ABSTRACT
Remote patient management (RPM) programs are one of the most crucial innovations in the peritoneal dialysis (PD) field that have been developed in the last decade. RPM programs are associated with favourable clinical outcomes by increasing the adherence of the patients to PD prescription. The literature supports that RPM is associated with increased blood pressure control and technique survival, and decreased hospitalization rate, length of hospital stay and health costs. RPM programs also facilitate patient follow-up during the coronavirus disease 2019 pandemic, increase treatment adherence and lead to better clinical outcomes. However, published data remain scarce and mainly consist of observational or retrospective studies with relatively low numbers of patients. Therefore, randomized controlled trial results will be more informative to demonstrate the effect of RPM programs on clinical outcomes.
Keywords: adherence, clinical outcome, COVID-19, peritoneal dialysis, remote patient management
INTRODUCTION
Peritoneal dialysis (PD) represents a home-based kidney replacement therapy that offers greater patient independence and autonomy compared with facility-based haemodialysis. Success of PD depends on adherence to treatment prescription [1]. However, details regarding whether a treatment was fully implemented rely on information provided by patients and their caregivers. In home environments, patients may feel safer and more independent, but home dialysis patients should capture details of their treatment such as their weight, blood pressure, PD fluid fill/dwell/drain information, and share them with healthcare providers routinely (i.e. monthly) and when problems occur. Increased hospitalization rate, prolonged length of hospital stay and transfer to haemodialysis generally ensue when patients do not adhere to treatment prescription [2, 3].
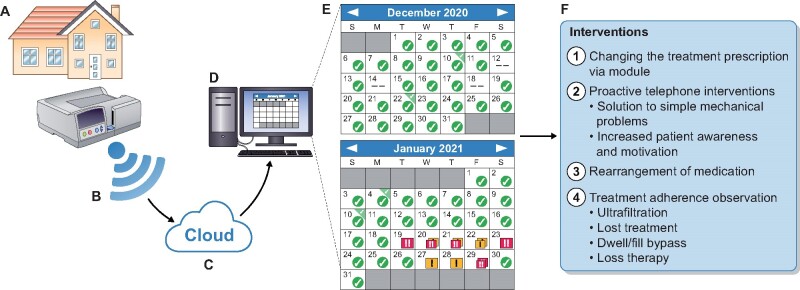
Adherence to treatment prescription must be maximized for the success of PD, particularly for better clinical outcomes and technique survival. Remote patient management (RPM) programs work with software using a cloud-based server and make it possible to collect and upload certain patient treatment data, including dwell, fill or drain bypass, treatment loss, non-adherence to prescription, blood pressure and weight, and to transmit them to the PD centre (Figure 1) [1, 4, 5]. One of these programs was launched in 2015 by Baxter (Homechoice Claria with platform Sharesource; Baxter Healthcare Corporation, Deerfield, IL, USA), and it is integrated with automated PD (APD) devices. In this manner, the awareness of patients about their treatment can be increased. Also, healthcare providers may be able to produce early solutions for some mechanical problems without a clinic visit, and most importantly, have a chance to change the treatment prescription through the system. RPM programs have become essential for increasing adherence.
FIGURE 1:
RPM programs provide bidirectional software-based communication system. (A–D) The data stream. (E) An example of a patient’s monthly data summary (Homechoice Claria with platform Sharesource; Baxter Healthcare Corporation). Yellow and red flags define the type of alarms. Flags and the alarms they represent can be changed on the module and flags identify filling, drain and dwell problems. Absent marks are identifying 20% non-adherence to the prescribed therapy. (F) The possible intervention that can be implemented by the PD team.
In this context, the integration of RPM programs with APD devices has been one of the most important technical developments in the last decade, and the objective of the present paper reviews published evidence on RPM with APD.
TREATMENT ADHERENCE
The most important limitation of home-based dialysis therapies is the lack of contact between the clinician and the patient. Thus, the clinician must believe that the prescribed treatment is fully executed and reflected in the patient’s statement. Non-adherence with the PD treatment is defined as the performance of less than 90% of the prescribed number of exchanges [3]. The overall non-adherence rates to PD prescription have been reported as 2.6–53% for PD prescription and 5–20% for APD prescription [3, 6–8]. Non-adherence to treatment was significantly associated with death, transfer to haemodialysis, increased hospitalization rate and prolonged hospital stay [2, 3].
