Abstract
Background
There are limited data on outcomes of moderate to severe coronavirus disease 2019 (COVID-19) among patients treated with remdesivir and dexamethasone in a real-world setting. We sought to compare the effectiveness of standard of care (SOC) alone versus SOC plus remdesivir and dexamethasone.
Methods
Two population-based nationwide cohorts of individuals hospitalized with COVID-19 during February through December 2020 were studied. Death within 30 days and need of mechanical ventilation (MV) were compared by inverse probability of treatment weighted (ITPW) logistic regression analysis and shown as odds ratio (OR) with 95% confidence interval (CI).
Results
The 30-days mortality rate of 1694 individuals treated with remdesivir and dexamethasone in addition to SOC was 12.6% compared to 19.7% for 1053 individuals receiving SOC alone. This corresponded to a weighted OR of 30-day mortality of 0.47 (95% CI: .38–.57) for patients treated with remdesivir and dexamethasone compared to patients receiving SOC alone. Similarly, progression to MV was reduced (OR 0.36; 95% CI: .29–.46).
Conclusions
Treatment of moderate to severe COVID-19 during June through December that included remdesivir and dexamethasone was associated with reduced 30-day mortality and need of MV compared to treatment in February through May.
Keywords: COVID-19, pneumonia, remdesivir, dexamethasone, survival
Treatment of moderate to severe coronavirus disease 2019 (COVID-19) with remdesivir and dexamethasone was associated with a lower risk of mechanical ventilation and a significant better overall 30-day survival as compared to standard care alone.
Within 6 months of the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), 2 pharmacological interventions were separately shown to impact clinical outcomes [1, 2]. The Adaptive COVID-19 Treatment Trial-1 (ACTT-1) showed that remdesivir improved time to recovery [1], whereas the RECOVERY Collaborative Group and others showed that corticosteroids improved 28-day survival for patients with COVID-19 [2, 3]. Later the World Health Organization advised against use of remdesivir following results of the Solidarity trial [4]. A meta-analysis suggested that remdesivir resulted in little to no mortality difference but improved the percentage recovered [5]. Only a few studies have reported on outcomes following treatment with remdesivir. Olender et al used data from a phase III trial and compared these to a retrospective cohort of patients receiving standard of care early in the pandemic [6]. Use of remdesivir was associated with improved 14-day recovery and reduced odds of death. Corticosteroid use was not reported. Garibaldi et al similarly showed that clinical improvement occurred faster with remdesivir, whereas use of remdesivir with or without corticosteroids did not reduce time to death [7].
Danish guidelines have recommended the use of remdesivir and dexamethasone for hypoxemic COVID-19 since May and June 2020, respectively. Reports from the United States and the United Kingdom suggest early improvements in outcomes of COVID-19 during first waves of the pandemic compatible with gains in experience [8–10]. Further improvements between waves appear to have occurred [11]. Studies that compare outcomes during second to first waves of COVID-19 are few.
Using causal inference, we report outcomes of individuals hospitalized with COVID-19, who were treated with remdesivir and dexamethasone and compare outcomes with a cohort of individuals, who received standard of care (SOC) without remdesivir or corticosteroids.
METHODS
Setting
Healthcare in Denmark is universal and free of cost for all residents. Initially, remdesivir was administered through ACTT-1 at 7 sites (March and April) and later through an early access program at two sites (May and June). Remdesivir became widely available in August. To ensure equal distribution and access to remdesivir across the country, an overarching body of the 5 regions each responsible for healthcare provision (Danish Regions) established a task force in May to oversee the distribution and use of COVID-19 treatments. Criteria for treatment with remdesivir were confirmed SARS-CoV-2 infection with a new onset pulmonary radiographic infiltrate, oxygen saturation ≤94%, need of supplemental oxygen, and a symptom duration of <10 days, which was later extended to 12 days. Patients with evidence of multiorgan failure, those receiving more than one pressor for septic shock, plasma alanine transaminase 5 times above the upper limit of normal, renal failure or dialysis or continuous veno-venous hemofiltration, pregnant or lactating women, and those with a history of hypersensitivity to remdesivir, were ineligible for treatment with remdesivir, but the use of dexamethasone could be considered. Remdesivir was given for 5 days with few exceptions [12]. On 17 April 2020, the Danish Society of Thrombosis and Hemostasis introduced a guideline on thromboprophylaxis suggesting intermediate doses of low-molecular weight heparin (LMWH) to patients in intensive care units and low dose LMWH to all ward patients.
