Abstract
Background
We investigated the association of vitamin K and vitamin D with coronavirus disease 2019 (COVID-19) outcomes.
Methods
Levels of inactive vitamin K–dependent dephosphorylated uncarboxylated matrix Gla protein (dp-ucMGP; marker of vitamin K status) and 25-hydroxyvitamin D (25(OH)D; vitamin D status) were measured in plasma samples from participants with confirmed acute COVID-19 and were age- and sex-matched to healthy controls. Unadjusted odds ratios and adjusted odds ratios (AORs) with 95% CIs were computed using cumulative logistic regression.
Results
One hundred fifty subjects were included, 100 COVID-19+ and 50 controls. The median age (interquartile range) was 55 (48–63) years, and 50% were females. Thirty-four percent had mild COVID-19 disease, 51% moderate disease, and 15% severe. Dp-ucMGP levels were higher (ie, worse K status) in COVID-19+ vs controls (776.5 ng/mL vs 549.8 ng/mL; P < .0001) with similar 25(OH)D between groups (25.8 vs 21.9 ng/mL; P = .09). Participants who were vitamin D deficient (<20 ng/mL) had the worse vitamin K status (dp-ucMGP >780 ng/mL) and experienced the most severe COVID-19 outcomes. In adjusted models, every 1-unit increase in the log2 dp-ucMGP nearly doubled the odds of acute critical disease or death (AOR, 1.84; 95% CI, 1.01–3.45), and every 1-unit decrease in the natural log 25(OH)D was associated with >3 times the likelihood of severe COVID-19 disease (AOR, 0.29; 95% CI, 0.11–0.67).
Conclusions
Early in acute COVID-19, both vitamin K and vitamin D deficiency were independently associated with worse COVID-19 disease severity, suggesting a potential synergistic interplay between these 2 vitamins in COVID-19.
Keywords: COVID-19, SARS-CoV-2, vitamin D, vitamin K
Since the emergence of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2019, there has been a substantial worldwide effort in understanding the pathogenesis of coronavirus disease 2019 (COVID-19), treatment modalities, and prevention. Those infected with this virus may have a wide range of symptoms from an asymptomatic or mild state to severe pneumonia, coagulopathy, and death [1]. The ability to predict severe disease and response to treatment remains elusive [2]. Several risk factors have been identified for disease severity, including advanced age, male sex, non-White race, obesity, vitamin and mineral deficiencies (specifically vitamin D, vitamin C, and selenium), diabetes, and hypertension. Severe outcomes have been associated with multiorgan failure, which may correspond to the underlying inflammatory response postinfection. Host factors triggered by the virus can lead to an exaggerated inflammatory response or “cytokine storm,” leading to pulmonary damage through calcification of pulmonary interstitial arteriolar walls, hypercoagulation, and disseminated intravascular coagulation [3].
Vitamin K is the name given to a group of essential fat-soluble vitamins, phylloquinone (vitamin K1) and menaquinones (vitamin K2), which are co-factors for several proteins involved in coagulation homeostasis and calcium homeostasis. Phylloquinone and menaquinones share the same head group (2-methyl-napthoquinone) but have different compositions of the poly-isoprenoid side chain (at the 3-position). In the circulation, there is a differential distribution of vitamin K to lipoproteins, where the majority of short-chain vitamin K (phylloquinone) is associated with triacylglycerol-rich lipoproteins, and the longer menaquinones are more associated with low-density lipoproteins (LDLs). The associations with different lipoproteins allow different distribution in the body, where the long-chain menaquinones reach extrahepatic tissues that possess LDL receptors [4]. Vitamin K activates both hepatic coagulation factors and extrahepatic endothelial anticoagulant protein S, which are required for thrombosis prevention. Other important extrahepatic vitamin K–dependent proteins are osteocalcin, matrix gla-protein (MGP), Gla-rich protein (GRP), and growth arrest–specific protein 6 (Gas6). Deficiency in vitamin K is linked to vascular calcification and osteoporosis. Vitamin K is also believed to have anti-inflammatory properties, mediated by the reduction of circulating inflammatory mediators [5].
Recent research has reported a poor vitamin K status in hospitalized adults with COVID-19 infection [6, 7]. Anastasi et al. and Dofferhoff et al. suggested that deficiency in vitamin K may be associated with more severe outcomes, although all patients in these studies were hospitalized with more severe disease. The biomarker that has been mostly used for extrahepatic vitamin K status is dp-ucMGP. High levels of dp-ucMGP (poor vitamin K status) have been associated with many additional clinical outcomes, like cardiovascular risk, arterial stiffness, endothelial function, and chronic obstructive pulmonary disease (COPD) [8–12].
