Abstract
Background
Patients with chronic illness are at increased risk for traumatic stress because of medical trauma. Initial studies of posttraumatic stress (PTS) in patients with inflammatory bowel disease (IBD) have found that approximately one-third of patients may experience significant PTS symptoms including flashbacks, nightmares, hypervigilance, disrupted sleep, and low mood. We aim to better characterize PTS in IBD and its relationship with patient outcomes in a large cohort of patients with IBD.
Methods
Adult patients registered with the Crohn’s & Colitis Foundation/University of North Carolina IBD Partners database were invited to complete a supplementary survey between February and July 2020. The Post Traumatic Stress Disorder Checklist-5th edition was administered as a supplemental survey. Additional data from IBD Partners included disease severity, surgery and hospital history, demographics, and health care utilization.
Results
A total of 797 patients participated (452 with Crohn disease, 345 with ulcerative colitis). No impacts on response patterns because of the COVID-19 pandemic were found. Although 5.6% of the sample reported an existing PTS diagnosis because of IBD experiences, 9.6% of participants met the full IBD-related PTS diagnostic criteria per the Post Traumatic Stress Disorder Checklist-5th edition. Female patients, younger patients, those with less educational attainment, non-White patients, and Hispanic patients reported higher levels of PTS symptoms. Patients with higher PTS symptoms were more likely to have been hospitalized, have had surgery, have more severe symptoms, and not be in remission. Increased PTS was also associated with increased anxiety, depression, pain interference, fatigue, and health care utilization.
Conclusions
The present findings support prior research that approximately one-quarter to one-third of patients with IBD report significant symptoms of PTS directly from their disease experiences, and certain demographic groups are at higher risk. In addition, PTS is associated with several IBD outcomes. Patients with higher PTS symptoms are less likely to be in remission and may utilize more outpatient gastrointestinal services. Intervention trials to mitigate PTS symptoms in patients with IBD are warranted.
Keywords: inflammatory bowel disease, post-traumatic stress, medical trauma
Introduction
Chronic psychological sequelae from a traumatic experience, including post-traumatic stress disorder (PTSD), are widely studied phenomena.1 At-risk groups include combat veterans, victims of crime or terrorism, and those surviving natural disasters. Traumatic stress reactions may become pervasive and chronic without proper treatment, with approximately 10% of the U.S. population diagnosed with PTSD.2-4 Trauma can also occur with medical events, such as a hospitalization in an intensive care unit5 or a near-death experience from severe illness. In medical patients, posttraumatic stress (PTS) is the most widely accepted and studied construct.
The symptoms of PTS mirror those of PTSD but may be less severe (ie, subclinical) or may not include every criterion from the Diagnostic and Statistical Manual for Mental Disorders, 5th edition (DSM-5). However, people with PTS may experience pervasive flashbacks, nightmares, emotional numbing or irritability, avoidance of triggering situations, and physiological hyperarousal.1 Traumatic stress occurs in 9% to 27% of patients who experience an acute medical event. Medical PTS is most often explored among patients with cancers,6, 7 cardiovascular disease,8, 9 and HIV/AIDS,10, 11 with multiple studies showing persistent trauma symptoms. Because patients with severe inflammatory bowel disease (IBD) often have repeated emergency department visits and emergency surgeries and may utilize treatments with rare but serious adverse effects, it is possible that a proportion of these patients will develop PTS.
Only 2 studies to date have evaluated PTS in patients with IBD. In 2010, a study of a large Swiss cohort of 597 patients with Crohn disease (CD) found that 19.5% of patients had symptoms consistent with PTSD.12 Those patients were 4.3 times more likely to experience disease exacerbation than patients with milder traumatic stress symptoms and 13 times more likely to have an IBD flare-up than those reporting no symptoms of PTSD. In 2019, our study of 131 patients with IBD in the United States found that 32% reported significant PTS symptoms, with patients with CD more likely to experience PTS than those with ulcerative colitis (UC).13 Risk factors for PTS included IBD severity, negative hospital experiences, and surgery leading to an ileostomy.
Chronic traumatic stress is associated with poor outcomes in other diseases. These include decreases in health-related quality of life and treatment adherence and increases in health care utilization (HCU). Anxiety, depression, substance abuse, and suicide14, 15 are also possible. In IBD, very little is known about PTS and IBD outcomes. For example, untreated PTS can affect disease severity. Chronic inflammation is consistently implicated in PTSD via elevations in acute-phase proteins, pro- and anti- inflammatory cytokines (eg, interleukin-6, interleukin-1β, tumor necrosis factor α, and interferon γ), innate immune cells, and white blood cell counts.16-20 Many of the inflammatory mechanisms in PTSD overlap with those in IBD. In addition, the brain-gut-microbiome axis is implicated in chronic PTSD,21, 22 suggesting that PTS may have multifactorial, deleterious effects on outcomes for patients with IBD.
This study aims to look at a national, large, representative sample from the Crohn’s & Colitis Foundation IBD Partners database. Specifically, we aim to (1) measure the prevalence of PTS symptoms with a validated measure using DSM-5 criteria; (2) determine how PTS symptoms may relate to IBD, disease activity, and 2 specific outcomes that drive the costs of care—namely, the number of hospitalizations and surgeries; and (3) evaluate how PTS symptoms relate to psychological distress, pain interference, sleep and fatigue, and HCU via visits to primary care or gastroenterology clinics in the past year. We hypothesize that PTS symptoms are associated with poorer patient outcomes and increased HCU.