On the other hand, a case report showed that patients’ treatment adherence reached 90% with the RPM program [5]. In two studies, it was demonstrated that patients’ treatment adherence rate following the integration of the RPM program was 85% and 90% [9, 10]. As a result, the integration of RPM to APD therapy significantly contributed to the improvement in patients’ treatment adherence, because notifications about treatments that patients skipped or could not complete are directly transmitted to the PD team. Accordingly, healthcare providers communicate with patients to solve problems and emphasize the regularity of treatment, and the awareness of patients that someone is supervising them increases adherence with the treatment. Although there are no randomized clinical trials, the positive clinical contributions in all of the studies discussed below are attributed to increased treatment adherence with the initiation of RPM programs.
CLINICAL OUTCOMES
Most evidence supports that RPM programs facilitate treatment intervention, increase adherence and decrease health costs. However, there are limited data on the clinical outcomes. Also, current literature consists of relatively small observational studies (Table 1).
Table 1.
Studies in the literature on RPM in PD
| Study, reference | Design | Participants | Follow-up time | Outcome | Result |
|---|---|---|---|---|---|
| Corzo et al. [11] |
Retrospective Multicentre Observational |
148 patients with RPM and 410 patients without RPM | 1.1 ± 0.6 years | Technique survival | RPM associated with higher technique survival |
| Bunch et al. [12] | Observational | 1023 patients with RPM and effect of COVID-19 | 4 months |
Adherence Peritonitis Blood pressure |
During the COVID-19 pandemic, increased patients’ adherence and blood pressure control similar peritonitis rate |
| Walker et al. [13] | Qualitative, interview | 27 patients with RPM and 7 caregivers | – | Expectation experience | Increased patient knowledge on the disease; enhanced partnership with clinician |
| Walker et al. [14] | Qualitative, interview | 13 nurses, 12 nephrologists | – |
Perspective experience |
Enhanced patients focused care; emphasized patient privacy and boundaries |
| Manani et al. [15] | Retrospective | 35 patients with RPM and 38 patients without RPM | 6 months |
HRQoL Hospitalization Urgent visit Peritonitis |
Decreased in disease-specific hospitalization and length of stay and urgent visit; similar peritonitis and HRQoL |
|
Yeter et al. [7] |
Observational | 15 patients pre- and post-RPM | 6 months |
Adherence Blood pressure Adequacy Drug burden HRQoL Sleep quality |
Increased adherence and dialysis adequacy; better blood pressure control; decreased drug burden; similar sleep quality and HRQoL |
|
Yeter et al. [16] |
Observational cross-sectional | 15 CAPD, 20 RPM–APD and 38 healthy control | 12 months |
Hypervolaemia, Central haemodynamics and peripheral blood pressure Drug burden |
RPM was associated with better control of haemodynamic parameters with less antihypertensive drugs via controlling the excess body water |
| Ariza et al. [17] | Amorkov projection model | 100 APD patients | – |
Health cost Hospitalization Peritonitis |
Decreased $121 233 in annual health cost; 27 fewer hospitalizations; 518 fewer hospitalization days and 6 fewer peritonitis episodes |
|
Yeter et al. [18] |
Observational cross-sectional | 53 CAPD, 40 RPM–APD and 30 APD and effect of COVID-19 | 97 ± 31 days |
Laboratory parameters Blood pressure Depression |
RPM–APD provided better laboratory parameters; similar blood pressure control; depression may affect the accuracy of clinical assessment. |
| Sanabria et al. [6] | Retrospective | 63 patients with and without RPM; 1:1 propensity score matching | 12 months | Hospitalization | 0.36 fewer hospitalizations per patient-year and 6.57 fewer days per patient-year with RPM |
| Manani et al. [1] | Observational | 43 patients with RPM and 42 patients without RPM | 12 months |
Health cost Hospital visit QoL |
RPM–APD was cost-effective; decreased hospital visit; increased QoL according to the internal questionnaire |
|
Bunch et al. [10] |
Observational | 49 patients pre- and post-RPM | 2 months |
Adherence Peritonitis Blood pressure |
85% treatment adherence; similar peritonitis and technique failure; decreased diastolic blood pressure |
|
Uchiyama et al. [19] |
Simulation | 12 patients | – |
Health cost Hospital visit Hospitalization |
Decreased health cost and hospital visit; similar hospitalization |
| Manani et al. [20] | Observational | 37 patients pre- and post-RPM | 12 months |
Hospital visit Treatment adequacy |
Decreased hospital visit, similar treatment adequacy |
| Makhija et al. [2] | Simulation | 12 patients | – | Health cost | Decreased health cost |
|
Sanabria et al. [9] |
Cross-sectional | 396 patients with RPM | – |
Adherence Blood pressure |
90.1% adherence; 55.5% blood pressure <140/90 mmHg |
RPM–APD, RPM with APD; QoL, quality of life.
