Abstract
On May 10, 2021, the Emergency Use Authorization of the Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) was expanded to include adolescents (May 10, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use). We describe clinical characteristics of 8 adolescents who presented over the course of 36 days to Nicklaus Children’s Hospital with perimyocarditis within 4 days of receiving a dose of BNT162b2 vaccine.
Keywords: BNT162b2, COVID-19 vaccine, myocarditis, pericarditis
PATIENT 1
A 17-year-old male with a past medical history of vitiligo presented to the emergency department (ED) with retrosternal pressure-like chest pain. He denied shortness of breath, fever, vomiting, rhinorrhea, abdominal pain, or diarrhea. The patient tested positive for SARS-CoV-2 10 months before. He received the second dose of the BNT162b2 vaccine 4 days before presentation. Chest X-ray was negative, and electrocardiogram (ECG) demonstrated normal sinus rhythm with ST-segment depressions in V1 and V2. There were no ST-segment elevations or PR-segment depressions. Initial laboratories were unremarkable aside from elevated creatine phosphokinase-MB (CPK-MB) level of 13 ng/mL and troponin level of 3.34 ng/mL. The peak troponin was 4.85 ng/mL. The patient was transferred to our hospital. Transthoracic echocardiogram (TTE) showed no structural abnormalities, qualitatively low normal left ventricular systolic function (LVSF), and no pericardial effusion. Patient was managed with ibuprofen with no recurrence of pain. Patient had a negative respiratory pathogen panel (RPP), adenovirus plasma PCR, cytomegalovirus (CMV) antibodies, enterovirus plasma PCR, Epstein-Barr virus (EBV) titers, and Parvovirus B19 antibodies. SARS-CoV-2 nucleocapsid immunoglobulin G (IgG) was positive.
PATIENT 2
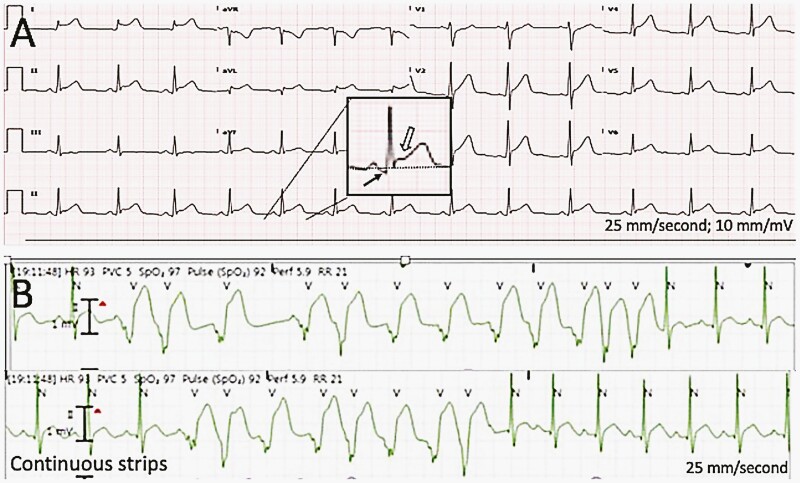
A 16-year-old male with no past medical history presented to the ED with left-sided chest pain and intermittent palpitations for approximately 1 day. He denied fever, shortness of breath, or recent illness. There was no history of SARS-CoV-2 infection. Patient had received the second dose of BNT162b2 vaccine 3 days prior to presentation. His troponin-I was elevated at 4.82 ng/mL, and ECG showed diffuse ST elevation and PR-segment depression (Figure 1A).
Figure 1.
(A) Twelve lead electrocardiogram from patient 2, demonstrating sinus rhythm with normal axis and intervals. Diffuse PR depressions and horizontal ST-segment elevations are illustrated in the magnified inset as black and white arrows, respectively. The dotted line represents the baseline. These findings are typical in patients with pericarditis. (B) A continuous telemetry strip demonstrating 2 episodes of irregular, polymorphic ventricular tachycardia from patient 4. N represents the normal QRS, and V represents the ventricular ectopic beats.
The patient was managed with ibuprofen with resolution of pain. TTE showed no structural abnormalities and qualitatively normal LVSF. Cardiac MRI findings were consistent with acute myocarditis, showing late gadolinium enhancement pattern and myocardial edema, normal biventricular size and function, and pericardial thickening.