In addition, there is currently an ongoing debate about the relevance of vitamin D supplementation in COVID-19. Vitamin D has been shown to reduce the incidence of viral respiratory tract infections, in particular within persons who have a low vitamin D status [13]. However, it is not clear if vitamin D status also plays a role in COVID-19 and whether that role is independent of vitamin K status. In this study, we investigated the status of vitamin K and vitamin D early in the course of COVID-19 and their ability to independently predict COVID outcomes, as well as the potential synergistic interplay between these 2 vitamins and inflammatory chemical mediators. We hypothesized that COVID-19 participants would show a lower vitamin K status and that a lower status would be associated, independently of vitamin D status and other known risk factors, with poor COVID-19 outcomes.
METHODS
This was a prospective observational cohort study. Participants were enrolled from April 21, 2020, to November 5, 2020, at University Hospitals Cleveland Medical Center in Cleveland, Ohio. Patients were confirmed to have SARS CoV-2 infection by nasopharyngeal swab real-time polymerase chain reaction (PCR) testing. Participants varied in disease presentation from asymptomatic to life-threatening and even death. Additional participants who were asymptomatic had been selected to match by age and sex to the COVID-19 cohort. These healthy controls had been enrolled prior to November 2019 as “healthy controls” for an ongoing HIV study. The COVID-19 participants were enrolled as part of a COVID-19 biorepository, which enrolled any willing patient who presented to University Hospitals Health System, Cleveland, Ohio, with documented positive SARS-CoV-2, whether they presented to the ambulatory clinic, emergency department, or were hospitalized. In addition, patients self-referred for biorepository enrollment, including some who were (and remained) asymptomatic but tested positive by PCR after a known exposure. Blood was collected at multiple time points throughout the disease course. We used blood at an early time point (within the first 7 days after symptoms) for this study. Detailed clinical history was available on these participants, both before the illness and throughout their illness course.
This study was approved by the Institutional Review Board at University Hospitals Case Medical Center, Cleveland, Ohio. All subjects gave written informed consent for collection of blood samples. Patient demographics for age, sex, and race were collected. In addition, body mass index (BMI) at the time of initial presentation and comorbidities and laboratory data for creatinine, complete blood count, C-reactive protein (CRP), and liver function were assessed on all subjects.
COVID-19 Outcomes
Participants’ acute illness severity was assessed according to the World Health Organization’s ordinal scale for clinical improvement (OSCI) for COVID-19 [14]. Acute illness severity ranges from having no clinical signs of infection (low score) to death. The highest score during hospitalization was used for this analysis. These scores were collapsed into 3 categories according to disease severity and dedicated treatments. The acute mild disease group included those with mild disease and no assistance to those with moderate disease and no oxygen therapy. The acute moderate disease group included those with moderate disease requiring oxygen therapy to those with severe disease requiring noninvasive oxygen therapy. The acute severe disease group included severe disease, any form of invasive ventilation, and death.
Dephosporylated Uncarboxylated Matrix Gla Protein
Due to inability to directly measure naturally occurring blood vitamin K levels, measuring vitamin K–dependent protein in circulation is a valuable method to measure vitamin K deficit. Circulating dephosporylated uncarboxylated matrix Gla protein (dp-ucMGP) level was used to indirectly quantify extrahepatic vitamin K status [9]. High dp-ucMGP levels are considered the best marker of low vitamin K status. Plasma samples that were never previously thawed were processed at the Maine Medical Center Research Institute. For the analysis, vitamin K (dp-ucMGP) was categorized by distribution tertiles, defined as low vitamin K (dp-ucMGP >897 ng/mL), mid vitamin K (dp-ucMGP between 520 and 897 ng/mL), and high vitamin K (dp-ucMGP <520 ng/mL).
25-Hydroxyvitamin D Level
Vitamin D status was assessed through measurement of 25-hydroxyvitamin D (25(OH)D) levels [15]. Plasma samples never previously thawed were processed at the Maine Medical Center Research Institute. For the analysis, vitamin D (25-hydroxyvitamin D) was categorized by distribution tertiles and defined as optimal vitamin D status (>30 ng/mL), insufficient (20–30 ng/mL), and deficient (<20 ng/mL).
Statistical Analysis
Characteristics of study participants were described using median and interquartile range for continuous variables and frequency and percentage for categorical variables. Differences between COVID-19-positive and healthy controls were computed using either nonparametric Wilcoxon rank-sum (Mann-Whitney U) for continuous variables or χ 2 for categorical variables. Log transformations were used to stabilize the error variance. Independent associations between demographics, comorbidities, lab values, and inflammation markers with dp-ucMGP and 25(OH)D were computed using generalized linear models by method of least squares. To assess if suboptimal vitamin K and vitamin D levels are associated with worse COVID-19 outcomes, estimated unadjusted odds ratios (UORs) and adjusted odds ratios (AORs) with 95% profile likelihood CIs were computed using cumulative logistic regression and adjusted for age, sex, race, comorbidities, and marker of systemic inflammation. Variance inflation factor and tolerance (or 1-R2) was used to assess linear dependencies among explanatory variables, and the likelihood ratio chi-square statistic was used to test the proportional odds assumption. P values less than alpha < .05 were considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Inc., Cary, NC, USA).