MATERIALS AND METHODS
A cross-sectional observational study was conducted with patients registered in the IBD Partners database (https://ccfa.med.unc.edu/) between February and August 2020. IBD Partners is a research-dedicated national database of the Crohn’s & Colitis Foundation of >13,000 adult patients with IBD with a robust existing dataset designed to supplement additional surveys. After completing the standard questionnaires for IBD Partners, patients were given the option to participate in the present study. Participants were invited to participate if they lived in the United States and had a record of at least 1 surgery or 1 hospitalization because of IBD or had ever scored higher than 220 on the Simple Crohn’s Disease Activity Index for CD or higher than 5 on the Simple Colitis Clinical Activity Index (SCCAI) for UC or indeterminate IBD. Before completing the study questions, participants were screened for pre-existing PTSD not related to IBD. Those who endorsed a non-IBD-related PTSD diagnosis were excluded, and participants who endorsed a PTS diagnosis related to IBD were included.
Those who agreed and met the inclusion criteria completed the following additional questionnaire. The PTSD Checklist-5 (PCL-5)23 is a standardized questionnaire that measures symptoms of PTSD outlined in the DSM-5 over the past month using the prompt “In the past month, how much were you been bothered by….” The PCL-5 contains 20 items (eg, “Repeated, disturbing, and unwanted memories of the stressful experience?” and “Feeling jumpy or easily startled”) that encompass the 4 DSM-5 symptom groups of PTSD (intrusive memories, avoidance, negative changes in thinking and mood, changes in physical and emotional reactions). Responses are ranked on a 5-point Likert scale (0 = not at all to 4 = extremely). Participants are instructed to think about IBD-related traumatic events and avoid thinking about traumatic events that are not related to their disease when answering the questions. A total score of 80 is obtained by summing the 20 items. A DSM-5 symptom group severity score is obtained by summing items within that given criterion (B: intrusion/re-experiencing = items 1-5; C: avoidant symptoms = items 6-7; D: negative mood/thoughts = items 8-14; E: increased arousal = items 15-20). A provisional diagnosis of PTS is made by counting the number of items in each cluster rated as “moderately” or higher and following the DSM-5 criteria: 1 B item, 1 C item, 2 D items, and 2 E items. A suggested cutoff score of 31 is indicative of PTSD but is not fully validated and was therefore not used in this study.
Data Extracted From the Main IBD Partners Database
Disease severity
For patients with CD, the total score of the patient version of the Harvey-Bradshaw Index (pHBI) was computed with a cutoff for remission as pHBI < 5. For patients with UC, the total score of the SCCAI was calculated with a cutoff for remission as SCCAI < 5. Two additional categorical questions about disease activity in the last 6 months (constant, most days, 1-2 days a week, 1-2 days a month, few days, no symptoms) and in the last week (severely active, moderately active, mildly active, minimal, remission) were included.
Hospitalizations and surgeries
Any history of hospitalization or surgery for IBD was dichotomized (yes, no), and the number of hospitalizations or surgeries was reported on a continuous scale.
HCU
Patients self-reported the number of visits they had to their primary care physician and to their gastroenterologist in the past year. Responses were categorical (never, 1-2, 3-4, 5 or more) for each variable.
Additional IBD Partners variables
Sex, age, age at diagnosis, race, ethnicity, highest level of education, state of residence, type of gastrointestinal (GI) clinic (university, private practice, Veterans Administration), anxiety, depression, sleep quality, fatigue, and pain interference were also recorded.
Ethical Considerations
This study was approved by the Institutional Review Board of Northwestern University and the University of North Carolina/IBD Partners. All participants completed an online consent before completing the study questionnaires.
Statistical Analyses
A priori power analyses for the planned statistics, assuming a power of 0.80, a Cohen’s d effect size of 0.30, and an error probability of P < 0.01, indicated that a minimum sample of 526 patients was needed. Data were exported from the IBD Partners database into an Microsoft Excel workbook and were then imported into SPSS v.26 for Macintosh (Chicago, IL) for analyses. Total scores for the PCL-5 and the DSM-5 symptom groups were calculated. Participant responses were coded 1 = yes and 0 = no for each criterion rated as “moderately” or higher. Tests for normal distribution and nonparametric tests were conducted using skewness/kurtosis cutoffs of ±2.0. Given the small number of the cohort, the patients with indeterminate IBD were pooled with the UC group.
Descriptive statistics are presented as mean (SD) for continuous and percentage (frequency) for categorical variables. States were organized into the 4 geographic regions of the U.S. Census: northeast, south, midwest, and west. The PCL-5 total score was nonnormally distributed. Therefore, DSM-5 symptom group scores were dichotomized to yes/no for each criterion (re-experiencing, avoidance, negative mood/thoughts, and increased arousal) and to meeting the full DSM-5 criteria for PTS as outlined above. Chi-square analyses with the Fisher exact test (where appropriate) evaluated differences when we compared categorical variables (eg, IBD diagnosis, sex, education, prior surgery). Independent-samples t tests examined relationships between PTSD symptom groups for continuous variables (age, number of hospitalizations, number of surgeries). The number of hospitalizations was also nonnormally distributed. As such, differences between groups were evaluated using the Mann-Whitney U test with median and interquartile range (IQR) reported.
Binary logistic regression models were used to evaluate the relationship of PTS symptoms and hospitalization history and PTS symptoms and surgical history (each dichotomized as no = 0, yes = 1). Any demographic or clinical variables also associated with these 2 outcomes were also entered into the regressions model to control for their effects. The models were organized by demographic and clinical variables, where appropriate, with each PTS symptom category and full IBD-related PTS diagnosis. Odds ratios (ORs) with 95% confidence intervals (CIs) are reported with forest plots.
Separate hierarchical linear regression models were used to evaluate the relationship between PTS symptoms and the following outcome variables: (1) symptom severity in the past week, (2) symptom severity in the past 6 months, (3) primary care physician (PCP) visits in the past year, (4) GI clinic visits in the past year. Any demographic or clinical variables also associated with each outcome were entered into the regression model first to control for their effects. Standardized beta weights are reported for each model. Statistical significance was set to P < 0.05 for all analyses.
