through intention to work increases the likelihood that someone will strive to overcome their disability and work, thereby contributing to active cognitive reserve and further strengthening the intention to work
There are a number of important exogenous variables that create the environment in which a person is dealing with the disease, for example, childhood or initial socioeconomic status, marital/family status versus being on one’s own, ethnicity, cultural norms, country of origin, gender. These exogenous variables set the stage for how endogenous factors (e.g., individual and societal characteristics) interact, but are not dynamic themselves. In the interest of reducing the un-readability of the causal loop diagrams, we restricted the number of exogenous variables described and show them drawn with a boundary around them coming from outside the causal loop diagram. The diagrams are meant to focus on changes that occur in an individual’s life (endogenous to our model), and which interact to influence disease trajectories.
The concept of cognitive reserve [96] is very helpful for understanding how patients internalize that history. Cognitive reserve [96] is the concept that the brain can compensate for progressive injury by shifting function from areas of injury to other areas through the neuronal network. Both cognitive and physical activities appear to increase the interconnectedness of the neuronal network allowing for more compensation and later onset of disease progression [97–100]. Cognitive reserve theory [96] posits two components: passive and active. Whereas passive reserve reflects past and premorbid indicators of brain reserve (e.g., intelligence quotient, educational and occupational attainment, and childhood enrichment activities), active reserve encompasses activity concurrent with the comorbidity (e.g., stimulating leisure and cultural activities, and exercise).
Cognitive reserve is a concept that brings both history and current activities into focus. Active cognitive reserve has been found to have important relationships with patient-reported outcomes [101], symptom experience [102], and ways of thinking about QOL [103]. It has also been found to relate to patients’ ways of interacting with those around them [104]. These relationships form an additional set of feedback loops. Figure 2 introduces a balancing loop in which the standard for QOL helps to stabilize perceived QOL and resist deterioration due to consequences of diabetes. Figure 2 also shows two new reinforcing loops. To the extent that people are not able to work or engage in social activities, their active cognitive reserve is diminished and subsequently their standard for QOL is lowered. This leads to deterioration in their perceived QOL [87, 105]. The degree of diabetes disability is affected both by their clinical health status and also by their perceived QOL, which disables them as well [106]. Conversely, these reinforcing loops show that, to the extent that they can increase their lifestyle activities to include more active cognitive reserve, over time they would be able to reduce the rate of their diabetes-related distress, deterioration, and disability.
Due to the American arrangement of employment-based health insurance, one’s ability to work affects the ability to afford health care. This relationship adds another reinforcing loop because if one cannot work, one may not afford health care and thus may not get health care or have access to higher quality care. This loop can lead to a downward trajectory of clinical health status (Fig. 3). It is plausible that people with higher active cognitive reserve engage in more focused efforts to push themselves to stay employed and thus enhance or maintain their employability [107] despite symptom burden or clinical health status.
Fig. 3.

A reinforcing loop showing that if one cannot work, one cannot afford and access health care, clinical health status will worsen and lead to greater disability
In addition to participation in medical care, there are lifestyle factors that are relevant to patient participation. Specifically, Fig. 4 shows a balancing loop created by developing habits that follow recommended dietary and exercise guidelines. This loop shows an important component of adherence [108]. For example, diabetes patients who adopt a low-fat, low-sugar diet are better able to maintain a healthy hemoglobin A1c level [106, 109]. Similarly, engaging in regular physical activity has clear benefits for blood glucose as well as metabolism function [110]. These lifestyle factors are key components to patient participation [111] and flow directly from perceived QOL and standard for QOL. If there is a gap between one’s perceived QOL and one’s standard for QOL, then one is more likely to seek out and adhere to lifestyle changes that improve one’s clinical health status as well as feeding back to affect perceived QOL. Similarly, one’s standard for QOL is determined by both passive reserve and active cognitive reserve. There is an activation process: If one maintains a strong standard for QOL, one is creating a gap that motivates or activates lifestyle changes that support better clinical health status.
Fig. 4.
