Abstract
A 55-year-old man with hypogammaglobulinemia due to previous rituximab treatment developed persistent coronavirus disease 2019 pneumonia. Treatment with REGN-COV2 (casirivimab and imdevimab) resulted in the clearance of the infection. Targeted antiviral antibodies may be an important weapon in the management of immunocompromised patients infected with severe acute respiratory syndrome coronavirus 2 who fail to mount an immune response.
Keywords: COVID-19, hypogammaglobulinemia, REGN-COV2, rituximab
Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a global pandemic with significant morbidity and mortality. Although age, sex, comorbidities, and ethnicity are risk factors for adverse outcomes [1], various preexisting conditions, including hematological cancers, have also been reported to correlate with poor outcomes [2]. Anti-CD20 monoclonal antibodies such as rituximab represent the cornerstone of treatment of most patients with B-cell malignancies and, to a lesser extent, patients with autoimmune disease [3, 4]. Anti-CD20 treatment is associated with a risk of prolonged humoral immunodeficiency, which can last years and can hamper the ability to mount an antibody response to infection [5]. Nonsustained hypogammaglobulinemia after treatment with rituximab can persist for 42 months, while around 4% of patients had never recovered CD20 cell function [6]. A treatment standard for patients who fail to mount an antibody response to COVID-19 is yet to be defined. Convalescent plasma has been reported to be used in such patients with limited benefits [7]. We report a case of a 55-year-old man with severe hypogammaglobulinemia, following treatment for mantle cell lymphoma with a rituximab-containing regimen, who developed persistent SARS-CoV-2 infection and was treated successfully with REGN-COV2 antibody after failing to clear the infection.
CASE REPORT
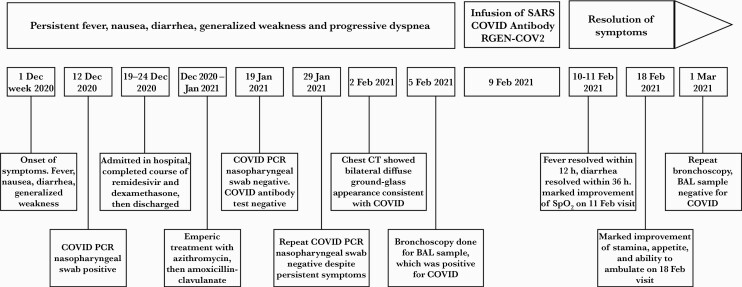
Our patient is a 55-year-old man with a history of mantle cell lymphoma in remission after treatment with hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose cytarabine and high-dose methotrexate (Hyper-CVAD) and rituximab maintenance concluded in 2015. He subsequently developed hypogammaglobulinemia managed with monthly intravenous immunoglobulin (Gamunex-C 65 g). He developed fever, nausea, diarrhea, and generalized weakness in the first week of December 2020. On 12 December 2020, he tested positive for SARS-CoV-2 by high-sensitivity polymerase chain reaction (PCR) (nasal swab) and was subsequently admitted to hospital for COVID-19 pneumonia where he completed a course of remdesivir and dexamethasone. His hospital stay was uneventful, and he was discharged after 5 days.
However, the patient continued to have fever, diarrhea, and weakness and lost 13.6 kg (30 lbs) over the next 4 weeks. He developed progressive dyspnea with exertion and his blood oxygen saturation decreased to 91% on room air. Empiric therapy with azithromycin and then amoxicillin-clavulanate did not improve his symptoms. A SARS-CoV-2 antibody test (Abbott anti-nucleocapsid immunoglobulin G) performed on 19 January 2021 was negative. Serum levels of immunoglobulins A, G, and M at that time were <10 mg/dL, 479 mg/dL, and <20 mg/dL, respectively. COVID-19 high-sensitivity reverse-transcription PCR tests from nasopharyngeal swabs done on 2 different platforms on 22 and 29 January 2021 were negative. Contrast-enhanced computed tomography of the chest, abdomen, and pelvis done on 3 February 2021 showed bilateral diffuse ground-glass appearance consistent with COVID-19 pneumonia and no evidence of recurrence of lymphoma. Clostridioides difficile was ruled out given persistent diarrhea. To rule out other pathogens, a bronchoscopy was done on 5 February 2021. Legionella, Pneumocystis jirovecii, fungal pathogens, acid-fast bacilli, influenza, parainfluenza, respiratory syncytial virus, Bordetella pertussis, Chlamydia pneumoniae, and Mycoplasma pneumoniae were ruled out. Other coronaviruses were excluded as well. The bronchoalveolar lavage sample was positive for SARS-CoV-2 by high-sensitivity PCR with cycle numbers of 29.9 of 40 cutoff, performed on Cepheid GeneXpert, thus confirming the diagnosis of COVID-19 pneumonia. At that time his oxygen saturation was 90% at rest and decreased to 85% with ambulation of 50 feet.
