Abstract
Background
Expression of angiotensin-converting enzyme 2 (ACE2) and type II transmembrane serine protease (TMPRSS2), host molecules required for viral entry, may underlie sex differences in vulnerability to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We investigated whether placental ACE2 and TMPRSS2 expression vary by fetal sex in the presence of maternal SARS-CoV-2 infection.
Methods
Placental ACE2 and TMPRSS2 expression was quantified by quantitative reverse transcription polymerase chain reaction (RT-PCR) and by Western blot in 68 pregnant women (38 SARS-CoV-2 positive, 30 SARS-CoV-2 negative) delivering at Mass General Brigham from April to June 2020. The impact of fetal sex and maternal SARS-CoV-2 exposure on ACE2 and TMPRSS2 was analyzed by 2-way analysis of variance (ANOVA).
Results
Maternal SARS-CoV-2 infection impacted placental TMPRSS2 expression in a sexually dimorphic fashion (2-way ANOVA interaction, P = .002). We observed no impact of fetal sex or maternal SARS-CoV-2 status on ACE2. TMPRSS2 expression was significantly correlated with ACE2 expression in males (Spearman ρ = 0.54, P = .02) but not females (ρ = 0.23, P = .34) exposed to maternal SARS-CoV-2.
Conclusions
Sex differences in placental TMPRSS2 but not ACE2 were observed in the setting of maternal SARS-CoV-2 infection, which may have implications for offspring vulnerability to placental infection.
Keywords: SARS-CoV-2, vertical transmission, transplacental transmission, COVID-19, angiotensin-converting enzyme 2, type II transmembrane serine protease, sex differences, placenta, infection
More than 97 000 pregnant women in the United States have tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Reported rates of vertical transmission of SARS-CoV-2—the passage of SARS-CoV-2 from mother to baby during pregnancy or childbirth—range from 1% to 3% [2–4]. Case reports and case series documenting placental SARS-CoV-2 infection in the second and third trimesters suggest that SARS-CoV-2 infection of the placenta may be an intermediate step in transplacental transmission to the fetus [5–11]. The relative rarity of placental SARS-CoV-2 infection and transplacental transmission may be driven by a low incidence of maternal viremia and by protective patterns of SARS-CoV-2 entry receptors within the placenta [12].
Infection of host cells by SARS-CoV-2, the novel coronavirus responsible for coronavirus disease 2019 (COVID-19), requires the presence of 2 molecules: the transmembrane receptor angiotensin-converting enzyme 2 (ACE2) and the type II transmembrane serine protease (TMPRSS2) [13, 14]. TMPRSS2 primes the SARS-CoV-2 spike protein, which subsequently binds to ACE2 as the point of entry into the host cell [14]. Immunohistochemical analyses in placentas exposed to maternal SARS-CoV-2 demonstrate strong expression of ACE2 in syncytiotrophoblast and cytotrophoblast across gestation, but weak expression of TMPRSS2, most often confined to the fetal endothelium or unquantifiable by conventional immunohistochemical methods [12, 15, 16]. Single cell placental atlases from samples predating the COVID-19 pandemic demonstrate single cell gene expression of ACE2, but offer conflicting results regarding co-transcription of TMPRSS2, and coexpression of ACE2 and TMPRSS2 within the same cell type at the maternal–fetal interface [17–20]. There is therefore a lack of information on how maternal infection with SARS-CoV-2 impacts the expression of ACE2 and TMPRSS2 in the placenta, at the level of both transcript and protein.