Blood pressure
It is well known that elevated blood pressure is a risk factor for left ventricular hypertrophy, cardiovascular events, stroke and mortality [21]. Blood pressure control becomes more difficult with chronic kidney disease progression. Also, salt restriction, dialysis adequacy and adherence, preservation of residual renal function and achieving dry weight become fundamental factors controlling blood pressure in patients receiving dialysis [21–23]. In general, patients had better blood pressure control after the initiation of RPM [7, 10, 16]. Possible explanations could be the increased treatment adherence, higher awareness of blood pressure and improved volume control [7, 10, 16]. In 2019, Bunch et al. [10] observed a significant decrease in diastolic blood pressure of approximately 5 mmHg after RPM initiation. However, the number of patients requiring antihypertensive medicine and the number of medicines were significantly higher after the introduction of RPM. They conducted a preliminary study and the main limitation was a short follow-up period of 2 months. One year later, we published data of 15 patients with RPM initiation with 6-months results [7]. We recorded the daily blood pressure, important alarms (bypass drain, bypass dwell/fill, treatment time lost), ultrafiltration rate and body weight via the RPM module. We also recorded the number of antihypertensive medications and the dosage of the drugs was titrated to achieve target blood pressure (<140/90 and >110/70 mmHg). According to our results, mean arterial blood pressure and the number of antihypertensive medications used were significantly reduced after the RPM. However, both studies did not have control groups and only included comparisons before and after switching to RPM. We recently conducted a study consisting of three groups, including RPM–APD, continuous ambulatory PD (CAPD) and healthy controls [16]. We found that RPM provides better control of peripheral diastolic blood pressure, central systolic and diastolic blood pressure via controlling the excess body water, which has been demonstrated by bioimpedance spectroscopy. However, there are no randomized clinical trials to confirm the effect of the RPM on blood pressure and change in daily antihypertensive drug counts. Also, the long-term reflection of better blood pressure control, such as reducing major adverse cardiovascular events, needs to be confirmed.
Technique survival
Technique failure is an inevitable outcome of PD therapy. It is defined by the minimum number of days the patient received haemodialysis after cessation of PD, ranging from >30 to 180 days [24]. Only 24% of patients who require haemodialysis for at least 30 days could return to PD. The chance to return PD is similar when using the need for haemodialysis at least 180 days as the technique failure definition [24, 25]. Theoretically, increased adherence to therapy and blood pressure control, rapid detection and solving of treatment-related problems and decreased hospitalization should be associated with increased technique survival. Peritonitis is the most common risk factor for technique failure. Other well-defined risk factors are inadequate dialysis, mechanical problems and social reasons [26]. Also, non-adherence to PD treatment could facilitate the development of peritonitis [27]. RPM may increase technique survival via increasing PD prescription adherence and contributing to early identification of mechanical problems [6, 9]. However, there are limited data in the literature regarding the effect of RPM on technique survival. Firstly, Manani et al. [1] reported that RPM is associated with a reduced technique failure in a relatively small-sized sample. Recently, Corzo et al. [11] showed that the RPM program was associated with higher technique survival in a multicentre, retrospective observational cohort study. Although this study was conducted with a large number of patients (148 patients with RPM and 410 patients without), it does not allow inference of causality due to the retrospective nature of the study. Furthermore, the authors only analysed the causes of the technique failure and there were no data on possible contributor factors to technique failure such as dialysis adequacy (Kt/V, amount of ultrafiltration and residual kidney function) and the number of previous peritonitis episodes [28]. Thus, randomized clinical trials are needed to confirm the effect of the RPM on technique survival.