RESULTS
Baseline Characteristics
One hundred fifty participants were included in the final analysis, 100 with SARS-CoV-2 and 50 age- and sex-matched healthy controls. Demographic data are summarized in Table 1. Overall, the median age (interquartile range [IQR]) was 54.9 (48–63) years, and 50% were female. Compared with healthy controls, BMI (32.48 vs 27.12; P = .001), creatinine values (1.05 vs 0.82 mg/dL; P < .0001), neutrophil percentage (78.3 vs 58.6; P < .0001), and aspartate aminotransferase (AST) values (29.5 vs 17 U/L; P < .0001) were higher in the COVID-19-positive cohort. However, there were no differences in total white blood cell count (8000 vs 5800; P = .14), lymphocyte percentage (30.8 vs 29.5; P = .6), or alanine aminotransferase (19 vs 20.5 U/L; P = .73).
Table 1.
Baseline Characteristics of Participants
| Overall (n = 150) | COVID-19 Positive (n = 100) | COVID-19 Negative (n = 50) | P Value | |
|---|---|---|---|---|
| Median (IQR) or No. (%) | ||||
| Vitamin D and K status | ||||
| Vitamin D (25-hydroxyvitamin D), ng/mL | 24.40 (15.43–31.58) | 25.78 (15.65–35.63) | 21.88 (14.32–26.58) | .09 |
| Vitamin K (dp-ucMGP), ng/mL | 638.10 (519.79–897.12) | 776.51 (570.92–1228.52) | 549.76 (468.86–635.05) | <.0001 |
| Demographics | ||||
| Age, y | 54.90 (48.00–63.00) | 57 (41.00–68.50) | 53.36 (50.74–56.30) | .25 |
| Female | 75.00 (50.00) | 49 (19.60) | 26 (10.40) | .73 |
| Non-Whitea | 89.00 (59.33) | 65 (26.00) | 24 (9.60) | .05 |
| BMI, kg/m2 | 29.86 (25.04–35.63) | 32.48 (26.61–38.60) | 27.12 (23.48–30.58) | .001 |
| Comorbidities | ||||
| Any comorbidityb | - - | 60 | - - | - - |
| Hypertension | - - | 48 | - - | - - |
| Pulmonaryc | - - | 31 | - - | - - |
| Diabetes | - - | 28 | - - | - - |
| Cardiovascular diseased | - - | 16 | - - | - - |
| Chronic kidney disease | - - | 12 | - - | - - |
| Cancer or immunosuppression | - - | 10 | - - | - - |
| Laboratory data | ||||
| Creatinine, mg/dL | 0.95 (0.78–1.17) | 1.05 (0.86–1.44) | 0.82 (0.73–0.97) | <.0001 |
| White blood cell count, ×10E9/L | 6.30 (4.60–9.20) | 8.00 (3.00–13.10) | 5.8 (5.00–6.70) | .14 |
| Neutrophil, % | 73.10 (62.00–82.50) | 78.3 (68.60–85.50) | 58.60 (46.60–62.75) | <.0001 |
| Lymphocyte, % | 30.70 (21.70–38.10) | 30.8 (20.00–39.00) | 29.45 (25.80–37.20) | .6 |
| Hemoglobin, g/dL | 13.30 (12.20–14.40) | 12.35 (11.30–14.05) | 13.9 (13.20–14.90) | .001 |
| Aspartate aminotransferase, U/L | 21.0 (17.00–31.00) | 29.50 (19.00–45.00) | 17.0 (15.00–22.00) | <.0001 |
| Alanine aminotransferase, U/L | 23.0 (16.00–32.00) | 19.00 (13.00–32.00) | 20.5 (13.00–27.00) | .73 |
| C-reactive protein, mg/dL | 1.02 (0.20–9.18) | 4.64 (1.06–13.24) | 0.18 (0.10–0.43) | <.0001 |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 3019; dp-ucMGP, dephosphorylated uncarboxylated matrix Gla protein; IQR, interquartile range.
aIncludes African American, Asian, Hispanic, and other.
bIncludes hypertension, pulmonary diseases, diabetes, cardiovascular diseases, chronic kidney disease, and cancer or immunosuppression.
cIncludes chronic pulmonary disease, asthma, and pulmonary circulation disorder.
dIncludes coronary artery disease, congestive heart failure, and valvular heart disease.
Among the COVID-19-positive cohort, 52% (52/100) had at least 1 comorbidity, with hypertension being the most prevalent, at 48% (48/100). The most common presenting symptoms at admission included nausea/vomiting (41%), followed by cough (33%) and fatigue (32%). Thirty-four had mild disease (4 asymptomatic and 30 symptomatic, not requiring hospitalization or oxygen), 51 had moderate disease (all hospitalized, requiring oxygen by nasal cannula, face mask, or other noninvasive oxygen therapy), and 15 were classified as severe (8 requiring mechanical ventilation and 7 deaths). There were no gender or racial differences in disease severity (P = .75). In terms of treatments received, only 2 participants received remdesivir, 1 in the moderate disease category and 1 severe. Tocilizumab was given to only 1 participant, who died. Steroid therapy was given to 3 participants (dexamethasone to 2 and prednisone to 1), all in the moderate category.
