Abstract
A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines
Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Geriatrics Society, the American Society of Preventive Cardiology, and the Preventive Cardiovascular Nurses Association
Keywords: ACC/AHA Clinical Practice Guidelines, guidelines, antihypertensive agents, aspirin, atherosclerosis, atherosclerotic cardiovascular disease, atrial fibrillation, behavior modification, behavior therapy, blood cholesterol, blood pressure, body mass index, cardiovascular team-based care, cardiovascular, cardiovascular disease, cholesterol, chronic kidney disease, coronary artery calcium score, coronary disease, coronary heart disease, cost, diet, dietary patterns, dietary fats, dietary sodium, dyslipidemia, e-cigarettes, exercise, healthcare disparities, health services accessibility, heart failure, hypertension, LDL-cholesterol, diabetes mellitus, lifestyle, lipids, measurement, myocardial infarction, nicotine, nonpharmacological treatment, nutrition, physical activity, prejudice, primary prevention, psychosocial deprivation, public health, quality indicators, quality measurement, risk assessment, risk-enhancing factors, risk factors, risk reduction, risk reduction discussion, risk treatment discussion, secondhand smoke, sleep, smoking, smoking cessation, social determinants of health, socioeconomic factors, statin therapy, systems of care, tobacco, tobacco smoke pollution, treatment adherence, treatment outcomes, type 2 diabetes mellitus, waist circumference, weight loss
TOP 10 TAKE-HOME MESSAGES FOR THE PRIMARY PREVENTION OF CARDIOVASCULAR DISEASE
The most important way to prevent atherosclerotic vascular disease, heart failure, and atrial fibrillation is to promote a healthy lifestyle throughout life.
A team-based care approach is an effective strategy for the prevention of cardiovascular disease. Clinicians should evaluate the social determinants of health that affect individuals to inform treatment decisions.
Adults who are 40 to 75 years of age and are being evaluated for cardiovascular disease prevention should undergo 10-year atherosclerotic cardiovascular disease (ASCVD) risk estimation and have a clinician–patient risk discussion before starting on pharmacological therapy, such as antihypertensive therapy, a statin, or aspirin. The presence or absence of additional risk-enhancing factors can help guide decisions about preventive interventions in select individuals, as can coronary artery calcium scanning.
All adults should consume a healthy diet that emphasizes the intake of vegetables, fruits, nuts, whole grains, lean vegetable or animal protein, and fish and minimizes the intake of trans fats, red meat and processed red meats, refined carbohydrates, and sweetened beverages. For adults with overweight and obesity, counseling and caloric restriction are recommended for achieving and maintaining weight loss.
Adults should engage in at least 150 minutes per weekof accumulated moderate-intensity physical activity or 75 minutes per week of vigorous-intensity physical activity.
For adults with type 2 diabetes mellitus, lifestylechanges, such as improving dietary habits and achieving exercise recommendations are crucial. If medication is indicated, metformin is first-line therapy, followed by consideration of a sodium-glucose cotransporter 2 inhibitor or a glucagon-like peptide-1 receptor agonist.
All adults should be assessed at every healthcare visitfor tobacco use, and those who use tobacco should be assisted and strongly advised to quit.
Aspirin should be used infrequently in the routineprimary prevention of ASCVD because of lack of net benefit.
Statin therapy is first-line treatment for primary prevention of ASCVD in patients with elevated low-density lipoprotein cholesterol levels (≥190 mg/dL), those with diabetes mellitus, who are 40 to 75 years of age, and those determined to be at sufficient ASCVD risk after a clinician–patient risk discussion.
Nonpharmacological interventions are recommended for all adults with elevated blood pressure or hypertension. For those requiring pharmacological therapy, the target blood pressure should generally be <130/80 mm Hg.
PREAMBLE
Since 1980, the American College of Cardiology (ACC) and American Heart Association (AHA) have translated scientific evidence into clinical practice guidelines with recommendations to improve cardiovascular health. These guidelines, which are based on systematic methods to evaluate and classify evidence, provide a foundation for the delivery of quality cardiovascular care. The ACC and AHA sponsor the development and publication of clinical practice guidelines without commercial support, and members volunteer their time to the writing and review efforts.
Clinical practice guidelines provide recommendations applicable to patients with or at risk of developing cardiovascular disease (CVD). The focus is on medical practice in the United States, but these guidelines are relevant to patients throughout the world. Although guidelines may be used to inform regulatory or payer decisions, the goals are to improve quality of care and align with patients’ interests. Guidelines are intended to define practices meeting the needs of patients in most but not all circumstances and should not replace clinical judgment.
Recommendations for guideline-directed management and therapy, which encompasses clinical evaluation, diagnostic testing, and both pharmacological and procedural treatments, are effective only when adopted by both practitioners and patients. Adherence to recommendations can be enhanced by shared decision-making between clinicians and patients, with patient engagement in selecting interventions on the basis of individual values, preferences, and associated conditions and comorbidities.
The ACC/AHA Task Force on Clinical Practice Guidelines strives to ensure that the guideline writing committee includes requisite expertise and is representative of the broader medical community by selecting experts from a broad array of backgrounds, representing different geographic regions, sexes, races, ethnicities, intellectual perspectives/biases, and scopes of clinical practice. The ACC and AHA have rigorous policies and methods to ensure that documents are developed without bias or improper influence. The complete policy on relationships with industry and other entities (RWI) can be found online.
Beginning in 2017, numerous modifications to the guidelines have been and continue to be implemented to make guidelines shorter and enhance “user friendliness.” Guidelines are written and presented in a modular knowledge chunk format, in which each chunk includes a table of recommendations, a brief synopsis, recommendation-specific supportive text and, when appropriate, flow diagrams or additional tables. Hyperlinked references are provided for each modular knowledge chunk to facilitate quick access and review. More structured guidelines—including word limits (“targets”) and a web guideline supplement for useful but noncritical tables and figures—are 2 such changes. This Preamble is an abbreviated version, with the detailed version available online.
Patrick T. O’Gara, MD, MACC, FAHA
Chair, ACC/AHA Task Force on Clinical Practice Guidelines
1. INTRODUCTION
Although there has been substantial improvement in atherosclerotic cardiovascular disease (ASCVD) outcomes in recent decades, ASCVD remains the leading cause of morbidity and mortality globally (S1-1–S1-3). In the United States, it is also the leading cause of death for people of most racial/ethnic groups, with an estimated cost of >$200 billion annually in healthcare services, medications, and lost productivity. Much of this is attributable to suboptimal implementation of prevention strategies and uncontrolled ASCVD risk factors in many adults (S1-2).
Most Americans who have had a myocardial infarction (MI) had unfavorable levels of at least 1 cardiovascular risk factor before their ASCVD event (S1-4). In 2010, the AHA defined a new model of “ideal cardiovascular health,” referred to as Life’s Simple 7 (S1-5). Clinicians will find the 2018 Journal of American College of Cardiology (JACC) Cardiovascular Health Promotion Series very helpful in approaching the various aspects of prevention with patients (S1-6). An increasing number of ideal cardiovascular health factors have been associated with a lower prevalence and incidence of ASCVD events, heart failure, atrial fibrillation, cancer, depression, and cognitive impairment (S1-7). Therefore, moving individuals toward ideal cardiovascular health is critically important for prevention of many important health conditions.
The ACC/AHA Task Force on Clinical Practice Guidelines has commissioned this guideline to consolidate existing recommendations and various recent scientific statements, expert consensus documents, and clinical practice guidelines into a single guidance document focused on the primary prevention of ASCVD. However, this guideline also includes newly generated recommendations for aspirin use, exercise and physical activity, and tobacco use, in addition to recommendations related to team-based care, shared decision-making, and assessment of social determinants of health, to create a comprehensive yet targeted ACC/AHA guideline on the prevention of ASCVD. This guideline has been formatted in the modular chunk format to facilitate readability and future updating.
Prevention strategies occur at the population level but must also engage individual adults to slow the development of ASCVD. The most important way to prevent ASCVD is to promote a healthy lifestyle throughout life. Prevention strategies must include a strong focus on lifestyle optimization (improvements in diet, physical activity, and avoidance of tobacco use and exposure to secondhand smoke) to minimize the risk of future ASCVD events.
A comprehensive patient-centered approach that addresses all aspects of a patient’s lifestyle habits and estimated risk of a future ASCVD event is the first step in deciding on where there may be a need for pharmacotherapy. Even if a blood pressure (BP)–reducing medication, lipid-lowering medication, or diabetes medication is ultimately prescribed, lifestyle goals should be emphasized on a regular basis. Only when a person’s risk is sufficiently high should medications to reduce ASCVD risk be considered as part of a shared decision-making process for optimal treatment. In summary, clinicians and individuals should focus attention on living a healthy lifestyle by referring to these evidence-based recommendations to help prevent ASCVD.
1.1. Methodology and Evidence Review
This guideline continues the ACC and AHA effort to design a comprehensive yet succinct compilation of practical guidance for the primary prevention of ASCVD and to promote optimal dissemination of information by using concise language and formatting. The recommendations listed in this guideline are evidence based and supported by an extensive evidence review. A search for literature derived from research involving human subjects, published in English, and indexed in Ovid MEDLINE, PubMed, Cochrane Library, National Institute for Health and Care Excellence (NICE), and other selected databases relevant to this guideline, was conducted between May and July 2018. For specific search terms used and years searched per section, please see Appendix 1.
Randomized controlled trials (RCTs), systematic reviews of RCTs, meta-analyses, and large, United States–based, high-quality cohort studies, as well as observational studies and systematic reviews of observational studies, were evaluated for their content on the prevention of ASCVD outcomes related to the following 9 topic areas: risk assessment, diet, exercise/physical activity, obesity and weight loss, type 2 diabetes mellitus (T2DM), blood cholesterol, hypertension, smoking cessation, and aspirin use. Previous ACC/AHA guidelines, as well as U.S. Preventive Services Task Force (USPSTF) reviews and other guidance relevant to this guideline, were also assessed. The final evidence tables included in the Online Data Supplement summarize the evidence used to formulate recommendations. References selected and published in this document are representative and not all-inclusive.
Avalere Health, a healthcare advisory services firm contracted by ACC/AHA, served as the document manager for this guideline to facilitate its development process. As document manager, Avalere facilitated the deliberations of the Writing Committee and led the modified Delphi process for establishing the Class of Recommendation and the Level of Evidence. In parallel, an independent health data and epidemiology expert, Lee Ann Prebil, conducted a systematic evidence review for the key topic of exercise and physical activity and conducted targeted literature searches to support this document’s discussion of patient-centered approaches, including team-based care, shared decision-making, and assessment of social determinants of health. A targeted literature search was also conducted for this guideline’s cost and value considerations. These searches are available as downloadable Excel files (http://jaccjacc.acc.org/Clinical_Document/Prevention_GL_Targeted_Literature_Searches.pdf).
Recommendations and supportive text relevant to cardiovascular risk, blood cholesterol, and high BP were taken directly from 2 recently released ACC/AHA guidelines, the 2017 Hypertension Clinical Practice Guidelines (S1.1-1) and the 2018 Cholesterol Clinical Practice Guideline (S1.1-2), and were adapted for the present guideline, which aims to provide an overview of the primary prevention of ASCVD among adults. Recommendations that were adapted from previous publications are noted in the recommendation tables, and both the original published recommendation and the adapted version are provided in the guideline Web Supplement.
The results of these evidence reviews were evaluated by the writing committee for incorporation into the present guideline. (See Table S1 in the Web Supplement for a list of relevant publications and statements used in support of the guideline’s recommendations.) Each topic area was assigned a primary writer, as well as a primary, and sometimes secondary, reviewer. These assignments were based on areas of particular expertise of writing committee members. All recommendations were fully reviewed and discussed among the full committee to allow for diverse perspectives and considerations for this guideline. Recommendations were then voted upon, with a modified Delphi process used to reach consensus.
1.2. Organization of the Writing Committee
The writing committee consisted of clinicians, cardiologists, health services researchers, epidemiologists, internists, nurses, and a lay representative. The writing committee included representatives from the ACC and AHA. Appendix 2 of the present document lists writing committee members’ relevant RWI. For the purposes of full transparency, the writing committee members’ comprehensive disclosure information is available online.
1.3. Document Review and Approval
This document was reviewed by 5 official reviewers nominated by the ACC and AHA (1 reviewer from the ACC/AHA Task Force for Practice Guidelines, 2 reviewers from the AHA, and 2 reviewers from the ACC); 3 reviewers on behalf of the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Society for Nutrition, and the American Society of Preventive Medicine; and 23 individual content reviewers. Reviewers’ RWI information was distributed to the writing committee and is published in this document (Appendix 3). This document was approved for publication by the governing bodies of the ACC and AHA.
1.4. Scope of the Guideline
This guideline is intended to be a resource for the clinical and public health practice communities. It addresses the primary prevention of CVD in adults (≥18 years of age), focused on outcomes of ASCVD (i.e., acute coronary syndromes, MI, stable or unstable angina, arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease of atherosclerotic origin), as well as heart failure and atrial fibrillation. The guideline presents recommendations to prevent CVD that are related to lifestyle factors (e.g., diet and exercise or physical activity), other factors affecting CVD risk (e.g., obesity, diabetes, blood cholesterol, high BP, smoking, aspirin use), patient-centered approaches (e.g., team-based care, shared decision-making, assessment of social determinants of health), and considerations of the cost and value of primary prevention.
1.5. Class of Recommendation and Level of Evidence
Recommendations are designated with both a Class of Recommendation (COR) and a Level of Evidence (LOE). The COR indicates the strength of recommendation, encompassing the estimated magnitude and certainty of benefit in proportion to risk. The LOE rates the quality of scientific evidence supporting the intervention on the basis of the type, quantity, and consistency of data from clinical trials and other sources (Table 1) (S1.5-1).
TABLE 1.