Peritonitis
Peritonitis is one of the leading causes of technique failure [28]. APD is an established PD modality offering daytime dialysis independence and medical advantages, including lower peritonitis rates and improved fluid balance [6, 29]. One of the main advantages of integrating RPM to APD is increased treatment adherence, and it is known that non-adherence to treatment is associated with more frequent peritonitis episodes [7, 27, 30]. However, current data in the literature contain conflicting results regarding the effect of RPM programs with APD on the frequency of peritonitis episodes, but, in general, RPM had no significant effect on the development of peritonitis [6, 10, 11, 15]. While Sanabria et al. [6] reported fewer peritonitis episodes in patients with RPM than those without RPM, Manani et al. [15] and Corzo et al. [11] reported higher but not significant peritonitis episodes in the RPM group. However, the frequency of peritonitis was not the primary endpoint of these studies and there is no study focusing directly on peritonitis. Thus, it is difficult to mention a direct relationship between the frequency of peritonitis and the RPM program use.
Hospitalization
Treatment non-adherence is a significant risk factor not only for peritonitis, short technique survival and poor volume control, but also for hospitalization [3, 6]. As mentioned above, non-adherence to prescription is a common problem in PD patients, and less contact of patients with the clinician and being utterly independent from the hospital causes non-adherence. Theoretically, the adaptation of RPM programs to APD treatment is expected to have a favourable contribution to the number of hospitalizations and the length of hospital stay, since it increases treatment adherence. In 2018, a simulation study was designed to reflect the effect of RPM on health source utilization, and the study showed that RPM programs significantly contributed to the reduction of health costs by reducing hospitalization [2]. In 2019, Sanabria et al. [6] conducted a study which was the first clinical observation that evaluated the effect of RPM on hospitalization, with 126 patients with APD (63 patients with RPM and 63 patients without RPM), and they showed that RPM programs led to a decrease in both hospitalization rate (decrease in 0.36 episodes per patient-year) and the length of hospital stay (decrease in 6.57 hospital days per patient-year). Later, in a retrospective study consisting of 35 patients with RPM–APD and 35 patients without RPM–APD, the RPM group had lower hospitalization rate and shorter length of hospital stay [15]. However, the difference was not statistically significant. The 6-month follow-up period and the relatively small number of patients may have caused the difference not to reach statistical significance.
QUALITY OF LIFE
PD, a home-based therapy, provides similar survival to facility-based haemodialysis, but fewer clinic and emergency room visits and higher quality of life [9]. Furthermore, APD was associated with better health-related quality of life (HRQoL) in several domains measured in randomized studies [31]. Most studies assess the quality of life using the Kidney Disease Quality of Life-Short Form (KDQOL-SF). In 2017, He et al. [32] conducted a systematic review on the effect of remote home management for chronic kidney disease. They showed that patients with remote management received more staff encouragement, and their pain was improved. However, other aspects of quality of life did not change. Nevertheless, it is important to emphasize two crucial points. First, most of the studies in this review did not directly evaluate the patient treatment adherence. Also, patients were managed with teleconsultation, videoconferencing and smartphones, not with a remote management program, which provides treatment adherence data. Thus, it is not possible to fully evaluate the effect of RPM programs on HRQoL with these studies. Secondly, the KDQOL-SF scale is an instrument developed for patients with chronic kidney disease, but no specific questions assess the effect of home dialysis monitoring daily activities and patient satisfaction. In 2020, Manani et al. [15] detected similar results in patients with RPM with APD in accordance with a previous systematic review. Unlike other studies, they designed an internal questionnaire to evaluate the effect of RPM on HRQoL to a greater extent, and found that RPM–APD indicated an excellent acceptance and satisfaction when monitored closely and frequently. As a result, current data are insufficient to evaluate the effect of RPM on HRQoL.