Vitamin K Status Between Groups and Relationship to COVID-19 Outcomes
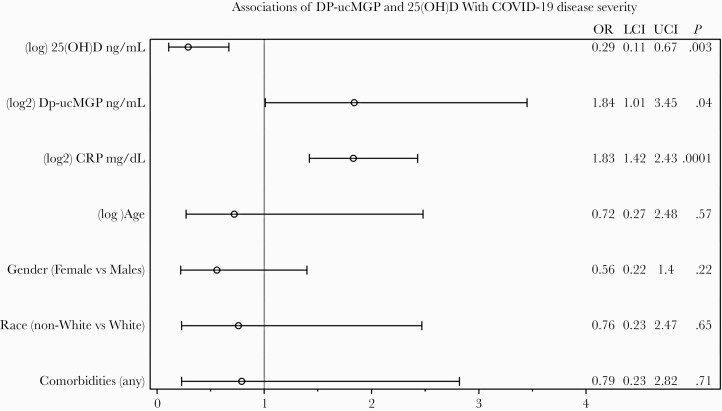
The overall median dp-ucMGP (IQR) was 638.10 (519.79–897.12) ng/mL and was higher (ie, worse vitamin K status) in the COVID-19-positive cohort compared with controls (776.5 ng/mL vs 549.8 ng/mL; P < .0001). Chronic kidney disease (P < .0001), cardiovascular disease (P = .001), hypertension (P = .02), cancer/immunosuppression (P = .0002), creatinine (P < .0001), lymphocyte percentage (P = .02), and CRP (P ≤ .0001) were independently associated with dp-ucMGP (Table 2). As shown in Table 3 and Figure 1, in the adjusted model, every 1-unit increase in the log2 dp-ucMGP nearly doubled the odds of acute critical disease or death (AOR, 1.84; 95% CI, 1.01–3.45).
Table 2.
Independent Associations of Participant Characteristics With 25(OH)D and dp-ucMGP Among Patients With COVID-19
| 25(OH)D | MGP | |
|---|---|---|
| P > .05 | ||
| Age, y | <.0001 | - - |
| Female | .02 | - - |
| Non-White | .01 | - - |
| Chronic kidney disease | - - | <.0001 |
| Cardiovascular disease | - - | .001 |
| HTN | - - | .02 |
| Cancer or immunosuppression | - - | .0002 |
| Creatinine, mg/dL | - - | <.0001 |
| Lymphocyte, % | - - | .02 |
| CRP, mg/dL | - - | <.0001 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; COVID-19, coronavirus disease 3019; CRP, C-reactive protein; dp-ucMGP, dephosphorylated uncarboxylated matrix Gla protein; HTN, hypertension; MGP, matrix Gla protein.
Table 3.
UOR, AOR, and 95% CI
| COVID-19 Outcome | ||
|---|---|---|
| UOR (95% CI) | AOR (95% CI) | |
| Vitamin D (25OH), ng/mLa | 0.43 (0.22–0.82) | 0.29 (0.11–0.67) |
| MGP (dp-ucMGP), ng/mLb | 2.07 (1.29–3.48) | 1.84 (1.01–3.45) |
| C-reactive protein, mg/dLb | 1.83 (1.47–2.34) | 1.83 (1.42–2.43) |
| Age, ya | 1.02 (0.99–1.04) | 0.72 (0.27–2.48) |
| Female | 0.84 (0.40–1.78) | 0.56 (0.22–1.40) |
| Non-White | 0.61 (0.27–1.35) | 0.76 (0.23–2.47) |
| Comorbidities (any) | 1.35 (0.58–3.18) | 0.79 (0.23–2.82) |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; AOR, adjusted odds ratio; COVID-19, coronavirus disease 3019; dp-ucMGP, dephosphorylated uncarboxylated matrix Gla protein; UOR, unadjusted odds ratio.
aNatural log transformed.
bLog(base)2 transformed.
Figure 1.
Association of vitamin K (Dp-ucMGP) and vitamin D (25(OH)D) with COVID-19 disease severity. Abbreviations: 25(OH)D, 25-hydroxyvitamin D; COVID-19, coronavirus disease 3019; dp-ucMGP, dephosphorylated uncarboxylated matrix Gla protein; LCI, lower CI; OR, adjusted odds ratio; UPI, upper CI.