There is an urgent need to identify factors, including biological sex, that may influence ACE2 and TMPRSS2 expression and functionality in human tissues. Epidemiologic data point to a male bias in susceptibility to severe COVID-19 disease and mortality [21–25], with sex differences observed in outcomes across the lifespan [25–28]. More severe disease in male infants and children has been demonstrated in population level and cohort data reporting a male predominance in children with multisystem inflammatory syndrome in children, a severe form of COVID-19 disease in children, and in reported infections in the age range from birth to 2 years [27–31]. Sex differences in the expression of ACE2 and TMPRSS2 in respiratory and cardiovascular tissues has been hypothesized to underlie these population-level observations [25, 32–36]. Recent exploratory human and animal model data have identified relative increases in ACE2 and TMPRSS2 levels in male respiratory cells, tissues, and plasma compared to females [37–43], although this pattern is not consistently observed across all studies [41, 44, 45]. Examining sex differences in placental expression of ACE2 and TMPRSS2 in the setting of maternal SARS-CoV-2 infection has the potential to yield insights into placental defenses against transplacental transmission.
Whether male offspring are more susceptible to vertical transmission is not well-characterized, as vertical transmission is extremely rare and sex-disaggregated data are not routinely reported [2, 3]. Given available data supporting a connection between COVID-19 outcomes and sex differences in ACE2 and TMPRSS2 expression levels [32, 36, 37, 46], and the paucity of information on ACE2 and TMPRSS2 expression during pregnancy, we sought to investigate whether placental expression of ACE2 and TMPRSS2 varies by fetal sex in the presence and absence of maternal SARS-CoV-2 infection.
METHODS
Study Design and Participant Enrollment
These experiments include 68 pregnant women (38 SARS-CoV-2 positive, 30 SARS-CoV-2 negative) enrolled in a cohort study at Massachusetts General Hospital and Brigham and Women’s Hospital from April to June 2020, coincident with universal screening for SARS-CoV-2 infection by nasopharyngeal real-time polymerase chain reaction (RT-PCR) on the Labor and Delivery unit. This study was approved by the Mass General Brigham Institutional Review Board (number 2020P003538). All participants provided written informed consent.
Pregnant women were eligible for inclusion if they were (1) 18 years of age or older; (2) able to provide informed consent or had a named healthcare proxy to do so; and (3) diagnosed with SARS-CoV-2 infection or known to be negative for SARS-CoV-2 by nasopharyngeal swab RT-PCR. Maternal SARS-CoV-2 positivity was defined as a positive nasopharyngeal swab RT-PCR at any time during pregnancy. Identification of eligible women and participant enrollment have been described in previous publications [12, 47] and are summarized here. Participants positive for SARS-CoV-2 were identified either on admission to the Labor and Delivery unit through universal SARS-CoV-2 screening (initiated early April 2020) by nasopharyngeal RT-PCR, or by a prior documented positive SARS-CoV-2 nasopharyngeal RT-PCR during pregnancy for a COVID-19–related illness. Participants negative for SARS-CoV-2 on admission to Labor and Delivery were enrolled as a convenience sample, recruited on the same days as enrolled positive cases. Newborns born to women positive for SARS-CoV-2 at delivery were screened by nasopharyngeal RT-PCR per American Academy of Pediatrics guidelines [48]; no neonates tested positive for SARS-CoV-2. Demographic and clinical outcomes data were abstracted from the electronic medical record using REDCap electronic data capture tools [49]. Included participants were selected to balance groups for fetal sex, and to distribute maternal disease severity evenly across fetal sex categories. COVID-19 disease severity was defined according to National Institutes of Health criteria [50].
Sample Collection and Processing
Sample collection protocols have been described in a previous publication [47]. In brief, at the time of delivery, 2 approximately 0.5-cm3 placental biopsies were collected from the maternal side of the placenta and 2 from the fetal side of the placenta, at least 4 cm from the cord insertion and the placental edge, after dissecting off the overlying amnion and chorion or decidua. Biopsies were placed in 5 mL RNALater and stored at 4°C for at least 24 hours. Biopsies were then flash frozen and stored at –80°C.