This study was approved by the Danish Board of Health (record no. 31–1522–84 and 31–1521–309), the Capital Regional Data Protection Center (record no. P-2020–492), the Region Zealand Data Protection Agency (record no. 070–2020), the Region of Southern Denmark (record no. 10.960 and 20/16169) and the legal authorities in North Denmark Region (record no. 2020–045). By Danish legislation, register-based studies are exempted from ethical committee approval.
Cohorts
Hospitalized individuals at 8 centers were included in the SOC cohort from February through May 2020. Details have been described previously [13–16]. Data on age, sex, comorbidity, chest X-ray infiltrate, use of supplemental oxygen and outcomes were extracted from electronic health records.
From June through December 2020, hospitalized individuals at 13 centers across Denmark were included. For every individual, a case report was completed at discharge. Data included age, sex, comorbidity, chest X-ray infiltrate, use of supplemental oxygen, and outcomes.
Included individuals in both cohorts had confirmed SARS-CoV-2 infection by reverse-transcriptase polymerase- chain-reaction. Chest X-ray findings and use of supplemental oxygen was ascertained within 24 hours of admission.
Statistics
Baseline characteristics are reported as frequencies with percentages or medians with an interquartile range (IQR). Comparisons were performed using χ 2test, Fisher exact test or Mann-Whitney U test, as appropriate.
Stabilized inverse probability of treatment weighting (IPTW) was computed using a multiple logistic regression model on the probability of receiving remdesivir. Covariates in the model included age, sex, presence of any comorbidity (hypertension, diabetes mellitus, cardiovascular disease, chronic obstructive pulmonary disease, asthma, cancer or other), need of supplemental oxygen and presence of radiographic infiltration. Weights were computed as stabilized inverse probability of treatment selection and used to create a pseudo-population in which covariates were independent of treatment selection. We computed normal and weighted absolute standardized mean difference between treatment groups to validate the weighting procedure. Weighted logistic regression models were used to assess any association with 30-day mortality or mechanical ventilation (MV) reported as odds ratio (OR) with 95% CI. Survival curves were constructed by the method of Kaplan and Meier; for MV with death as a competing risk. Follow-up was from the date of admission and until day 30. P values < .05 were considered statistically significant. Data analysis was performed using IBM SPSS (version 25.0, SPSS Inc., Chicago, IL, USA) and R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
From February through May, 1053 individuals receiving SOC and from June through December, 1694 individuals receiving SOC with remdesivir and dexamethasone were included; 1593 (91.7%) of the latter were treated after 1 October 2020. Characteristics of study subjects are shown in Table 1. Individuals treated with remdesivir and dexamethasone were more often male, who presented 1 day earlier, more often had pulmonary radiographic infiltration and were more likely to receive supplemental oxygen at baseline compared to the individuals receiving only SOC. The distribution of comorbidity was comparable for both periods, except for “other” comorbidity that was more common in the remdesivir and dexamethasone treated. Unweighted standardized mean differences (SMDs) ranged from 0.025 to 1.315 for age, sex, comorbidities, infiltration, and supplemental use of oxygen. After IPTW, the SMDs ranged from 0.004 to 0.017.
Table 1.