Further workup showed negative SARS-CoV-2 nucleocapsid IgG, negative human immunodeficiency virus (HIV) antigen/antibody, adenovirus PCR, enterovirus PCR, Lyme antibody titers, Leptospira immunoglobulin M (IgM), Trypanosoma cruzi IgG, and Toxocara canis IgG. EBV antibody panel was consistent with past infection. CMV IgM was borderline-positive, but IgG was negative. RPP was negative. Mycoplasma IgM was positive; however, nasopharyngeal PCR was negative. The decision was made to treat with doxycycline for Mycoplasma infection, although the IgM was suspected to be a false-positive. The patient was discharged home on doxycycline, ibuprofen, and lansoprazole.
PATIENT 3
A 17-year-old male with a history of mild perimyocarditis in 2014, presented to the ED with 1 day of retrosternal pressure. In the ED, his troponin was 0.45 ng/mL, and the ECG demonstrated normal sinus rhythm with no other abnormalities. TTE showed normal biventricular function. Patient had received his first BNT162b2 vaccine 14 days earlier. He was discharged with a 2-week course of ibuprofen with complete resolution of symptoms. He had no history of SARS-CoV-2 infection.
Patient re-presented to the ED with retrosternal chest pressure 19 days after his first presentation. He received the second BNT162b2 dose 4 days before. Troponin-I level at his second presentation was 34.5 ng/mL. ECG demonstrated sinus rhythm with occasional premature ventricular contractions (PVCs), diffuse ST-segment elevations, and no PR depression. He was treated with 1 dose of intravenous immunoglobulin (IVIG). On telemetry, he had a single 4 beat episode of monomorphic ventricular tachycardia. TTE showed no significant valvular dysfunction, qualitatively normal biventricular systolic function, and no significant pericardial effusion. Cardiac MRI showed a late gadolinium enhancement pattern and edema consistent with acute myocarditis (Supplementary Figures 1 and 2). The patient was found to be SARS-CoV-2 nucleocapsid IgG-positive and SARS-CoV-2 PCR-negative. Extensive infectious workup was negative, including adenovirus plasma PCR, CMV antibodies, enterovirus plasma PCR, EBV titers, Leptospira antibodies, Lyme antibodies, Mycoplasma IgM, Parvovirus B19 PCR, Trypanosoma cruzi antibodies, and HIV antigen/antibody.
PATIENT 4
A 15-year-old male with Marfan syndrome and aortic root dilation presented to the ED with sudden onset of retrosternal chest pain, fatigue, and abdominal pain for 1 day. He denied fever, shortness of breath, or recent illness. The patient had a history of SARS-CoV-2 infection in January 2021 with mild symptoms. He had received the first dose of the BNT162b2 vaccine 3 days prior to admission. In the ED, his troponin was 7.2 ng/mL and ECG demonstrated normal sinus rhythm with no other changes. Chest X-ray was normal. The peak troponin level was 11.8 ng/mL. Pain resolved while he was still in ED.
The patient was asymptomatic at the time of admission. TTE showed patent foramen ovale, mild mitral valve prolapse with mild-to-moderate mitral regurgitation, mild aortic root dilation (unchanged), and qualitatively low normal LVSF. Cardiac MRI was significant for focal edema at the basal inferolateral segments, normal biventricular size and global systolic function, and stable aortic root dilation. The patient had 12- and 8-beat runs of irregular polymorphic ventricular tachycardia (Figure 1B). The runs occurred within 1 minute of each other, and the patient was asymptomatic at the time. He remained hemodynamically stable. Infectious workup was negative, including adenovirus plasma PCR, CMV antibodies, enterovirus plasma PCR, EBV titers, Leptospira IgM, Parvovirus B19 PCR, and RPP.
PATIENT 5
A 16-year-old male with no past medical history presented to the ED with intermittent retrosternal chest pressure over 2 days. He denied fever, shortness of breath, or recent illness. He had received his second dose of the BNT162b2 vaccine 1 day before symptom onset. Post-vaccination symptoms included mild body aches and headache. Family history was positive for vitiligo in the patient’s mother. Troponin-I was elevated at 1.92 ng/mL, and the peak level obtained was 2.42 ng/mL. ECG demonstrated ST-segment elevation. Chest X-ray was negative. TTE showed no structural abnormalities and qualitatively normal LVSF. He had no recurrence of chest pain while admitted. Patient recovered with no medical intervention. Infectious workup was negative, including RPP, adenovirus plasma PCR, CMV antibodies, enterovirus plasma PCR, EBV titers, and Parvovirus B19 antibodies. Additional workup showed SARS-CoV-2 nucleocapsid IgG-negative.