Vitamin D Status Between Groups and Relationship to COVID-19 Outcomes
The overall median vitamin D (IQR) was 24.40 (15.43–31.58) ng/mL. Although vitamin D levels were higher in the COVID-19-positive cohort, there was not enough evidence to suggest that these values were different when compared with the control group (25.8 vs 21.9; P = .09). Age (P < .0001), sex (P = .02), and race (P = .01) were independently associated with 25(OH)D (Table 2). In adjusted models, as shown in Table 3 and Figure 1, every 1-unit decrease in the natural log 25(OH)D was associated with >3 times the likelihood of COVID-19 disease severity (AOR, 0.29; 95% CI, 0.11–0.67).
Interplay of Vitamin K and Vitamin D and Inflammatory Mediators
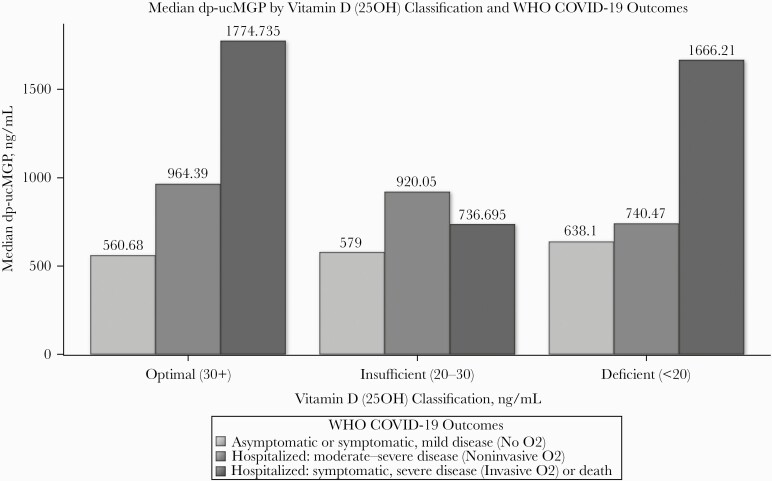
Although there was not enough evidence to suggest that 25(OH)D was independently associated with dp-ucMGP (P = .26), participants with sufficient vitamin D (>30 ng/mL) as well as those who were vitamin D deficient (<20 ng/mL) had the worse vitamin K status (dp-ucMGP >780 ng/mL) and experienced the most severe COVID-19 disease outcomes (Figure 2).
Figure 2.
Median dp-ucMGP by vitamin D (25OH) classification and WHO COVID-19 outcomes. Abbreviations: COVID-19, coronavirus disease 3019; dp-ucMGP, dephosphorylated uncarboxylated matrix Gla protein; WHO, World Health Organization.
CRP (mg/dL) was independently associated with dp-ucMGP (P ≤ .0001) and acute severe COVID outcome (P < .0001) and was not associated with 25(OH)D (P = .66). As shown in Table 3 and Figure 1, after adjusting for demographics, comorbidities, dp-ucMGP, and 25(OH)D, every 1-unit increase in log2 CRP nearly doubled the odds of acute critical disease or death (AOR, 1.83; 95% CI, 1.42–2.43). There is some evidence to suggest that CRP may confound the relationship of dp-ucMGP on COVID-19 outcomes.
In separate models, we also adjusted for lymphocyte and creatinine, which did not remain associated with acute critical disease (P ≥ .36 for both), and neither lymphocytes nor creatinine changed the effect of log2 dp-ucMGP on COVID-19 disease severity (AOR, 2.66; 95% CI, 1.49–5.12; and AOR, 2.51; 95% CI, 1.32–5.09; for models adjusting for log lymphocytes and log creatinine, respectively).
DISCUSSION
This is the first study to measure both vitamin D and vitamin K status in COVID-19. Our study suggests an independent role of both poor vitamin K and D status in COVID-19 disease severity. Furthermore, vitamin K was associated with factors that have been described to be associated with COVID-19 outcomes, including demographics, BMI, inflammation marker CRP, and comorbidities. However, after accounting for vitamin K status, the association between these known risk factors and COVID-19 outcome was no longer present, suggesting that the role of demographics and comorbidities may be mediated by their association with vitamin K (and possibly also vitamin D status).