Applying Class of Recommendation and Level of Evidence to Clinical Strategies, Interventions, Treatments, or Diagnostic Testing in Patient Care (Updated August 2015)

|
2. OVERARCHING RECOMMENDATIONS FOR ASCVD PREVENTION EFFORTS
2.1. Patient-Centered Approaches to Comprehensive ASCVD Prevention
Recommendations for Patient-Centered Approaches to Comprehensive ASCVD Prevention
Referenced studies that support recommendations are summarized in Online Data Supplements 1 and 2.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| I | A | 1. A team-based care approach is recommended for the control of risk factors associated with ASCVD (S2.1-1–S2.1-14). |
| I | B-R | 2. Shared decision-making should guide discussions about the best strategies to reduce ASCVD risk (S2.1-15–S2.1-18). |
| I | B-NR | 3. Social determinants of health should inform optimal implementation of treatment recommendations for the prevention of ASCVD (S2.1-19–S2.1-25). |
Table 2 outlines key considerations related to social determinants of health and ASCVD prevention.
TABLE 2.
Example Considerations for Addressing Social Determinants of Health to Help Prevent ASCVD Events
| Topic/Domain | Example Considerations |
|---|---|
| Cardiovascular risk | |
| Diet |
|
| Exercise and physical activity | |
| Obesity and weight loss |
|
| Diabetes mellitus | |
| High blood pressure |
|
| Tobacco treatment |
Advanced age generally refers to age ≥75 years.
ASCVD indicates atherosclerotic cardiovascular disease.
2.2. Assessment of Cardiovascular Risk
Recommendations for Assessment of Cardiovascular Risk
Referenced studies that support recommendations are summarized in Online Data Supplement 3.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| I | B-NR | 1. For adults 40 to 75 years of age, clinicians should routinely assess traditional cardiovascular risk factors and calculate 10-year risk of ASCVD by using the pooled cohort equations (PCE) (S2.2-1,S2.2-2). |
| IIa | B-NR | 2. For adults 20 to 39 years of age, it is reasonable to assess traditional ASCVD risk factors at least every 4 to 6 years (S2.2-1–S2.2-3). |
| IIa | B-NR | 3. In adults at borderline risk (5% to <7.5% 10-year ASCVD risk) or intermediate risk (≥7.5% to <20% 10-year ASCVD risk), it is reasonable to use additional risk-enhancing factors to guide decisions about preventive interventions (e.g., statin therapy) (S2.2-4–S2.2-14). |
| IIa | B-NR | 4. In adults at intermediate risk (≥7.5% to <20% 10-year ASCVD risk) or selected adults at borderline risk (5% to <7.5% 10-year ASCVD risk), if risk-based decisions for preventive interventions (e.g., statin therapy) remain uncertain, it is reasonable to measure a coronary artery calcium score to guide clinician–patient risk discussion (S2.2-15–S2.2-31). |
| IIb | B-NR | 5. For adults 20 to 39 years of age and for those 40 to 59 years of age who have <7.5% 10-year ASCVD risk, estimating lifetime or 30-year ASCVD risk may be considered (S2.2-1,S2.2-2,S2.2-32–S2.2-35). |
Among adults at borderline (5% to <7.5%) and intermediate (≥7.5% to <20%) risk, one may consider additional individual risk-enhancing clinical factors (Table 3) that can be used to revise the 10-year ASCVD risk estimate (S2.2-4). These factors may include having a family history of premature ASCVD (S2.2-5), chronic inflammatory disease [rheumatoid arthritis (S2.2-6), lupus (S2.2-7), or HIV infection (S2.2-12)], South Asian ancestry (S2.2-13), a history of preeclampsia (S2.2-8) or preterm delivery (S2.2-9), early menopause (S2.2-10), erectile dysfunction (S2.2-11), chronic kidney disease (CKD), metabolic syndrome, persistently elevated inflammatory markers (S2.2-14), or elevated lipid biomarkers (S2.2-4). After these clinically available risk-enhancing factors have been considered, if there is still uncertainty about the reliability of the risk estimate for individuals in the borderline- or intermediate-risk categories, further testing to document subclinical coronary atherosclerosis is reasonable to more accurately reclassify the risk estimate upward or downward (S2.2-17–S2.2-19,S2.2-36).
TABLE 3.
Risk-Enhancing Factors for Clinician–Patient Risk Discussion
| Risk-Enhancing Factors |
|---|
|
Optimally, 3 determinations.
ABI indicates ankle-brachial index; AIDS, acquired immunodeficiency syndrome; apoB, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); and RA, rheumatoid arthritis.
Reproduced with permission from Grundy et al. (S2.2-4). Copyright © 2018, American Heart Association, Inc., and American College of Cardiology Foundation.
3. LIFESTYLE FACTORS AFFECTING CARDIOVASCULAR RISK
3.1. Nutrition and Diet
Recommendations for Nutrition and Diet
Referenced studies that support recommendations are summarized in Online Data Supplements 4 and 5.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| I | B-R | 1. A diet emphasizing intake of vegetables, fruits, legumes, nuts, whole grains, and fish is recommended to decrease ASCVD risk factors (S3.1-1–S3.1-11). |
| IIa | B-NR | 2. Replacement of saturated fat with dietary monounsaturated and polyunsaturated fats can be beneficial to reduce ASCVD risk (S3.1-12,S3.1-13). |
| IIa | B-NR | 3. A diet containing reduced amounts of cholesterol and sodium can be beneficial to decrease ASCVD risk (S3.1-9,S3.1-14–S3.1-16). |
| IIa | B-NR | 4. As a part of a healthy diet, it is reasonable to minimize the intake of processed meats, refined carbohydrates, and sweetened beverages to reduce ASCVD risk (S3.1-17–S3.1-23). |
| III-Harm | B-NR | 5. As a part of a healthy diet, the intake of trans fats should be avoided to reduce ASCVD risk (S3.1-12, S3.1-17, S3.1-25–S3.1-27). |
3.2. Exercise and Physical Activity
Recommendations for Exercise and Physical Activity
Referenced studies that support recommendations are summarized in Online Data Supplements 6 and 7.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| I | B-R | 1. Adults should be routinely counseled in healthcare visits to optimize a physically active lifestyle (S3.2-1, S3.2-2). |
| I | B-NR | 2. Adults should engage in at least 150 minutes per week of accumulated moderate-intensity or 75 minutes per week of vigorous-intensity aerobic physical activity (or an equivalent combination of moderate and vigorous activity) to reduce ASCVD risk (S3.2-3–S3.2-8). |
| IIa | B-NR | 3. For adults unable to meet the minimum physical activity recommendations (at least 150 minutes per week of accumulated moderate-intensity or 75 minutes per week of vigorous-intensity aerobic physical activity), engaging in some moderate- or vigorous-intensity physical activity, even if less than this recommended amount, can be beneficial to reduce ASCVD risk (S3.2-5,S3.2-6). |
| IIb | C-LD | 4. Decreasing sedentary behavior in adults may be reasonable to reduce ASCVD risk (S3.2-3,S3.2-9–S3.2-11). |
Other activity states that comprise a 24-hour period for an average individual include sleep, light-intensity physical activity, and sedentary behavior (Figure 1). Sedentary behavior refers to waking behavior with an energy expenditure of ≤1.5 metabolic equivalents while in a sitting or reclining posture (Table 4) (S3.2-12).
FIGURE 1. Hours Per Day Spent in Various States of Activity.

U.S. adults spend >7 h/d on average in sedentary activities. Replacing sedentary time with other physical activity involves increasing either moderate- to vigorous-intensity physical activity or light-intensity physical activity. Data modified from Young et al. (S3.2-12).
TABLE 4.
Definitions and Examples of Different Intensities of Physical Activity
| Intensity | METs | Examples |
|---|---|---|
| Sedentary behavior* | 1–1.5 | Sitting, reclining, or lying; watching television |
| Light | 1.6–2.9 | Walking slowly, cooking, light housework |
| Moderate | 3.0–5.9 | Brisk walking (2.4–4 mph), biking (5–9mph), ballroom dancing, active yoga, recreational swimming |
| Vigorous | ≥6 | Jogging/running, biking (≥10 mph), singles tennis, swimming laps |
Sedentary behavior is defined as any waking behavior characterized by an energy expenditure ≤1.5 METs while in a sitting, reclining, or lying posture. Standing is a sedentary activity in that it involves ≤1.5 METs, but it is not considered a component of sedentary behavior.
MET indicates metabolic equivalent; mph, miles per hour.
4. OTHER FACTORS AFFECTING CARDIOVASCULAR RISK
4.1. Adults With Overweight and Obesity
Recommendations for Adults With Overweight and Obesity
Referenced studies that support recommendations are summarized in Online Data Supplements 8 and 9.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| I | B-R | 1. In individuals with overweight and obesity, weight loss is recommended to improve the ASCVD risk factor profile (S4.1-1). |
| I | B-R | 2. Counseling and comprehensive lifestyle interventions, including calorie restriction, are recommended for achieving and maintaining weight loss in adults with overweight and obesity (S4.1-1,S4.1-2). |
| I | C-EO | 3. Calculating body mass index (BMI) is recommended annually or more frequently to identify adults with overweight and obesity for weight loss considerations. |
| IIa | B-NR | 4. It is reasonable to measure waist circumference to identify those at higher cardiometabolic risk (S4.1-3–S4.1-6). |
4.2. Adults With Type 2 Diabetes Mellitus
See Figure 2 for an algorithm for treatment of T2DM for primary prevention of cardiovascular disease.
FIGURE 2. Treatment of T2DM for Primary Prevention of Cardiovascular Disease.

CVD indicates cardiovascular disease; GLP-1R, glucagon-like peptide-1 receptor; HbA1c, hemoglobin A1c; SGLT-2, sodium-glucose cotransporter 2; and T2DM, type 2 diabetes mellitus.
Recommendations for Adults With Type 2 Diabetes Mellitus
Referenced studies that support recommendations are summarized in Online Data Supplement 10.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| I | A | 1. For all adults with T2DM, a tailored nutrition plan focusing on a heart-healthy dietary pattern is recommended to improve glycemic control, achieve weight loss if needed, and improve other ASCVD risk factors (S4.2-1,S4.2-2). |
| I | A | 2. Adults with T2DM should perform at least 150 minutes per week of moderate-intensity physical activity or 75 minutes of vigorous-intensity physical activity to improve glycemic control, achieve weight loss if needed, and improve other ASCVD risk factors (S4.2-3,S4.2-4). |
| IIa | B-R | 3. For adults with T2DM, it is reasonable to initiate metformin as first-line therapy along with lifestyle therapies at the time of diagnosis to improve glycemic control and reduce ASCVD risk (S4.2-5–S4.2-8). |
| IIb | B-R | 4. For adults with T2DM and additional ASCVD risk factors who require glucose-lowering therapy despite initial lifestyle modifications and metformin, it may be reasonable to initiate a sodium-glucose cotransporter 2 (SGLT-2) inhibitor or a glucagon-like peptide-1 receptor (GLP-1R) agonist to improve glycemic control and reduce CVD risk (S4.2-9–S4.2-14). |
4.3. Adults With High Blood Cholesterol
Recommendations from the 2018 Cholesterol Clinical Practice Guidelines (S4.3-1) are included and adapted below.
Recommendations for Adults With High Blood Cholesterol
Referenced studies that support recommendations are summarized in Online Data Supplements 11 and 12.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| I | A | 1. In adults at intermediate risk (≥7.5% to <20% 10-year ASCVD risk), statin therapy reduces risk of ASCVD, and in the context of a risk discussion, if a decision is made for statin therapy, a moderate-intensity statin should be recommended (S4.3-2–S4.3-9). Adapted from recommendations in the 2018 Cholesterol Clinical Practice Guidelines (S4.3-1). |
| I | A | 2. In intermediate risk (≥7.5% to <20% 10-year ASCVD risk) patients, LDL-C levels should be reduced by 30% or more, and for optimal ASCVD risk reduction, especially in patients at high risk (≥20% 10-year ASCVD risk), levels should be reduced by 50% or more (S4.3-2,S4.3-5–S4.3-10). Adapted from recommendations in the 2018 Cholesterol Clinical Practice Guidelines (S4.3-1). |
| I | A | 3. In adults 40 to 75 years of age with diabetes, regardless of estimated 10-year ASCVD risk, moderate-intensity statin therapy is indicated (S4.3-11–S4.3-19). Included from recommendations in the 2018 Cholesterol Clinical Practice Guidelines (S4.3-1). Included from recommendations in the 2018 Cholesterol Clinical Practice Guidelines (S4.3-1). |
| I | B-R | 4. In patients 20 to 75 years of age with an LDL-C level of 190 mg/dL (≥4.9 mmol/L) or higher, maximally tolerated statin therapy is recommended (S4.3-2,S4.3-20–S4.3-25). Included from recommendations in the 2018 Cholesterol Clinical Practice Guidelines (S4.3-1). |
| IIa | B-R | 5. In adults with diabetes mellitus who have multiple ASCVD risk factors, it is reasonable to prescribe high-intensity statin therapy with the aim to reduce LDL-C levels by 50% or more (S4.3-2,S4.3-7). Included from recommendations in the 2018 Cholesterol Clinical Practice Guidelines (S4.3-1). |
| IIa | B-R | 6. In intermediate-risk (≥7.5% to <20% 10-year ASCVD risk) adults, risk-enhancing factors favor initiation or intensification of statin therapy (S4.3-7,S4.3-26–S4.3-33). Adapted from recommendations in the 2018 Cholesterol Clinical Practice Guidelines (S4.3-1). |
| IIa | B-NR | 7. In intermediate-risk (≥7.5% to <20% 10-year ASCVD risk) adults or selected borderline-risk (5% to <7.5% 10-year ASCVD risk) adults in whom a coronary artery calcium score is measured for the purpose of making a treatment decision, AND
|
| IIb | B-R | 8. In patients at borderline risk (5% to <7.5% 10-year ASCVD risk), in risk discussion, the presence of risk-enhancing factors may justify initiation of moderate-intensity statin therapy (S4.3-28,S4.3-35). Adapted from recommendations in the 2018 Cholesterol Clinical Practice Guidelines (S4.3-1). |
Primary ASCVD prevention requires attention to ASCVD risk factors beginning early in life (Figure 3). The benefit from statin therapy is related to both global risk and intensity of treatment (S4.3-2), and no RCTs of high-intensity statin therapy have been carried out in cohorts of patients exclusively with diabetes. On the basis of these considerations and the fact that patients with diabetes have a higher trajectory of lifetime risk than do those without diabetes, high-intensity statin therapy is preferred in patients with diabetes as they develop risk modifiers (Table 5).
FIGURE 3. Primary Prevention.