HEALTH ECONOMICS
Chronic kidney disease causes a significant burden on patients, healthcare systems and society. Treating chronic kidney disease and managing its complications cost billions of euros annually and it is essential to note that renal replacement therapies account for more than half of the cost [2]. For instance, a study estimated the annual spending on dialysis in Germany in 2006 to be €4.9 billion, and another study estimated an annual cost of €2.1 billion in Italy [33, 34]. Therefore, any attempt to reduce the cost will alleviate the burden on healthcare systems. It is a fact that the effect of RPM programs on clinical outcomes such as hospital visits, hospitalization, blood pressure control and technique survival will reduce health expenses. First, in a simulation study conducted by Italian, German and US experts revealed that the integration of the RPM programs to APD could save €538, €871 and $1947 in expenses per patient in Italy, Germany and the USA, respectively [2]. While the top three saving resources were hospitalization, emergency room visits and transfer to haemodialysis in the USA, they were hospitalization, transfer to haemodialysis and clinical calls in Germany, and hospitalization, emergency room visits and unplanned clinical visits in Italy. Although the amount of resources saved differed among countries, the net effect pointed to decreased health expenditures. Another study was designed in Italy with 43 patients with RPM–APD and 42 without RPM and the study showed that the hospital saves €9130 in annual personnel expenses and €5810 per year in logistic expenses [1]. They also determined that patients with RPM–APD travel 1134 km less annually and save €9720 cumulatively. A study designed in Colombia to assess the net monetary cost of RPM integration to APD and used the Amarkov model to project costs showed that RPM programs would save $121 233 annually [17]. Also, probabilistic sensitivity analysis found a 91% chance for the RPM program to be cost-saving. In line with these results, it can be concluded that the integration of RPM to PD will reduce the financial burden in the health system.
PATIENTS', CAREGIVERS' AND CLINICIANS' PERSPECTIVE
RPM programs increase treatment success as well as reducing the burden of the patients in the management of the treatment. A qualitative study conducted in 2019, reflecting the patient perspective, determined that the participants welcomed the RPM technology [35]. The authors concluded that this technology should become widespread in order to observe the effect on patients. Walker et al. [13, 14] designed a qualitative study using face-to-face interviews. They showed that RPM might increase patients’ awareness about treatment, enhance partnership with the healthcare providers, and improve treatment and timely care. According to their interviews, patients felt more relaxed and supported; they no longer needed to record their dialysis information. Also, the anxiety about problems with the treatment was reduced.
Similarly, sharing the data and responsibility reduces the burden of caregivers. However, some patients and caregivers would have concerns about data sharing. Patients may fear that their privacy is being invaded and this belief may pose a barrier to using RPM [36]. Therefore, the PD team should explain the boundaries of information sharing and the independence of the patients at home should not be restricted with the information obtained. Another contribution is increased accountability to the dialysis team. RPM motivates the patients to adhere to prescriptions since they know that dialysis data are being delivered to the dialysis team.
On the other hand, there were some differences in perspectives between nurses and nephrologists. Nurses considered that RPM was a successful tool and increased patients’ self-management [14]. However, even if nurses spend a few minutes on each patient daily, it imposes a serious workload, especially in busy clinics, and nurses often felt they did not have enough time to dedicate to monitoring data. Nurses’ workload can be reduced by monitoring the patients’ data every other day or every 3 days. However, randomized controlled trials are needed to show that it is not inferior to daily monitoring to adopt such a workflow.
Walker et al. [14] suggested that the nephrologists were concerned about data privacy similar to patients. However, the benefits of RPM far outweigh this concern. Also, nephrologists reported a greater understanding of patient adherence and developed empathy for patients, facilitating an improved patient–provider relationship.
CORONAVIRUS DISEASE 2019 PANDEMIC
Coronavirus disease 2019 (COVID-19) caused a pandemic that emerged at the end of 2019, causing more than 100 million cases and more than 2 million deaths worldwide [37]. A short time after the beginning of the pandemic, home-based dialysis therapies were recommended as the first-line treatment option by the International Society of PD (ISPD) to provide patients isolation and protection [38]. Under normal conditions, PD patients access the PD unit monthly or bimonthly for routine follow-up visits. However, hospital access has been restricted under current circumstances.
Ronco et al. [39] suggested maintaining a close follow-up of the PD population, using every available communication strategy: phones, e-mail, electronic medical records and software platforms embedded into the cyclers. Clinical management should focus on prevention and early treatment of complications. Novel symptoms could be previously discussed by phone to reduce the number of accesses to the hospital. When necessary, the patient is invited to an in-person visit with personal protection equipment (facial mask) and after hospital COVID-19 triage.
In particular, specific recommendations were implemented to care for the patients at a distance. Those undergoing CAPD were invited to send a weekly report of dialysis exchanges, body weight and blood pressure values by e-mail. The APD treatments were monitored daily through RPM programs [39].
