Abstract
Anti-melanoma differentiation-associated gene 5 juvenile dermatomyositis (anti-MDA5 JDM) is associated with high risk of developing rapidly progressive interstitial lung disease (RP-ILD). Here we report an 11-year-old girl with anti-MDA5 JDM and RP-ILD which led to a fatal outcome, further aggravated by SARS-CoV-2 infection. She was referred to our hospital after being diagnosed with anti-MDA5 JDM and respiratory failure due to RP-ILD. On admission, fibrobronchoscopy with bronchoalveolar lavage (BAL) revealed Pneumocystis jirovecii infection so treatment with intravenous trimethoprim-sulfamethoxazole was initiated. Due to RP-ILD worsening, immunosuppressive therapy was intensified using methylprednisolone pulses, cyclophosphamide, tofacitinib and intravenous immunoglobulin without response. She developed severe hypoxemic respiratory failure, pneumomediastinum and pneumothorax, further complicated with severe RP-ILD and cervical subcutaneous emphysema. Three real-time RT-PCR for SARS-CoV-2 were made with a negative result. In addition, she was complicated with a secondary hemophagocytic lymphohistiocytosis and a fourth real-time PCR for SARS-CoV-2 performed in BAS sample was positive. Despite aggressive treatment of RP-ILD due to anti-MDA5 JDM, there was no improvement of respiratory failure in the following days and patient developed refractory septic shock and died. Anti-MDA5 JDM patients with RP-ILD have a poor prognosis with a high mortality rate. For this reason, intensive immunosuppressive therapy is essential including the use of promising drugs such as tofacitinib. COVID-19 in children with underlying health conditions like anti-MDA5 JDM may still be at risk for disease and severe complications.
Keywords: Anti-MDA5, juvenile dermatomyositis, interstitial lung disease (ILD), COVID-19, tofacitinib
Introduction
Anti-melanoma differentiation-associated gene 5 juvenile dermatomyositis (anti-MDA5 JDM) consists of clinically amyopathic dermatomyositis (CADM). It is characterised by minor muscle involvement and main distinctive clinical features, such as cutaneous and oral ulceration, painful palmar papules and significant risk of developing interstitial lung disease (ILD) [1].
Anti-MDA5 antibodies are detected in up to 70% of JDM patients with ILD, which is an important systemic complication and a major cause of death when rapidly progressive ILD (RP-ILD) develops [2, 3]. Moreover, pneumomediastinum and pneumothorax may occur with RP-ILD and are considered a serious pulmonary life-threatening complication in patients with anti-MDA5 JDM [4]. In this regard, intensive immunosuppressive therapy is mandatory in order to improve the prognosis of RP-ILD [5]. On the other hand, aggressive treatment of these patients has been associated with opportunistic infections due to severe immunosuppression [6].
Hereby, we report the case of an 11-year-old girl with anti-MDA5 JDM and fatal rapidly progressive interstitial lung disease, further complicated by SARS-CoV-2 infection.
Case presentation
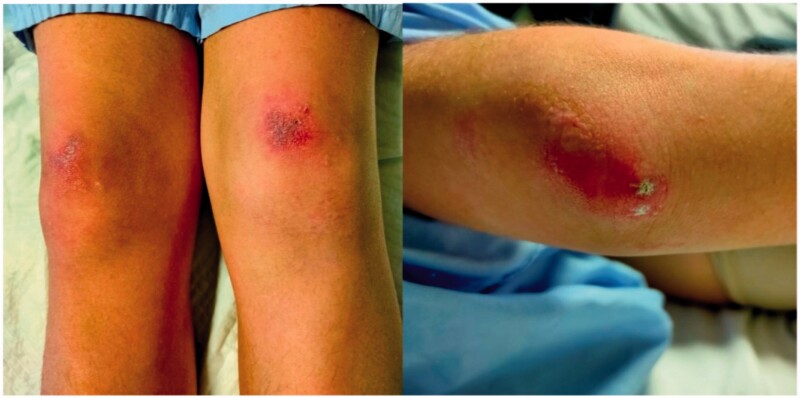
An 11-year-old Spanish female presented with skin rash and dry cough and was admitted to a local hospital. Four months before admission, she noticed palmar erythema, periorbital rash and papular skin lesions on elbows and knees. In addition, the patient referred weight loss, wrist arthritis and generalised arthromyalgias without proximal muscle weakness. Gottron’s papules with calcinosis, heliotrope rash and painful palmar papules were found (Figures 1 and 2). Initial laboratory findings are summarised in Table 1. Immunology study revealed a positive ANA (tittle 1/160) and extended myositis antibody determination (Myositis 12 IgG DOT for BlueDiver Instrument, Alphadia, Wavre, Belgium) showed positive anti Ro/SS-A and anti-MDA5 antibodies. She was diagnosed with juvenile dermatomyositis. Respiratory function tests revealed a moderate restrictive ventilatory impairment. Spirometry data according to Global Lung Function Initiative (GLI) reference values were: forced expiratory volume in one second (FEV1) 1.06 L (44.24%, Z-score −4.45); forced vital capacity (FVC) 0.97 L (35.56%, Z-score −5.89); FEV1/FVC 109% (Z-score 5.52) [7]. Lung volume measurements using plethysmography were: total lung capacity (TLC) 1.60 L (45%); residual volume (RV) 0.65 L (71%); RV/TLC 40.25%.