Colors correspond to Class of Recommendation in Table 1. ABI indicates ankle-brachial index; apoB, apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CHD, coronary heart disease; HIV, human immunodeficiency virus; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; and Lp(a), lipoprotein (a). Reproduced with permission from Grundy et al. (S4.3-1). Copyright © 2018, American Heart Association, Inc., and American College of Cardiology Foundation.
TABLE 5.
Diabetes-Specific Risk Enhancers That Are Independent of Other Risk Factors in Diabetes Mellitus
| Risk Enhancers in Diabetic Patients |
|---|
ABI indicates ankle-brachial index; eGFR, estimated glomerular filtration rate; and T2DM, type 2 diabetes mellitus.
Reproduced with permission from Grundy et al. (S4.3-1). Copyright © 2018, American Heart Association, Inc., and American College of Cardiology Foundation.
Selected examples of candidates who might benefit from knowing their coronary artery calcium score is zero are listed in Table 6.
TABLE 6.
Selected Examples of Candidates for Coronary Artery Calcium Measurement Who Might Benefit From Knowing Their Coronary Artery Calcium Score Is Zero
| Coronary Artery Calcium Measurement Candidates Who Might Benefit from Knowing Their Coronary Artery Calcium Score Is Zero |
|---|
|
Caveats: If patient is at intermediate risk and if a risk decision is uncertain and a coronary artery calcium score is obtained, it is reasonable to withhold statin therapy unless higher-risk conditions, such as cigarette smoking, family history of premature ASCVD, or diabetes mellitus, are present and to reassess the coronary artery calcium score in 5 to 10 years. Moreover, if coronary artery calcium is recommended, it should be performed in facilities that have current technology and expertise to deliver the lowest radiation possible.
ASCVD indicates atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; and PCE, pooled cohort equations.
Reproduced with permission from Grundy et al. (S4.3-1). Copyright © 2018, American Heart Association, Inc., and American College of Cardiology Foundation.
4.4. Adults With High Blood Pressure or Hypertension
Recommendations from the 2017 Hypertension Clinical Practice Guidelines (S4.4-1) are adapted below.
Recommendations for Adults With High Blood Pressure or Hypertension
Referenced studies that support recommendations are summarized in Online Data Supplements 13 and 14.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| I | A | 1. In adults with elevated blood pressure (BP) or hypertension, including those requiring antihypertensive medications nonpharmacological interventions are recommended to reduce BP. These include:
|
| I |
SBP: A |
2. In adults with an estimated 10-year ASCVD risk* of 10% or higher and an average systolic BP (SBP) of 130 mm Hg or higher or an average diastolic BP (DBP) of 80 mm Hg or higher, use of BP-lowering medications is recommended for primary prevention of CVD (S4.4-30–S4.4-38). Adapted from recommendations in the 2017 Hypertension Clinical Practice Guidelines (S4.4-1). |
|
DBP: C-EO | ||
| I |
SBP: B-RSR |
3. In adults with confirmed hypertension and a 10-year ASCVD event risk of 10% or higher, a BP target of less than 130/80 mm Hg is recommended (S4.4-33,S4.4-39–S4.4-42). Adapted from recommendations in the 2017 Hypertension Clinical Practice Guidelines (S4.4-1). |
|
DBP: C-EO | ||
| I |
SBP: B-RSR |
4. In adults with hypertension and chronic kidney disease, treatment to a BP goal of less than 130/80 mm Hg is recommended (S4.4-43–S4.4-48). Adapted from recommendations in the 2017 Hypertension Clinical Practice Guidelines (S4.4-1). |
|
DBP: C-EO | ||
| I |
SBP: B-RSR |
5. In adults with T2DM and hypertension, antihypertensive drug treatment should be initiated at a BP of 130/80 mm Hg or higher, with a treatment goal of less than 130/80 mm Hg (S4.4-33, S4.4-47, S4.4-49–S4.4-54). Adapted from recommendations in the 2017 Hypertension Clinical Practice Guidelines (S4.4-1). |
|
DBP: C-EO | ||
| I |
C-LD |
6. In adults with an estimated 10-year ASCVD risk <10% and an SBP of 140 mm Hg or higher or a DBP of 90 mm Hg or higher, initiation and use of BP-lowering medication are recommended (S4.4-36,S4.4-55–S4.4-58). Adapted from recommendations in the 2017 Hypertension Clinical Practice Guidelines (S4.4-1). |
| IIb |
SBP: B-NR |
7. In adults with confirmed hypertension without additional markers of increased ASCVD risk, a BP target of less than 130/80 mm Hg may be reasonable (S4.4-59–S4.4-62). Adapted from recommendations in the 2017 Hypertension Clinical Practice Guidelines (S4.4-1). |
|
DBP: C-EO | ||
ACC/AHA pooled cohort equations to estimate 10-year risk of ASCVD.
See Figure 4 for the BP thresholds and treatment recommendations algorithm and refer to the 2017 Hypertension Clinical Practice Guidelines for comprehensive details (S4.4-1).
FIGURE 4. BP Thresholds and Recommendations for Treatment.
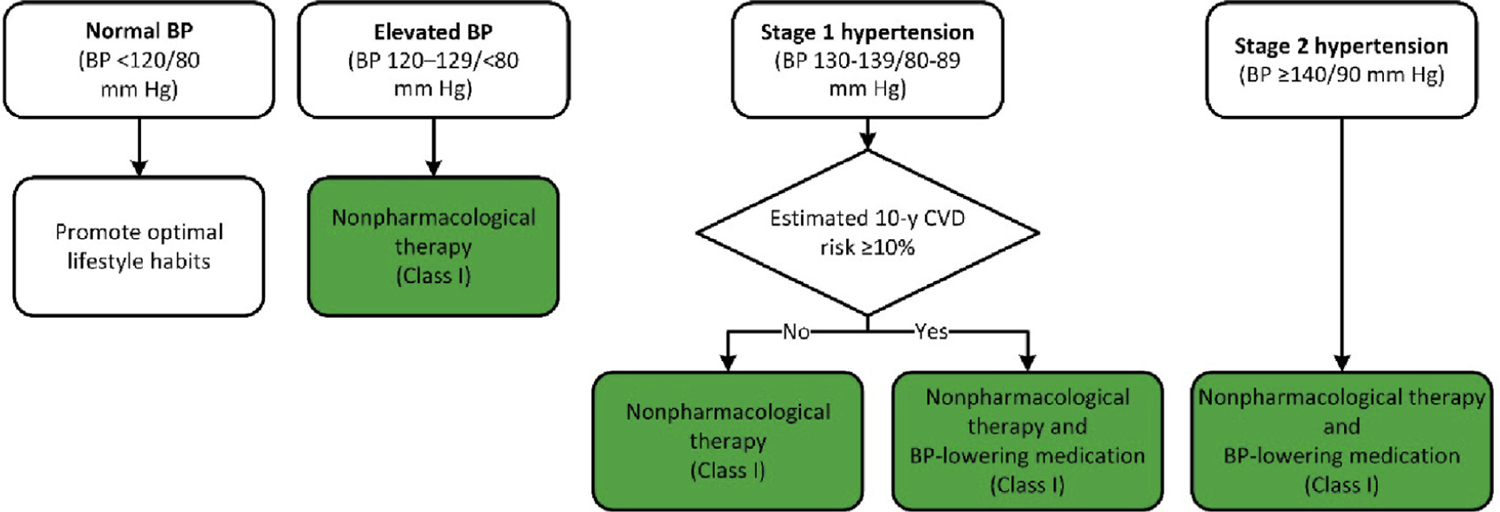
Colors correspond to Class of Recommendation in Table 1. BP indicates blood pressure; and CVD, cardiovascular disease. Adapted with permission from Whelton et al. (S4.4-1). Copyright © 2017, American College of Cardiology Foundation and the American Heart Association, Inc.
See Table 7 for recommended dose and approximate impact on SBP.
TABLE 7.
Best Proven Nonpharmacological Interventions for Prevention and Treatment of Hypertension*
| Nonpharmacological Intervention | Goal | Approximate Impact on SBP | |||
|---|---|---|---|---|---|
| Hypertension | Normotension | Reference | |||
| Weight loss | Weight/body fat | Best goal is ideal body weight, but aim for at least a 1-kg reduction in body weight for most adults who are overweight. Expect about 1 mm Hg for every 1-kg reduction in body weight. | −5 mm Hg | −2/3 mm Hg | (S4.4-2) |
| Healthy diet | DASH dietary pattern† | Consume a diet rich in fruits, vegetables, whole grains, and low-fat dairy products, with reduced content of saturated and total fat. | −11 mm Hg | −3 mm Hg | (S4.4-7, S4.4-8) |
| Reduced intake of dietary sodium | Dietary sodium | Optimal goal is <1500 mg/d, but aim for at least a 1000-mg/d reduction in most adults. | −5/6 mm Hg | −2/3 mm Hg | (S4.4-12, S4.4-10) |
| Enhanced intake of dietary potassium | Dietary potassium | Aim for 3500–5000 mg/d, preferably by consumption of a diet rich in potassium. | −4/5 mm Hg | −2 mm Hg | (S4.4-14) |
| Physical activity | Aerobic |
|
−5/8 mm Hg | −5/8 mm Hg | (S4.4-19, S4.4-20) |
| Dynamic resistance |
|
−4 mm Hg | −4 mm Hg | (S4.4-19) | |
| Isometric resistance |
|
−5 mm Hg | −5 mm Hg | (S4.4-21, S4.4-63) | |
| Moderation in alcohol intake | Alcohol consumption | In individuals who drink alcohol, reduce alcohol‡ to:
|
−4 mm Hg | −4 mm Hg | (S4.4-20, S4.4-24, S4.4-25) |
Type, dose, and expected impact on BP in adults with a normal BP and with hypertension.
Detailed information about the DASH diet is available via the NHLBI (S4.4-65) and Dashdiet.org (S4.4-66).
In the United States, 1 “standard” drink contains roughly 14 g of pure alcohol, which is typically found in 12 oz of regular beer (usually about 5% alcohol), 5 oz of wine (usually about 12% alcohol), and 1.5 oz of distilled spirits (usually about 40% alcohol) (S4.4-64).
BP indicates blood pressure; DASH, Dietary Approaches to Stop Hypertension; NHLBI, National Heart, Lung, and Blood Institute; and SBP, systolic blood pressure.
Reproduced with permission from Whelton et al. (S4.4-1). Copyright © 2017, American College of Cardiology Foundation and the American Heart Association, Inc.
4.5. Treatment of Tobacco Use
Recommendations for Treatment of Tobacco Use
Referenced studies that support recommendations are summarized in Online Data Supplements 15 and 16.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| I | A | 1. All adults should be assessed at every healthcare visit for tobacco use and their tobacco use status recorded as a vital sign to facilitate tobacco cessation (S4.5-1). |
| I | A | 2. To achieve tobacco abstinence, all adults who use tobacco should be firmly advised to quit (S4.5-2). |
| I | A | 3. In adults who use tobacco, a combination of behavioral interventions plus pharmacotherapy is recommended to maximize quit rates (S4.5-2,S4.5-3). |
| I | B-NR | 4. In adults who use tobacco, tobacco abstinence is recommended to reduce ASCVD risk (S4.5-4,S4.5-5). |
| IIa | B-R | 5. To facilitate tobacco cessation, it is reasonable to dedicate trained staff to tobacco treatment in every healthcare system (S4.5-1). |
| III: Harm | B-NR | 6. All adults and adolescents should avoid secondhand smoke exposure to reduce ASCVD risk (S4.5-6). |
In alignment with previous expert consensus regarding strategies for tobacco cessation (S4.5-7), Table 8 summarizes recommended behavioral interventions and pharmacotherapy for tobacco treatment. There are 7 FDA-approved cessation medications, including 5 forms of nicotine replacement. Note that the black box warnings about neuropsychiatric events have been removed by the FDA (S4.5-8, 4.5-9).
TABLE 8.
Highlights of Recommended Behavioral and Pharmacotherapy Tobacco Treatment Modalities for Prescribers*
| Timing of Behavioral Interventions† | |||
|---|---|---|---|
| <3 min of tobacco status assessment with cessation counseling at each clinic encounter | >3–10 min of tobacco status assessment with cessation counseling at each clinic encounter | >10 min of tobacco status assessment with cessation counseling at each clinic encounter | |
| Treatment | Dosing‡ | Precautions | |
| NRT* | |||
| Patch | 21 mg, 14 mg, or 7 mg | Starting dose: 21 mg for ≥10 CPD; 14 mg for <10 CPD | Local irritation possible; avoid with skin disorders; may remove for sleep if needed |
| Gum Lozenge |
2 mg or 4 mg 2 mg or 4 mg |
Starting dose: 4 mg if first tobacco use is ≤30 min after waking; 2 mg if first tobacco use is >30 min after waking; maximum of 20 lozenges or 24 pieces of gum/d. Chew and park gum* |
Hiccups/dyspepsia possible; avoid food or beverages 15 min before and after use |
| Nasal spray | 10 mg/mL | Starting dose: 1–2 doses/h (1 dose=1 spray each nostril) maximum of 40 doses/d | Local irritation possible; avoid with nasal or reactive airway disorders |
| Oral inhaler | 10-mg cartridge | Starting dose: Puff for 20 min/cartridge every 1–2 h; maximum 16 cartridges/d | Cough possible; avoid with reactive airway disorders |
| Other§ | |||
| Bupropion (Zyban [GlaxoSmithKline], Wellbutrin SR [GlaxoSmithKline]) | 150 mg SR | 150 mg once daily (am) for 3 d; then 150 mg twice daily; may use in combination with NRT (S4.5-9) | Avoid with history/risk of seizures, eating disorders, MAO inhibitors, or CYP 2D6 inhibitor |
| Varenicline (Chantix [Pfizer]) | 0.5 mg or 1 mg | 0.5 mg once daily (am) for 3 d; then 0.5 mg twice daily for 4 d; then 1 mg twice daily (use start pack followed by continuation pack) for 3–6 mo | Nausea common; take with food. Renal dosing required. Very limited drug interactions; near-exclusive renal clearance. |
CPD can guide dosing. 1 CPD is ≈1–2 mg of nicotine. Note: Use caution with all NRT products for patients with recent (≤2 wk) MI, serious arrhythmia, or angina; patients who are pregnant or breastfeeding; and adolescents.