PATIENT 6
A 15-year-old male with obesity, insulin resistance, and dyslipidemia presented to outside hospital ED with 3 days of sharp, intermittent, retrosternal pain, with associated fever (38°C), headache, loose bowel movement, and cough. Patient denied any trauma or recent viral illnesses. No prior history of SARS-CoV-2 infection. He had received the second dose of the BNT162b2 vaccine 2 days prior to symptom onset. On physical examination, there was diffuse reproducible chest wall tenderness. Laboratories unremarkable aside from elevated peak troponin-I of 5.1 ng/mL and Creatine Kinase Myocardial Band of 19.9 ng/mL. ECG demonstrated normal sinus rhythm, with no significant ST-segment changes. Chest X-ray was normal. TTE showed no structural abnormalities and qualitatively normal LVSF. He recovered with no medical intervention. Infectious workup was negative but limited to RPP, adenovirus plasma PCR, enterovirus plasma PCR, SARS-CoV-2 nucleocapsid IgG, blood culture, and gastrointestinal panel.
PATIENT 7
A 15-year-old male with no past medical history presented with a 2-day history of intermittent, nonradiating, retrosternal pain. He denied recent fever, shortness of breath, or recent illness. No history of SARS-CoV-2 infection. Three days prior to presentation, the patient received his second BNT162b2 vaccine. Upon further interviewing, he reported 2 episodes of self-resolving chest pain 3 days after the first dose of the BNT162b2 vaccine of unclear significance given he did not seek medical care. Chest X-ray was normal. Initial laboratories were unremarkable aside from elevated CPK-MB level of 44.5 ng/mL with a peak troponin level of 22.05 ng/mL. ECG showed intraventricular conduction delay with transient ST-segment depression. TTE showed no structural abnormalities, qualitatively normal LVSF, and no significant pericardial effusion. A 24-hour Holter showed 13 multiform PVCs, including 1 couplet. Patient was managed with ibuprofen with resolution of pain on day 3 of admission. RPP was negative, and Mycoplasma IgG and IgM were positive; however, nasopharyngeal PCR for Mycoplasma was negative. The decision was made to treat with azithromycin for Mycoplasma infection.
PATIENT 8
A 17-year-old male with no past medical history presented to the ED with 3 days of sharp, retrosternal pain. He denied fever, shortness of breath, or recent illness. There was no history of SARS-CoV-2 infection. Patient had received the second dose of the BNT162b2 vaccine 1 day prior to symptom onset. His troponin-I was elevated with a peak of 5.09 ng/mL. ECG demonstrated mild diffuse ST-segment elevation. TTE showed no structural abnormalities and qualitatively normal LVSF. The patient was managed with ibuprofen with resolution of pain. Infectious workup was negative but limited to RPP and SARS-CoV-2 nucleocapsid IgG.
Discussion
Herein described is the first case series from a single institution of 8 adolescent males with a diagnosis of perimyocarditis in the setting of recent BNT162b2 vaccination with no other identifiable cause. All patients presented with acute onset chest pain and an elevated troponin level; 7 of the 8 had ST-segment changes on ECG. None of the patients demonstrated signs of concomitant systemic illness or had a history suggestive of recent illness. Two patients, patients 1 and 3, were SARS-CoV-2 nucleocapsid IgG-positive suggesting a prior infection with SARS-CoV-2. In 7 of the 8 patients, their symptoms were either self-limited or resolved after nonsteroidal anti-inflammatory medication. Two patients experienced isolated, nonsustained ventricular tachycardia without hemodynamic instability, and they were asymptomatic. TTE showed qualitatively normal LVSF in all 8 patients. In the 3 patients who underwent cardiac MRI, findings demonstrated myocardial edema and late gadolinium enhancement with normal biventricular size and global systolic function, consistent with acute nonischemic inflammation as can be seen in myocarditis. The mean length of stay was 57 hours. All patients were discharged home with strict physical activity restrictions and cardiology follow-up.
Patients 3, 4, and 7 were unique in that they experienced chest pain after the first dose of the BNT162b2 vaccine. Patient 3 had a remote history of mild perimyocarditis with no sequalae, he presented 14 days after his first dose of vaccine with mild chest pain, a small increase in troponin and a normal EKG and then with severe chest pain (Table 1), significantly elevated troponin and abnormal EKG 4 days after his second dose; additional work up showed serological evidence of previous SARS-CoV-2 infection demonstrated by positive nucleocapside antibodies. Patient 4 had a prior history of mild symptomatic SARS-CoV-2 4 months before receiving the vaccine (IgG nucleocapsid was negative). It was decided not to recommend the second dose of BNT162b2 vaccine to patient 4. Patient 7 reported chest pain after first vaccine although he did not seek medical care.
Table 1.