Figure 1.
Gottron’s papules with calcinosis over knees and elbows.
Figure 2.
Painful palmar papules.
Table 1.
Results of blood tests at diagnosis.
| Test | Result | Test | Result | Test | Result |
| WBCs | 10,400/µL (4800–15,000/µL) | Urea | 6.9 mg/dL (11–49 mg/dL) | ANA | Positive (1/160) |
| Neutrophils | 4720/µL (1500–8700/µL) | Creatinine | 0.27 mg/dL (0.3–0.7 mg/dL) | Anti-DsDNA | Negative |
| Lymphocytes | 4070/µL (1500–9000/µL) | ALT | 98 U/L (<35 U/L) | C3 | 17,900 mg/dL (normal) |
| Haemoglobin | 14.9 g/dL (10.2–14.6 g/dL) | AST | 47 U/L (<95 U/L) | C4 | 39,7 mg/dL (normal) |
| Platelets | 255,000/µL (180,000–490,000/µL) | LDH | 436 U/L (110–295 U/L) | ANCA | Negative |
| ESR | 40 mm/h (0–20 mm/h) | CPK | 27 U/L (40–180 U/L) | Anti-SS-A | Positive |
| CRP | 0.3 mg/L (0–5 mg/L) | Albumin | 4.2 g/dL (3.8–5.4 g/dL) | Anti-SS-B | Negative |
| Ferritin | 878 ng/mL (10–291 ng/mL) | Urinalysis | Negative | Anti-MDA5 | Positive |
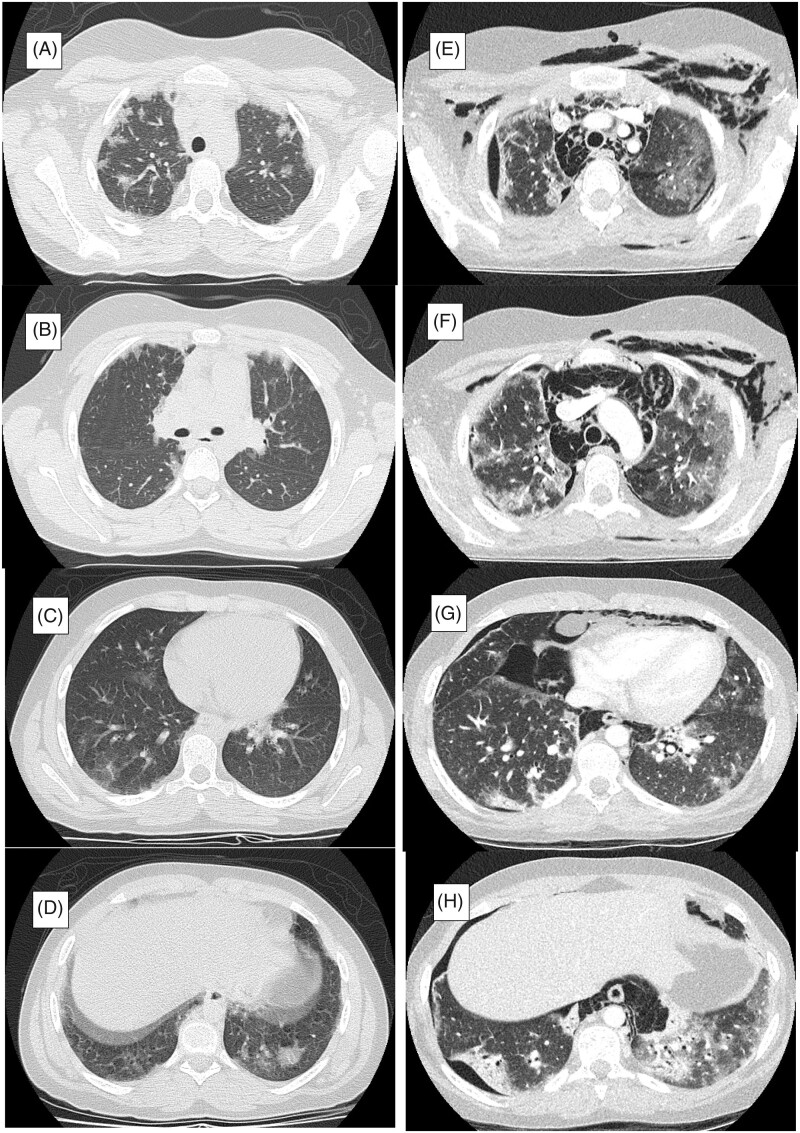
A computerised tomography (CT) of the chest showed interstitial lung disease (Figure 3(A–D)). She was initially treated with oral prednisone (1 mg/kg/day) combined with mycophenolate mofetil (MMF) and tacrolimus. On 11 March 2020, four weeks after initiation of treatment, she developed dysphagia, dysphonia with shortness of breath and was referred to our hospital.