However, there are limited data in the literature on the reliability and success of RPM programs during the COVID-19 era. One of the studies conducted with 1023 PD patients on the RPM program showed that patients’ treatment adherence was improved, the proportion of poorly controlled hypertension decreased and the peritonitis rate did not change significantly [12]. In another study, PD modalities were compared during the lockdown period [18]. The study results showed that CAPD, APD and RPM–APD had a similar effect on hypervolaemia, blood pressure control, residual renal function, hospitalization and peritonitis episodes. On the other hand, RPM–APD provided a more stable bone mineral metabolism in controlling serum parathyroid hormone, calcium and phosphorus. Both studies confirmed the reliability of using RPM programs during the COVID-19 pandemic.
In the PD centre at San Bortolo Hospital, in Vicenza, Italy, from 15 February 2020 to 31 December 2020, the prevalent PD dialysis population consisted of 130 patients. In this period, nine patients were COVID-19 positive (6.9%). Three positive patients remained at home and were able to self-manage the dialysis. They were monitored at a distance by the PD team through the new tools. Six patients were admitted to the hospital for COVID-19 symptoms and they were treated with RPM–APD. This modality made it possible to reduce the access of PD nurses to the COVID-19 area to twice a day (connection and disconnection). Furthermore, physicians’ daily check of the RPM platform allowed an early identification and timely troubleshooting of PD-related issues. None of the patients had a disease-specific complication during the hospitalization, such as peritonitis, exit-site infection, overload and dialysis access dysfunction.
Therefore, RPM programs have become even more crucial and allow the physicians to ensure optimal care of PD patients, avoiding the increased complications or technique failure, even in the COVID-19 area.
FUTURE DIRECTIONS
The success of innovations in the PD field will depend on its simplicity, degree of automation, biocompatibility, comfort, efficiency and safety. In this context, wearable PD technology, which uses sorbent technology to generate dialysate in miniaturized portable systems, will revolutionize the PD treatment [40]. Studies on wearable PD device prototypes that work with continuous flow or automated devices are ongoing [40, 41]. RPM modules can also be integrated with these devices in the future and could make wearable PD devices more automated, comfortable and safe.
Nowadays, RPM technologies are available integrated with APD devices. This technology provides many advantages and will be an integral part of PD in the near future. However, this technology also has points that need to be improved. For example, installing programs that enable video conferencing to RPM systems or making a face-to-face call through smartphone applications integrated with these systems may be an important step in strengthening the bond between the healthcare providers and the patient in the future.
Furthermore, systems where biochemical blood sample analysis of patients can be evaluated with strips and delivered to healthcare providers via RPM will increase patients’ comfort and make the management of the treatment easier.
On the other hand, not every patient is suitable for APD. The integration of this technology with CAPD will be another important step in the future. Maybe software-operated and server-using hangers will be designed to weigh the filling and drainage fluids, record how many times the patient makes connections per day and create a face-to-face interview via the monitor.
CONCLUSION
The increasing literature knowledge about RPM programs that are widely used in a short time indicates that RPM programs are associated with favourable clinical outcomes and could lead to a reduction in health expenses.
PD, as a home-based therapy, reduces exposure to the hospital. Moreover, telemedicine links patients at home with the caregivers in the hospital, avoiding patient isolation and integrating the usual follow-up. Therefore, particularly, during the COVID-19 time, ISPD strongly recommends RPM as a major way to manage PD patients [42].
However, current literature reports mainly from relatively small observational studies and no results from a randomized controlled clinical trial have been reported yet. Therefore, randomized controlled trial results will be more informative to demonstrate the effect of RPM programs on clinical outcomes (NCT04034966 and NCT04157764).
CONFLICT OF INTEREST STATEMENT
All of the authors declare that they have no conflict of interest. The evaluation in this paper has not been published previously elsewhere, and it is not under consideration in other journals.
Contributor Information
Haci Hasan Yeter, Department of Nephrology Dialysis and Transplantation, Gazi University Faculty of Medicine, Ankara, Turkey.
Sabrina Milan Manani, Department of Nephrology Dialysis and Transplantation, San Bortolo Hospital, Vicenza, Italy.
Claudio Ronco, Department of Nephrology Dialysis and Transplantation, San Bortolo Hospital, Vicenza, Italy.