Demographic and Clinical Characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age (yr) | 17.6 | 16.7 | 17.2 | 15.9 | 16.6 | 15.8 | 15.2 | 17.9 |
| Sex (male/female) | Male | Male | Male | Male | Male | Male | Male | Male |
| Race | White | White | White | White | White | White | White | White |
| Ethnicity | Hispanic | Hispanic | Hispanic | Hispanic | Hispanic | Hispanic | Hispanic | Hispanic |
| Weight (kg) | 69.3 | 77.6 | 107.5 | 51.4 | 104.8 | 97 | 48.75 | 85 |
| Body mass index (kg/m2) | 24.2 | 25.1 | 39.7 | 16.8 | 30.8 | 29.5 | 17.3 | N/A (no height) |
| Past medical history (PMH) | Vitiligo | No PMH | Myocarditis (2014) | Marfan syndrome | No PMH | Insulin resistance dyslipidemia | No PMH | No PMH |
| History of prior COVID-19 infection | Yes | No | No | Yes | No | No | No | No |
| BNT162b2 vaccine dose # | #2 | #2 | #1/#2 | #1 | #2 | #2 | #2 | #2 |
| Days between vaccine and symptom onset | 4 | 3 | 3 | 2 | 1 | 2 | 3 | 1 |
| Length of stay (hours) | 48 | 71 | 95 | 71 | 34 | 37 | 65 | 34 |
| Laboratories | ||||||||
| White blood cell count (Ref: 5-10) | 7.2 | 4.3 | 10.4 | 6.6 | 10 | 5.9 | 8.9 | N/A |
| Peak troponin-I (Ref: 0.00-0.08 ng/mL) | 4.85 | 5.56 | 34.50 | 11.80 | 2.42 | 5.10 | 22.05 | 5.09 |
| Discharge troponin-I (Ref: 0.00-0.08 ng/mL) | 3.15 | 0.72 | 0.72 | 0.28 | 0.81 | 1.30 | 11.56 | 2.86 |
| CKMB (Ref:0-3.5ng/mL) | 13 | 61.5 | N/A | N/A | N/A | 19.9 | 44.5 | 33.9 |
| CPK (Ref: 33-145 IU/L) | 317 | N/A | 523 | 312 | N/A | N/A | N/A | N/A |
| d-dimer (Ref: 0.27-0.41 µg/mL) | 0.2 | 0.2 | N/A | <0.19 | 0.52 | 0.53 | 0.11 | N/A |
| COVID-19 testing | ||||||||
| SARS-CoV-2 PCR | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| SARS-CoV-2 spike IgM | Negative | Negative | Negative | Negative | Positive | N/A | N/A | N/A |
| SARS-CoV-2 nucleocapsid IgG | Positive | Negative | Positive | Negative | Negative | Negative | Negative | N/A |
| EKG | ||||||||
| EKG findings | Abnormal T waves, ST-segment depression | PR depression, diffuse ST-segment elevation | PR depression, diffuse ST-segment elevation | Normal sinus rhythm | Diffuse ST-segment elevation | Normal sinus rhythm | Intraventricular conduction delay, transient ST-segment depression | Mild diffuse ST-segment elevation |
| Imaging | ||||||||
| Chest radiograph (cardiopulmonary findings) | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Left ventricular ejection fraction on ECHO | 56 | 55 | 58 | 57 | 60 | 62 | 60 | 61 |
| Pericardial effusion present | No | No | No | No | No | No | No | No |
| Cardiac MRI performed | No | Yes | Yes | Yes | No | No | No | No |
| Left ventricular ejection fraction on MRI | 60 | 61 | 61 | |||||
| Myocardial edema on MRI (yes/no) | Yes | Yes | Yes | |||||
| Late gadolinium enhancement on MRI (yes/no) | Yes | Yes | Yes | |||||
| Treatment/intervention | ||||||||
| IVIG | No | No | Yes | No | No | No | No | No |
| Scheduled NSAIDs | Yes | Yes | Yes | No | No | No | Yes | Yes |
Abbreviations: CKMB,creatine kinase myocardial band; CPK, creatine phosphokinase; ECHO, echocardiogram; EKG, electrocardiogram; IVIG, intravenous immunoglobulin; MRI, magnetic resonance imaging; NSAIDS, nonsteroidal anti-inflammatory drugs.