Figure 3.
Time course of chest CT images. (A–D) Multifocal, bilateral and peripheral predominant ground glass opacities were found on chest computed tomography (CT) at the first admission in the previous hospital. (E–H) Chest CT at PICU admission in our hospital. Severe RP-ILD (peripleural ground-glass opacity and patchy distribution of areas of consolidation accompanied by traction bronchiectasis) further complicated with pneumomediastinum, pneumothorax and cervical subcutaneous emphysema. PICU: Paediatric Intensive Care unit; RP-ILD: rapidly progressive interstitial lung disease.
On admission, fibrobronchoscopy was performed, ruling out tracheal or bronchial perforation. The bronchoalveolar lavage (BAL) revealed a positive culture for Pneumocystis jirovecii, so treatment with intravenous trimethoprim-sulfamethoxazole was initiated and immunosuppressive therapy (tacrolimus and MMF) was interrupted. Five days after admission the patient exhibited severe dyspnoea, hypoxaemia, and chest X-ray showed right pneumothorax and pneumomediastinum. She required high-flow oxygen therapy up to 15 L/min and insertion of right thoracic drainage tube. Because of worldwide COVID-19 pandemic, a first screening for SARS-CoV-2 in nasopharyngeal sample with real-time PCR (RT-PCR) was made with a negative result.
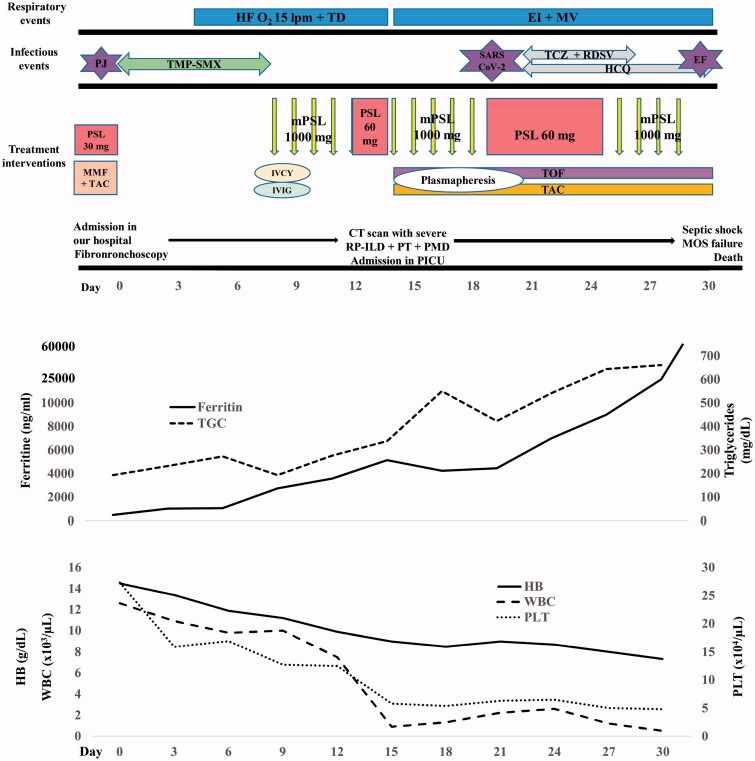
After completing an eight-day antibiotic course for P. jirovecii her RP-ILD worsened. Immunosuppressive therapy was intensified using a combination of methylprednisolone pulses (30 mg/kg/day for 5 days followed by 2 mg/kg/day), cyclophosphamide (IV-CYC; 500 mg/m2) and intravenous immunoglobulin (2 gr/kg) (Figure 4). Seven days after IV-CYC therapy, she was admitted to paediatric intensive care unit (PICU) with severe hypoxemic respiratory failure requiring endotracheal intubation and mechanical ventilation. New chest CT showed progression of pneumomediastinum and pneumothorax, further complicated with severe RP-ILD and cervical subcutaneous emphysema (Figure 3(E–H)). Laboratory data revealed: haemoglobin 9 gr/dl, WBCs 900/µL, neutrophils 680/µL, lymphocytes 110/µL, platelets 58,000/µL, reticulocyte count 0.5%, ferritin 5134 ng/mL, triglycerides 338 mg/dl, AST 98 UI/L, ALT 40 UI/L, CRP 35 mg/L, ESR 50 mm/h, D-dimer 3380 ng/mL and fibrinogen 200 mg/dL. Microbiological tests for bacteria, virus and fungus were negative, including negative culture and RT-PCR for P. jirovecii in BAL. Two more RT-PCR for SARS-CoV-2 were ordered in nasopharyngeal and broncoaspirate (BAS) samples being both negative.
Figure 4.