Timing of assessment relates to ICD-10 coding.
Dose and duration can be titrated on the basis of response (S4.5-9).
am indicates morning; CPD, cigarettes smoked per day; FDA, U.S. Food and Drug Administration; ICD-10, International Classification of Diseases, Tenth Revision; MAO, monoamine oxidase; NRT, nicotine replacement; and SR, sustained release.
4.6. Aspirin Use
Recommendations for Aspirin Use
Referenced studies that support recommendations are summarized in Online Data Supplements 17 and 18.
| COR | LOE | RECOMMENDATIONS |
|---|---|---|
| IIb | A | 1. Low-dose aspirin (75–100 mg orally daily) might be considered for the primary prevention of ASCVD among select adults 40 to 70 years of age who are at higher ASCVD risk but not at increased bleeding risk (S4.6-1–S4.6-8). |
| III: Harm | B-R | 2. Low-dose aspirin (75–100 mg orally daily) should not be administered on a routine basis for the primary prevention of ASCVD among adults >70 years of age (S4.6-9). |
| III: Harm | C-LD | 3. Low-dose aspirin (75–100 mg orally daily) should not be administered for the primary prevention of ASCVD among adults of any age who are at increased risk of bleeding (S4.6-10). |
5. COST AND VALUE CONSIDERATIONS
The growing need to consider value stems directly from the goal of achieving the best possible health outcomes with finite healthcare resources in the primary prevention of CVD (S5-1). Value in healthcare can be defined as the incremental health benefits of a therapy or procedure relative to its incremental net long-term costs. The consideration of cost and value in the guideline development process supports key goals, including: 1) enhancing overall value in the delivery of cardiovascular care and 2) involving healthcare professionals in the challenging care decisions that must be made to increase value in the U.S. healthcare system (S5-2).
The integration of value assessments into our national guidelines involves inherent methodological challenges, including: 1) variability in costs across different healthcare settings; 2) variability in costs and benefits across different patient subgroups; 3) variability over time; 4) variability in who bears the burden of the health outcome (i.e., typically the individual patient) versus who bears the burden of the healthcare cost (e.g., often spread beyond the individual to third-party payers, taxpayers); and 5) an inadequate literature base on which to render a sound, evidence-based assessment of certain specific therapies (S5-1,S5-2).
There are additional challenges specific to the prevention realm. As described in the 2011 AHA policy statement, “Value of Primordial and Primary Prevention in CVD” (S5-1):
“Assessing the value of prevention in apparently healthy patients is generally more difficult than evaluating therapy for established disease because the time horizon to the clinical manifestation of disease is generally long—many decades in the young. Thus, it is difficult, perhaps impossible, to assess long-term effectiveness in terms of survival or quality-adjusted life-years (QALYs) or associated costs because of increasing uncertainty about outcome the further one tries to look into the future.”
Furthermore, the principle of discounting, which places relative emphasis on current costs and benefits while deemphasizing downstream costs and benefits, creates disadvantages for prevention because costs often accrue in the present while the benefit may only be fully realized long into the future. These methodological challenges notwithstanding, prior AHA statements have highlighted the public policies, community efforts, and pharmacological interventions that are likely to be cost-effective and, at times, cost-saving prevention tactics compared with common benchmarks. For example, robust evidence suggests that both antihypertensive therapy (S5-3–S5-6) and statin therapy (S5-7–S5-9), particularly with low-cost generic drug formulations, are high-value interventions across a wide spectrum of risk and age strata.
The incorporation of the value category into clinical practice guidelines is one of several considerations in medical decision-making and resource allocation. Clinicians, researchers, and policymakers alike must continue to place cost-effective analyses in the proper context, extracting key value determinations while acknowledging the challenges in fully characterizing and incorporating the downstream benefits of a given therapeutic prevention tactic. Further research and methodological advances are needed to comprehensively characterize the full spectrum of benefits produced by the prevention approach, thereby rendering cost-effectiveness assessments more consequential to clinical practice.
6. CONCLUSION
Most ASCVD events are avoidable through primordial prevention (i.e., the prevention of risk factor development) and control of traditional cardiovascular risk factors. Tobacco avoidance is critically important for ASCVD prevention, and all adults should strive to engage in regular brisk physical activity most days of the week and adhere to a healthy dietary pattern to help lower future ASCVD risk. A diet high in fruits, vegetables, and whole grains is best. Fish, legumes, and poultry are the preferred sources of protein. Minimizing the consumption of trans fats, added sugars (including sugar-sweetened beverages), red meats, sodium, and saturated fats is also important. Clinicians should work in partnership with patients to assess their readiness for sustained lifestyle improvements, identify potential barriers to change, and encourage them to try to achieve measurable goals and continue to monitor their progress (S6-1). Finally, social determinants of ASCVD risk—and their impact on the patient’s ability to prevent or treat risk factors—must be taken into account. Clinicians need to consider patients’ health literacy and education levels and assess patients’ motivation to improve their lifestyle habits.
The goal of the clinician is to match the intensity of preventive efforts with an individual’s absolute risk of a future ASCVD event and with the individual’s willingness and capacity to implement preventive strategies. Risk estimation is imperfect and based on group averages that are then applied to individual patients. The clinician must balance an understanding of a patient’s estimated ASCVD risk with potential benefits and adverse risk from pharmacological therapy in the context of a risk discussion. To determine the appropriateness of pharmacological therapy after quantitative risk estimation in cases that are unclear, risk-enhancing factors or selective use of a coronary artery calcium measurement can inform decision-making for cholesterol-lowering or antihypertensive medication use in intermediate-risk individuals.
This primary-prevention guideline strives to provide clinicians with the information they need to help their patients reduce their risk of ASCVD and encourage them to make healthier lifestyle changes when needed.
Supplementary Material
1.6. Abbreviations
| Abbreviation | Meaning/Phrase |
|---|---|
| ASCVD | atherosclerotic cardiovascular disease |
| AU | Agatston units |
| BMI | body mass index |
| BP | blood pressure |
| CHD | coronary heart disease |
| CKD | chronic kidney disease |
| CVD | cardiovascular disease |
| DASH | Dietary Approaches to Stop Hypertension |
| DBP | diastolic blood pressure |
| DM | diabetes mellitus |
| ENDS | electronic nicotine delivery systems |
| FDA | U.S. Food and Drug Administration |
| GLP-1R | glucagon-like peptide-1 receptor |
| HbA1c | hemoglobin A1c |
| HDL-C | high-density lipoprotein cholesterol |
| HbA1c | hemoglobin A1c |
| LDL-C | low-density lipoprotein cholesterol |
| MI | myocardial infarction |
| PCE | pooled cohort equations |
| RCT | randomized controlled trial |
| SBP | systolic blood pressure |
| SGLT-2 | sodium-glucose cotransporter 2 |
| T2DM | type 2 diabetes mellitus |
| USPSTF | U.S. Preventive Services Task Force |
APPENDIX 1. SEARCH CRITERIA
The rapid review conducted by the Evidence-based Practice Center to complete this literature search, in the limited timeframe provided, built on existing systematic reviews conducted on behalf of the USPSTF.
| Medical Subject Headings (MeSH) Terms | Key Words |
|---|---|
| Nutrition and Diet | |
| Search since the 2017 review (1) | |
| exp Diet/ | diet* |
| exp Diet Therapy/ | cardiovascular |
| Healthy Diet | coronary |
| Primary Prevention/ | heart |
| myocardial infarction | |
| MI | |
| CVD | |
| CHD | |
| cerebrovascular | |
| stroke | |
| microvascular | |
| mortality | |
| prevent* | |
| Obesity and Weight Loss | |
| Search since the 2018 review (2) | |
| exp Obesity/ | obes* |
| exp Weight Loss | overweight |
| Primary Prevention/ | weight |
| cardiovascular | |
| coronary | |
| heart | |
| myocardial infarction | |
| MI | |
| CVD | |
| CHD | |
| cerebrovascular | |
| stroke | |
| microvascular | |
| mortality | |
| prevent* | |
| Type 2 Diabetes Mellitus | |
| Search since the 2015 review (3) | |
| exp Diabetes Mellitus, Type 2/ | impaired fasting glucose |
| Prediabetic State/ | impaired glucose tolerance |
| Glucose Intolerance/ | Ifg |
| Primary Prevention/ | Igt |
| prediabetes* | |
| type 2 diabet* | |
| DM | |
| cardiovascular | |
| coronary | |
| heart | |
| myocardial infarction | |
| MI | |
| CVD | |
| CHD | |
| cerebrovascular | |
| stroke | |
| microvascular | |
| mortality | |
| prevent* | |
| Tobacco Use | |
| Search since the 2015 review (4) | |
| Smoking/ | smoking |
| exp “Tobacco Use Cessation”/ | cigarette* |
| Tobacco Use Disorder/ | tobacco |
| Electronic Cigarettes/ | nicotine |
| Primary Prevention/ | vape |
| vaping | |
| e-cigarette | |
| electronic cigarette | |
| electronic nicotine delivery system* | |
| ENDS | |
| cardiovascular | |
| coronary | |
| heart | |
| myocardial infarction | |
| MI | |
| CVD | |
| CHD | |
| cerebrovascular | |
| stroke | |
| microvascular | |
| mortality | |
| prevent* | |
| Aspirin Use | |
| Search since the 2016 review (5) | |
| Aspirin | aspirin |
| exp Cerebrovascular Disorders/ | acetylsalicylic acid |
| exp Cardiovascular Diseases/ | clopidogrel |
| Primary Prevention/ | cardiovascular |
| coronary | |
| heart | |
| myocardial infarction | |
| MI | |
| CVD | |
| CHD | |
| cerebrovascular | |
| stroke | |
| microvascular | |
| mortality | |
| prevent* | |
| Social Determinants of Health | |
|
Search limited to English. No date restrictions (conducted 7/11/2018) Similar articles searches were also conducted where potentially highly relevant papers were found | |
| NONE SPECIFIED, BUT DUE TO AUTOMATIC TERM MAPPING IN PUBMED, SOME MeSH TERMS MAY HAVE BEEN EMPLOYED | Social determinants of health |
| Equity | |
| Social status | |
| Social deprivation | |
| Neighborhood | |
| Neighborhood conditions | |
| Uninsured | |
| Housing | |
| Immigration | |
| Adverse childhood events | |
| Social gradient | |
| Educational status | |
| Inequalities | |
| Sexuality | |
| Atherosclerosis | |
| cardiovascular | |
| Team Based Care | |
|
Search limited to English, 1/1/2010–10/14/2018 (though earlier articles may have been identified through related articles search) Related articles searches were also conducted where potentially highly relevant papers were found | |
| NONE SPECIFIED, BUT DUE TO AUTOMATIC TERM MAPPING IN PUBMED, SOME MeSH TERMS MAY HAVE BEEN EMPLOYED | team |
| Team care | |
| Collaborative care | |
| Multidisciplinary | |
| “team based” | |
| “team approach” | |
| prevention | |
| Primary prevention | |
| Cardiovascular disease, | |
| Cholesterol | |
| Aspirin | |
| Smoking | |
| Obesity | |
| Heart disease | |
| Atherosclerosis | |
| stroke | |
| Shared Decision Making | |
|
Search limited to English, 1/1/2010–10/24/2018 (though earlier articles may have been identified through related articles search) Related articles searches were also conducted where potentially highly relevant papers were found | |
| NONE SPECIFIED, BUT DUE TO AUTOMATIC TERM MAPPING IN PUBMED, SOME MeSH TERMS MAY HAVE BEEN AUTOMATICALLY EMPLOYED | Shared decision making |
| Prevention | |
| Cardiovascular | |
| Atherosclerosis | |
| Stroke | |
| Heart | |
| Hypertension | |
| Lipids | |
| Cholesterol | |
| diabetes | |
| Exercise & Physical Activity | |
|
Search limits: Not ACP Journal Club OR Summaries for patients OR Editorial OR case-report OR letter OR letter OR abstract OR newspaper article OR comment OR baseline characteristics OR study design OR methodology Terms to identify clinical trials/SRs/Mas: Filters: Meta-Analysis, Systematic Reviews, Clinical Trial, Controlled Clinical Trial, Randomized Controlled Trial, From 2011/01/01 to 2018/05/25, Humans, English, Adult: 19+ years Terms to identify observational studies: 2011/01/01 to 2018/12/31, Humans, English, Epidemiologic Studies, Case-Control Studies, Cohort Studies, Cross-Sectional Studies, epidemiolog* AND stud*, case control, cohort stud*, cross sectional, cohort analys*, follow up stud*, longitudinal, retrospective, prospective, observational AND stud* Filters: Adult: 19+ years | |
| Waist Circumference | |
| Search limited to adult populations, 01/01/2010–10/3/18, English language | |
| Acute Coronary Syndrome | Acute coronary syndromes |
| Angina Unstable | Unstable angina?, “Angina Unstable” |
| Myocardial infarction | Myocardial infarctions |
| Shock cardiogenic | “shock cardiogenic” |
| Myocardial Stunning | “myocardial stunning” |
| No Reflow Phenomenon | |
| Heart Arrest | |
| St elevation myocardial infarction | STEMI |
| Non-st elevated myocardial infarction | NSTEMI |
| “death/sudden cardiac” | |
| Stroke | |
| Brain Infarction | |
| Brain Stem Infarctions | |
| Lateral Medullary Syndrome | |
| Cerebral Infarction | |
| Myocardial ischemia | |
| “Dementia Multi infarct” | |
| “infarction anterior cerebral artery” | |
| “infarction middle cerebral artery” | |
| “infarction posterior cerebral artery” | |
| Myocardial revascularization | |
| Coronary artery bypass | |
| Internal mammary coronary artery anastomosis | |
| Angioplasty | “angioplasty transluminal percutaneous coronary” |
| Heart failure | |
| Hospitalization | Hospitalization? OR rehospitalization? |
| “atherectomy coronary” | |
| Coronary stent | |
| CABG | |
| “bypass grafts” | |
| “Carotid” | |
| pathology | |
| physiopathology | |
| Non-coronary revascularization procedure | |
| Carotid revascularization? | |
| Lower extremity revascularization? | |
| Percutaneous transluminal angioplast? | |
| Stent placement? | |
| Abdominal aortic aneurysm repair? | |
| AAA repair? | |
| complications | |
| Event? OR outcome? OR episode? | |
| Risk score | |
| Coronary risk modification | |
| Cardiovascular diseases | Cardiovascular OR CVD |
| Cardiovascular disease | |
| Coronary disease | coronary |
| Coronary artery disease | |
| Myocardial infarction | |
| Heart failure | CHF OR CHD |
| Cerebrovascular disorders | |
| “dyspnea paroxysmal” | |
| “edema cardiac” | |
| Physical fitness | |
| Motor activity | |
| Exercise tolerance | Metabolic equivalent |
| Metabolic equivalent | Graded exercise test OR gxt |
| Exercise test | |
| Life style or lifestyle | |
| Exercise | |
| Training | |
| Walking | |
| Vo2 | |
| Maximal met | |
| Mets | |
| Physical activity | |
| Maximal metabolic? | |
| Acute Coronary Syndrome | Acute coronary syndromes |
| Angina Unstable | Unstable angina?, “Angina Unstable” |
| Myocardial infarction | Myocardial infarctions |
| Shock cardiogenic | “shock cardiogenic” |
| Myocardial Stunning | “myocardial stunning” |
| No Reflow Phenomenon | |
| Heart Arrest | |
| St elevation myocardial infarction | STEMI |
| Non-st elevated myocardial infarction | NSTEMI |
| “death/sudden cardiac” | |
| Stroke | |
| Brain Infarction | |
| Brain Stem Infarctions | |
| Lateral Medullary Syndrome | |
| Cerebral Infarction | |
| Myocardial ischemia | |
| “Dementia Multi infarct” | |
| “infarction anterior cerebral artery” | |
| “infarction middle cerebral artery” | |
| “infarction posterior cerebral artery” | |
| Myocardial revascularization | |
| Coronary artery bypass | |
| Internal mammary coronary artery anastomosis | |
| Angioplasty | “angioplasty transluminal percutaneous coronary” |
| Heart failure | |
| Hospitalization | Hospitalization? OR rehospitalization? |
| “atherectomy coronary” | |
| Coronary stent | |
| CABG | |
| “bypass grafts” | |
| “Carotid” | |
| pathology | |
| physiopathology | |
| Non-coronary revascularization procedure | |
| Carotid revascularization? | |
| Lower extremity revascularization? | |
| Percutaneous transluminal angioplast? | |
| Stent placement? | |
| Abdominal aortic aneurysm repair? | |
| AAA repair? | |
| complications | |
| Event? OR outcome? OR episode? | |
| Risk score | |
| Coronary risk modification | |
| Cardiovascular diseases | Cardiovascular OR CVD |
| Cardiovascular disease | |
| Coronary disease | coronary |
| Coronary artery disease | |
| Myocardial infarction | |
| Heart failure | CHF OR CHD |
| Cerebrovascular disorders | |
| “dyspnea paroxysmal” | |
| “edema cardiac” | |
Because of automatic term mapping in PubMed, some MeSH terms may have been used even when not explicitly specified.