REFERENCES
- 1. Manani SM, Rosner MH, Virzì GM et al. Longitudinal experience with remote monitoring for automated peritoneal dialysis patients. Nephron 2019; 142: 1–9 [DOI] [PubMed] [Google Scholar]
- 2. Makhija D, Alscher MD, Becker S et al. Remote monitoring of automated peritoneal dialysis patients: assessing clinical and economic value. Telemed J E Health 2018; 24: 315–323 [DOI] [PubMed] [Google Scholar]
- 3. Bernardini J, Nagy M, Piraino B. Pattern of noncompliance with dialysis exchanges in peritoneal dialysis patients. Am J Kidney Dis 2000; 35: 1104–1110 [DOI] [PubMed] [Google Scholar]
- 4.Blaauw M. Use of sharesource in remote patient management in peritoneal dialysis: a UK nurse’s perspective. In: Ronco C, Crepaldi C, and Rosner MH (eds). Remote Patient Management in Peritoneal Dialysis. Basel: Karger, 2019, 73–83 [DOI] [PubMed] [Google Scholar]
- 5. Drepper VJ, Martin P-Y, Chopard CS et al. Remote patient management in automated peritoneal dialysis: a promising new tool. Perit Dial Int 2018; 38: 76–78 [DOI] [PubMed] [Google Scholar]
- 6. Sanabria M, Buitrago G, Lindholm B et al. Remote patient monitoring program in automated peritoneal dialysis: impact on hospitalizations. Perit Dial Int 2019; 39: 472–478 [DOI] [PubMed] [Google Scholar]
- 7. Yeter HH, Akcay OF, Ronco C et al. Automated remote monitoring for peritoneal dialysis and its impact on blood pressure. Cardiorenal Med 2020; 10: 198–208 [DOI] [PubMed] [Google Scholar]
- 8. Griva K, Lai AY, Lim HA et al. Non-adherence in patients on peritoneal dialysis: a systematic review. PLoS ONE 2014; 9: e89001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanabria M, Rosner M, Vesga J et al. A remote management program in automated peritoneal dialysis patients in Colombia. Nefrol Latinoam 2018; 15: 48–51 [Google Scholar]
- 10. Bunch A, Vesga JI, Camargo DO et al. Remote automated peritoneal dialysis management in Colombia. Kidney Int Rep 2019; 4: 873–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corzo L, Wilkie M, Vesga JI et al. Technique failure in remote patient monitoring program in patients undergoing automated peritoneal dialysis: A retrospective cohort study. Perit Dial Int 2020; 0896860820982223. [DOI] [PubMed] [Google Scholar]
- 12. Bunch A, Ardila F, Castaño R et al. Through the storm: Automated peritoneal dialysis with remote patient monitoring during COVID-19 pandemic. Blood Purif 2020; 50: 279–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walker RC, Tong A, Howard K et al. Patients’ and caregivers’ expectations and experiences of remote monitoring for peritoneal dialysis: A qualitative interview study. Perit Dial Int 2020; 40: 540–547 [DOI] [PubMed] [Google Scholar]
- 14. Walker RC, Tong A, Howard K et al. Clinicians’ experiences with remote patient monitoring in peritoneal dialysis: A semi-structured interview study. Perit Dial Int 2020; 40: 202–208 [DOI] [PubMed] [Google Scholar]
- 15. Manani SM, Baretta M, Giuliani A et al. Remote monitoring in peritoneal dialysis: benefits on clinical outcomes and on quality of life. J Nephrol 2020; 33: 1301–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeter HH, Karacalik C, Eraslan E et al. Effect of remote patient management in peritoneal dialysis on haemodynamic and volume control. Nephrology 2020; 25: 856–864 [DOI] [PubMed] [Google Scholar]
- 17. Ariza JG, Walton SM, Sanabria M et al. Evaluating a remote patient monitoring program for automated peritoneal dialysis. Perit Dial Int 2020; 40: 377–383 [DOI] [PubMed] [Google Scholar]
- 18. Yeter HH, Gok Oguz E, Akcay OF et al. The reliability and success of peritoneal dialysis during the COVID‐19 pandemic. Semin Dial 2021; 34: 147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uchiyama K, Washida N, Yube N et al. The impact of a remote monitoring system of healthcare resource consumption in patients on automated peritoneal dialysis (APD): A simulation study. Clin Nephrol 2018; 90: 334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manani SM, Crepaldi C, Giuliani A et al. Remote monitoring of automated peritoneal dialysis improves personalization of dialytic prescription and patient’s independence. Blood Purif 2018; 46: 111–117 [DOI] [PubMed] [Google Scholar]