RNA Extraction
To control for regional differences in placental gene expression, 2 maternal-side and 2 fetal-side placental samples (~50 mg tissue per side) were processed per placenta. Total RNA was obtained following homogenization of tissue sample in Trizol (100 µL/10 mg). Resulting suspension was then centrifuged at 12 000g for 10 minutes after which the pellet was discarded. Chloroform was added to supernatant at a ratio of 100 µL chloroform/50 mg tissue. Tubes were shaken vigorously for 15 seconds, allowed to stand at room temperature for 10 minutes, and then centrifuged at 12 000g for 15 minutes. The aqueous phase was collected and the remainder of the RNA extraction procedure was performed using an RNeasy Mini Kit with on-column DNase I treatment (Qiagen) according to the manufacturer’s instructions. RNA quantity and purity were assessed using a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific).
Complementary DNA Synthesis and Quantitative RT-PCR
Complementary DNA (cDNA) synthesis was performed using iScriptTM cDNA Synthesis Kit (Bio-Rad) per manufacturer instructions using a MiniAmp Plus Thermal Cycler (Thermo Fisher Scientific). No template control and no reverse transcriptase controls were prepared. Quantitative RT-PCR (RT-qPCR) was then performed using TaqMan gene expression assays on a QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific). Gene expression was normalized to the placental reference gene tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ) and expressed relative to female SARS-CoV-2–negative control samples to yield a relative quantity value (2-ΔΔCt). YWHAZ was selected due to its prior validation as a stably expressed reference gene in placental tissue [51–53], and no changes in YWHAZ expression were noted by fetal sex or maternal SARS-CoV-2 infection. Gene expression assays used include ACE2 Hs01085333_m1, TMPRSS2 Hs01122322_m1, and YWHAZ Hs01122445_g1 (TaqMan, Thermo Fisher Scientific). ACE2 and TMPRSS2 TaqMan gene expression assays used FAM-MGB dye, the YWHAZ assay used VIC_PL dye. Cases and controls were run on the same plates using the same mastermix for all samples, and reactions were performed in triplicate.
Quantification of SARS-CoV-2 Viral Load by RT-PCR
SARS-CoV-2 viral load was quantified from extracted placental RNA using the US Centers for Disease Control and Prevention (CDC) 2019-nCoV_N1 primers and probe set [54]. Viral copy numbers were quantified using N1 qPCR standards in 16-fold dilutions to generate a standard curve. The assay was run in triplicate for each sample. Positive controls, negative controls, and 2 no template control wells were included as negative controls. Quantification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) reference gene RNA level was performed to determine the efficiency of RNA extraction and qPCR amplification (Bio-Rad PrimePCR assay number qHsaCED0038674). SARS-CoV-2 viral loads <40 RNA copies/mL were categorized as undetectable and set at 1.0 log10 RNA copies/mL.
Western Blots
Fifty-six cases (28 female, 28 male) were examined for expression of ACE2 and TMPRSS2 by Western blot. Thirty micrograms of protein was prepared in sodium dodecyl sulfate polyacrylamide gel electrophoresis sample loading buffer (G Biosciences) with 5 mM dithiothreitol and heated for 10 minutes at 80°C, and loaded on Mini-PROTEAN 10% TGX Stain-Free Protein Gels (Bio-Rad). Gels were run at 200V for 30 minutes, after which they were transferred onto low-fluorescence polyvinylidene difluoride membrane using a Trans-Blot Turbo Transfer System (Bio-Rad). Blots were then washed 3 × 10 minutes in tris-buffered saline (TBS) followed by imaging of Stain-Free Total Protein signal with a ChemiDoc MP System (Bio-Rad). Following stain-free imaging, blots were washed another 3 × 10 minutes in TBS, followed by blocking in Intercept (TBS) Blocking Buffer (Li-Cor Biosciences) for 1 hour at room temperature. ACE2 and TMPRSS2 primary antibodies were then incubated in Intercept T20 (TBS) Antibody Diluent (Li-Cor Biosciences) overnight at 4°C. Membranes were then washed 6 × 10 minutes in tris-buffered saline with 0.1% Tween 20 (TBS-T), followed by incubation with secondary antibodies at 1:2500 dilution for 1 hour in Intercept T20 (TBS) antibody diluent. Primary and secondary antibodies, manufacturer, and dilution conditions used for immunoblot are detailed in Table 1. Blots were washed 6 × 10 minutes in TBS-T, after which they were briefly rinsed in TBS prior to imaging. Band intensity was then imaged using a ChemiDoc MP System and quantified using Image Lab Software (Bio-Rad). Data are presented as the volume of bands of protein of interest relative to the volume of total protein quantified using stain-free imaging.