APPENDIX 2. AUTHOR RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES (RELEVANT)—2019 ACC/AHA GUIDELINE ON THE PRIMARY PREVENTION OF CARDIOVASCULAR DISEASE
| Committee Member | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|
| Donna K. Arnett (Co-Chair) | University of Kentucky College of Public Health—Dean and Professor of Epidemiology | None | None | None | None | None | None |
| Roger S. Blumenthal (Co-Chair) | Johns Hopkins University—Professor of Medicine and Director, Ciccarone Center for the Prevention of Heart Disease | None | None | None | None | None | None |
| Michelle A. Albert | UCSF School of Medicine—Professor of Medicine and Director, UCSF NURTURE Center | None | None | None | None | None | None |
| Andrew B. Buroker | Faegre Baker Daniels LLP, Partner | None | None | None | None | None | None |
| Zachary D. Goldberger | University of Wisconsin School of Medicine and Public Health—Associate Professor of Medicine, Division of Cardiology | None | None | None | None | None | None |
| Ellen J. Hahn | University of Kentucky College of Nursing—Professor & Director, BREATHE, Deputy Director, UK-CARES & Leader, and Community Engagement Core; Marcia A. Dake Professor of Nursing | None | None | None | None | None | None |
| Cheryl Dennison Himmelfarb | Johns Hopkins School of Nursing—Professor; Associate Dean Research, Office for Science and Innovation; and Deputy Director, Johns Hopkins Institute for Clinical and Translational Research | None | None | None | None | None | None |
| Amit Khera | UT Southwestern School of Medicine—Professor of Internal Medicine and Director, Preventive Cardiology Program | None | None | None | None | None | None |
| Donald Lloyd-Jones | Northwestern University—Eileen M. Foell Professor; Senior Associate Dean for Clinical and Translational Research; Chair, Department of Preventive Medicine; and Director, Clinical and Translational Sciences Institute | None | None | None | None | None | None |
| J. William McEvoy | National University of Ireland, Galway Campus—Professor of Preventive Cardiology; National Institute for Preventive Cardiology, Galway—Medical and Research Director; and University Hospital Galway, Ireland—Consultant Cardiologist. | None | None | None | None | None | None |
| Erin D. Michos | Johns Hopkins School of Medicine—Associate Professor of Medicine and Associate Director of Preventive Cardiology, Ciccarone Center for the Prevention of Heart Disease; Johns Hopkins Bloomberg School of Public Health—Associate Professor of Epidemiology | None | None | None | None | None | None |
| Michael D. Miedema | Minneapolis Heart Institute—Research Cardiologist | None | None | None | None | None | None |
| Daniel Muñoz | Vanderbilt University Medical Center—Assistant Professor of Medicine, Division of Cardiology, Medical Director for Quality, Vanderbilt Heart & Vascular Institute, and Associate Medical Director, Cardiovascular ICU | None | None | None | None | None | None |
| Sidney C. Smith, Jr | University of North Carolina, Chapel Hill—Professor of Medicine, Division of Cardiology | None | None | None | None | None | None |
| Salim S. Virani | Baylor College of Medicine—Professor, Section of Cardiovascular Research and Director for Research, Cardiology Fellowship Training Program; Michael E. DeBakey VA Medical Center—Staff Cardiologist and Investigator, Health Policy, Quality & Informatics Program, Center for Innovations in Quality, Effectiveness and Safety | None | None | None | None | None | None |
| Kim A. Williams, Sr | Rush Medical College—James B. Herrick Professor and Chief, Division of Cardiology, Department of Internal Medicine | None | None | None | None | None | None |
| Joseph Yeboah | Wake Forest Baptist Health—Associate Professor, Internal Medicine, Cardiovascular | None | None | None | None | None | None |
| Boback Ziaeian | University of California at Los Angeles/U.S. Department of Veterans Affairs Greater Los Angeles Healthcare System, David Geffen School of Medicine–Assistant Professor, Division of Cardiology | None | None | None | None | None | None |
This table represents the relationships of committee members with industry and other entities that were determined to be relevant to this document. These relationships were reviewed and updated in conjunction with all meetings and/or conference calls of the writing committee during the document development process. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5,000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted.
According to the ACC/AHA, a person has a relevant relationship IF: a) the relationship or interest relates to the same or similar subject matter, intellectual property or asset, topic, or issue addressed in the document; or b) the company/entity (with whom the relationship exists) makes a drug, drug class, or device addressed in the document or makes a competing drug or device addressed in the document; or c) the person or a member of the person’s household, has a reasonable potential for financial, professional or other personal gain or loss as a result of the issues/content addressed in the document.
ACC indicated American College of Cardiology; AHA, American Heart Association; ICU, Intensive Care Unit; LLP, Limited Liability Partnership; UCSF, University of California, San Francisco; UT, University of Texas; and VA, Veterans Affairs.
APPENDIX 3. REVIEWER RELATIONSHIPS WITH INDUSTRY AND OTHER ENTITIES (COMPREHENSIVE)—2019 ACC/AHA GUIDELINE ON THE PRIMARY PREVENTION OF CARDIOVASCULAR DISEASE
| Reviewer | Representation | Employment | Consultant | Speakers Partnership/ | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness | Salary |
|---|---|---|---|---|---|---|---|---|---|
| Amy Peterson | Official Reviewer—AHA | Hospital Affiliations: American Family Children’s Hospital; UnityPoint Health—Meriter; UW School of Medicine and Public Health Department of Pediatrics | None | None | None | None | None | None | None |
| Kim K. Birtcher | Official Reviewer— ACC/AHA Task Force on Clinical Practice Guidelines Lead Reviewer | University of Houston, College of Pharmacy, Clinical Professor |
|
None | None | None |
|
None | None |
| Sanjay Gandhi | Official Reviewer—ACC | Metro Health Medical Center Cleveland, Associate Professor, Case Western Reserve University School of Medicine | None | None | None |
|
|
None | None |
| Andrea Price | Official Reviewer—ACC Science and Quality Committee | Quality Databases at Indiana University Health, Director | None | None | None | None |
|
None | None |
| Jennifer E. Sanner Beauchamp | Content Reviewer—AHA | University of Texas Health Science Center, Cizik School of Nursing, Associate Professor | None | None | None | None | None | None | None |
| Glenn N. Levine | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Professor of Medicine at Baylor College of Medicine in Houston, Texas | None | None | None | None | None | None | |
| Patrick T. O’Gara | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Director of Strategic Planning for the Cardiovascular Division at Brigham and Women’s Hospital, the Watkins Family Distinguished Chair in Cardiology and Professor of Medicine at Harvard Medical School | None | None | None | None | None | None | |
| Joshua A. Beckman | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Director, Vascular Medicine; Professor of Medicine at Vanderbilt University |
|
None |
|
None | None | ||
| Anita Deswal | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Chief, Cardiology, Michael E. DeBakey VA Medical Center & Baylor College of Medicine, Professor, Baylor College of Medicine | None | None | None |
|
None | None | |
| Federico Gentile | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Centro Medico Diagnostico—Director, Cardiovascular Disease | None | None | None | None | None | None | None |
| José A. Joglar | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Program Director, Clinical Cardiac Electrophysiology Fellowship Program; Professor, UT Southwestern Medical Center | None | None | None | None | None | None | None |
| Duminda N. Wijeysundera | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Associate Professor Anesthesia, University of Toronto | None | None | None |
|
None |
|
|
| Eileen M. Handberg | Content Reviewer—ACC | Research Professor of Medicine; Director, Clinical Trials Program; Program Director, Florida CARES, UF Health |
|
None | None |
|
|
None | None |
| Prem Soman | Content Reviewer—ACC | Associate Professor of Medicine (Cardiology), Director, Nuclear Cardiology, UPMC |
|
None |
|
|
None | None | None |
| Eric Stecker | Content Reviewer—ACC | Associate Professor of Medicine, Division of Cardiovascular Medicine School of Medicine, OHSU | None | None |
|
None | None | None | |
| Pamela Morris | Content Reviewer—ACC | Professor, Medical University of South Carolina |
|
None | None | None | None | None | None |
| Andrew Freeman | Content Reviewer—ACC | Director, Clinical Cardiology and Operations; Co-Director, Nuclear Cardiology, National Jewish Health | None |
|
None | None | None | None | None |
| Carl J. Lavie | Content Reviewer—ACC | Medical Director, Cardiac Rehabilitation and Prevention, Ochsner Clinic Foundation | None | None | None | None | None | None | |
| James Stein | Content Reviewer—ACC | Director, UW Health Preventive Cardiology Program, Robert Turell Professor in Cardiovascular Research, UW School of Medicine and Public Health |
|
None | None | None |
|
None | None |
| Heather Johnson | Content Reviewer—ACC | Associate Professor in the Division of Cardiovascular Medicine at the University of Wisconsin School of Medicine and Public Health | None | None | None | None |
|
None | None |
| Nanette Wenger | Content Reviewer—ACC | Professor of Medicine, Division of Cardiology, Emory University School of Medicine |
|
None | None | None | None | None | |
| Michael Blaha | Content Reviewer—AHA | Director of Clinical Research, Ciccarone Center for the Prevention of Heart Disease Associate Professor of Medicine, Johns Hopkins Medicine | None | None | None | None | None | ||
| Laurence Sperling | Content Reviewer—ACC/AHA | Founder and Director of Preventive Cardiology at the Emory Clinic, Co-Director of the Cardiovascular Disease Fellowship Program at Emory, Professor of Medicine (Cardiology) at the Emory University School of Medicine | None | None | None | None | None | None | None |
| Seth Martin | Content Reviewer—ACC/AHA | Director, Advanced Lipid Disorders Program of the Ciccarone Center; Associate Professor of Medicine at Johns Hopkins Medicine |
|
None | None | None | None | ||
| Samia Mora | Content Reviewer—ACC/AHA | Associate Professor of Medicine, Harvard Medicine School Director, Center for Lipid Metabolomics, Brigham and Women’s Hospital |
|
None | None | None | None | None | |
| Clyde Yancy | Content Reviewer—ACC/AHA | Chief of Cardiology in the Department of Medicine, Northwestern Medicine | None | None | None | None |
|
None | None |
| Quinn Pack | AACVPR | Assistant Professor of Medicine at University of Massachusetts Medical School | None | None | None | None | None | None | None |
| Frank Sacks | ASN | Professor of Cardiovascular Disease Prevention, Harvard School of Public Health | None | None | None | None | None | None | |
| Salvatore Lacagnina | ACPM | System Medical Director of Wellness & Employee Health, Lee Health | None | None | None | None | None | None | None |
| Ron Blankstein | ASPC | Co-Director, Cardiovascular Imaging Training Program, Associate Physician, Preventive Cardiology, Director, Cardiac Computed Tomography, Brigham Health, Associate Professor in Medicine and Radiology, Harvard Medical School |
|
None | None |
|
None | None | |
| Jo-Ann Eastwood | PCNA | Associate Professor, UCLA School of Nursing | None | None | None | None | None | None | None |
| Stuart Haines | Content Reviewer—ACC/AHA | Professor of Pharmacy Practice, University of Mississippi | None | None |
|
None |
|
None | None |
| Michael Rich | AGS | Professor of Medicine, Washington University School of Medicine in St. Louis | None | None | None | None | None | None | None |
This table represents all relationships of reviewers with industry and other entities that were reported at the time of peer review, including those not deemed to be relevant to this document, at the time this document was under review. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥5,000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Names are listed in alphabetical order within each category of review. Please refer to http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/relationships-with-industry-policy for definitions of disclosure categories or additional information about the ACC/AHA Disclosure Policy for Writing Committees.