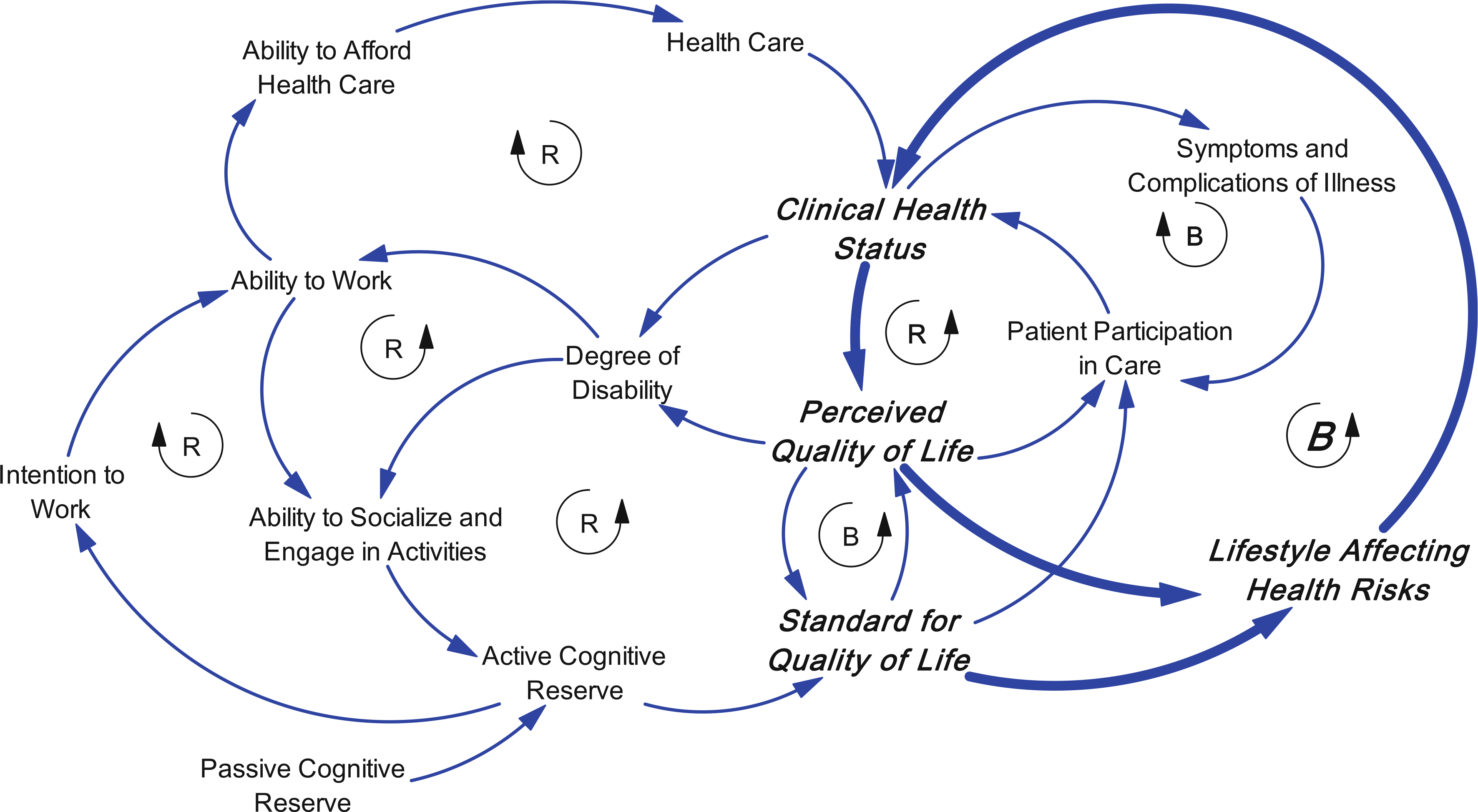
A balancing loop showing that lifestyle factors are key components to patient participation, and flow directly from perceived QOL and standard for QOL. There is an activation process: If one maintains a strong standard for QOL, one is creating a gap that motivates or activates lifestyle changes that support better clinical health status
Three other causal loops add to the dynamic complexity (Fig. 5). Emotional state and depression are affected by the ability to socialize and engage in activities [80, 84], and can directly affect perceived QOL [89]. Further they can affect caregiver relationship and support, which affects patient participation in care as well as lifestyle [112]. Lifestyle in turn affects health risks and clinical health status [109]. These are reinforcing loops in which declining clinical health status can be accelerated by emotional reactions and depression, which have a direct effect on patient participation in care as well as an indirect effect through the loss of caregiver support as caregivers become alienated by the patient’s emotional reactions to his or her illness.
Fig. 5.
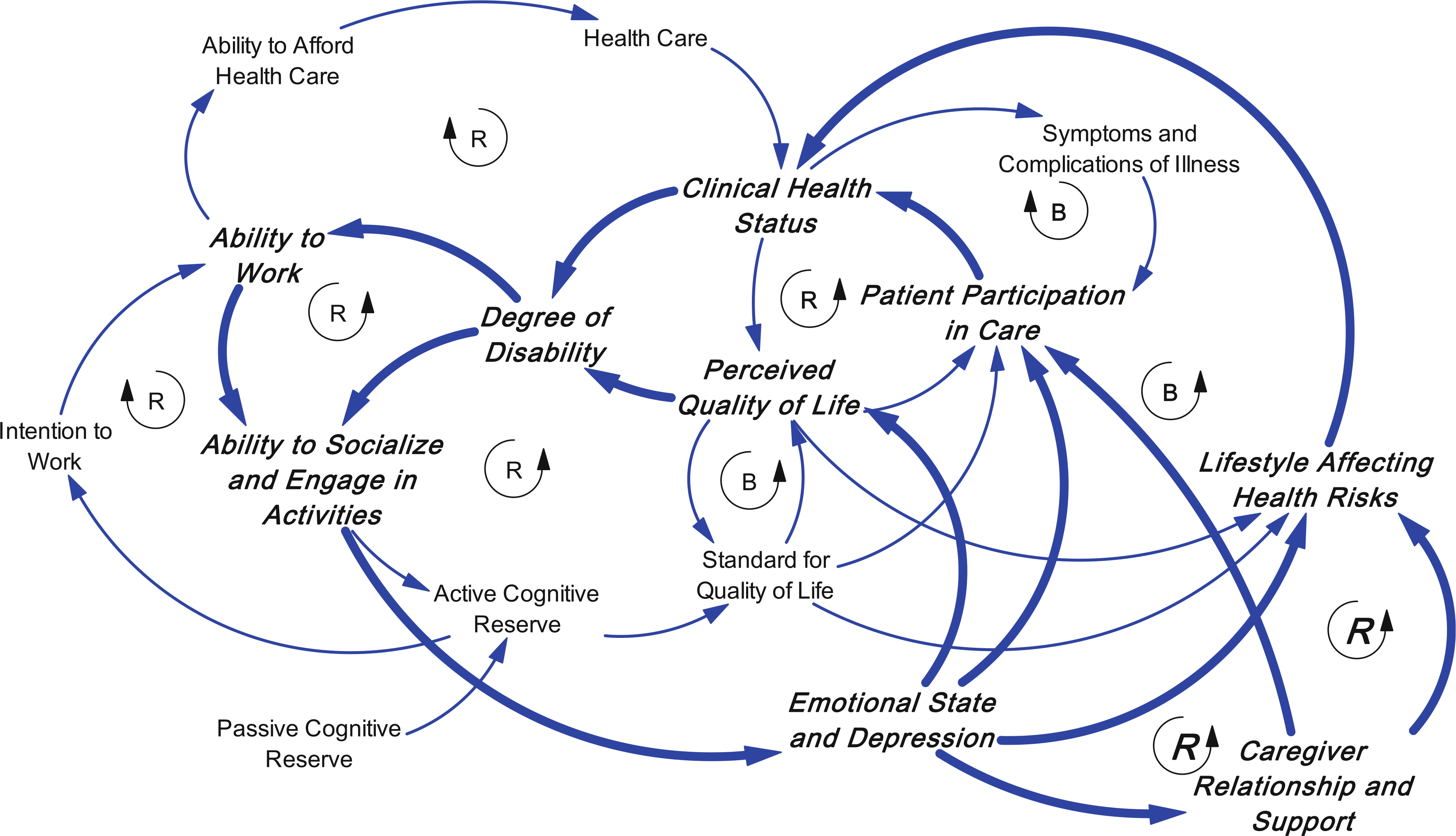
Three other causal loops are added: Emotional state and depression are affected by the ability to socialize and engage in activities and can directly affect perceived QOL. Further they can affect caregiver relationship and support, which affects patient participation in care as well as lifestyle
Dynamic and policy implications of the causal structure
Disease and QOL trajectories
What does this causal structure imply for different trajectories that might be experienced by patients with diabetes? One possibility that is unfortunately seen in many patients is a poor trajectory in which these reinforcing loops amplify the effects of declines in clinical health status and cause gradual deterioration over time where the disease process affects the patients’ QOL, reduces their social participation and employment, and leads to poor participation in care and further progression of the disease. A variation in this trajectory is a stair-step series of temporary improvements stimulated by the balancing loop reacting to symptoms and complications, but with a long-term trend downward. These downward trajectories may be partially the result of limited cognitive reserve and less stable standards for QOL that ultimately result in weak reactions in terms of patient participation in care and having a lifestyle that affects health risk. Reinforcing loops through work and social activities, emotional state, and caregiver relationships can accelerate the downward trend and make it difficult to recover to better states of health and QOL.