There are 20 described vitamin K–dependent proteins (VKDP) in the human body, where vitamin K serves as a co-factor to activate these proteins through a carboxylation process [16]. We measured the inactive form of MGP, dephosphorylated uncarboxylated MGP (dp-ucMGP), as it is the biomarker that has been most often used for extrahepatic vitamin K status. High levels of dp-ucMGP have been associated with many clinical outcomes, like cardiovascular risk, arterial stiffness, endothelial function, and COPD [8–12]. In an active, carboxylated state, MGP is a strong binder of calcium. A low vitamin K status leads to higher levels of the inactive form, dp-ucMGP, which do not bind calcium. The role of vitamin K in COPD was discussed by Piscaer and colleagues in 2019, who proposed that decreased activation of MGP leads to calcification of elastin, something that further leads to increased elastase activity, accelerated elastolysis, and elevated plasma desmosine levels [10]. The importance of vitamin K in lung health was also recently discussed by another research group. Using NHANES 2007–2016 data (n = 17 681), Shen et al. showed that consumption of recommended amounts of vitamin K was associated with a 39% decrease in odds of emphysema, and they concluded that vitamin K is important for lung health [17]. Recently, 2 independent European research groups reported vitamin K status as a potentially modifiable risk factor for COVID-19 [6, 7]. Dofferhoff and colleagues used 2 assays to measure vitamin K status in COVID-19 patients: [7] dp-ucMGP to measure extrahepatic vitamin K and protein induced by vitamin K absence (PIVKA)-II to detect hepatic vitamin K status. The vitamin K status in 135 hospitalized COVID-19 patients was compared with controls. Similar to our findings, the extrahepatic vitamin K status was lower in the COVID-19 patients compared with the controls, and it was even lower in the poor outcome patients (invasive ventilation and/or death). In another study, in 62 COVID-19 patients, 72% of the males and 36% of the females were vitamin K deficient. Our study extends these findings to a US cohort, and also to a cohort with largely varying clinical presentation (varying from asymptomatic status to death), in which we report a significant association between vitamin K status and COVID-19 disease severity.
A state of heightened inflammation, usually measured by soluble cytokines, seems to play an important role in the pathophysiology of COVID-19. Some cytokines are potentially beneficial (type-I interferon, interleukin [IL]-7), and others harmful (tumor necrosis factor [TNF]-α, IL-1β, IL-6) [18]. Anastasi and colleagues have shown a positive correlation between low hepatic vitamin K status and elevated interleukin 6 (IL-6). The anti-inflammatory action of vitamin K is shown in several studies outside of COVID-19. Using human monocyte–derived macrophages, Pan and colleagues showed inhibition of cytokine release (TNF-α, IL-1α, IL-1β) when the cells were pretreated with vitamin K2 (menaquinone-7), and the scientists were able to show a dose–response relationship [19]. Similar inhibitory effects on IL-6 have also been shown by other research groups [20, 21]. The release of pro-inflammatory cytokines is mainly regulated through the NF-kB signaling pathway, and vitamin K has been shown to inhibit the release of IkB from NF-kB to allow its entry into the nucleus [22–24]. We were able to show that CRP was associated with more severe COVID-19 outcome and with lower vitamin K status. In addition, when adjusting for CRP, we saw an attenuation in the relationship between vitamin K and COVID-19 outcomes. These observations suggest that the association between vitamin K, but not vitamin D, and COVID-19 severity outcome is likely partially due to vitamin K’s anti-inflammatory effect. In addition, vitamin K status may unfavorably modify COVID-19 pathogenesis by several other mechanisms, including lower extrahepatic activation of the anticoagulant protein S, leading to accelerated thrombosis formation, and lower activation of MGP, reducing inhibition of mineralization of the elastic fibers in the lung, leading to accelerated elastic fiber damage.
There is currently ongoing debate about the relevance of vitamin D supplementation in COVID-19. Vitamin D has been shown to reduce the incidence of viral respiratory tract infections, in particular within persons who have a low vitamin D status. However, it is not clear if vitamin D status also plays a role in COVID-19 and whether that role is independent of vitamin K status. Recently, Meltzer and colleagues documented an inverse association between vitamin D status and increased risk of COVID-19 [25]. In addition, patients admitted to the intensive care unit have lower vitamin D levels than non–intensive care unit patients [26]. Vitamin D could potentially modulate the pathogenesis of COVID-19 early by lowering the entry rate of the virus, and/or at a later stage by lowering the release of pro-inflammatory cytokines [27]. Although vitamin D was not different between those with and without COVID-19 in our study, vitamin D status was independently associated with disease severity in COVID-19.
Vitamin D is mainly synthesized in the skin after exposure to UVB, with a part being derived from dietary sources [28]. Endogenous vitamin D status highly depends on several factors including nutritional habits, latitude, season, skin pigmentation, and lifestyle [15]. It has important pleiotropic immunomodulatory effects. In a meta-analysis of 25 randomized controlled trials, vitamin D supplementation reduced the risk of developing acute respiratory tract infections, with a higher protective effect in those with low levels of vitamin D at baseline (25-hydroxyvitamin D levels <25 nmol/L) [13]. We have previously investigated the role of vitamin D supplementation in other diseases associated with ongoing inflammation; specifically we focused on markers of T-cell activation/exhaustion and monocyte activation in HIV-infected youth whose HIV was controlled with antiretrovirals [29]. In a randomized, active-control, double-blind trial investigating 3 different vitamin D doses in HIV-infected youth on ART with serum 25-hydroxyvitamin D concentrations ≤30 ng/mL, we found that all measured markers decreased including CD4+ and CD8+ T-cell activation, CD4+ exhaustion, and inflammatory mediators.