COVID-19 Pandemic Impact
Participants were coded by the study date/time stamp for survey completion as “pre-COVID” and “post-COVID” using a date of March 31, 2020. Mann-Whitney U test and chi-square analyses evaluated differences between the pre- and post-COVID groups for PCL-5 scores and symptom cluster. No significant differences existed based on survey timing during the COVID-19 pandemic for the PCL-5 score (P = 0.894), the proportion meeting the PTS diagnostic cutoff (P = 0.333), and the proportion scoring moderately or higher on any PTS symptom criterion (P = 0.121-0.404).
RESULTS
Study Sample
During the study period, 1352 patients were presented with the option to enroll. Of these, 914 (68%) agreed to participate. An existing PTS/PTSD diagnosis was reported by 107 people (94 women, 13 men). Sixty-two out of 107 people indicated that their PTSD was not related to IBD and were therefore excluded from the study. Fifty-five participants did not complete the PCL-5, which left a final sample of 797 participants (452 with CD, 345 with UC). Demographic and clinical characteristics of the sample are outlined in Tables 1 and 2, respectively. Patients in the sample were predominantly White, non-Hispanic, middle aged (mean [SD] = 49.22 [15.14]), female, and college educated.. Participants were evenly divided across the 4 major geographic regions of the United States (west, south, midwest, northeast), and no significant differences existed for PTS. Participants tended to be diagnosed with IBD in their late 20s. The majority (80%) had at least 1 prior hospitalization, and half (54%) had at least 1 prior surgery because of IBD. On average, patients reported 4.15 prior surgeries and 3.03 prior hospitalizations. Less than 10% had an ostomy or J-Pouch. Almost all participants had both a primary care physician (PCP) and a gastroenterologist, with most patients receiving treatment from a private practice or university/academic hospital clinic.
Table 1.
Demographic Information for Study Sample
| N = 797 | |
|---|---|
| Age (y) | 49.22 (15.14) |
| Sex | |
| Male | 27.6% (220) |
| Female | 72.4% (577) |
| Race | |
| White | 95.1% (723) |
| Black/African American | 1.4% (11) |
| Asian | 1.1% (8) |
| Multiracial | 1.8% (14) |
| Other | 0.5% (4) |
| Unknown | 4.6% (37) |
| Ethnicity | |
| Hispanic | 2.3% (18) |
| Non-Hispanic | 97.7% (754) |
| Education | |
| Less than college | 22.3% (173) |
| College graduate or higher | 77.8% (604) |
| Unknown | 2.5% (20) |
Table 2.
Clinical Characteristics of Study Sample
| N = 797 | |
|---|---|
| Age at diagnosis (y) | 28.53 (13.47) |
| CD | 56.7% (452) |
| UC | 43.3% (345) |
| Ostomy | 7.9% (63) |
| J-Pouch | 9.7% (77) |
| Prior surgery for IBD | 53.8% (429) |
| Number of prior surgeries | 4.15 (2.31); range, 1-14 |
| Prior hospitalization for IBD | 80.2% (639) |
| Number of prior hospitalizations | 3.03 (2.37); range: 1-10 |
| Duration, d (most stressful hospitalization) | |
| 1-3 | 19.5% (128) |
| 4-7 | 35.6% (233) |
| 8-14 | 22.6% (148) |
| >14 | 19.8% (130) |
| Unknown | 2.4% (16) |
| Has PCP | 90.9% (720) |
| PCP visits in past year | |
| None | 7.9% (57) |
| 1-2 | 63.1% (454) |
| 3-4 | 22.1% (159) |
| >5 | 6.9% (50) |
| Unknown | 9.7% (77) |
| Has gastroenterologist | 96.8% (768) |
| Type of GI clinic | |
| University/academic | 29.4% (226) |
| Private practice | 60.3% (463) |
| Veterans Administration | 0.4% (3) |
| Other | 8.6% (66) |
| Unknown | 4.9% (39) |
| GI visits in past year | |
| None | 7.2% (55) |
| 1-2 | 60.2% (462) |
| 3-4 | 23.2% (178) |
| >5 | 9.1% (70) |
| Unknown | 3.9% (32) |
Prevalence of PTS Symptoms in IBD
Based on the self-report item “Have you ever been diagnosed with PTS/PTSD and is this because of your IBD?” 5.6% of participants in the sample had a diagnosis of IBD-related PTS. However, 9.6% of participants met the full diagnostic criteria for PTS, or all symptom criteria having the minimum number of items rated at “moderately” or higher on the PCL-5 (Fig. 1). When examining each symptom group (re-experiencing, avoidance, negative mood, increased arousal), we found that the proportion of participants with a positive score ranged from 21% to 26%; no significant differences existed between CD and UC. The younger a patient’s age, the more PTS symptoms reported across all symptom groups and were more likely to meet the full criteria for IBD-related PTS (all P < 0.001).
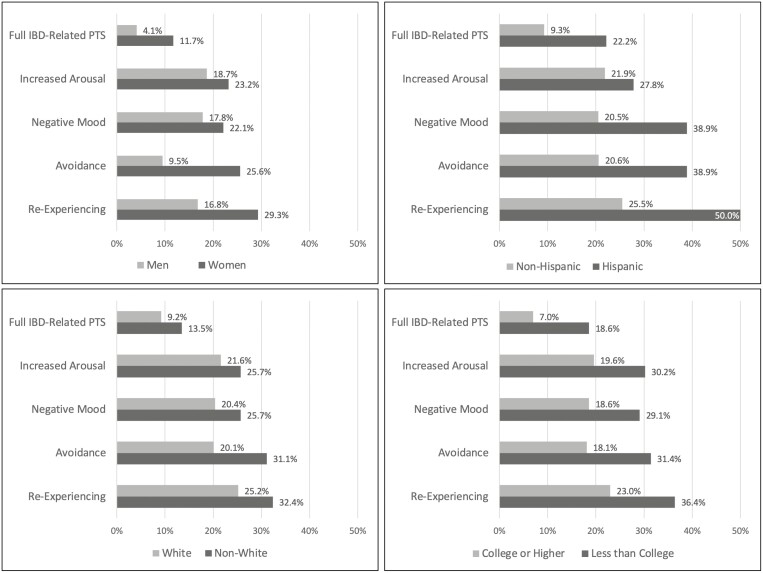
Figure 1.