Characteristics of Individuals Hospitalized With Coronavirus Disease 2019 (COVID-19) Over Two Time Periods
| February–May n = 1053 |
June–December n = 1694 |
P value | SMD | SMD After IPTW | |
|---|---|---|---|---|---|
| Age, years | 71 (57–80) | 69 (57–79) | .20 | 0.030 | 0.011 |
| Female, no. (%) | 474 (45.0%) | 621 (36.7%) | .001 | 0.171 | 0.006 |
| Comorbidity, no. (%) | 838 (79.6%) | 1343 (79.3%) | .88 | … | … |
| Coexisting comorbidity | |||||
| Hypertension, no. (%) | 410 (38.9%) | 624 (36.8%) | .27 | 0.043 | 0.010 |
| Diabetes mellitus, no. (%) | 209 (19.8%) | 384 (22.7%) | .09 | 0.069 | 0.009 |
| Cardiovascular disease, no. (%) | 289 (27.4%) | 446 (26.3%) | .53 | 0.025 | 0.018 |
| COPD, no. (%) | 154 (14.6%) | 221 (13.0%) | .25 | 0.046 | 0.004 |
| Malignancy, no. (%) | 127 (12.1%) | 173 (10.2%) | .13 | 0.060 | 0.017 |
| Other, no. (%) | 360 (35.9%) | 808 (47.7%) | <.001 | 0.240 | 0.015 |
| Symptom duration, days | 7 (3–10) | 6 (3–9) | <.001 | … | … |
| Radiographic evidence of pneumonic infiltration, no. (%) | 810 (80.8%) | 1566 (92.4%) | <.001 | 0.346 | 0.005 |
| Supplemental oxygen, no. (%) | 464 (44.6%) | 1610 (95.0%) | <.001 | 1.315 | 0.003 |
| Duration of hospitalization before first dose of remdesivir, days | … | 1 (0–1) | … | … | … |
| Duration of symptoms before first dose of remdesivir, days | … | 7 (4–10) | … | … | … |
| Duration of hospitalization before first dose of dexamethasone, days | … | 1 (0–1) | … | … | … |
| Duration of symptoms before first dose of dexamethasone, days | … | 7 (4–10) | … | … | … |
| Length of stay, days | 7 (3–13) | 7 (5–14) | <.001 | … | … |
| Time to discharge, days | 6 (2–13) | 7 (5–11) | <.001 | ||
| 0–3 days | 292 (34.5%) | 186 (12.6%) | |||
| 4–6 days | 153 (18.1%) | 480 (32.4%) | |||
| 7–12 days | 170 (20.1%) | 436 (29.4%) | |||
| ≥13 days | 231 (27.3%) | 379 (25.6%) | <.001 | ||
| Time to death, days | 8 (5–13) | 14 (8–20) | <.001 | ||
| 0–3 days | 30 (14.5%) | 16 (7.5%) | |||
| 4–6 days | 43 (20.8%) | 25 (11.7%) | |||
| 7–12 days | 82 (39.6%) | 59 (27.7%) | |||
| ≥13 days | 52 (25.1%) | 113 (53.1%) | <.001 | ||
| Instigation of mechanical ventilation | |||||
| ≤24 hours | 29 (2.8%) | 20 (1.2%) | 0.004 | ||
| ≤30 days | 154 (14.6%) | 153 (9.0% | <.001 | ||
| Survival | |||||
| Deceased ≤30 days | 207 (19.7%) | 213 (12.6%) | <.001 |
Values are frequencies with percentages or medians with an interquartile range (IQR).
Abbreviations: COPD, chronic obstructive pulmonary disease; IPTW, inverse probability of treatment; SMD, standard mean difference.
Median time to initiation of remdesivir and dexamethasone treatment was similar at 1 (IQR 0–1) day after admission and 7 (IQR 4–10) days after symptom onset.
The median length of hospital stay was 7 days in both periods. The median time to discharge increased by 1 day. However, the time to discharge varied markedly with more individuals discharged within 3 days during the early period (34.5%) compared to the later period (12.6%) (Table 1).