The alternative, more desirable, trajectory is a stable one that avoids this sort of deterioration and results in a more stable patient who has a better outcome. Why do some patients do better? It starts with a strong standard for QOL based on cognitive reserve that activates balancing loops and works against the deterioration process. Adaptations activated by this balancing loop lead to stable changes in lifestyle which improve patient participation in care and clinical health status. These beneficial changes then engage the reinforcing loops in a positive direction so that they are supporting improvement rather than accelerating deterioration. As indicated earlier, reinforcing loops can work in an upward as well as downward direction. The result will be more stable clinical health status and perceived QOL and resiliency that provides for more rapid recoveries from clinical setbacks.
Policy implications
Looking more broadly at the aspects of QOL that interact with the disease process and clinical care has added value [113]. It also suggests additional leverage points for affecting the progression of a chronic illness such as diabetes that would not be apparent from a narrower clinical perspective. Many years of experience with system dynamics indicates that policy leverage comes from strengthening balancing loops that maintain stability in systems, and reducing the strength of reinforcing loops that can produce vicious cycles. For example, employment counseling that helps a person maintain their ability to work in the face of disease has an impact on a number of variables that influence disease trajectory (e.g., ability to afford health care, preventing further disability, and enhancing participation in medical care). In doing so, it weakens the reinforcing loops that would otherwise isolate the patient, reduce their access to health care, and lessen their participation in their care. Although the Affordable Care Act may reduce the impact of this reinforcing loop, it is still likely to be a problem, especially in states that do not fully participate in the Act’s implementation (e.g., will not expand Medicaid programs to accommodate lower- income working people).
Similarly paying attention to patient’s emotional state and their relationship to caregivers would lead to making counseling available to deal with those aspects of QOL that affect patients’ engagement in care. If they are not getting caregiver support because they have alienated their caregivers due to their own emotional problems, patients will be less likely to participate in their own care. Counseling focused on these emotional and family issues will thereby weaken loops in which emotional distress and deteriorating caregiver relationships contribute to a downward trajectory. Providing meaningful support for lifestyle changes can strengthen the balancing loop that produces improvements in lifestyle and clinical outcomes, and leads to a more stable trajectory.