The clinical course of the patient. The severe cytopenia, the levels of ferritin and tryglicerides, the treatment interventions, the respiratory and infectious events are shown. HF O2: high-flow oxygen therapy; TD: thoracic drainage; EI + MV: endotracheal intubation and mechanical ventilation; PJ: Pneumocystis jirovecii; TMP-SMX: trimethoprim-sulfamethoxazole; TZZ: tocilizumab; RDSV: remdesivir; HCQ: hydroxychloroquine; EC: Enterococcus faecium; mPSL: methylprednisolone pulses; MMF: mycophenolate mofetil; TAC: tacrolimus; IVCY: intravenous cyclophosphamide; IVIG: intravenous immunoglobulin; TOF: tofacitinib; CT: computerised tomography; RP-ILD: rapidly progressive interstitial lung disease; PT: pneumothorax; PMD: pneumomediastinum; PICU: paediatric intensive care unit; MOS: multi-organ system failure.
Given the high systemic inflammation and suspected secondary hemophagocytic lymphohistiocytosis (calculated Hemophagocytic Score [8] 226), soluble IL-2 receptor (sIL-2R) was determined (205 U/mL, normal value) and methylprednisolone pulses (30 mg/kg/day) were initiated. In addition, she received tofacitinib (10 mg/day) and tacrolimus (0.2 mg/kg/day) for her RP-ILD. Moreover, therapeutic plasma exchange for 5 days was associated with the aim of removing multiple proinflammatory cytokines concurrently. No fever was observed despite apparent inflammatory disease. Twenty days after admission, a fourth real-time PCR for SARS-CoV-2 performed in BAS sample was positive at this time. She was diagnosed with COVID-19 infection starting treatment with hydroxychloroquine (400 mg/day), remdesivir (200 mg/day for 5 days) and tocilizumab (single dose 8 mg/kg). Despite aggressive treatment, there was no improvement of respiratory failure in the following days (PaO2/FiO2 67–100), serum ferritin levels increased to a maximum value of 60,456 ng/mL and IL-6 > 1000 pg/mL. Enterococcus faecium was isolated in blood culture, and patient developed refractory septic shock with acute kidney injury that required vasoactive drugs and continuous hemofiltration. The patient died 31 days after admission in hospital due to a multi-organ system failure.
Discussion
To the best of our knowledge, this is the first report of anti-MDA5 JDM patient with severe RP-ILD complicated with SARS-CoV-2 infection. Anti-MDA5 JDM is occasionally accompanied by fatal RP-ILD which is associated with a high mortality rate [9]. Due to the poor prognosis of RP-ILD in anti-MDA5 JDM patients, combined aggressive immunosuppressive therapy with high-dose steroids, cyclophosphamide and tacrolimus is required to improve survival [5].
Recent report has suggested early intervention with multi-drug therapy should be started at the diagnosis of ILD associated with anti-MDA5 JDM [10]. Based on these facts, recently developed clinical practice guidance for JDM 2018 in Japan, where anti-MDA5 JDM is prevalent, recommends screening for ILD by CT scan in all cases of JDM [11].