Percentage of patients within each demographic group meeting criteria for each PTS symptom cluster and full PTSD.
Demographic and Illness Traits Associated With PTS
Women were significantly more likely to meet the full IBD-related PTS criteria than men, including re-experiencing the event(s) through dreams, flashbacks or intrusive thoughts, and avoidance behaviors (all P < 0.001; Fig. 1). Both non-White patients and non-Hispanic patients reported more avoidance behaviors than White patients (P = 0.023) and Hispanic patients (P = 0.024); these results should be interpreted with caution because of the small sample sizes for racial and ethnic minorities. Patients whose education was less than a college degree were more likely to meet the full criteria for IBD-related PTS and all symptom groups (re-experiencing, avoidance, negative mood, increased arousal; all P < 0.004).
Certain illness traits were also associated with IBD-related PTS. Patients with CD and a history of abscess reported more re-experiencing (P = 0.03) and low mood symptoms (P = 0.05). For patients with UC, those who reported “immediate” or higher bowel urgency reported significantly more re-experiencing, avoidance, low mood, and hypervigilance (all P < 0.01), whereas those who reported more frequent blood in their stool also reported more avoidance (P = 0.023) and low mood symptoms (P = 0.033). The only extraintestinal symptom associated with elevations in PTS symptoms was joint pain (all PTS symptom groups, P < 0.05). Those with a history of fistula or stricture did not differ regarding PTS than those without.
PTS and IBD Symptom Severity
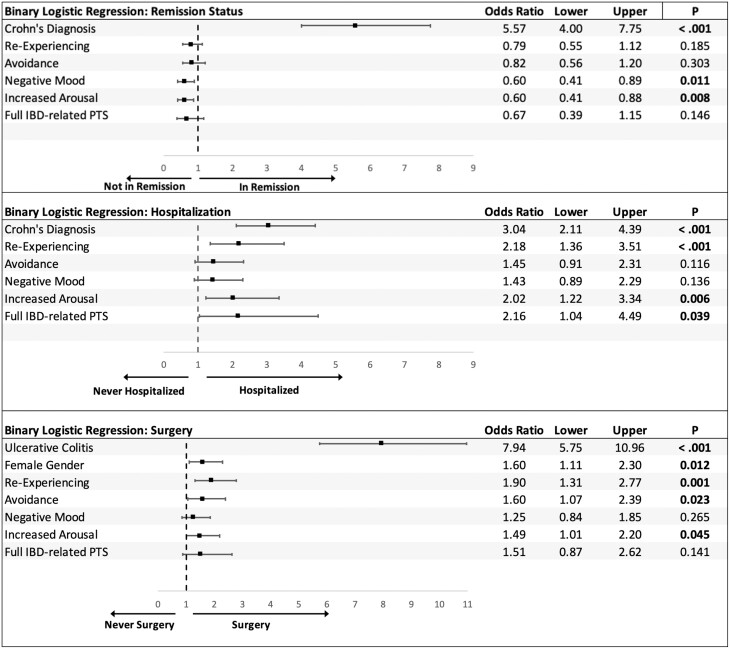
Based on the pHBI or SCCAI scores, those with UC were less likely to be in remission compared to those with CD (P < 0.001). As such, patients with CD and patients with UC were evaluated separately for the relationship between PTS and remission. For CD, patients with more PTS symptoms in all categories were significantly associated with having active disease (all P ≤ 0.01). For UC, only elevations for the re-experiencing category of PTS symptoms were associated with active disease (P = 0.009); avoidance, negative mood, and increased arousal were not significant. According to the logistic regression, having a CD diagnosis remained a substantial predictor of being in remission (OR = 5.57; 95% CI, 4.0-7.75; Fig. 2). The PTS symptoms of negative mood (OR = 0.60; 95% CI, 0.41-0.89) and increased arousal (OR = 0.60; 95% CI, 0.41-0.88) were associated with lower odds of being in clinical remission when controlling for IBD type (Fig. 2).
Figure 2.
Relationship between PTS symptoms and remission status, history of IBD-related hospitalization, or surgery. Remission: 0 = no, 1 = yes. Diagnosis: CD = 1, UC = 2. Sex: female = 1, male = 2. Each PTS variable: 0 = no, 1 = yes.
Patients with UC were more likely to report more severe and frequent symptoms over the last 6 months, compared with patients with CD (P < 0.001). When controlling for diagnosis in the regression models, all PTS symptom groups and those participants who met the full diagnostic criteria for IBD-related PTS were associated with more severe IBD symptoms (Table 3). Similar findings existed for symptom severity in the past week when controlling for diagnosis, sex, and race. Although a diagnosis of CD remained a predictor, the relationship between each PTS symptom group and IBD severity was considerably larger; sex and race were removed from regression models as nonsignificant predictors.
Table 3.