Next steps
The relationships in the causal diagrams can be further validated and expanded upon to include other factors, and can be quantified to become the basis for a system dynamics simulation model. Such a model can be used to more rigorously investigate different trajectories and effects of policies and programs on those trajectories and help to design comprehensive programs for managing diabetes and other chronic illnesses. The programs would benefit from a perspective that goes beyond clinical factors and integrates other aspects of a patient’s life in a strategy for dealing effectively with both the disease and the quality of the patient’s life. These simulation models can also become learning environments that practitioners in the field can use to develop their own sense of more effective ways of managing the care of patients [113].
Using system dynamics modeling facilitates understanding about not only how things change, but also why things change, as time-dependent interdependencies between variables are explicitly represented in the structure of the model. Such analyses are critical for identifying robust program strategies and policies that work in the real world. System dynamics simulation modeling provides a novel approach for evaluating how implementation and outcome variables are related to contextual factors that impact the functioning for patients, their caregivers, clinical settings (e.g., primary care facilities and hospitals), as well as health care institutions that govern access to and quality of health services.
As a first step, balancing and reinforcing causal loops are an effective method to elucidate the connections, or “feedback structures,” among factors that influence a given problem. After iterative testing and refinement, a simulation model becomes a useful tool to guide strategic planning and policy implementation. Stakeholders at every level can and should play a substantive role in helping to validate the utility of the tool, by bringing their tacit knowledge of the problem at hand as well as by facilitating access to sources of evidence or data that can inform key assumptions and reliable estimates of important model parameters.
In the case of diabetes, expansive bodies of useful empirical research and treatment guidelines have already been developed. Some prior efforts have also been created using simulation models to identify and assess policies to curb the burden of chronic illnesses like diabetes. The future simulation modeling proposed here should build upon both of these existing resources, focusing on ways to promote individual (e.g., patient, caregiver, clinician) as well as organizational learning (e.g., primary care facility, hospital, payor) by studying how delays in processes or procedures contribute to performance barriers [19, 21, 114]. For example, time delays in a diabetes patients’ taking action to build active cognitive reserve, or in a health care system’s development of capacity to promote self-care for patients, may impede effective learning. System dynamics simulations of these delays, ubiquitous in real-world settings, have been found to be relevant to understanding how decisions were made between long-term and short-term organizational objectives [115].
Summary
System science approaches can raise awareness of important factors that influence health and QOL and the relationships between them. By taking this broader view, it identifies different leverage points that could affect diabetes trajectories that are not as immediately apparent when thinking in narrower clinical terms. This thinking thus leads to policy levers for improving the lives of people with diabetes and positively influencing the course of their disease. For QOL researchers, this thinking could lead to more contextualized research, characterized not only by more comprehensive analytic models but also by the inclusion of measures that tap the psychosocial and behavioral factors that influence health outcomes.
Acknowledgments
We gratefully acknowledge Joel Zonszein, M.D., and Judith Wylie-Rosett, Ed.D., for helpful discussions during the writing of this manuscript, and Brian Quaranto for assistance with manuscript preparation.
Contributor Information
David W. Lounsbury, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA
Gary B. Hirsch, Creator of Learning Environments, Wayland, MA, USA
Chawntel Vega, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Carolyn E. Schwartz, DeltaQuest Foundation, Inc., 31 Mitchell Road, Concord, MA 01742, USA Departments of Medicine and Orthopaedic Surgery, Tufts University School of Medicine, Boston, MA, USA.
References
- 1.Ahmed S, et al. (2012). The use of patient-reported outcomes (PRO) within comparative effectiveness research: Implications for clinical practice and health care policy. Medical Care, 50(12), 1060–1070. [DOI] [PubMed] [Google Scholar]
- 2.Fleming BB, et al. (2001). The Diabetes Quality Improvement Project: Moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care, 24(10), 1815–1820. [DOI] [PubMed] [Google Scholar]
- 3.Velikova G, et al. (2004). Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 22(4), 714–724. [DOI] [PubMed] [Google Scholar]
- 4.FDA, Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims, F.a.D. Administration, Editor. 2009, U.S. Dept. of Health and Human Services: Washington, D.C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner EH, et al. (2001). Improving chronic illness care: Translating evidence into action. Health Affairs, 20(6), 64–78. [DOI] [PubMed] [Google Scholar]