The incidence and prevalence of myocarditis have not been established, although it is suspected to be in the range of 1-10 cases per 100 000 persons. The causes of myocarditis are diverse and include infectious and autoimmune etiologies. In children, the most common causes of viral myocarditis are enterovirus, adenovirus, Parvovirus B19, EBV, CMV, and HHV-6 [1]. The majority of cases of myocarditis are idiopathic, typically with no definitive cause identified despite extensive workup [2]. Vaccine-associated perimyocarditis following smallpox vaccination has also been described with an incidence of 0.01%. Typically, a majority of patients make a spontaneous recovery [3]. Su et al [4] described the reports of perimyocarditis in the United States after vaccination from 1990 to 2018 utilizing the Vaccine Adverse Event Reporting System. Among those less than 18 years of age, most reported cases were male (56%), and the reported time to symptom onset was 14 days or less (61%). The most frequently reported vaccines to be associated with perimyocarditis were Haemophilus influenzae type b and hepatitis B [4].
Data on the characteristics and clinical features of pediatric patients with post-BNT162b2 vaccine perimyocarditis are limited with to date only 2 case series in print at the time of this publication [5, 6]. Marshall et al described 7 adolescent males from various institutions who presented with chest pain within 4 days after the second dose of the Pfizer-BioNTech COVID-19 vaccination, however, none developed symptoms after the first dose [5]. Sinapiri et al reported 1 patient that developed symptoms after the first dose of vaccine [6] but there were no data regarding prior evidence of SARS-CoV-2 infection or whether the patient had received the second dose of vaccine. On May 17, 2021, the Center for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices COVID-19 Vaccine Safety Technical Work Group (VaST) reviewed post-authorization BNT162b2 vaccine safety data, including presentations of myocarditis following mRNA vaccines, and are undergoing further investigation [7]. VaST concluded that the reports of myocarditis occurred predominantly in adolescents and young adults, more often in males, and more often following the second dose than the first similar to our patients [5, 6]. On May 28, 2021, CDC released guidelines for diagnosing, managing, and reporting these cases [8] and as of June 2021, they continue to recommend SARS-CoV-2 vaccination for all those 12 years and older because of the risk of SARS-CoV-2 infection and its complications are greater [8].
Pediatricians should consider myocarditis in the differential diagnosis of patients presenting with chest pain after receiving the BNT162b2 vaccine and be aware of the clinical implications and the need to report this potential adverse event. Currently, the association between perimyocarditis and the BNT162b2 vaccine is unproven but recent reports suggest there may be causality. This case series raises additional questions regarding appropriate recommendations for COVID-19 vaccination in adolescents with a prior history of perimyocarditis, of SARS CoV-2 infection (clinically or by serology), and of multisystem inflammatory syndrome associated with SARS-CoV-2 (MIS-C). It also poses the issue of whether patients who experience perimyocarditis post-BNT162b2 vaccine should receive booster SARS-CoV-2 vaccines in the future.
Our case series indicates the need for further investigation and close follow-up of these patients to guide future COVID-19 vaccine recommendations in the pediatric population.
Supplementary Material
Notes
Acknowledgments. The authors thank the patients, their parents, and the clinical staff of Nicklaus Children’s Hospital. The authors would also like to thank Dr. Ronald Kanter and Dr. Luisa Cervantes for their expertise and support.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Canter CE, Simpson KE, Simpson KP. Diagnosis and treatment of myocarditis in children in the current era. Circulation 2014; 129:115–28. [DOI] [PubMed] [Google Scholar]
- 2. Manda YR. Myopericarditis. StatPearls [Internet]. 2020. https://www.ncbi.nlm.nih.gov/books/NBK534776/
- 3. Halsell JS, Riddle JR, Atwood JE, et al. ; Department of Defense Smallpox Vaccination Clinical Evaluation Team . Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA 2003; 289:3283–9. [DOI] [PubMed] [Google Scholar]
- 4. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine 2021; 39:839–45. [DOI] [PubMed] [Google Scholar]
- 5. Marshall M, Ferguson I, Lewis P, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics 2021:148:e2021052478. [DOI] [PubMed] [Google Scholar]
- 6. Sinapiri O, Danzinger C, Shirman N, et al. Transient cardiac injury in adolescents receiving BNT162b mRNA COVID-19 vaccine [published online ahead of print June 2, 2021]. Pediatr Infect Dis J. doi: 10.1097/INF.0000000000003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. COVID-19 VaST Technical Report May 17, 2021. Centers for Disease Control and Prevention. 2021. https://www.cdc.gov/vaccines/acip/work-groups-vast/technical-report-2021-05-17.html. Accessed 21 June 2021. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines among Adolescents and Young Adults. 2021. https://www.cdc.gov/vaccines/covid-19/clinicalconsiderations/myocarditis.html. Accessed 21 June 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.