Hierarchical Multiple Regression Analysis of Relationship Between PTS and Symptom Severity Over Last Week and Last 6 Months
| Symptom Severity—Past Week* | |||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| χ2 | P | B | β | P | |
| Non-White | 11.233 | 0.024 | –0.104 to –0.223 | –0.048 to –0.057 | All > 0.05 |
| Female | 9.845 | 0.043 | –0.188 to –0.203 | –0.041 to –0.063 | All > 0.10 |
| CD | 20.448 | <0.001 | 0.234 | 0.102 | 0.003 |
| Re-experiencing | 38.064 | <0.001 | 0.529 | 0.203 | <0.001 |
| CD | 20.448 | <0.001 | 0.261 | 0.113 | 0.001 |
| Avoidance | 41.178 | <0.001 | 0.568 | 0.204 | <0.001 |
| CD | 20.448 | <0.001 | 0.245 | 0.106 | 0.002 |
| Negative mood | 80.510 | <0.001 | 0.871 | 0.311 | <0.001 |
| CD | 20.448 | <0.001 | 0.230 | 0.100 | 0.004 |
| Increased arousal | 45.268 | <0.001 | 0.622 | 0.226 | <0.001 |
| CD | 20.448 | <0.001 | 0.267 | 0.116 | 0.001 |
| Full IBD-related PTS | 42.039 | <0.001 | 0.721 | 0.186 | <0.001 |
| Symptom Severity—Past 6 Months† | |||||
| Univariate | Multivariate | ||||
| χ2 | P | B | β | P | |
| UC | 29.836 | <0.001 | 0.335 | 0.101 | 0.004 |
| Re-experiencing | 40.608 | <0.001 | 0.752 | 0.201 | <0.001 |
| UC | 29.836 | <0.001 | 0.376 | 0.114 | 0.001 |
| Avoidance | 38.413 | <0.001 | 0.821 | 0.204 | <0.001 |
| UC | 29.836 | <0.001 | 0.346 | 0.105 | 0.002 |
| Negative mood | 75.620 | <0.001 | 1.195 | 0.297 | <0.001 |
| UC | 29.836 | <0.001 | 0.326 | 0.099 | 0.005 |
| Increased arousal | 45.218 | <0.001 | 0.828 | 0.210 | <0.001 |
| UC | 29.836 | <0.001 | 0.378 | 0.114 | 0.001 |
| Full IBD-related PTS | 43.841 | <0.001 | 1.028 | 0.185 | <0.001 |
*Model: diagnosis (step 1), sex + race (step 2), and each symptom cluster (step 3). Sex: female = 1, male = 2. Race: non-White = 1, White = 2. Diagnosis: CD = 1, UC = 2.
†Model: Diagnosis (step 1) and each symptom cluster (step 2). Diagnosis: CD = 1, UC = 2.
PTS and Hospitalizations
Patients with CD were more likely to ever be hospitalized for IBD than for UC (P < 0.001). The PTS symptoms of re-experiencing and increased arousal, along with meeting the full diagnostic criteria for IBD-related PTS, were associated with having at least 1 IBD-related hospitalization (Fig. 2). When controlling for diagnosis, patients with elevations in re-experiencing PTS symptoms were 2.18 times more likely to have been hospitalized for IBD (95% CI, 1.36-3.51), with elevations in increased arousal 2.02 times more likely (95% CI, 1.22-3.3.4) and meeting the full criteria for IBD-related PTS 2.16 times more likely (95% CI, 1.04-4.49).
Patients with CD were also likely to have a higher number of hospitalizations for IBD than were patients with UC (median, 3.0; IQR, 1-5 vs median, 2.0; IQR, 1-3; P < 0.001). Elevations in all PTS symptom categories were significantly associated with a higher number of hospitalizations for IBD (Table 4). When controlling for diagnosis, these symptoms remained significant predictors for the number of hospitalizations. However, having CD indicated a larger relationship with the number of hospitalizations than PTS symptoms.
Table 4.
Hierarchical Multiple Regression Analysis of Relationship Between PTS and HCU
| Primary Care Visits—Past Year* | |||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| χ2 | P | B | β | P | |
| Less than college degree | 9.824 | 0.020 | –0.095 to –0.073 | –0.056 to –0.043 | All > 0.10 |
| Re-experiencing | 17.386 | <0.001 | 0.225 | 0.139 | <0.001 |
| Avoidance | 17.293 | <0.001 | 0.190 | 0.109 | 0.004 |
| Negative mood | 21.469 | <0.001 | 0.284 | 0.164 | <0.001 |
| Increased arousal | 29.414 | <0.001 | 0.271 | 0.159 | <0.001 |
| Full IBD-related PTS | 23.645 | <0.001 | 0.390 | 0.161 | <0.001 |
| GI Clinic Visits—Past Year† | |||||
| Univarixxate | Multivariate | ||||
| χ2 | P | B | β | P | |
| CD | 19.434 | <0.001 | –0.153 | –0.099 | 0.005 |
| Re-experiencing | 24.723 | <0.001 | 0.266 | 0.153 | <0.001 |
| CD | 19.434 | <0.001 | –0.167 | –0.109 | 0.002 |
| Avoidance | 16.830 | <0.001 | 0.255 | 0.136 | <0.001 |
| CD | 19.434 | <0.001 | –0.162 | –0.105 | 0.003 |
| Negative mood | 17.641 | <0.001 | 0.272 | 0.146 | <0.001 |
| CD | 19.434 | <0.001 | –0.155 | –0.101 | 0.005 |
| Increased arousal | 14.222 | <0.001 | 0.223 | 0.122 | 0.001 |
| CD | 19.434 | <0.001 | –0.172 | –0.112 | 0.002 |
| Full IBD-related PTS | 15.726 | <0.001 | 0.346 | 0.135 | <0.001 |
| Total IBD Hospitalizations ‡ | |||||
| Univariate | Multivariate | ||||
| t | P | B | β | P | |
| CD | –7.208 | <0.001 | –1.201 | –0.248 | <0.001 |
| Re-experiencing | –2.231 | 0.026 | 0.481 | 0.092 | 0.017 |
| CD | –7.208 | <0.001 | –1.223 | –0.253 | <0.001 |
| Avoidance | –2.237 | 0.026 | 0.591 | 0.104 | 0.007 |
| CD | 7.208 | <0.001 | –1.204 | –0.249 | <0.001 |
| Negative mood | –2.073 | 0.039 | 0.509 | 0.090 | 0.020 |
| CD | 7.208 | <0.001 | –1.174 | –0.244 | <0.001 |
| Increased arousal | –3.294 | 0.001 | 0.705 | 0.129 | 0.001 |
| CD | 7.208 | <0.001 | –1.241 | –0.256 | <0.001 |
| Full IBD-related PTS | –2.726 | 0.007 | 0.982 | 0.129 | 0.001 |
*Model: education (step 1) and each symptom cluster (step 2). Education: less than college = 1, college or higher = 2.