Given the deteriorating clinical picture, an emergency authorization was obtained from the US Food and Drug Administration (FDA) for the use of REGN-COV2, and the patient was given the infusion of casirivimab 1200 mg and imdevimab 1200 mg on 9 February 2021 and was under observation for 1 hour without any complications or adverse effects during or following the therapy. His fevers resolved within 12 hours of treatment. Within 36 hours he no longer had diarrhea. On 11 February 2021, his oxygen saturation was 99% at rest and dropped to 92% while ambulating 200 feet but returned to 99% within 2 minutes of rest. On 18 February 2021, he reported marked improvement in his stamina, appetite, and ability to ambulate. He was able to walk 200 feet and climb 2 flights of stairs without desaturation below 95%. On 1 March 2021, repeat bronchoscopy was negative for SARS-CoV-2 by high-sensitivity PCR. The patient reported complete recovery to preinfection baseline (Figure 1).
Figure 1.
Course of illness. Abbreviations: BAL, bronchoalveolar lavage; COVID, coronavirus disease 2019; CT, computed tomography; PCR, polymerase chain reaction; RGEN-COV2, casirivimab and imdevimab; SARS, severe acute respiratory syndrome coronavirus 2; SpO2, oxygen saturation.
DISCUSSION
As the SARS-CoV-2 pandemic swept across the globe, there was an effort to find effective treatments for COVID-19. Although thousands of papers were published, the experience with managing severely immunocompromised patients is still scant. We report a case of successful treatment of persistent COVID-19 infection in a patient with acquired humoral immune deficiency due to treatment with anti-CD20 antibody rituximab for a diagnosis of mantle cell lymphoma, who failed to mount an antibody response to COVID-19 infection.
Anti-CD20 monoclonal antibodies such as rituximab not only deplete malignant B lymphocytes but also their normal counterparts, and therefore impair humoral immunity [5]. Patients with prior rituximab exposure are known to be poor responders to various types of vaccinations including influenza viruses, Haemophilus influenzae, and Streptococcus pneumoniae [8, 9]. In general, these patients have a higher risk of infections, both bacterial and viral, as demonstrated previously [10]. While some individuals with COVID-19 have a mild prolonged course and eventually develop antibodies [11], others may develop a more ominous course. While the dynamics of T-cell and B-cell responses in successfully controlling this viral infection are not fully understood, there may be lack of coordination within the adaptive immune system with rituximab affecting the naive B cells in the blood (which seem to be the source of neutralizing antibodies in COVID-19), resulting in an ineffective response [12].
The impact of COVID-19 on immunodeficient individuals and the optimal treatment modality is still unclear. There have been reports of patients after B-cell depletion therapy or with hypogammaglobulinemia who cleared SARS-CoV-2 infection on their own; however, other patients undergoing recent rituximab therapy had severe and prolonged COVID-19 infections with persistent detectable virus [5].
Treatment modalities such as remdesivir, dexamethasone, and convalescent plasma [13–15] have shown benefit in the general population. Convalescent plasma therapy has also been used in the general population of COVID-19 patients with a benefit when used with high antibody titer early (within 3 days of symptoms) [7], but did not show a mortality benefit when given late or as low titer [16, 17]. Since, in immunocompetent patients, higher antigen load would drive up the antibody titers to control infection, the monoclonal antibodies might provide a much-needed booster in immunocompromised patients.
REGN-COV2, manufactured by Regeneron Pharmaceuticals, is a combination of 2 SARS-CoV-2 monoclonal antibodies, casirivimab and imdevimab. These antibodies target the receptor-binding domain of the SARS-CoV-2 spike protein, thereby preventing viral entry into human cells through the angiotensin-converting enzyme 2 receptor. REGN-COV2 is a synthetic drug, unlike convalescent plasma, which is obtained from a human donor, thus eliminates transfusion-related complications. REGN-COV2 is in phase 3 clinical trials and has been authorized by FDA for emergency use for patients with mild to moderate COVID who have a high risk of propagating to severe disease and hospitalization [18]. REGN-COV2 is not authorized for use in patients who are hospitalized, or in those who require oxygen therapy. REGN-COV2 is not approved yet for routine clinical use. Efficacy and safety are not yet fully established [18].