- 6.Wagner EH, et al. (1999). A survey of leading chronic disease management programs: are they consistent with the literature? Managed Care Quarterly, 7(3), 56–66. [PubMed] [Google Scholar]
- 7.Stellefson M, Dipnarine K, & Stopka C (2013). The chronic care model and diabetes management in US primary care settings: A systematic review. Preventing Chronic Disease, 10, 120180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen I HHS Secretary calls on corporate America and government to help fight obesity. Center for the Advancement of Health. [Google Scholar]
- 9.Huang TT, et al. (2009). A systems-oriented multilevel framework for addressing obesity in the 21st century. Preventing Chronic Disease: Public Health Research, Practice, and Policy, 6(3), 1–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Milstein B, Homer J, & Hirsch GB (2010). Analyzing national health reform strategies with a dynamic simulation model. American Journal of Public Health, 100(5), 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roux AVD (2011). Complex systems thinking and current impasses in health disparities research. American Journal of Public Health, 101(9), 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalf SS, Northridge ME, & Lamster IB (2011). A systems perspective for dental health in older adults. American Journal of Public Health, 101(10), 1820–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luke DA, & Stamatakis KA (2012). Systems science methods in public health: Dynamics, networks, and agents. Annual Review of Public Health, 33, 357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mabry PL, et al. (2008). Interdisciplinarity and systems science to improve population health: A view from the NIH office of behavioral and social sciences research. American Journal of Preventive Medicine, 35(S2), S211–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch GB, Levine R, & Miller RL (2007). Using system dynamics modeling to understand the impact of social change initiatives. American Journal of Community Psychology, 39(3–4), 239–253. [DOI] [PubMed] [Google Scholar]
- 16.Homer J, et al. (2004). Models for collaboration: How system dynamics helped a community organize cost-effective care for chronic illness. System Dynamics Review, 20(3), 199–222. [Google Scholar]
- 17.Forrester JW (1987). Nonlinearity in high-order models of social systems. European Journal of Operational Research, 30(2), 104–109. [Google Scholar]
- 18.Maani KE, & Cavana RY (2000). Systems thinking modeling: Understanding change and complexity. Auckland: Pearson Education New Zealand Limited. [Google Scholar]
- 19.Sterman J (1994). Learning in and about complex systems. System Dynamics Review, 10(2–3), 291–330. [Google Scholar]
- 20.Forrester JW (1987). The model versus a modeling process. System Dynamics Review, 1(1), 133–134. [Google Scholar]
- 21.Repenning NA (2002). A simulation-based approach to understanding the dynamics of innovation implementation. Organization Science, 13, 109–127. [Google Scholar]
- 22.Richardson GP (1991). Feedback thought in social science and systems theory. Waltham: Pegasus Communications, Inc. [Google Scholar]
- 23.Richardson GP, & Pugh AL I. I. I. (1981). Introduction to system dynamics modeling. Portland: Productivity Press. [Google Scholar]
- 24.Homer JB, & Hirsch G (2006). System dynamics modeling for public health: Background and opportunities. American Journal of Public Health, 96(3), 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arboleda CA, Abraham DM, & Lubitz R (2007). Simulation as a tool to assess the vulnerability of the operation of a health care facility. Journal of Performance of Constructed Facilities, 21(4), 302–312. [Google Scholar]
- 26.Cavana RY, et al. (1999). Drivers of quality in health services: Different worldviews of clinicians and policy managers revealed. System Dynamics Review, 15(3), 331–340. [Google Scholar]
- 27.Hirsch G, & Immediato CS (1999). Microworlds and generic structures as resources for integrating care and improving health. System Dynamics Review, 15(3), 315–330. [Google Scholar]
- 28.Hirsch G, & Miller S (1974). Evaluating HMO Policies with a Computer Simulation Model. Medical Care, 12(8), 668–681. [DOI] [PubMed] [Google Scholar]
- 29.Hovmand PS, & Gillespie DF (2010). Implementation of evidence-based practice and organizational performance. Journal of Behavioral Health Services and Research, 37(1), 79–94. [DOI] [PubMed] [Google Scholar]
- 30.Royston G, et al. (1999). Using system dynamics to help develop and implement policies and programmes in health care in England. System Dynamics Review, 15(3), 293–313. [Google Scholar]
- 31.Wolstenholme E, et al. (2007). Coping but not coping in health and social care: Masking the reality of running organisations beyond safe design capacity. System Dynamics Review, 23(4), 371–389. [Google Scholar]
- 32.Gonzalez-Busto B, & Garcia R (1999). Waiting lists in Spanish public hospitals: A system dynamics approach. System Dynamics Review, 15(3), 201–224. [Google Scholar]
- 33.Lane DC, & Husemann E (2008). System dynamics mapping of acute patient flows. Journal of the Operational Research Society, 59(2), 213–224. [Google Scholar]