- 21. Goldfarb-Rumyantzev AS, Baird BC, Leypoldt JK et al. The association between BP and mortality in patients on chronic peritoneal dialysis. Nephrol Dial Transplant 2005; 20: 1693–1701 [DOI] [PubMed] [Google Scholar]
- 22. Vaios V, Georgianos PI, Liakopoulos V et al. Assessment and management of hypertension among patients on peritoneal dialysis. Clin J Am Soc Nephrol 2019; 14: 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ataş N, Erten Y, Okyay GU et al. Left ventricular hypertrophy and blood pressure control in automated and continuous ambulatory peritoneal dialysis patients. Ther Apher Dial 2014; 18: 297–304 [DOI] [PubMed] [Google Scholar]
- 24. Lan PG, Clayton PA, Johnson DW et al. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Perit Dial Int 2016; 36: 623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan S, Cho Y, Koh YH et al. Association of socio-economic position with technique failure and mortality in Australian non-indigenous peritoneal dialysis patients. Perit Dial Int 2017; 37: 397–406 [DOI] [PubMed] [Google Scholar]
- 26. Chen JH, Johnson DW, Hawley C et al. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci Rep 2018; 8: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mawar S, Gupta S, Mahajan S. Non-compliance to the continuous ambulatory peritoneal dialysis procedure increases the risk of peritonitis. Int Urol Nephrol 2012; 44: 1243–1249 [DOI] [PubMed] [Google Scholar]
- 28. Davies SJ, Phillips L, Griffiths AM et al. What really happens to people on long-term peritoneal dialysis? Kidney Int 1998; 54: 2207–2217 [DOI] [PubMed] [Google Scholar]
- 29. Rodríguez-Carmona A, Fontán MP, Falcón TG et al. A comparative analysis on the incidence of peritonitis and exit-site infection in CAPD and automated peritoneal dialysis. Perit Dial Int 1999; 19: 253–258 [PubMed] [Google Scholar]
- 30. Segal JH, Messana JM. Prevention of peritonitis in peritoneal dialysis. Semin Dial 2013; 26: 494–502 [DOI] [PubMed] [Google Scholar]
- 31. Rabindranath KS, Adams J, Ali TZ et al. Automated vs continuous ambulatory peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2007; 22: 2991–2998 [DOI] [PubMed] [Google Scholar]
- 32. He T, Liu X, Li Y et al. Remote home management for chronic kidney disease: a systematic review. J Telemed Telecare 2017; 23: 3–13 [DOI] [PubMed] [Google Scholar]
- 33. Icks A, Haastert B, Gandjour A et al. Costs of dialysis—a regional population-based analysis. Nephrol Dial Transplant 2010; 25: 1647–1652 [DOI] [PubMed] [Google Scholar]
- 34. Cicchetti A, Ruggeri M, Codella P et al. The health and social costs of chronic kidney disease in Italy. Farmeconomia 2011; 12: 21–28 [Google Scholar]
- 35. Subramanian L, Kirk R, Cuttitta T et al. Remote management for peritoneal dialysis: A qualitative study of patient, care partner, and clinician perceptions and priorities in the United States and the United Kingdom. Kidney Med 2019; 1: 354–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wallace EL, Rosner MH, Alscher MD et al. Remote patient management for home dialysis patients. Kidney Int Rep 2017; 2: 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. WHO. Coronavirus disease 2019 (COVID-19) weekly epidemiological update, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-27-january-2021
- 38. Wilkie M, Davies S. Peritoneal dialysis in the time of COVID-19. Perit Dial Int 2020; 40: 357–358 [DOI] [PubMed] [Google Scholar]
- 39. Ronco C, Manani SM, Giuliani A et al. Remote patient management of peritoneal dialysis during COVID-19 pandemic. Perit Dial Int 2020; 40: 363–367 [DOI] [PubMed] [Google Scholar]
- 40. Foo MW, Htay H. Innovations in peritoneal dialysis. Nat Rev Nephrol 2020; 16: 548–549 [DOI] [PubMed] [Google Scholar]
- 41. Salani M, Roy S, Fissell IW. Innovations in wearable and implantable artificial kidneys. Am J Kidney Dis 2018; 72: 745–751 [DOI] [PubMed] [Google Scholar]
- 42. Brown E, Arteaga J, Chow J et al. ISPD: strategies regarding COVID-19 in PD patients adapted from Peking University First Hospital. Março, 2020. (4 April 2020, date last accessed)