†,‡ Model: diagnosis (step 1) and each symptom cluster (step 2). Diagnosis: CD = 1, UC = 2.
PTS and Surgical History
Patients with UC were more likely to report past surgery for IBD than were patients with CD (P < 0.001), with women more likely than men (P = 0.012). The PTS symptoms of re-experiencing and increased arousal were associated with having at least 1 IBD surgery (Fig. 2), whereas negative mood and meeting the full criteria for IBD-related PTS were nonsignificant. Patients reporting greater re-experiencing symptoms were 1.9 times more likely to have had an IBD surgery (95% CI, 1.31-2.77), when we controlled for diagnosis and sex. Similarly, patients with elevated avoidance or elevated arousal symptoms were 1.6 times more likely (95% CI, 1.07-2.39) and 1.49 times more likely (95% CI, 1.01-2.20), respectively, to have had surgery for IBD. No differences existed by demographic or clinical variables for the number of prior IBD surgeries, and no particular PTS symptoms were associated with the number of surgeries (all P > 0.10). Patients with past ostomy surgery (P = 0.036) or J-Pouch (P = 0.025) reported more of the re-experiencing type of PTS symptoms. No other surgery types (resection, strictureplasty, perianal repair) were associated with PTS.
PTS and HCU
People with less than a college degree reported more PCP visits (P = 0.015). All other demographic or clinical variables were not related to visits to PCPs. All PTS symptoms and meeting the full criteria for IBD-related PTS were associated with more PCP visits (Table 4). When controlling for education level, we found that all PTS symptoms were predictors of the utilization of primary care. Patients with CD had more outpatient GI visits in the past year than patients with UC (P < 0.001). All PTS symptoms and meeting the full criteria for IBD-related PTS were associated with more GI visits (Table 4). When controlling for diagnosis, we found that all PTS symptoms remained significant predictors of more GI clinic utilization.
PTS and Psychosocial Outcomes
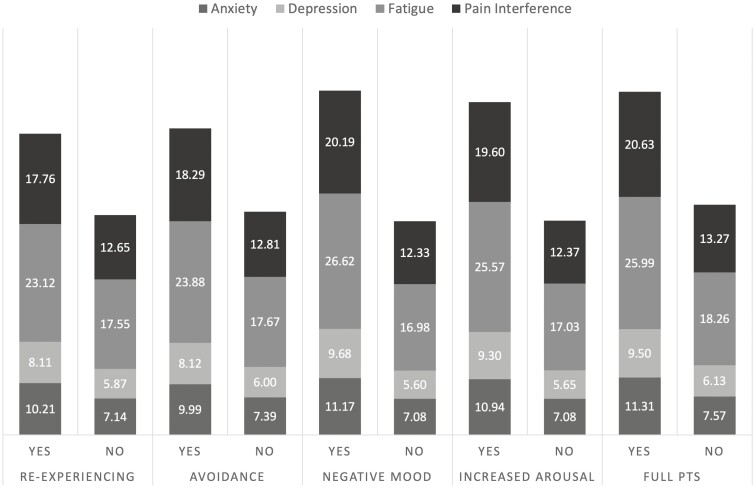
As expected, increased PTS symptoms were associated with increased anxiety, depression, fatigue, and pain interference (Fig. 3). Patients with elevations across any of the symptom groups and those meeting the full criteria for IBD-related PTS scored significantly higher across these 4 domains of psychosocial function (all P < 0.001). Although sleep quality was slightly related to fatigue (r = –0.12; P = 0.024), only fatigue was substantially higher in patients with more PTS symptoms. This difference remained when controlling for symptom severity over the past week. Patients meeting the full criteria for IBD-related PTS had poorer sleep, albeit negligible, than those who did not (yes: 11.53 (1.87) vs no: 11.03 (1.67); P = 0.028).
Figure 3.
Mean scores for psychosocial patient-reported outcomes by PTS symptom group.
Discussion
The current study sought to replicate our previous data regarding the prevalence of significant PTS symptoms with a larger cohort of patients with IBD. As hypothesized, PTS symptoms were associated with hospitalizations, IBD-related surgery, HCU, and poorer psychosocial outcomes. We found that 9.5% of patients met the full DSM-5 diagnostic criteria for IBD-related PTS, based on PCL-5 criteria. Of particular concern is the finding that 5.6% of participants reported a prestudy diagnosis of PTS because of IBD-related experiences. Our findings suggest that traumatic stress is underdiagnosed in patients with IBD. A similar percentage of patients in this study reported significant PTS symptoms for the 4 symptom categories as in earlier research.13
As a whole, approximately one-quarter of patients had moderate to severe symptoms of re-experiencing the event(s), avoidance, negative mood, and increased arousal. Certain demographic groups seemed to be at increased risk, including those with less than a college education and patients of Hispanic ethnicity. Women also scored significant higher across all PTS domains than men. These findings were also consistent with the PTSD literature, which finds that women, racial and ethnic minorities, and those from lower socioeconomic backgrounds are more likely to have PTSD than comparable groups. Unfortunately, even with purposeful sampling, our cohort of participants was 95% White and 98% non-Hispanic. As such, the findings on race and ethnicity should be interpreted with caution. Future studies should focus on non-White patients with IBD to better understand the rates of PTS in these groups.