Significant relationship.
No financial benefit.
AACVPR indicates American Association of Cardiovascular and Pulmonary Rehabilitation; ACC, American College of Cardiology; ACPM, American College of Preventive Medicine; AGS, American Geriatrics Society; AHA, American Heart Association; ASN, American Society for Nutrition; American Society of Preventive Cardiology; DSMB, Data and Safety Monitoring Board; FDA, U.S. Food and Drug Administration; FEBS, Federation of European Biochemical Societies; JAMA, Journal of the American Medical Association; LDL-C, low-density lipoprotein cholesterol; NHLBI, National Heart, Lung, and Blood Institute; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIH, National Institutes of Health; OHSU, Oregon Health & Science University; PCNA, Preventive Cardiovascular Nurses Association; PCORI, Patient-Centered Outcomes Research Institute; UCLA, University of California, Los Angeles; UF, University of Florida; UPMC, University of Pittsburgh Medical Center; UT, University of Texas; UW, University of Wisconsin; and VA, Veterans Affairs.
Footnotes
This document was approved by the American College of Cardiology Clinical Policy Approval Committee, the American Heart Association Science Advisory and Coordinating Committee, and the American Heart Association Executive Committee in February 2019.
Copies: This document is available on the websites of the American College of Cardiology (www.acc.org) and the American Heart Association (professional.heart.org/). For copies of this document, please contact the Elsevier Inc.
REFERENCES
1. INTRODUCTION
- S1-1.Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths—trends and projections in the United States, 1969–2020. Prev Chronic Dis 2016;13:E157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S1-2.Johnson NB, Hayes LD, Brown K, et al. CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors—United States, 2005–2013. MMWR Suppl 2014;63:3–27. [PubMed] [Google Scholar]
- S1-3.Xu J, Murphy SL, Kochanek KD, et al. Mortality in the United States, 2015. NCHS Data Brief 2016:1–8. [PubMed] [Google Scholar]
- S1-4.Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 2003;290:891–7. [DOI] [PubMed] [Google Scholar]
- S1-5.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- S1-6.Turco JV, Inal-Veith A, Fuster V. Cardiovascular health promotion: an issue that can no longer wait. J Am Coll Cardiol 2018;72:908–13. [DOI] [PubMed] [Google Scholar]
- S1-7.Younus A, Aneni EC, Spatz ES, et al. A systematic review of the prevalence and outcomes of ideal cardiovascular health in US and non-US populations. Mayo Clin Proc 2016;91:649–70. [DOI] [PubMed] [Google Scholar]
1.1. Methodology and Evidence Review
- S1.1-1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–248. [DOI] [PubMed] [Google Scholar]
- S1.1-2.Grundy SM, Stone NJ, Bailey AL, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018 Nov 10 [E-pub ahead of print]. [Google Scholar]
1.5. Class of Recommendation and Level of Evidence
- S1.5-1.Halperin JL, Levine GN, Al-Khatib SM, et al. Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;67:1572–4. [DOI] [PubMed] [Google Scholar]
2. OVERARCHING RECOMMENDATIONS FOR ASCVD PREVENTION EFFORTS
2.1. Patient-Centered Approaches for Providing Comprehensive ASCVD Prevention
- S2.1-1.Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med 2009;169:1748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-2.Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care 2010;48:923–33. [DOI] [PubMed] [Google Scholar]
- S2.1-3.Fazel MT, Bagalagel A, Lee JK, et al. Impact of diabetes care by pharmacists as part of health care team in ambulatory settings: a systematic review and meta-analysis. Ann Pharmacother 2017;51:890–907. [DOI] [PubMed] [Google Scholar]
- S2.1-4.Mills KT, Obst KM, Shen W, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med 2018;168: 110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-5.Proia KK, Thota AB, Njie GJ, et al. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med 2014;47: 86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-6.Chen Z, Ernst ME, Ardery G, et al. Physician-pharmacist co-management and 24-hour blood pressure control. J Clin Hypertens (Greenwich) 2013;15: 337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-7.Hirsch JD, Steers N, Adler DS, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther 2014;36:1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-8.Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med 2008;23: 1966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-9.Isetts BJ, Buffington DE, Carter BL, et al. Evaluation of pharmacists’ work in a physician-pharmacist collaborative model for the management of hypertension. Pharmacotherapy 2016;36:374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-10.McLean DL, McAlister FA, Johnson JA, et al. A randomized trial of the effect of community pharmacist and nurse care on improving blood pressure management in patients with diabetes mellitus: study of cardiovascular risk intervention by pharmacists-hypertension (SCRIP-HTN). Arch Intern Med 2008; 168:2355–61. [DOI] [PubMed] [Google Scholar]
- S2.1-11.Polgreen LA, Han J, Carter BL, et al. Cost-effectiveness of a physician-pharmacist collaboration intervention to improve blood pressure control. Hypertension 2015;66:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-12.Chen EH, Thom DH, Hessler DM, et al. Using the Teamlet Model to improve chronic care in an academic primary care practice. J Gen Intern Med 2010; 25 suppl 4:S610–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-13.Kravetz JD, Walsh RF. Team-based hypertension management to improve blood pressure control. J Prim Care Community Health 2016;7:272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-14.Wan EYF, Fung CSC, Jiao27 FF, et al. Five-year effectiveness of the Multidisciplinary Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM) on diabetes-related complications and health service uses—a population-based and propensity-matched cohort study. Diabetes Care 2018;41:49–59. [DOI] [PubMed] [Google Scholar]
- S2.1-15.Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open 2015;5: e009116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-16.Cooper LA, Roter DL, Carson KA, et al. A randomized trial to improve patient-centered care and hypertension control in underserved primary care patients. J Gen Intern Med 2011;26:1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-17.Olomu A, Hart-Davidson W, Luo Z, et al. Implementing shared decision making in federally qualified health centers, a quasi-experimental design study: the Office-Guidelines Applied to Practice (Office-GAP) program. BMC Health S24erv Res 2016;16:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-18.Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med 2010;8: 410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-19.Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2015;132:873–98. [DOI] [PubMed] [Google Scholar]
- S2.1-20.Vilhelmsson A, Östergren P-O. Reducing health inequalities with interventions targeting behavioral factors among individuals with low levels of education—a rapid review. PLoS ONE 2018;13:e0195774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-21.Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation 2018;137: 2166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-22.Backholer K, Peters SAE, Bots SH, et al. Sex differences in the relationship between socioeconomic status and cardiovascular disease: a systematic review and meta-analysis. J Epidemiol Community Health 2017;71:550–7. [DOI] [PubMed] [Google Scholar]
- S2.1-23.Beauchamp A, Peeters A, Tonkin A, et al. Best practice for prevention and treatment of cardiovascular disease through an equity lens: a review. Eur J Cardiovasc Prev Rehabil 2010;17:599–606. [DOI] [PubMed] [Google Scholar]
- S2.1-24.Khaing W, Vallibhakara SA, Attia J, et al. Effects of education and income on cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol 2017;24:1032–42. [DOI] [PubMed] [Google Scholar]
- S2.1-25.Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health 2005;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-26.DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017;38:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-27.Magnani JW, Mujahid MS, Aronow HD, et al. Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation 2018;138:e48–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-28.Powell TM, de Lemos JA, Banks K, et al. Body size misperception: a novel determinant in the obesity epidemic. Arch Intern Med 2010;170:1695–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-29.Padgett J, Biro FM. Different shapes in different cultures: body dissatisfaction, overweight, and obesity in African-American and caucasian females. J Pediatr Adolesc Gynecol 2003;16:349–54. [DOI] [PubMed] [Google Scholar]
- S2.1-30.U.S. Department of Health and Human Services. Healthy People 2020 Washington, DC: 2010. Available at: https://www.healthypeople.gov. Accessed January 3, 2019.
- S2.1-31.Malambo P, Kengne AP, De Villiers A, et al. Built environment, selected risk factors and major cardiovascular disease outcomes: a systematic review. PLoS ONE 2016;11:e0166846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-32.Bird EL, Ige JO, Pilkington P, et al. Built and natural environment planning principles for promoting health: an umbrella review. BMC Public Health 2018; 18:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-33.Kaiser P, Diez Roux AV, Mujahid M, et al. Neighborhood environments and incident hypertension in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2016;183:988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-34.Kumanyika SK, Gary TL, Lancaster KJ, et al. Achieving healthy weight in African-American communities: research perspectives and priorities. Obes Res 2005;13:2037–47. [DOI] [PubMed] [Google Scholar]
- S2.1-35.Grandner MA. Addressing sleep disturbances: an opportunity to prevent cardiometabolic disease? Int Rev Psychiatry 2014;26:155–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-36.Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardiometabolic disease risk. Soc Sci Med 2013;79:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-37.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363:1589–97. [DOI] [PubMed] [Google Scholar]
- S2.1-38.Alam R, Sturt J, Lall R, et al. An updated meta-analysis to assess the effectiveness of psychological interventions delivered by psychological specialists and generalist clinicians on glycaemic control and on psychological status. Patient Educ Couns 2009;75:25–36. [DOI] [PubMed] [Google Scholar]
- S2.1-39.Bolen SD, Chandar A, Falck-Ytter C, et al. Effectiveness and safety of patient activation interventions for adults with type 2 diabetes: systematic review, meta-analysis, and meta-regression. J Gen Intern Med 2014;29:1166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-40.Gonzalez JS, Tanenbaum ML, Commissariat PV. Psychosocial factors in medication adherence and diabetes self-management: Implications for research and practice. Am Psychol 2016;71: 539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-41.Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest 2017;152:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-42.Samuel LJ, Dennison Himmelfarb CR, Szklo M, et al. Social engagement and chronic disease risk behaviors: the Multi-Ethnic Study of Atherosclerosis. Prev Med 2015;71:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.1-43.Verbiest M, Brakema E, van der Kleij R, et al. National guidelines for smoking cessation in primary care: a literature review and evidence analysis. NPJ Prim Care Respir Med 2017;27:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
2.2. Assessment of Cardiovascular Risk
- S2.2-1.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63: 2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-2.American College of Cardiology, American Heart Association. ASCVD Risk Estimator Available at: https://tools.acc.org/ldl/ascvd_risk_estimator/index.html#!/calulate/estimator. Accessed September 21, 2018.
- S2.2-3.Ference BA, Graham I, Tokgozoglu L, et al. Impact of lipids on cardiovascular health: JACC Health Promotion Series. J Am Coll Cardiol 2018;72:1141–56. [DOI] [PubMed] [Google Scholar]
- S2.2-4.Grundy SM, Stone NJ, Bailey AL, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018 Nov 10 [E-pub ahead of print]. [Google Scholar]
- S2.2-5.Patel J, Al Rifai M, Scheuner MT, et al. Basic vs more complex definitions of family history in the prediction of coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. Mayo Clin Proc 2018;93: 1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-6.del Rincón ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 2001;44:2737–45. [DOI] [PubMed] [Google Scholar]
- S2.2-7.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145:408–15. [DOI] [PubMed] [Google Scholar]
- S2.2-8.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017;10:e003497. [DOI] [PubMed] [Google Scholar]
- S2.2-9.Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation 2017;135: 578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-10.Wellons M, Ouyang P, Schreiner PJ, et al. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause 2012;19:1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-11.Uddin SMI, Mirbolouk M, Dardari Z, et al. Erectile dysfunction as an independent predictor of future cardiovascular events. Circulation 2018;138: 540–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-12.Triant VA, Perez J, Regan S, et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation 2018;137:2203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-13.Volgman AS, Palaniappan LS, Aggarwal NT, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation 2018;138:e1–34. [DOI] [PubMed] [Google Scholar]
- S2.2-14.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- S2.2-15.DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015;162:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-16.Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC primary prevention guidelines. J Am Coll Cardiol Img 2017;10:143–53. [Google Scholar]
- S2.2-17.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur Heart J 2018;39:2401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-18.McClelland RL, Jorgensen NW, Budoff M, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66:1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-19.Kavousi M, Desai CS, Ayers C, et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA 2016;316:2126–34. [DOI] [PubMed] [Google Scholar]
- S2.2-20.Carr JJ, Jacobs DR Jr, Terry JG, et al. Association of coronary artery calcium in adults aged 32 to 46 years with incident coronary heart disease and death. JAMA Cardiol 2017;2:391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-21.Mortensen MB, Fuster V, Muntendam P, et al. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the BioImage Study. J Am Coll Cardiol 2016;68:881–91. [DOI] [PubMed] [Google Scholar]
- S2.2-22.Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-23.Patel J, Al Rifai M, Blaha MJ, et al. Coronary artery calcium improves risk assessment in adults with a family history of premature coronary heart disease: results from the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging 2015;8:e003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-24.Pursnani A, Massaro JM, D’Agostino RB Sr., et al. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA 2015; 314:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-25.Valenti V, Ó Hartaigh B, Heo R, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. J Am Coll Cardiol Img 2015;8: 900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-26.Yano Y, O’Donnell CJ, Kuller L, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol 2017; 2:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-27.Yeboah J, Young R, McClelland RL, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol 2016;67:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-28.Multi-Ethnic Study of Atherosclerosis. MESA 10-Year CHD Risk with Coronary Artery Calcification Available at: https://www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed September 21, 2018.