Thirty-day Mortality
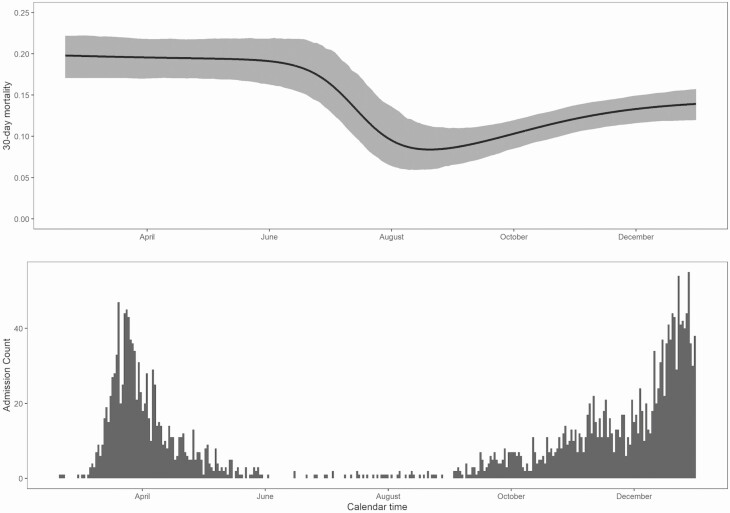
Survival status at day 30 after admission was ascertained for 100% of the 2 cohorts. Figures 1 and 2 show changes in overall 30-day mortality during the study as well as changes in the number of daily admissions. During February through May, the overall 30-day mortality was 19.7%, from June through July it declined to below 10%, and from August it stabilized below 15%. The median time to death among those who died increased significantly during the later period (14 days [IQR 8–20]) compared to the early period (8 [IQR 5–13]) corresponding to 46.9% of death occurring before day 14 in the later period, whereas 74.9% had occurred in the early period by day 14 (Figure 2). The crude OR of 30-day mortality was 0.58 (95% CI: .46–.74) for SOC with remdesivir and dexamethasone compared to SOC alone. After weighting, the OR was 0.47 (95% CI: .38–.57, P < .0001).
Figure 1.
Thirty-day survival for hospitalized patients (upper panel) and daily admissions (lower panel) with COVID-19 according to calendar time period. Abbreviation: COVID-19, coronavirus disease 2019.
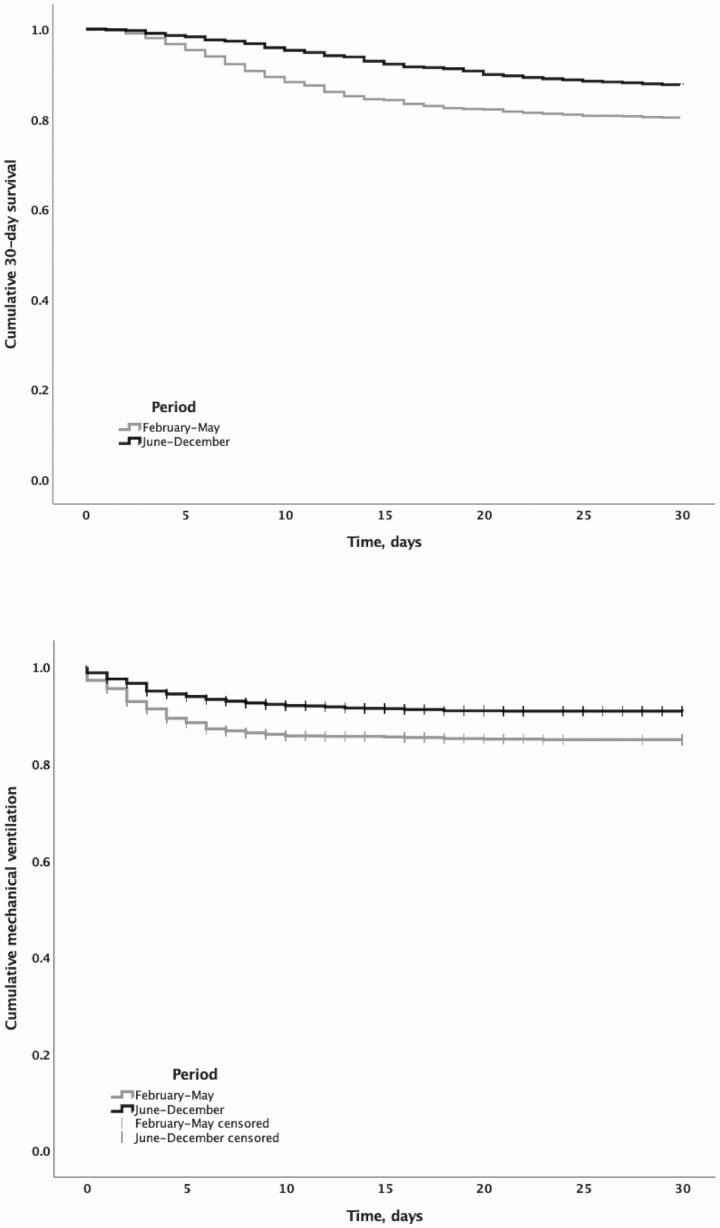
Figure 2.
Thirty-day survival (upper panel) and risk of mechanical ventilation (lower panel) for hospitalized patients with COVID-19 during February through May compared to June through December 2020. Abbreviation: COVID-19, coronavirus disease 2019.
Mechanical Ventilation
From February through May, 154 (14.6%) individuals had respiratory failure requiring MV, and from June through December, 153 (9.0%) required MV (Table 1 and Figure 2). The crude OR was 0.58 (95% CI: .46–.74). After weighting, the OR of MV was 0.36 (95% CI: .29–.46, P < .0001) for individuals treated with remdesivir and dexamethasone compared to SOC alone.
DISCUSSION
Here we show that 30-day mortality and need of MV was significantly lower in individuals receiving SOC with remdesivir and dexamethasone compared to individuals treated before remdesivir and dexamethasone was SOC. The benefit appeared to occur 4–5 days after admission. Time to death changed over the 2 periods with significantly more deaths occurring early in the early period compared to the later period. This is the first study to our knowledge to report a survival benefit of patients treated with remdesivir and dexamethasone in addition to initial SOC for COVID-19.