We also sought to understand how PTS symptoms relate to several self-reported patient outcomes. We found that all PTS symptoms were more likely to be associated with active disease, as measured by the pHBI, in patients with CD, whereas only patients with UC with re-experiencing symptoms more often reported active IBD per the SCCAI score. When controlling for these disease differences, we found that patients with greater levels of negative mood and increased arousal had significantly lower odds of being in clinical remission. Alterations in immune regulation associated with PTSD, including elevated levels of proinflammatory markers that may overlap with IBD (eg, C-reactive protein),24 may explain the association between PTSD and disease activity in IBD. However, inflammation itself may be a risk factor for developing PTSD, which would place individuals with IBD at greater risk for developing PTSD or IBD-related PTS after a traumatic event. Additional research related to the relationship between inflammation, PTSD, and IBD involving longitudinal data is warranted.
We also evaluated how longer-term symptom severity (past 6 months) and short-term (past week) may be associated with PTS symptoms. Patients with UC reported greater symptoms in the past week, whereas patients with CD reported more symptoms in the past 6 months. Regardless of this difference, PTS symptoms were substantially larger contributors to symptom severity in both the short and long term when controlling for diagnosis. In earlier research,13 symptom severity was only predictive of PTS in patients with UC, accounting for roughly one-fifth of the variance. The present findings suggest that symptom severity has an important relationship with PTS symptoms in patients with CD and with UC.
Research into the relationship of PTSD and HCU is mixed, with some studies finding a decrease in clinic visits (likely because of avoidance behaviors) and others showing increased HCU.10, 25 Either scenario is going to increase direct costs, because deferring care, especially in the context of IBD, can lead to more severe disease and emergent treatments. Patients with CD did not differ from patients with UC for PCP visits, but patients with CD reported more GI clinic visits in the past year and more lifetime hospitalizations. Patients with less than a college degree also visited their PCP more often than those with more education. This finding is consistent with previous studies highlighting socioeconomic status–based differences in HCU, surgical therapy, health-related quality of life, and clinical outcomes in patients with IBD.26 However, PTS symptoms remained significant predictors of PCP use in multivariate models where education level did not. Higher PTS symptoms were predictive of more GI clinic visits and lifetime hospitalizations when controlling for IBD type and remission status. This relationship seemed to be stronger for clinic visits than hospitalizations. Attending appointments with a health care provider may provide patients with PTSD a sense of control over their symptoms or a sense that their physician is a source of safety, care, or comfort. Several studies have highlighted lack of social support as a risk factor for developing PTSD.27, 28 For some patients, especially those who lack social support in the home, appointments with their medical providers may offer a sense of emotional support while being cared for by someone else.
Surgery is a common occurrence in IBD management. In this cohort, the median number of surgeries was 4, ranging from 1 to 14. Certain PTS symptoms, specifically patients with more re-experiencing of a traumatic event and arousal were 1.5 to 2 times more likely to have at least 1 IBD-related surgery. However, PTS symptoms were not associated with the number of surgeries. This finding suggests that the number of surgeries is less important than a particularly stressful or traumatic surgical experience in the development of PTS. Thus, patients undergoing ostomy or ileal pouch-anal anastomosis surgeries may be at higher risk compared to patients undergoing other types of IBD surgery.
Finally, PTS symptoms were associated with increased psychological distress, poorer sleep, fatigue, and pain interference. Interestingly, sleep quality was not associated with PTS, with the exception of patients meeting the full criteria for IBD-related PTS. However, fatigue was substantially higher in patients with elevations in each PTS symptom group even when controlling for IBD symptom severity over the past week. Based on this discrepancy, it seems that fatigue resulting from PTS symptoms could be a significant contributor to unexplained fatigue in IBD given a relationship that is independent of sleep quality and recent disease severity. In part, this condition may be attributable to the chronic autonomic arousal inherent in PTS.
This study has several strengths, including a large, representative national U.S. sample with a validated diagnosis of IBD via the IBD Partners database. We utilized the gold-standard measure of PTSD to evaluate IBD-related PTS and excluded individuals with a prior PTS/PTSD diagnosis not associated with their disease. This study also has some limitations. The sample was predominantly White, non-Hispanic, and female, and therefore the results may not be generalizable. Future studies of PTS should include more diverse groups. Although IBD Partners comprises a large pool of patients with IBD, these patients are motivated to participate in research and are not likely representative of the general IBD population. Because these data were collected cross-sectionally, we cannot state the true predictive nature of PTS for patient outcomes. Prospective, longitudinal research in this area is necessary. Finally, although we used specific criteria outlined for the PCL-5, a definitive diagnosis of PTSD cannot be made without a diagnostic interview. Thus, the prevalence of PTS in this study may be overestimated because some previous studies have found higher rates of PTSD symptoms when they are measured via questionnaire vs clinician interview.28
Conclusions
Results from this study suggest that approximately one-quarter of patients with IBD report substantial PTS symptoms because of their illness experiences, with women, racial and ethnic minorities, and those with less than a college degree possibly at increased risk. As expected, PTS was associated with more severe IBD, higher odds of hospitalizations and surgeries, increased outpatient HCU, and increased anxiety, depression, fatigue, and pain interference. The findings of this study underscore the need for the early detection of PTS in patients with IBD. Accordingly, patients need to be referred to appropriate mental health clinicians who can provide evidence-based treatment for PTS. In this context, referral to a clinician with expertise in trauma is preferable to referral to a GI psychologist. We recommend that IBD practices regularly screen for PTS, particularly after diagnosis, a hospitalization, or surgery and identify appropriate psychologists in their communities for referral purposes. Future studies should target which psychological therapies for IBD-related trauma are best suited for patients with CD and with UC. Perhaps, more important, additional studies should aim to prevent PTS in patients with IBD through clinician education and training.
Supported by: Digestive Health Foundation of Northwestern Medicine.
Conflicts of interest: TT: speaker for Abbvie, ownership interest in Oak Park Behavioral Medicine LLC, consultant for Healthline. SQ: speaker, Pri-Md. SBH: advisor or consultant for Centocor, Procter & Gamble, Otsuka, Abbott Labs, Protein Design Labs, and Shire; grants for clinical research from Centocor, Otsuka, Procter & Gamble, Abbott Labs, Protein Design Labs, and Shire; speaker for Centocor, Procter & Gamble, and Shire. SJ, MS, EAM: Nothing to disclose.