The pivotal study by Weinreich et al, which led to the approval of REGN-COV2, showed that REGN-COV2 enhanced clearance of virus, particularly in patients in whom an endogenous immune response had not yet been initiated or who had a high viral load at baseline [19]. REGN-COV2 has shown in a clinical trial to reduce COVID-19–related hospitalization of emergency room visits in patients at high risk for disease progression within 28 days after treatment when compared to placebo [18].
Our patient showed persistent symptoms of fever, diarrhea, weight loss, and generalized weakness. His symptoms persisted for >2 months and worsened even after initial hospitalization and treatment with remdesivir and dexamethasone. His lung function deteriorated to the point of requiring oxygen therapy and he lost 15% of his bodyweight, leading to severe generalized weakness and inability to function. Treatment with REGN-COV2 led to an immediate resolution of most symptoms, rapid improvement in respiratory function and functional capacity, and ultimately clearance of the infection.
This case demonstrates the potential of REGN-COV2 to significantly alter the course of illness in immunocompromised patients. We believe REGN-COV2 is a promising treatment option for immunocompromised patients who are unable to mount a humoral immune response. It should be deployed early in the course of the illness or as soon as it is established that a patient is unable to develop antibodies against SARS-CoV-2. It is not yet known if this product would provide any long-term protection from reinfection. In this regard, it may be useful to evaluate the potential for REGN-COV2 to affect long-term passive immunity to SARS-CoV-2 in patients with humoral immunity defects.
Notes
Author contributions. All authors drafted the manuscript. P. L. produced the figure. D. V. and A. G. revised the manuscript, and A. G. submitted the manuscript for publication.
Patient consent statement. The patient provided written consent. All information was anonymized as much as possible. The Amita Health institutional review board (IRB) approved the treatment plan. Local standards do not require IRB approval of case reports.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aries JA, Davies JK, Aue RL, et al. . Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br J Haematol 2020; 190:e64–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salles G, Barrett M, Foà R, et al. . Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther 2017; 34:2232–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawker K, O’Connor P, Freedman MS, et al. ; OLYMPUS Trial Group . Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009; 66:460–71. [DOI] [PubMed] [Google Scholar]
- 5.Yasuda H, Tsukune Y, Watanabe N, et al. . Persistent COVID-19 pneumonia and failure to develop anti-SARS-CoV-2 antibodies during rituximab maintenance therapy for follicular lymphoma. Clin Lymphoma Myeloma Leuk 2020; 20:774–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts DM, Jones RB, Smith RM, et al. . Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun 2015; 57:60–5. [DOI] [PubMed] [Google Scholar]
- 7.Joyner MJ, Senefeld JW, Klassen SA, et al. . Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv [Preprint]. Posted online 12 August 2020. doi: 10.1101/2020.08.12.20169359. [DOI] [Google Scholar]
- 8.Eisenberg RA, Jawad AF, Boyer J, et al. . Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol 2013; 33:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nazi I, Kelton JG, Larché M, et al. . The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood 2013; 122:1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salles G, Seymour JF, Offner F, et al. . Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 2011; 377:42–51. [DOI] [PubMed] [Google Scholar]
- 11.Fallet B, Kyburz D, Walker UA. Mild course of COVID-19 and spontaneous virus clearance in a patient with depleted peripheral blood B cells due to rituximab treatment. Arthritis Rheumatol 2020; 72:1581–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021; 184:861–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. . Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigel JH, Tomashek KM, Dodd LE.et al. . Remdesivir for the treatment of COVID-19—final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonovich VA, Burgos Pratx LD, Scibona P, et al. ; PlasmAr Study Group . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 2021; 384:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hueso T, Pouderoux C, Péré H, et al. . Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood 2020; 136:2290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Convalescent plasma in the management of moderate covid-19 in adults in India: open-label phase II multicentre randomized controlled trial (PLACID Trial) [erratum]. BMJ 2020; 371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov. Safety, tolerability, and efficacy of anti-spike (S) SARS-CoV-2 monoclonal antibodies for the treatment of ambulatory adult and pediatric patients with COVID-19 (NCTo4426695). 2020. https://clinicaltrials.gov/ct2/show/NCT04426695. Accessed 24 June 2021. [Google Scholar]
- 19.Weinreich DM, Sivapalasingam S, Norton T, et al. ; Trial Investigators . REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]