Our nonrandomized data do not permit any distinction between the individual effects of each of remdesivir and dexamethasone or other interventions on outcomes of COVID-19. SOC for moderate to severe COVID-19 in Denmark was initially supplemental oxygen, antipyretics, and thromboprophylaxis. Other changes in SOC may nevertheless have influenced our results although use of undocumented interventions remain infrequent outside clinical trials in Denmark. The analysis by Garibaldi et al suggested that remdesivir plus dexamethasone did not improve 28-day survival [7]. However, the cohort was smaller and experienced fewer deaths than our cohort, thus limiting power to detect differences. In the ACTT-1 trial patients receiving supplemental oxygen had a reduced 14-day mortality of 7.4 percentage points and 2.9 percentage points for patients receiving MV (although the latter not statistically significantly different) [1]. The Recovery trial reported a 2.9 percentage point reduction in 28-day mortality for patients receiving supplemental oxygen [2]. For patients on MV the reduction was 12.1 percentage points. In our study, <15% and 10% required MV, respectively. Although not directly comparable the crude reduction in mortality in our cohort of 7.1 percentage points was higher than expected based on the Recovery trial and in line with the ACTT-1 trial, suggesting a contribution of remdesivir and dexamethasone to the improvement in outcome. Should there by a contribution by remdesivir this would be in contrast to results of the randomized clinical trials [6]. The discrepancy would likely be explained by the fact that the remdesivir trials included individuals with a wider range of disease severities of COVID-19 where our population predominantly included individuals with hypoxemic COVID-19 similar to the subgroup of ACTT-1 that benefitted the most in terms of survival.