- 34.Fernandez MIT, Vasquez OC, & Martinic J (2010). Computer modeling and simulation of the patient-visit network within a Chilean public health service. Revista Panamericana De Salud Publica-Pan American Journal of Public Health., 27(3), 203–210. [DOI] [PubMed] [Google Scholar]
- 35.van Ackere A, & Smith PC (1999). Towards a macro model of national health service waiting lists. System Dynamics Review, 15(3), 225–252. [Google Scholar]
- 36.Vanderby S, & Carter MW (2010). An evaluation of the applicability of system dynamics to patient flow modelling. Journal of the Operational Research Society, 61(11), 1572–1581. [Google Scholar]
- 37.Wolstenholme E (1999). A patient flow perspective of UK Health Services: Exploring the case for new “intermediate care” initiatives. System Dynamics Review, 15(3), 253–271. [Google Scholar]
- 38.Bliss JR, Gillespie DF, & Gongaware NK (2010). Dynamics of caseworker turnover and clinical knowledge. Administration in Social Work, 34(1), 4–26. [Google Scholar]
- 39.McGregor M (2010). A system dynamics approach to jurisdictional conflict between a major and a minor healthcare profession. Systems Research and Behavioral Science, 27(6), 639–652. [Google Scholar]
- 40.Vanderby SA, et al. (2010). Modeling the cardiac surgery workforce in Canada. Annals of Thoracic Surgery, 90(2), 467–473. [DOI] [PubMed] [Google Scholar]
- 41.Braithwaite J, et al. (2009). The development, design, testing, refinement, simulation and application of an evaluation framework for communities of practice and social-professional networks. BMC Health Services Research, 9(1), 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elf M, Poutilova M, & Ohrn K (2007). A dynamic conceptual model of care planning. Scandinavian Journal of Caring Sciences, 21(4), 530–538. [DOI] [PubMed] [Google Scholar]
- 43.Taylor K, & Dangerfield B (2005). Modelling the feedback effects of reconfiguring health services. Journal of the Operational Research Society, 56(6), 659–675. [Google Scholar]
- 44.Homer J, et al. (2004). Models for collaboration: How system dynamics helped a community organize cost-effective care for chronic illness. System Dynamics Review, 20(3), 199–222. [Google Scholar]
- 45.Brailsford SC, et al. (2004). Emergency and on-demand health care: Modelling a large complex system. Journal of the Operational Research Society, 55(1), 34–42. [Google Scholar]
- 46.Lane DC, Monefeldt C, & Rosenhead JV (2000). Looking in the wrong place for healthcare improvements: A system dynamics study of an accident and emergency department. Journal of the Operational Research Society, 51(5), 518–531. [Google Scholar]
- 47.Storrow AB, et al. (2008). Decreasing lab turnaround time improves emergency department throughput and decreases emergency medical services diversion: A Simulation Model. Academic Emergency Medicine, 15(11), 1130–1135. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Hamid TK (2002). Modeling the dynamics of human energy regulation and its implications for obesity treatment. System Dynamics Review, 18(4), 431–471. [Google Scholar]
- 49.Karanfil O, & Barlas Y (2008). A dynamic simulator for the management of disorders of the body water homeostasis. Operations Research, 56(6), 1474–1492. [Google Scholar]
- 50.Liu H, & Shi P (2009). Maximum a posteriori strategy for the simultaneous motion and material property estimation of the heart. IEEE Transactions on Biomedical Engineering, 56(2), 378–389. [DOI] [PubMed] [Google Scholar]
- 51.Dangerfield BC, Fang YX, & Roberts CA (2001). Model-based scenarios for the epidemiology of HIV/AIDS: The consequences of highly active antiretroviral therapy. System Dynamics Review, 17(2), 119–150. [Google Scholar]
- 52.Flessa S (2003). Decision support for AIDS control programmes in eastern Africa. OR Spectrum, 25(2), 265–291. [Google Scholar]
- 53.Lebcir RM, Atun RA, & Coker RJ (2010). System dynamic simulation of treatment policies to address colliding epidemics of tuberculosis, drug resistant tuberculosis and injecting drug users driven HIV in Russia. Journal of the Operational Research Society, 61(8), 1238–1248. [Google Scholar]
- 54.Roberts C, & Dangerfield B (1990). Modeling the epidemiologic consequences of HIV-infection and aids—a contribution from operational-research. Journal of the Operational Research Society, 41(4), 273–289. [Google Scholar]
- 55.Roberts EB, et al. (1982). A systems view of the smoking problem: Perspective and limitations of the role of science in decision-making. International Journal of Bio-Medical Computing, 13(1), 69–86. [DOI] [PubMed] [Google Scholar]
- 56.Chick SE, Mamani H, & Simchi-Levi D (2008). Supply chain coordination and influenza vaccination. Operations Research, 56(6), 1493–1506. [Google Scholar]
- 57.Homer J, et al. (2000). Toward a dynamic theory of antibiotic resistance. System Dynamics Review, 16(4), 287–319. [Google Scholar]
- 58.Thompson KM, & Tebbens RJD (2008). Using system dynamics to develop policies that matter: Global management of poliomyelitis and beyond. System Dynamics Review, 24(4), 433–449. [Google Scholar]
- 59.Ahmad S (2005). The cost-effectiveness of raising the legal smoking age in California. Medical Decision Making, 25(3), 330–340. [DOI] [PubMed] [Google Scholar]
- 60.Ahmad S (2005). Closing the youth access gap: The projected health benefits and cost savings of a national policy to raise the legal smoking age to 21 in the United States. Health Policy, 75(1), 74–84. [DOI] [PubMed] [Google Scholar]