Differences in prevalence of ILD in JDM patients with anti-MDA5 depending on ethnicity have been reported. In this way, anti-MDA5 JDM patients in Europe and North America are reported to have few ILD (19% and 28%, respectively) [12, 13] and almost no RP-ILD, in comparison with those in East Asia (prevalence of ILD and RP-ILD: 63% and 36%, respectively) [3].
Prognostic markers for ILD and severe disease in patients with anti-MDA5 JDM have been described to date. Previous reports suggested that high titres of anti-MDA-5 antibodies seem to correlate with severe disease course and resistance to immunosuppressive therapy in patients with anti-MDA5 JDM [14]. Gono et al. reported that high levels of serum ferritin were associated with the development of RP-ILD in patients with JDM and its prognosis [15]. Additionally, Sabbagh et al. recently reported that anti-SS-A/Ro52 antibody is risk factor to have ILD in North America anti-MDA5 JDM patients [13]. Within the anti-MDA5 autoantibody positive subgroup in this study, anti-SS-A/Ro52 reactivity was even more strongly associated with ILD: 70% of those with coexisting anti-Ro52 autoantibodies had ILD compared with only 9% of those who were anti-Ro52 negative. Moreover, only 1 of 33 patients with ILD developed RP-ILD, and this patient was positive for both anti-MDA5 and anti-Ro52 autoantibodies. In line with the above-mentioned findings, our patient presented on admission elevated serum ferritin (878 ng/mL) and both positive anti-MDA5 and anti-Ro52 autoantibodies, which may worsen the prognosis.
Generally, aggressive immunosuppressive therapy is associated with a variable risk of opportunistic infections [6]. This is the case of our patient, who was diagnosed with P. jirovecii pneumonia at admission. This complication delayed intensive immunosuppressive treatment administration until the patient had received one week course of cotrimoxazole.
It has recently been described that tofacitinib might be useful in the management of refractory MDA5 ILD patients [16]. Due to rapid progression of ILD and appearance of life-threatening complications, such as pneumothorax and pneumomediastinum, it was decided to intensify her treatment adding this JAK inhibitor. Unfortunately, the clinical course of our patient did not allow to assess its potential benefits.
One of the main challenges regarding the management of our patient was to clarify whether her clinical deterioration was secondary to RP-ILD in anti-MDA5 JDM complicated with HLH/MAS (Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome) or due to SARS-CoV-2 infection. Indeed, as previously mentioned, hyperferritinemia might be related to anti-MDA5 JDM with RP-ILD [15] but it can also occur in secondary HLH [17]. Furthermore, COVID-19 may associate elevated serum ferritin [18–20], but no previous reports have described that SARS-CoV2 infection increase ferritin to the extremely high level found in our patient. However, further evidence about ferritin levels in COVID-19 patients is needed, especially in paediatric population with underlying rheumatic diseases.
Additionally, severe pancytopenia found in our patient suggest HLH/MAS based on hypercytokinemia (by JDM itself, COVID-19 or other etiology). Several mechanisms have been suggested in JDM-associated cytopenia such as macrophage activation, autoantibody formation or T-cell-mediated myelosuppression [21]. Other causes of pancytopenia in our patient might include myelosuppression by preceding IV-CYC or active infection. Although we did not perform a bone marrow aspirate due to the clinical severity of the patient, normal reticulocyte count found in our patient and negative microbiological tests at pancytopenia diagnosis would not support the previous causes. Thus, severe pancytopenia probably developed as a complication of JDM in our case.
sIL-2 receptor levels are usually elevated in the patients with infectious diseases including SARS-CoV-2 [19] or JDM complicated by HLH/MAS [22]. However, we found a normal value of sIL-2 receptor, although we didn’t test it more than once to confirm this result. In this regard, sIL-2R didn’t support the diagnosis of HLH/MAS.
Although SARS-CoV-2 was detected by RT-PCR late in the disease, her fatal clinical course is typical to severe case of RP-ILD in anti-MDA5 JDM without infectious complication. Particularly, patients who have already developed respiratory failure show poor response to intensive immunosuppressive therapy [3]. In addition, JDM-related MAS has been considered that the pathological conditions were different from HLH/MAS in other rheumatic diseases (systemic juvenile idiopathic arthritis or systemic lupus erythematosus), because of poorly responsive to HLH/MAS treatment [17]. In this regard, the fatal outcome of our patient can mainly be explained by anti-MDA5 JDM with RP-ILD, although COVID-19 might had a possible role in the rapidly worsening condition.