The population-based nationwide design with complete follow-up is a strength of the study. However, residual confounding by unmeasured variables may have influenced outcome estimates although results were robust using methodology to account for differences in cohort composition. Regardless, the results of a before-and-after study should be interpreted with caution.
In conclusion, individuals with COVID-19 who received remdesivir and dexamethasone in addition to SOC had reduced 30-day mortality and need of MV compared to individuals receiving SOC.
Notes
Potential conflict of interest. T. B. reports unrestricted grants from Novo Nordisk Foundation, unrestricted grants from Simonsen Foundation, unrestricted grants and personal fees for serving as an advisory board member from GlaxoSmithKline, unrestricted grants and personal fees for lecturing from Pfizer, personal fees for teaching from Boehringer Ingelheim, grants and personal fees for teaching from Gilead, personal fees for teaching/serving on advisory board from Merck, Sharp & Dohme, unrestricted grants from Lundbeck Foundation, unrestricted grants from Kai Hansen Foundation, personal fees for serving as a board member from Pentabase A/S/ApS, and unrestricted grants from Erik and Susanna Olesen’s Charitable Fund, outside the submitted work. J. L. reports grants from the National Institutes of Health, USA, during the conduct of the study; personal fees for participation in webinars from Critical Care Options, outside the submitted work. C. B. reports receiving honoraria outside this study (Nordic CLL meeting) from AstraZeneca. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. ACTT-1 Study Group. Remdesivir for the treatment of Covid-19: final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 : preliminary report [manuscript published online ahead of print 17 July 2020]. N Engl J Med 2020; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19: interim WHO solidarity trial results [manuscript published online ahead of print 2 December 2020]. N Engl J Med 2021; 384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaka AS, MacDonald R, Greer N, et al. Major update: remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians practice points. Ann Intern Med 2021; 174:663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olender SA, Perez KK, Go AS, et al. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin Infect Dis 2020:ciaa1041. doi: 10.1093/cid/ciaa1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garibaldi BT, Wang K, Robinson ML, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open 2021; 4:e213071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anesi GL, Jablonski J, Harhay MO, et al. Characteristics, outcomes, and trends of patients with Covid-19-related critical illness at a learning health system in the United States. Ann Intern Med 2021:M20–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med 2021; 49:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navaratnam AV, Gray WK, et al. Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: an observational study using administrative data. Lancet Respir Med 2021; 9:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med 2021; 16:90–2. [DOI] [PubMed] [Google Scholar]
- 12. Goldman JD, Lye DCB, Hui DS, et al. ; GS-US-540-5773 Investigators . Remdesivir for 5 or 10 Days in patients with severe Covid-19. N Engl J Med 2020; 383:1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Israelsen SB, Kristiansen KT, Hindsberger B, et al. Characteristics of patients with COVID-19 pneumonia at Hvidovre hospital, March–April 2020. Dan Med J 2020; 67:A05200313. [PubMed] [Google Scholar]
- 14. Meyer CN. Transmission, start of symptom and morbidity among Danish COVID-19 patients admitted to hospital. Dan Med J 2020; 67:A05200325. [PubMed] [Google Scholar]
- 15. Dalager-Pedersen M, Lund LC, Mariager T, et al. Venous thromboembolism and major bleeding in patients with COVID-19: a nationwide population-based cohort study [manuscript published online ahead of print 5 January 2021]. Clin Infect Dis 2021:ciab003. doi: 10.1093/cid/ciab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brieghel C, Ellekvist P, Lund M, et al. Prognostic factors of 90-day mortality in patients hospitalized with COVID-19. Dan Med J 2021; 68:A09200705. [PubMed] [Google Scholar]