References
- 1. Bryant RA. Post-traumatic stress disorder: a state-of-the-art review of evidence and challenges. World Psychiatry. 2019;18:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angel CM. Resilience, post-traumatic stress, and posttraumatic growth: veterans’ and active duty military members’ coping trajectories following traumatic event exposure. Nurse Educ Today. 2016;47:57–60. [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Zhou X, Zeng M, et al. Post-traumatic stress symptoms and post-traumatic growth: evidence from a longitudinal study following an earthquake disaster. PLoS One. 2015;10:e0127241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunn EC, Solovieff N, Lowe SR, et al. Interaction between genetic variants and exposure to Hurricane Katrina on post-traumatic stress and post-traumatic growth: a prospective analysis of low income adults. J Affect Disord. 2014;152–154:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Righy C, Rosa RG, da Silva RTA, et al. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: a systematic review and meta-analysis. Crit Care. 2019;23:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marziliano A, Tuman M, Moyer A. The relationship between post-traumatic stress and post-traumatic growth in cancer patients and survivors: a systematic review and meta-analysis. Psychooncology. 2020;29:604–616. [DOI] [PubMed] [Google Scholar]
- 7. Groarke A, Curtis R, Groarke JM, et al. Post-traumatic growth in breast cancer: how and when do distress and stress contribute? Psychooncology. 2017;26:967–974. [DOI] [PubMed] [Google Scholar]
- 8. Muller HHO, Czwalinna K, Wang R, et al. Occurrence of post-traumatic stress symptoms, anxiety and depression in the acute phase of transient ischemic attack and stroke. Psychiatr Q. Published online January 2, 2021. doi: 10.1007/s11126-020-09873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liyanage-Don N, Birk J, Cornelius T, et al. Medications as traumatic reminders in patients with stroke/transient ischemic attack-induced posttraumatic stress disorder. Stroke. 2021;52:321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ayano G, Duko B, Bedaso A. The prevalence of post-traumatic stress disorder among people living with HIV/AIDS: a systematic review and meta-analysis. Psychiatr Q. 2020;91:1317–1332. [DOI] [PubMed] [Google Scholar]
- 11. Tang C, Goldsamt L, Meng J, et al. Global estimate of the prevalence of post-traumatic stress disorder among adults living with HIV: a systematic review and meta-analysis. BMJ Open. 2020;10:e032435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cámara RJ, Gander ML, Begré S, et al. ; Swiss Inflammatory Bowel Disease Cohort Study Group . Post-traumatic stress in Crohn’s disease and its association with disease activity. Frontline Gastroenterol. 2011;2:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taft TH, Bedell A, Craven MR, et al. Initial assessment of post-traumatic stress in a US cohort of inflammatory bowel disease patients. Inflamm Bowel Dis. 2019;25:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coker KL, Stefanovics E, Rosenheck R. Correlates of improvement in substance abuse among dually diagnosed veterans with post-traumatic stress disorder in specialized intensive VA treatment. Psychol Trauma. 2016;8:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown PJ, Wolfe J. Substance abuse and post-traumatic stress disorder comorbidity. Drug Alcohol Depend. 1994;35:51–59. [DOI] [PubMed] [Google Scholar]
- 16. Kim YK, Amidfar M, Won E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:103–112. [DOI] [PubMed] [Google Scholar]
- 17. Sagarwala R, Nasrallah HA. Changes in inflammatory biomarkers before and after SSRI therapy in PTSD: a review. Ann Clin Psychiatry. 2019;31:292–297. [PubMed] [Google Scholar]
- 18. Olff M, van Zuiden M. Neuroendocrine and neuroimmune markers in PTSD: pre-, peri- and post-trauma glucocorticoid and inflammatory dysregulation. Curr Opin Psychol. 2017;14:132–137. [DOI] [PubMed] [Google Scholar]
- 19. Bam M, Yang X, Zhou J, et al. Evidence for epigenetic regulation of pro-inflammatory cytokines, interleukin-12 and interferon gamma, in peripheral blood mononuclear cells from PTSD patients. J Neuroimmune Pharmacol 2016;11:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gill JM, Saligan L, Woods S, et al. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45:262–277. [DOI] [PubMed] [Google Scholar]
- 21. Ng QX, Soh AYS, Loke W, et al. Systematic review with meta-analysis: the association between post-traumatic stress disorder and irritable bowel syndrome. J Gastroenterol Hepatol. 2019;34:68–73. [DOI] [PubMed] [Google Scholar]
- 22. Klaassens ER, Giltay EJ, Cuijpers P, et al. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology. 2012;37:317–331. [DOI] [PubMed] [Google Scholar]
- 23. Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for Diagnostic and Statistical Manual of Mental Disorders-fifth edition (PCL-5) in veterans. Psychol Assess. 2016;28:1379–1391. [DOI] [PubMed] [Google Scholar]
- 24. Hori H, Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci. 2019;73:143–153. [DOI] [PubMed] [Google Scholar]
- 25. Arigo D, Juth V, Trief P, et al. Unique relations between post-traumatic stress disorder symptoms and patient functioning in type 2 diabetes. J Health Psychol. 2020;25:652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sewell JL, Velayos FS. Systematic review: the role of race and socioeconomic factors on IBD healthcare delivery and effectiveness. Inflamm Bowel Dis. 2013;19:627–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paredes Molina CS, Berry S, Nielsen A, et al. PTSD in civilian populations after hospitalization following traumatic injury: a comprehensive review. Am J Surg. 2018;216:745–753. [DOI] [PubMed] [Google Scholar]
- 28. Davydow DS, Lease ED, Reyes JD. Posttraumatic stress disorder in organ transplant recipients: a systematic review. Gen Hosp Psychiatry. 2015;37:387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]