Our findings suggest a potential relationship between vitamins D and K at the time of acute COVID-19 and an association with worse disease severity. Interestingly, both vitamin D and vitamin K may display complementary effects on the cytokine storm, thrombosis, and lung damage during COVID-19. Specifically, they display similar inhibitory effects on inhibition of NF-kB and cytokine release, and vitamins D and K appear to work synergistically to help protect against calcification and damage in the lungs. While vitamin D promotes MGP synthesis, vitamin K is crucial for activation of MGP. Active MGP helps remove calcium from soft tissues. Without this action, accumulation of calcium on elastic fibers leads to degradation. Low active MGP results in a higher risk for elastic fiber damage in the lungs. As such, concomitantly measuring the status of both vitamins in COVID-19 and relating it to pro- and anti-inflammatory markers are of major importance in COVID-19. In addition, optimizing vitamins K and D before acute COVID-19 infection may help to regulate overwhelming calcification, inflammation, and coagulopathy, which may subsequently lead to improved clinical outcomes. As the long-chain menaquinones of vitamin K2 have an extrahepatic distribution, they possess the capacity to optimize vitamin K status in tissues that are deficient; however, large preventative clinical trials are needed.
Several factors are frequently cited as risk factors for severe COVID-19 outcomes, including race, BMI, and comorbidities. Black individuals have higher mortality in the United States, with an infection rate >3-fold higher in predominantly Black counties compared with predominantly White counties [30]. Hypertension has been identified as the most common comorbidity in COVID-19, and obesity is a risk factor for COVID-19 disease severity. In our study, being non-White and having a higher BMI were independently associated with worse vitamin D and K levels; however, neither race nor BMI was associated with COVID-19 outcome after adjusting for vitamin D and vitamin K status. Similarly, hypertension was independently associated with worse vitamin K status but not with COVID-19 severity when adjusting for vitamin D and K levels. Most African Americans lack normal serum levels of vitamin D; in addition, greater adiposity is associated with lower vitamin D levels, suggesting a role for vitamin D in abdominal fat storage and function. Race, BMI, and comorbidities, specifically hypertension, may be inter-related, and racial differences in COVID-19 disease severity may be due to additional socioeconomic and health care disparities; however, our findings suggest that vitamins D and K may play a role in modulating the associations between these factors and COVID-19 disease severity.
Our study has several strengths, including the detailed analysis of COVID-19 outcomes, the varying presentations of COVID-19 illness, the concomitant measurements of vitamins D and K, and the enrollment of participants in a narrow time frame starting at the beginning of the COVID-19 pandemic in Ohio. Clinical records and outcomes of COVID-19 illness were recorded for at least 28 days after the illness. All participants were enrolled in the same location in the northern hemisphere. Our limitations include that the blood testing was cross-sectional, and therefore we cannot exclude the possibility of residual confounding and show causation, as well as the lack of dietary information, which may affect vitamins D and K.
CONCLUSIONS
Early in acute COVID-19, both vitamin K deficiency and vitamin D deficiency were independently associated with worse COVID-19 disease severity, even after adjusting for demographics and comorbidities. This suggests a potential synergistic interplay between these 2 vitamins in COVID-19 as important modifiable risk factors. Further studies are needed to determine whether optimizing vitamin D and K status will improve clinical outcomes in those with COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by funding through University Hospitals Cleveland Medical Center (UHCMC) and the Clinical and Translational Science Collaborative of Cleveland, as well as UL1TR002548 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research. Funding supported the enrollment of participants. Findings in the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of UHCMC or the NIH. In addition, the study was partially funded by Kappa Bioscience AS; Kappa Bioscience AS funded the vitamin K and vitamin D assays by directly paying the Maine laboratory. Kappa Bioscience AS has no access to any raw data or any participants’ data besides what is described in this manuscript.
Potential conflicts of interest. G.M. has served as a scientific consultant for Gilead, GSK/ViiV, Merck, Theratechnologies, and Janssen and has received funding support from Gilead, ViiV, Tetraphase, Roche, Genentech, Vanda, Astellas, and Merck. All other authors have no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. G.M. conceptualized the study, obtained IRB consent, enrolled the patients, and oversaw the experiments and the analyses. J.D. led data analysis. H.T. and D.L. led participant enrollment and data acquisition. A.D. and S.D.F. wrote the draft report, and all other authors revised the report critically for important intellectual content. All authors have read and approved the final version of the report. G.M. and J.D. verified the data sets and have access to the raw data.
Data sharing. All of the individual participant data collected during the trial, after de-identification, will be shared upon request.
Patient consent. This study was approved by the Institutional Review Board at University Hospitals Case Medical Center, Cleveland, Ohio. All subjects gave written informed consent for collection of blood samples.