- S2.2-29.Astronaut Cardiovascular Health and Risk Modification (Astro-CHARM). 10-Year ASCVD Risk Calculator with Coronary Artery Calcium Available at: http://astrocharm.org/calculator-working. Accessed September 21, 2018. [DOI] [PubMed]
- S2.2-30.Gupta A, Lau E, Varshney R, et al. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacological and lifestyle preventive therapies: a systematic review and meta-analysis. J Am Coll Cardiol Img 2017;10: 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-31.Shah RV, Spahillari A, Mwasongwe S, et al. Subclinical atherosclerosis, statin eligibility, and outcomes in African American individuals: the Jackson Heart Study. JAMA Cardiol 2017;2:644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-32.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113:791–8. [DOI] [PubMed] [Google Scholar]
- S2.2-33.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-34.Wilkins JT, Ning H, Berry J, et al. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012;308:1795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-35.Pencina MJ, D’Agostino RB Sr., Larson MG, et al. Predicting the 30-year risk of cardiovascular disease: the Framingham Heart Study. Circulation 2009;119:3078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S2.2-36.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015;66:1657–68. [DOI] [PubMed] [Google Scholar]
3. LIFESTYLE FACTORS AFFECTING CARDIOVASCULAR RISK
3.1. Nutrition and Diet
- S3.1-1.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- S3.1-2.Kim H, Caulfield LE, Rebholz CM. Healthy plant-based diets are associated with lower risk of all-cause mortality in US adults. J Nutr 2018;148:624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-3.Reedy J, Krebs-Smith SM, Miller PE, et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr 2014;144:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-4.Satija A, Bhupathiraju SN, Spiegelman D, et al. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in US adults. J Am Coll Cardiol 2017;70:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-5.Sotos-Prieto M, Bhupathiraju SN, Mattei J, et al. Association of changes in diet quality with total and cause-specific mortality. N Engl J Med 2017;377: 143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-6.Whalen KA, Judd S, McCullough ML, et al. Paleolithic and Mediterranean diet pattern scores are inversely associated with all-cause and cause-specific mortality in adults. J Nutr 2017;147:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-7.Bao Y, Han J, Hu FB, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med 2013;369:2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-8.Bernstein AM, Sun Q, Hu FB, et al. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-9.Song M, Fung TT, Hu FB, et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med 2016;176: 1453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-10.Tharrey M, Mariotti F, Mashchak A, et al. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: the Adventist Health Study-2 cohort. Int J Epidemiol 2018;47:1603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-11.Martínez-González MA, Sánchez-Tainta A, Corella D, et al. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr 2014; 100 suppl 1:320S–8S. [DOI] [PubMed] [Google Scholar]
- S3.1-12.Wang DD, Li Y, Chiuve SE, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med 2016;176:1134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-13.Dehghan M, Mente A, Zhang X, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050–62. [DOI] [PubMed] [Google Scholar]
- S3.1-14.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- S3.1-15.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007;334:885–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-16.Micha R, Peñalvo JL, Cudhea F, et al. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 2017;317:912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-17.Kiage JN, Merrill PD, Robinson CJ, et al. Intake of trans fat and all-cause mortality in the Reasons for Geographical and Racial Differences in Stroke (REGARDS) cohort. Am J Clin Nutr 2013;97:1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-18.Löfvenborg JE, Andersson T, Carlsson P-O, et al. Sweetened beverage intake and risk of latent autoimmune diabetes in adults (LADA) and type 2 diabetes. Eur J Endocrinol 2016;175:605–14. [DOI] [PubMed] [Google Scholar]
- S3.1-19.Yang Q, Zhang Z, Gregg EW, et al. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 2014;174:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-20.Johnson RK, Lichtenstein AH, Anderson CAM, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation 2018;138:e126–40. [DOI] [PubMed] [Google Scholar]
- S3.1-21.Shikany JM, Safford MM, Newby PK, et al. Southern dietary pattern is associated with hazard of acute coronary heart disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Circulation 2015;132:804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-22.Seidelmann SB, Claggett B, Cheng S, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 2018;3:e419–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-23.Trichopoulou A, Psaltopoulou T, Orfanos P, et al. Low-carbohydrate-high-protein diet and long-term survival in a general population cohort. Eur J Clin Nutr 2007;61:575–81. [DOI] [PubMed] [Google Scholar]
- S3.1-24.Noto H, Goto A, Tsujimoto T, et al. Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PLoS ONE 2013;8:e55030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-25.Brandt EJ, Myerson R, Perraillon MC, et al. Hospital admissions for myocardial infarction and stroke before and after the trans-fatty acid restrictions in New York. JAMA Cardiol 2017;2:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.1-26.Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol 2009;5:335–44. [DOI] [PubMed] [Google Scholar]
- S3.1-27.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation 2016;133:187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
3.2. Exercise and Physical Activity
- S3.2-1.Orrow G, Kinmonth A-L, Sanderson S, et al. Effectiveness of physical activity promotion based in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ 2012;344:e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.2-2.Sanchez A, Bully P, Martinez C, et al. Effectiveness of physical activity promotion interventions in primary care: a review of reviews. Prev Med 2015;76 suppl:S56–67. [DOI] [PubMed] [Google Scholar]
- S3.2-3.Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016; 388:1302–10. [DOI] [PubMed] [Google Scholar]
- S3.2-4.Hamer M, Chida Y. Walking and primary prevention: a meta-analysis of prospective cohort studies. Br J Sports Med 2008;42:238–43. [DOI] [PubMed] [Google Scholar]
- S3.2-5.Kyu HH, Bachman VF, Alexander LT, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ 2016;354:i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.2-6.Sattelmair J, Pertman J, Ding EL, et al. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 2011; 124:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.2-7.Zheng H, Orsini N, Amin J, et al. Quantifying the dose-response of walking in reducing coronary heart disease risk: meta-analysis. Eur J Epidemiol 2009;24:181–92. [DOI] [PubMed] [Google Scholar]
- S3.2-8.Wahid A, Manek N, Nichols M, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc 2016;5: e002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.2-9.Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162: 123–32. [DOI] [PubMed] [Google Scholar]
- S3.2-10.Chomistek AK, Manson JE, Stefanick ML, et al. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women’s Health Initiative. J Am Coll Cardiol 2013; 61:2346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.2-11.Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol 2018;33:811–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S3.2-12.Young DR, Hivert M-F, Alhassan S, et al. Sedentary behavior and cardiovascular morbidity and mortality: a science advisory from the American Heart Association. Circulation 2016;134:e262–79. [DOI] [PubMed] [Google Scholar]
4. OTHER FACTORS AFFECTING CARDIOVASCULAR RISK
4.1. Obesity and Being Overweight
- S4.1-1.LeBlanc EL, Patnode CD, Webber EM, et al. Draft evidence review for weight loss to prevent obesity-related morbidity and mortality in adults: behavioral interventions Kaiser Permanente Research Affiliates Evidence-based Practice Center, Kaiser Permanente Center for Health Research: Portland, OR; 2018. AHRQ Publication No. 18–05239-EF-1. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review/obesity-in-adults-interventions1. Accessed January 5, 2019. [Google Scholar]
- S4.1-2.Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ 2017;359:j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-3.Canoy D, Cairns BJ, Balkwill A, et al. Coronary heart disease incidence in women by waist circumference within categories of body mass index. Eur J Prev Cardiol 2013;20:759–62. [DOI] [PubMed] [Google Scholar]
- S4.1-4.Warren TY, Wilcox S, Dowda M, et al. Independent association of waist circumference with hypertension and diabetes in African American women, South Carolina, 2007–2009. Prev Chronic Dis 2012;9:E105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-5.Czernichow S, Kengne A-P, Stamatakis E, et al. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev 2011;12: 680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.1-6.Flint AJ, Rexrode KM, Hu FB, et al. Body mass index, waist circumference, and risk of coronary heart disease: a prospective study among men and women. Obes Res Clin Pract 2010;4:e171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
4.2. Type 2 Diabetes Mellitus
- S4.2-1.Huo R, Du T, Xu Y, et al. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: a meta-analysis. Eur J Clin Nutr 2015;69:1200–8. [DOI] [PubMed] [Google Scholar]
- S4.2-2.Azadbakht L, Fard NRP, Karimi M, et al. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care 2011;34:55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-3.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care 2006;29:2518–27. [DOI] [PubMed] [Google Scholar]
- S4.2-4.Church TS, Blair SN, Cocreham S, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010;304:2253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-5.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854–65. [PubMed] [Google Scholar]
- S4.2-6.Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016;164:740–51. [DOI] [PubMed] [Google Scholar]
- S4.2-7.Hong J, Zhang Y, Lai S, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013;36:1304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-8.Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017;60:1620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-9.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- S4.2-10.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- S4.2-11.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.2-12.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- S4.2-13.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–29. [DOI] [PubMed] [Google Scholar]
- S4.2-14.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. [DOI] [PubMed] [Google Scholar]
4.3. High Blood Cholesterol
- S4.3-1.Grundy SM, Stone NJ, Bailey AL, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018 Nov 10 [E-pub ahead of print]. [Google Scholar]
- S4.3-2.Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-3.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-4.Cholesterol Treatment Trialists’ (CTT) Collaboration, Herrington W, Emberson J, et al. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol 2016;4:829–39. [DOI] [PubMed] [Google Scholar]
- S4.3-5.Chou R, Dana T, Blazina I, et al. Statin use for the prevention of cardiovascular disease in adults: a systematic review for the U.S. Preventive Services Task Force. Report No. 14–05206-EF-2 Rockville, MD: U.S. Agency for Healthcare Research and Quality, 2016. Available at: http://www.ncbi.nlm.nih.gov/books/NBK396415. Accessed January 5, 2019. [PubMed] [Google Scholar]
- S4.3-6.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279: 1615–22. [DOI] [PubMed] [Google Scholar]
- S4.3-7.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- S4.3-8.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013:CD004816. [DOI] [PMC free article] [PubMed]
- S4.3-9.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31. [DOI] [PubMed] [Google Scholar]
- S4.3-10.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;316:1289–97. [DOI] [PubMed] [Google Scholar]
- S4.3-11.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004; 364:685–96. [DOI] [PubMed] [Google Scholar]
- S4.3-12.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–16. [DOI] [PubMed] [Google Scholar]
- S4.3-13.de Vries FM, Denig P, Pouwels KB, et al. Primary prevention of major cardiovascular and cerebrovascular events with statins in diabetic patients: a meta-analysis. Drugs 2012;72:2365–73. [DOI] [PubMed] [Google Scholar]
- S4.3-14.Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 2006;29:1478–85. [DOI] [PubMed] [Google Scholar]
- S4.3-15.Mulnier HE, Seaman HE, Raleigh VS, et al. Risk of myocardial infarction in men and women with type 2 diabetes in the UK: a cohort study using the General Practice Research Database. Diabetologia 2008;51: 1639–45. [DOI] [PubMed] [Google Scholar]
- S4.3-16.Rana JS, Liu JY, Moffet HH, et al. Diabetes and prior coronary heart disease are not necessarily risk equivalent for future coronary heart disease events. J Gen Intern Med 2016;31:387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-17.Sever PS, Poulter NR, Dahlöf B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial—lipid-lowering arm (ASCOT-LLA). Diabetes Care 2005;28:1151–7. [DOI] [PubMed] [Google Scholar]
- S4.3-18.Soedamah-Muthu SS, Fuller JH, Mulnier HE, et al. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care 2006;29:798–804. [DOI] [PubMed] [Google Scholar]
- S4.3-19.Wong ND, Glovaci D, Wong K, et al. Global cardiovascular disease risk assessment in United States adults with diabetes. Diab Vasc Dis Res 2012;9:146–52. [DOI] [PubMed] [Google Scholar]
- S4.3-20.Besseling J, Hovingh GK, Huijgen R, et al. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all-cause mortality. J Am Coll Cardiol 2016;68:252–60. [DOI] [PubMed] [Google Scholar]
- S4.3-21.Khera AV, Won H-H, Peloso GM, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol 2016;67: 2578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-22.Nanchen D, Gencer B, Muller O, et al. Prognosis of patients with familial hypercholesterolemia after acute coronary syndromes. Circulation 2016;134:698–709. [DOI] [PubMed] [Google Scholar]
- S4.3-23.Perak AM, Ning H, de Ferranti SD, et al. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation 2016;134:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-24.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333:1301–7. [DOI] [PubMed] [Google Scholar]
- S4.3-25.Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ 2008; 337:a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-26.Mortensen MB, Fuster V, Muntendam P, et al. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the BioImage Study. J Am Coll Cardiol 2016; 68:881–91. [DOI] [PubMed] [Google Scholar]
- S4.3-27.Willeit P, Kiechl S, Kronenberg F, et al. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol 2014; 64:851–60. [DOI] [PubMed] [Google Scholar]
- S4.3-28.Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015;66:1657–68. [DOI] [PubMed] [Google Scholar]
- S4.3-29.Ridker PM, Mora S, Rose L, et al. Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J 2016;37:1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-30.Yano Y, O’Donnell CJ, Kuller L, et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol 2017; 2:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-31.Malik S, Zhao Y, Budoff M, et al. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the Multi-Ethnic Study of Atherosclerosis. JAMA Cardiol 2017;2:1332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-32.Sniderman AD, Tsimikas S, Fazio S. The severe hypercholesterolemia phenotype: clinical diagnosis, management, and emerging therapies. J Am Coll Cardiol 2014;63:1935–47. [DOI] [PubMed] [Google Scholar]
- S4.3-33.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes 2011;4:337–45. [DOI] [PubMed] [Google Scholar]
- S4.3-34.Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J 2018;39:2401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-35.Cholesterol Treatment Trialists’ (CTT) Collaborators, Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-36.Huo X, Gao L, Guo L, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol 2016;4:115–24. [DOI] [PubMed] [Google Scholar]
- S4.3-37.Svensson MK, Cederholm J, Eliasson B, et al. Albuminuria and renal function as predictors of cardiovascular events and mortality in a general population of patients with type 2 diabetes: a nation-wide observational study from the Swedish National Diabetes Register. Diab Vasc Dis Res 2013;10:520–9. [DOI] [PubMed] [Google Scholar]