In conclusion, we hereby report a case of anti-MDA5 RP-ILD JDM complicated with pneumothorax and pneumomediastinum. These patients have a poor prognosis with a high mortality rate and require promptly intensive immunosuppressive therapy, including the use of promising drugs such as tofacitinib when the disease is refractory to standard immunosuppressive drugs (high-dose corticosteroids, cyclophosphamide, tacrolimus or MMF). However, these treatments increase the risk of sepsis and opportunistic infections. COVID-19 in children often have a milder course of illness and a lower death rate when compared with adults. However, children with underlying health conditions, especially those with chronic lung disease, may still be at risk for disease and serious complications.
Author contributions
All authors in this manuscript have:
Contributed substantially to the conception and design of the study, the acquisition of data, or the analysis and interpretation.
Drafted or provided critical revision of the article.
Provided final approval of the version to publish.
Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Study design. Cristian Quintana-Ortega; Agustín Remesal; Marta Ruiz de Valbuena; Olga de la Serna.
Acquisition of data: María Laplaza-González; Elena Álvarez-Rojas; Marta Ruiz de Valbuena; Olga de la Serna.
Manuscript preparation. Cristian Quintana-Ortega; Agustín Remesal; Sara Murias; Clara Udaondo; Rosa Alcobendas.
Patient consent
A written informed consent for this case report has been obtained from patient’s parents.
Ethical approval
Not applicable.
Conflict of interest
None.
References
- Kurtzman DJB, Vleugels RA Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78(4):776–785. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Okura Y, Yamada M, et al. Anti-Melanoma differentiation-associated gene 5 antibody is a diagnostic and predictive marker for interstitial lung diseases associated with juvenile dermatomyositis. J Pediatr. 2011;158(4):675–677. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Takezaki S, Kobayashi I, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology. 2015;54(5):784–791. [DOI] [PubMed] [Google Scholar]
- Le Goff B, Chérin P, Cantagrel A, et al. Pneumomediastinum in interstitial lung disease associated with dermatomyositis and polymyositis. Arthritis Rheum. 2009; 61(1):108–118. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Mizumaki K, Kano M, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol. 2017;176(2):395–402. [DOI] [PubMed] [Google Scholar]
- Zampieri S, Ghirardello A, Iaccarino L, et al. Polymyositis-dermatomyositis and infections. Autoimmunity. 2006;39(3):191–196. [DOI] [PubMed] [Google Scholar]
- Stanojevic S Standardisation of lung function test interpretation: Global Lung Function Initiative. Lancet Respir Med. 2018; 6(1):10–12. [DOI] [PubMed] [Google Scholar]
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the Hscore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–2620. [DOI] [PubMed] [Google Scholar]
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 2009;60(7):2193–2200. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72(3):488–498. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Akioka S, Kobayashi N, et al. Clinical practice guidance for juvenile dermatomyositis (JDM) 2018-Update [published correction appears in Mod Rheumatol. 2020 May;30(3):607]. Mod Rheumatol. 2020;30(3):411–423. [DOI] [PubMed] [Google Scholar]
- Tansley SL, Betteridge ZE, Gunawardena H, et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther. 2014;16(4):R138–Published 2014 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh S, Pinal-Fernandez I, Kishi T, et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis. Ann Rheum Dis. 2019;78(7):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC, Casciola-Rosen L, Samedy LA, et al. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res. 2013;65(8):1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology. 2012;51(9):1563–1570. [DOI] [PubMed] [Google Scholar]
- Sabbagh S, Almeida de Jesus A, Hwang S, et al. Treatment of anti-MDA5 autoantibody-positive juvenile dermatomyositis using tofacitinib. Brain. 2019;142(11):e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddighe D, Dauyey K Macrophage activation syndrome in juvenile dermatomyositis: a systematic review. Rheumatol Int. 2020;40(5):695–702. [DOI] [PubMed] [Google Scholar]
- Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020; 58(7):1021–1028. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu Y, Yu J, et al. Retrospective analysis of laboratory testing in 54 patients with severe- or critical-type 2019 novel coronavirus pneumonia. Lab Invest. 2020;100(6):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, Kawamura N, Okano M, et al. Thrombocytopaenia in juvenile dermatomyositis. Scand J Rheumatol. 2006;35(1):79–80. [DOI] [PubMed] [Google Scholar]
- Yajima N, Wakabayashi K, Odai T, et al. Clinical features of hemophagocytic syndrome in patients with dermatomyositis. J Rheumatol. 2008; 35(9):1838–1841. [PubMed] [Google Scholar]