References
- 1. Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324:782–93. [DOI] [PubMed] [Google Scholar]
- 2. Zhang JJY, Lee KS, Ang LW, et al. Risk factors for severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis, and meta-regression analysis. Clin Infect Dis 2020; 71:2199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zabetakis I, Lordan R, Norton C, Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients 2020; 12:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta 2002; 1570:27–32. [DOI] [PubMed] [Google Scholar]
- 5. Suleiman L, Négrier C, Boukerche H. Protein S: a multifunctional anticoagulant vitamin K-dependent protein at the crossroads of coagulation, inflammation, angiogenesis, and cancer. Crit Rev Oncol Hematol 2013; 88:637–54. [DOI] [PubMed] [Google Scholar]
- 6. Anastasi E, Ialongo C, Labriola R, et al. Vitamin K deficiency and COVID-19. Scand J Clin Lab Invest 2020; 80:525–7. [DOI] [PubMed] [Google Scholar]
- 7. Dofferhoff ASM, Piscaer I, Schurgers LJ, et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fain ME, Kapuku GK, Paulson WD, et al. Inactive matrix Gla protein, arterial stiffness, and endothelial function in African American hemodialysis patients. Am J Hypertens 2018; 31:735–41. [DOI] [PubMed] [Google Scholar]
- 9. Jespersen T, Møllehave LT, Thuesen BH, et al. Uncarboxylated matrix Gla-protein: a biomarker of vitamin K status and cardiovascular risk. Clin Biochem 2020; 83:49–56. [DOI] [PubMed] [Google Scholar]
- 10. Piscaer I, van den Ouweland JMW, Vermeersch K, et al. Low vitamin K status is associated with increased elastin degradation in chronic obstructive pulmonary disease. J Clin Med 2019; 8:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Ballegooijen AJ, Beulens JWJ, Kieneker LM, et al. Combined low vitamin D and K status amplifies mortality risk: a prospective study. Eur J Nutr 2021; 60:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zwakenberg SR, Burgess S, Sluijs I, et al. ; EPIC-CVD consortium. Circulating phylloquinone, inactive matrix Gla protein and coronary heart disease risk: a two-sample Mendelian randomization study. Clin Nutr 2020; 39:1131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017; 356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. WHO R and D Blueprint and COVID-19. Geneva: World Health Organization; 2020. [Google Scholar]
- 15. Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–81. [DOI] [PubMed] [Google Scholar]
- 16. Simes DC, Viegas CSB, Araujo N, Marreiros C. Vitamin K as a diet supplement with impact in human health: current evidence in age-related diseases. Nutrients 2020; 12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen T, Bimali M, Faramawi M, Orloff MS. Consumption of vitamin K and vitamin A are associated with reduced risk of developing emphysema: NHANES 2007-2016. Front Nutr 2020; 7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev 2020; 19:102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan MH, Maresz K, Lee PS, et al. Inhibition of TNF-α, IL-1α, and IL-1β by pretreatment of human monocyte-derived macrophages with menaquinone-7 and cell activation with TLR agonists in vitro. J Med Food 2016; 19:663–9. [DOI] [PubMed] [Google Scholar]
- 20. Ohsaki Y, Shirakawa H, Hiwatashi K, et al. Vitamin K suppresses lipopolysaccharide-induced inflammation in the rat. Biosci Biotechnol Biochem 2006; 70:926–32. [DOI] [PubMed] [Google Scholar]
- 21. Reddi K, Henderson B, Meghji S, et al. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine 1995; 7:287–90. [DOI] [PubMed] [Google Scholar]
- 22. Ohsaki Y, Shirakawa H, Miura A, et al. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J Nutr Biochem 2010; 21:1120–6. [DOI] [PubMed] [Google Scholar]
- 23. Ozaki I, Zhang H, Mizuta T, et al. Menatetrenone, a vitamin K2 analogue, inhibits hepatocellular carcinoma cell growth by suppressing cyclin D1 expression through inhibition of nuclear factor kappaB activation. Clin Cancer Res 2007; 13:2236–45. [DOI] [PubMed] [Google Scholar]
- 24. Xia J, Matsuhashi S, Hamajima H, et al. The role of PKC isoforms in the inhibition of NF-κB activation by vitamin K2 in human hepatocellular carcinoma cells. J Nutr Biochem 2012; 23:1668–75. [DOI] [PubMed] [Google Scholar]
- 25. Meltzer DO, Best TJ, Zhang H, et al. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open 2020; 3:e2019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Panagiotou G, Tee SA, Ihsan Y, et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin Endocrinol (Oxf) 2020; 93:508–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu W, Zhang L, Xu HJ, et al. The anti-inflammatory effects of vitamin D in tumorigenesis. Int J Mol Sci 2018; 19:2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients 2013; 5:2502–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eckard AR, O’Riordan MA, Rosebush JC, et al. Vitamin D supplementation decreases immune activation and exhaustion in HIV-1-infected youth. Antivir Ther 2018; 23:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yancy CW. COVID-19 and African Americans. JAMA 2020; 323:1891–2. [DOI] [PubMed] [Google Scholar]