- S4.3-38.Guo VY, Cao B, Wu X, et al. Prospective association between diabetic retinopathy and cardiovascular disease—a systematic review and meta-analysis of cohort studies. J Stroke Cerebrovasc Dis 2016;25:1688–95. [DOI] [PubMed] [Google Scholar]
- S4.3-39.Brownrigg JRW, de Lusignan S, McGovern A, et al. Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart 2014;100:1837–43. [DOI] [PubMed] [Google Scholar]
- S4.3-40.Ogren M, Hedblad B, Engström G, et al. Prevalence and prognostic significance of asymptomatic peripheral arterial disease in 68-year-old men with diabetes. Results from the population study “Men born in 1914” from Malmö, Sweden. Eur J Vasc Endovasc Surg 2005;29:182–9. [DOI] [PubMed] [Google Scholar]
- S4.3-41.Pang X-H, Han J, Ye W-L, et al. Lower extremity peripheral arterial disease is an independent predictor of coronary heart disease and stroke risks in patients with type 2 diabetes mellitus in China. Int J Endocrinol 2017;2017:9620513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.3-42.Waheed S, Pollack S, Roth M, et al. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: The St Francis Heart Study. Atherosclerosis 2016;255:193–9. [DOI] [PubMed] [Google Scholar]
4.4. High Blood Pressure or Hypertension
- S4.4-1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–248. [DOI] [PubMed] [Google Scholar]
- S4.4-2.Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2003;42: 878–84. [DOI] [PubMed] [Google Scholar]
- S4.4-3.The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA 1992;267:1213–20. [DOI] [PubMed] [Google Scholar]
- S4.4-4.Whelton PK, Kumanyika SK, Cook NR, et al. Efficacy of nonpharmacologic interventions in adults with high-normal blood pressure: results from phase 1 of the Trials of Hypertension Prevention. Trials of Hypertension Prevention Collaborative Research Group. Am J Clin Nutr 1997;65:652S–60S. [DOI] [PubMed] [Google Scholar]
- S4.4-5.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med 1997;157:657–67. [PubMed] [Google Scholar]
- S4.4-6.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- S4.4-7.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- S4.4-8.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003;289: 2083–93. [DOI] [PubMed] [Google Scholar]
- S4.4-9.Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014;371:624–34. [DOI] [PubMed] [Google Scholar]
- S4.4-10.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- S4.4-11.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998;279:839–46. [DOI] [PubMed] [Google Scholar]
- S4.4-12.Aburto NJ, Ziolkovska A, Hooper L, et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-13.Graudal NA, Hubeck-Graudal T, Jürgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). Am J Hypertens 2012;25:1–15. [DOI] [PubMed] [Google Scholar]
- S4.4-14.Whelton PK, He J, Cutler JA, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 1997;277: 1624–32. [DOI] [PubMed] [Google Scholar]
- S4.4-15.Aburto NJ, Hanson S, Gutierrez H, et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-16.Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens 2003;17:471–80. [DOI] [PubMed] [Google Scholar]
- S4.4-17.World Health Association. Guideline: Potassium Intake for Adults and Children Geneva, Switzerland: World Health Organization, Department of Nutrition for Health and Development; 2012. [Google Scholar]
- S4.4-18.Whelton PK, He J. Health effects of sodium and potassium in humans. Curr Opin Lipidol 2014;25: 75–9. [DOI] [PubMed] [Google Scholar]
- S4.4-19.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-20.Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 2002; 136:493–503. [DOI] [PubMed] [Google Scholar]
- S4.4-21.Carlson DJ, Dieberg G, Hess NC, et al. Isometric exercise training for blood pressure management: a systematic review and meta-analysis. Mayo Clin Proc 2014;89:327–34. [DOI] [PubMed] [Google Scholar]
- S4.4-22.García-Hermoso A, Saavedra JM, Escalante Y. Effects of exercise on resting blood pressure in obese children: a meta-analysis of randomized controlled trials. Obes Rev 2013;14:919–28. [DOI] [PubMed] [Google Scholar]
- S4.4-23.Rossi AM, Moullec G, Lavoie KL, et al. The evolution of a Canadian Hypertension Education Program recommendation: the impact of resistance training on resting blood pressure in adults as an example. Can J Cardiol 2013;29:622–7. [DOI] [PubMed] [Google Scholar]
- S4.4-24.Roerecke M, Kaczorowski J, Tobe SW, et al. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Health 2017;2:e108–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-25.Xin X, He J, Frontini MG, et al. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2001; 38:1112–7. [DOI] [PubMed] [Google Scholar]
- S4.4-26.Stewart SH, Latham PK, Miller PM, et al. Blood pressure reduction during treatment for alcohol dependence: results from the Combining Medications and Behavioral Interventions for Alcoholism (COMBINE) study. Addiction 2008;103:1622–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-27.Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens 2006;24:215–33. [DOI] [PubMed] [Google Scholar]
- S4.4-28.Wallace P, Cutler S, Haines A. Randomised controlled trial of general practitioner intervention in patients with excessive alcohol consumption. BMJ 1988;297:663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-29.Lang T, Nicaud V, Darné B, et al. Improving hypertension control among excessive alcohol drinkers: a randomised controlled trial in France. The WALPA Group. J Epidemiol Community Health 1995;49:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-30.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-31.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–67. [DOI] [PubMed] [Google Scholar]
- S4.4-32.Sundström J, Arima H, Jackson R, et al. Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Ann Intern Med 2015;162:184–91. [DOI] [PubMed] [Google Scholar]
- S4.4-33.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387:435–43. [DOI] [PubMed] [Google Scholar]
- S4.4-34.SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-35.Czernichow S, Zanchetti A, Turnbull F, et al. The effects of blood pressure reduction and of different blood pressure-lowering regimens on major cardiovascular events according to baseline blood pressure: meta-analysis of randomized trials. J Hypertens 2011;29:4–16. [DOI] [PubMed] [Google Scholar]
- S4.4-36.Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 2014;384:591–8. [DOI] [PubMed] [Google Scholar]
- S4.4-37.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 2. Effects at different baseline and achieved blood pressure levels—overview and meta-analyses of randomized trials. J Hypertens 2014;32: 2296–304. [DOI] [PubMed] [Google Scholar]
- S4.4-38.Thompson AM, Hu T, Eshelbrenner CL, et al. Antihypertensive treatment and secondary prevention of cardiovascular disease events among persons without hypertension: a meta-analysis. JAMA 2011; 305:913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-39.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels—updated overview and meta-analyses of randomized trials. J Hypertens 2016;34:613–22. [DOI] [PubMed] [Google Scholar]
- S4.4-40.Verdecchia P, Angeli F, Gentile G, et al. More versus less intensive blood pressure-lowering strategy: cumulative evidence and trial sequential analysis. Hypertension 2016;68:642–53. [DOI] [PubMed] [Google Scholar]
- S4.4-41.Bangalore S, Toklu B, Gianos E, et al. Optimal systolic blood pressure target after SPRINT: insights from a network meta-analysis of randomized trials. Am J Med 2017;130:707–19.e8. [DOI] [PubMed] [Google Scholar]
- S4.4-42.Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017;2:775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-43.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet 2005;365:939–46. [DOI] [PubMed] [Google Scholar]
- S4.4-44.Wright JT Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–31. [DOI] [PubMed] [Google Scholar]
- S4.4-45.Upadhyay A, Earley A, Haynes SM, et al. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011;154:541–8. [DOI] [PubMed] [Google Scholar]
- S4.4-46.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med 2003;139:244–52. [DOI] [PubMed] [Google Scholar]
- S4.4-47.Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ 2013;185:949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-48.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 1994;330:877–84. [DOI] [PubMed] [Google Scholar]
- S4.4-49.Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015;313:603–15. [DOI] [PubMed] [Google Scholar]
- S4.4-50.Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev 2013: CD008277. [DOI] [PMC free article] [PubMed]
- S4.4-51.Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care 2014;37:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-52.Bress AP, Bellows BK, King JB, et al. Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med 2017;377: 745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-53.Soliman EZ, Byington RP, Bigger JT, et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with diabetes mellitus: Action to Control Cardiovascular Risk in Diabetes blood pressure trial. Hypertension 2015;66:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-54.ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-55.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- S4.4-56.Kassaï B, Boissel J-P, Cucherat M, et al. Treatment of high blood pressure and gain in event-free life expectancy. Vasc Health Risk Manag 2005; 1:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.4-57.van Dieren S, Kengne AP, Chalmers J, et al. Effects of blood pressure lowering on cardiovascular outcomes in different cardiovascular risk groups among participants with type 2 diabetes. Diabetes Res Clin Pract 2012;98:83–90. [DOI] [PubMed] [Google Scholar]
- S4.4-58.Montgomery AA, Fahey T, Ben-Shlomo Y, et al. The influence of absolute cardiovascular risk, patient utilities, and costs on the decision to treat hypertension: a Markov decision analysis. J Hypertens 2003;21:1753–9. [DOI] [PubMed] [Google Scholar]
- S4.4-59.Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374:2009–20. [DOI] [PubMed] [Google Scholar]
- S4.4-60.Neaton JD, Grimm RH Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA 1993;270:713–24. [PubMed] [Google Scholar]
- S4.4-61.Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006; 354:1685–97. [DOI] [PubMed] [Google Scholar]
- S4.4-62.Lawes CMM, Bennett DA, Lewington S, et al. Blood pressure and coronary heart disease: a review of the evidence. Semin Vasc Med 2002;2:355–68. [DOI] [PubMed] [Google Scholar]
- S4.4-63.Inder JD, Carlson DJ, Dieberg G, et al. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res 2016;39:88–94. [DOI] [PubMed] [Google Scholar]
- S4.4-64.National Institute on Alcohol Abuse and Alcoholism. What Is A Standard Drink? Available at: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed August 16, 2017.
- S4.4-65.National Heart, Lung, and Blood Institute. Your Guide to Lowering Your Blood Pressure With DASH—How Do I Make the DASH? Available at: https://health.gov/dietaryguidelines/dga2005/toolkit/DASH/how_make_dash.htm. Accessed January 6, 2019.
- S4.4-66.Top 10 Dash Diet Tips Available at: http://dashdiet.org/dash-diet-tips.html. Accessed January 6, 2019.
4.5. Tobacco Use
- S4.5-1.Carson KV, Verbiest MEA, Crone MR, et al. Training health professionals in smoking cessation. Cochrane Database Syst Rev 2012:CD000214. [DOI] [PMC free article] [PubMed]
- S4.5-2.Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S. Preventive Services Task Force. Ann Intern Med 2015; 163:608–21. [DOI] [PubMed] [Google Scholar]
- S4.5-3.Stead LF, Koilpillai P, Fanshawe TR1, et al. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2016;3:CD008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5-4.Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation 2015;132:1795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5-5.Mons U, Müezzinler A, Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ 2015;350:h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.5-6.Lv X, Sun J, Bi Y, et al. Risk of all-cause mortality and cardiovascular disease associated with secondhand smoke exposure: a systematic review and meta-analysis. Int J Cardiol 2015;199:106–15. [DOI] [PubMed] [Google Scholar]
- S4.5-7.Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC Expert Consensus Decision Pathway on Tobacco Cessation Treatment: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2018;72:3332–65. [DOI] [PubMed] [Google Scholar]
- S4.5-8.Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016;387:2507–20. [DOI] [PubMed] [Google Scholar]
- S4.5-9.Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2016;67:467–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
4.6. Aspirin Use
- S4.6-1.Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2016; 164:804–13. [DOI] [PubMed] [Google Scholar]
- S4.6-2.Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164:826–35. [DOI] [PubMed] [Google Scholar]
- S4.6-3.Lotrionte M,BiasucciL M,Peruzzi M,et al.Which aspirin dose and preparation is best for the long-term prevention of cardiovascular disease and cancer? Evidence from a systematic review and network meta-analysis. Prog Cardiovasc Dis 2016;58:495–504. [DOI] [PubMed] [Google Scholar]
- S4.6-4.Raju N, Sobieraj-Teague M, Bosch J, et al. Updated meta-analysis of aspirin in primary prevention of cardiovascular disease. Am J Med 2016;129: e35–6. [DOI] [PubMed] [Google Scholar]
- S4.6-5.Ogawa H, Nakayama M, Morimoto T, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008;300:2134–41. [DOI] [PubMed] [Google Scholar]
- S4.6-6.Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008;337:a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.6-7.Fowkes FGR, Price JF, Stewart MCW, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA 2010;303: 841–8. [DOI] [PubMed] [Google Scholar]
- S4.6-8.Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014;312:2510–20. [DOI] [PubMed] [Google Scholar]
- S4.6-9.McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018;379:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S4.6-10.García Rodríguez LA, Martín-Pérez M, Hennekens CH, et al. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PLoS ONE 2016;11:e0160046. [DOI] [PMC free article] [PubMed] [Google Scholar]
5. COST AND VALUE CONSIDERATIONS
- S5-1.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation 2011;124:967–90. [DOI] [PubMed] [Google Scholar]
- S5-2.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2304–22. [DOI] [PubMed] [Google Scholar]
- S5-3.Bress AP, Bellows BK, King JB, et al. Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med. 2017;377:745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-4.Moran AE, Odden MC, Thanataveerat A, et al. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med 2015;372: 447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-5.Park C, Wang G, Durthaler JM, et al. Cost-effectiveness analyses of antihypertensive medicines: a systematic review. Am J Prev Med 2017;53:S131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-6.Richman IB, Fairley M, Jørgensen ME, et al. Cost-effectiveness of intensive blood pressure management. JAMA Cardiol 2016;1:872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-7.Heller DJ, Coxson PG, Penko J, et al. Evaluating the impact and cost-effectiveness of statin use guidelines for primary prevention of coronary heart disease and stroke. Circulation 2017;136:1087–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-8.Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015; 162:533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S5-9.Heart Protection Study Collaborative Group. Statin cost-effectiveness in the United States for people at different vascular risk levels. Circ Cardiovasc Qual Outcomes 2009;2:65–72. [DOI] [PubMed] [Google Scholar]
6. CONCLUSION
- S6-1.Lehr AL, Driver SL, Stone NJ. The ABCDs of lifestyle counseling. JAMA Cardiol 2016;1:505–6. [DOI] [PubMed] [Google Scholar]
APPENDIX 1. SEARCH CRITERIA
- 1.Patnode CD, Evans CV, Senger CA, et al. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017;318:175–93. [DOI] [PubMed] [Google Scholar]
- 2.LeBlanc ES, Patnode CD, Webber EM, et al. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018;320:1172–91. [DOI] [PubMed] [Google Scholar]
- 3.Selph S, Dana T, Bougatsos C, et al. Screening for abnormal glucose and type 2 diabetes mellitus: a systematic review to update the 2008 U.S. Preventive Services Task Force Recommendation. Report No. 13–05190EF-1 Rockville, MD: U.S. Agency for Healthcare Research and Quality,2015.Availableat: http://www.ncbi.nlm.nih.gov/books/NBK293871. Accessed January 5, 2019. [PubMed] [Google Scholar]
- 4.Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S. Preventive ServicesTask Force.Ann Intern Med 2015;163:608–21. [DOI] [PubMed] [Google Scholar]
- 5.Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2016; 164:804–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