- 61.Cavana RY, & Clifford LV (2006). Demonstrating the utility of system dynamics for public policy analysis in New Zealand: the case of excise tax policy on tobacco. System Dynamics Review, 22(4), 321–348. [Google Scholar]
- 62.Tengs TO, Osgood ND, & Chen LL (2001). The cost- effectiveness of intensive national school-based anti-tobacco education: Results from the Tobacco Policy Model. Preventive Medicine, 33(6), 558–570. [DOI] [PubMed] [Google Scholar]
- 63.Homer JB (1993). A system dynamics model of national cocaine prevalence. System Dynamics Review, 9(1), 49–78. [Google Scholar]
- 64.Homer JB, & StClair CL (1991). A model of hiv transmission through needle sharing. Interfaces, 21(3), 26–49. [Google Scholar]
- 65.Smith PC, & van Ackere A (2002). A note on the integration of system dynamics and economic models. Journal of Economic Dynamics and Control, 26(1), 1–10. [Google Scholar]
- 66.Wakeland W, et al. (2011). System dynamics modeling as a potentially useful tool in analyzing mitigation strategies to reduce overdose deaths associated with pharmaceutical opioid treatment of chronic pain. Pain Medicine, 12, S49–S58. [DOI] [PubMed] [Google Scholar]
- 67.Huz S, et al. (1997). A framework for evaluating systems thinking interventions: An experimental approach to mental health system change. System Dynamics Review, 13(2), 149–169. [Google Scholar]
- 68.Smits M (2010). Impact of policy and process design on the performance of intake and treatment processes in mental health care: a system dynamics case study. Journal of the Operational Research Society, 61(10), 1437–1445. [Google Scholar]
- 69.Homer J, Hirsch G, & Milstein B (2007). Chronic illness in a complex health economy: The perils and promises of downstream and upstream reforms. System Dynamics Review, 23(2–3), 313–343. [Google Scholar]
- 70.Siegel CA, et al. (2011). Real-time tool to display the predicted disease course and treatment response for children with Crohn’s disease. Inflammatory Bowel Diseases, 17(1), 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wild S, et al. (2004). Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care, 27(5), 1047–1053. [DOI] [PubMed] [Google Scholar]
- 72.Shaw JE, Sicree RA, & Zimmet PZ (2010). Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice, 87(1), 4–14. [DOI] [PubMed] [Google Scholar]
- 73.Owen J, & Reisin E (2012). Non-communicable disease: A welcome and long needed addition to the WHO’s 2012 world heath statistics. Current Hypertension Reports, 14(6), 475–477. [DOI] [PubMed] [Google Scholar]
- 74.Prevention, C.f.D.C.a., National Diabetes Fact Sheet. 2011, Centers for Disease Control and Prevention: Atlanta, GA. [Google Scholar]
- 75.Rubin RR, & Peyrot M (1999). Quality of life and diabetes. Diabetes/Metabolism Research and Reviews, 15, 205. [DOI] [PubMed] [Google Scholar]
- 76.Kim M, Berger D, and Matte T, Diabetes in New York city: Public health burden and disparities, Hygiene D.o.H.a.M., Editor. 2006, New York City NY. [Google Scholar]
- 77.Glasgow R, et al. (1999). If diabetes is a public health problem, why not treat it as one? A population-based approach to chronic illness. Annals of Behavioral Medicine, 21(2), 159–170. [DOI] [PubMed] [Google Scholar]
- 78.Ayalon L, et al. (2008). Determinants of quality of life in primary care patients with diabetes: Implications for social workers. Health and Social Work, 33(3), 229–236. [DOI] [PubMed] [Google Scholar]
- 79.Hinder S, Greenhalgh T (2012). “This does my head in”. Ethnographic study of self-management by people with diabetes. BMC Health Services Research. 12(Journal Article), 83–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Primozic S, et al. (2012). Specific cognitive abilities are associated with diabetes self-management behavior among patients with type 2 diabetes. Diabetes Research and Clinical Practice, 95(1), 48–54. [DOI] [PubMed] [Google Scholar]
- 81.Brod M (1998). Pilot study—quality of life issues in patients with diabetes and lower extremity ulcers: Patients and care givers. Quality of Life Research, 7(4), 365–375. [DOI] [PubMed] [Google Scholar]
- 82.Chiu C, & Wray LA (2011). Healthy lifestyle and psychological well-being buffer diabetes-related cognitive decline: Findings from the taiwan longitudinal study on aging. Gerontologist, 51, 518. [Google Scholar]
- 83.Hernandez-Tejada MA, et al. (2012). Effect of perceived control on quality of life in indigent adults with type 2 diabetes. The Diabetes Educator, 38(2), 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wandell PE (2005). Quality of life of patients with diabetes mellitus. Scandinavian Journal of Primary Health Care, 23(2), 68–74. [DOI] [PubMed] [Google Scholar]
- 85.Bower P, et al. (2012). A cluster randomised controlled trial of the clinical and cost-effectiveness of a ‘whole systems’ model of self-management support for the management of long- term conditions in primary care: trial protocol. Implementation Science, 7(7), 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dickman K, et al. (2012). Behavior changes in patients with diabetes and hypertension after experiencing shared medical appointments. Journal of the American Academy of Nurse Practitioners, 24(1), 43–51. [DOI] [PubMed] [Google Scholar]
- 87.Gagliardino JJ, et al. (2012). Patients’ education, and its impact on care outcomes, resource consumption and working conditions: Data from the International Diabetes Management Practices Study (IDMPS). Diabetes and Metabolism, 38(2), 128–134. [DOI] [PubMed] [Google Scholar]
- 88.Hecht L (2012). Informed shared decision making. Where are we in diabetology? Diabetologe, 8(3), 222. [Google Scholar]
- 89.Jonkers CCM, et al. (2012). The effectiveness of a minimal psychological intervention on self-management beliefs and behaviors in depressed chronically ill elderly persons: A randomized trial. International Psychogeriatrics, 24(2), 288–297. [DOI] [PubMed] [Google Scholar]
- 90.Feinglass J, et al. (2012). How ‘preventable’ are lower extremity amputations? A qualitative study of patient perceptions of precipitating factors. Disability and Rehabilitation, 34(25), 2158–2165. [DOI] [PubMed] [Google Scholar]
- 91.Fransen MP, von Wagner C, & Essink-Bot ML (2012). Diabetes self-management in patients with low health literacy: Ordering findings from literature in a health literacy framework. Patient Education and Counseling, 88(1), 44–53. [DOI] [PubMed] [Google Scholar]
- 92.Richardson G (1986). Problems with causal loop diagrams. System Dynamics Review, 2(2), 158–170. [Google Scholar]
- 93.Hernandez-Tejada MA, et al. (2012). Effect of perceived control on quality of life in indigent adults with type 2 diabetes. The Diabetes Educator, 38(2), 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rubin RR, Peyrot M Quality of life and diabetes. Diabetes/ Metabolism Research and Reviews. 15(Journal Article), 205. [DOI] [PubMed] [Google Scholar]
- 95.Jelsness-Jorgensen L-P, et al. (2011). Measuring health-related quality of life in non-complicated diabetes patients may be an effective parameter to assess patients at risk of a more serious disease course: a cross-sectional study of two diabetes outpatient groups. Journal of Clinical Nursing. 20(Journal Article), 1255. [DOI] [PubMed] [Google Scholar]
- 96.Stern Y, ed. Cognitive Reserve: Theory and Applications. 2007, Taylor and Francis: New York. [Google Scholar]
- 97.Stern Y (2009). Cognitive Reserve. Neuropsychologia, 47, 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Penner I-K, et al. (2006). Therapy-induced plasticity of cognitive functions in MS patients: Insights from fMRI. Journal of Physiology—Paris, 99, 455–462. [DOI] [PubMed] [Google Scholar]
- 99.Perneczky R, et al. (2008). Activities of daily living, cerebral glucose metabolism, and cognitive reserve in Lewy body and Parkinson’s disease. Dementia and Geriatric Cognitive Disorders, 26(5), 475–481. [DOI] [PubMed] [Google Scholar]
- 100.Nithianantharajah J, & Hannan AJ (2009). The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Progress in Neurobiology, 89(4), 369–382. [DOI] [PubMed] [Google Scholar]
- 101.Schwartz CE, et al. (2013). Cognitive reserve and patient- reported outcomes. MS Journal, 19(1), 87–105. [DOI] [PubMed] [Google Scholar]
- 102.Schwartz CE, et al. (2013). Cognitive reserve and symptom experience in multiple sclerosis: A buffer to disability progression over time? Archives of Physical Medicine and Rehabilitation (in press). [DOI] [PubMed] [Google Scholar]
- 103.Schwartz CE, et al. (2013). Cognitive reserve and appraisal in multiple sclerosis. Multiple Sclerosis and Related Disorders, 2, 36–44. [DOI] [PubMed] [Google Scholar]
- 104.Schwartz CE, et al. (2013). Altruism and health outcomes in multiple sclerosis: The effect of cognitive reserve. Journal of Positive Psychology, 8(2), 144–152. [Google Scholar]
- 105.Gagliardino JJ, et al. (2000). Evaluation and cost of diabetes care. Medicina-Buenos Aires, 60(6), 880–888. [PubMed] [Google Scholar]
- 106.Venkatesh S, & Weatherspoon L (2013). Social and health care provider support in diabetes self-management. American Journal of Health Behavior, 37(1), 112–121. [DOI] [PubMed] [Google Scholar]
- 107.McFadden E, et al. (2012). Screening for the risk of job loss in multiple sclerosis (MS): Development of an MS-specific Work Instability Scale (MS-WIS). Multiple Sclerosis, 18, 862–870. [DOI] [PubMed] [Google Scholar]
- 108.IOM. (2012). Living well with chronic illness: A call for public health action. Washington, DC: National Academy of Science Press. [Google Scholar]
- 109.Zagarins SE, et al. (2012). Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in depressive symptoms. Journal of Behavioral Medicine, 35(3), 299–304. [DOI] [PubMed] [Google Scholar]
- 110.Jacobs-Van DB, et al. (2007). Lifestyle interventions are cost- effective in people with different levels of diabetes risk. Diabetes Care, 30(1), 128–134. [DOI] [PubMed] [Google Scholar]
- 111.van der Wulp I, et al. (2012). Effectiveness of peer-led self-management coaching for patients recently diagnosed with Type 2 diabetes mellitus in primary care: A randomized controlled trial. Diabetic Medicine, 29(10), e390–e397. [DOI] [PubMed] [Google Scholar]
- 112.Osborn CY, & Egede LE (2012). The relationship between depressive symptoms and medication nonadherence in type 2 diabetes: The role of social support. General Hospital Psychiatry, 34(3), 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Homer JB, & Hirsch GB (2006). System dynamics modeling for public health: Background and opportunities. American Journal of Public Health, 96(3), 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Forrester JW (1987). Lessons from system dynamics modelling. System Dynamics Review, 3, 136–149. [Google Scholar]
- 115.Rahmandad H, Repenning N, & Sterman J (2009). Effects of feedback delay on learning. System Dynamics Review, 25, 309–338. [Google Scholar]