Table 1.
Primary and Secondary Antibodies, Manufacturer, and Dilution Conditions Used for Western Blot
| Antibody | Manufacturer | Catalog Number | Dilution |
|---|---|---|---|
| ACE2 antibody | Abcam | ab108252 | 1:500 |
| TMPRSS2 | Novus | NBP1-20984 | 1:2500 |
| Donkey anti-rabbit (HRP) | Abcam | ab205722 | 1:2500 |
| Donkey anti-goat (HRP) | ThermoFisher | A15999 | 1:2500 |
Abbreviations: ACE2, angiotensin-converting enzyme 2; HRP, horseradish peroxidase; TMPRSS2, type II transmembrane serine protease.
Statistical Analysis
All data are presented as mean ± standard error of the mean in the text and figure legends unless otherwise noted. Relative gene expression values (relative to YWHAZ) are expressed as arbitrary units. Two-way analysis of variance (2-way analysis of variance [ANOVA]) with Bonferroni post hoc testing was used to compare gene expression by fetal sex and maternal SARS-CoV-2 infection, and Western immunoblot staining intensity by fetal sex and maternal SARS-CoV-2 infection. Correlations between ACE2 and TMPRSS2 messenger RNA (mRNA) levels were assessed using Spearman rank-order correlation. P < .05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 9.
RESULTS
Participant Characteristics
Maternal demographic and clinical data of study participants are depicted in Table 2. Of the 68 included participants, 34 were pregnant with females and 34 with males, with 19 SARS-CoV-2 positive and 15 SARS-CoV-2 negative in each group. There were no significant differences in maternal age, gravidity, parity, race, or ethnicity. Of the 38 women with SARS-CoV-2 infection during pregnancy, there were no differences between male and female pregnancies with respect to gestational age at diagnosis of SARS-CoV-2 infection, days from SARS-CoV-2 diagnosis to delivery, or severity of COVID-19 illness. Women with SARS-CoV-2 infection during pregnancy were more likely to be Hispanic compared to uninfected controls, consistent with a prior report of ethnic disparities in COVID-19 vulnerability in our patient population [55].
Table 2.
Demographic and Clinical Characteristics of Pregnancy Cohort by Fetal Sex and Maternal Severe Acute Respiratory Syndrome Coronavirus 2 Status
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Characteristic | All (N = 68) | SARS-CoV-2 Negative (n = 15) | SARS-CoV-2 Positive (n = 19)a | SARS-CoV-2 Negative (n = 15) | SARS-CoV-2 Positive (n = 19)a | P Valueb |
| Maternal age, y, median (IQR) | 33 (28–38) | 33 (30–36) | 34 (30–40) | 30 (27–37) | 31 (26–36) | .34 |
| Parity, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | .34 |
| Race | .19 | |||||
| White | 40 (59) | 11 (73) | 8 (42) | 12 (80) | 9 (59) | |
| Black | 5 (7) | 1 (7) | 3 (16) | 0 (0) | 1 (7) | |
| Asian | 1 (1) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | |
| Other | 12 (18) | 3 (20) | 4 (21) | 1 (7) | 4 (8) | |
| Not reported | 10 (15) | 0 (0) | 4 (21) | 1 (7) | 5 (15) | |
| Ethnicity | .01 | |||||
| Hispanic | 32 (47) | 4 (27) | 11 (58) | 2 (13) | 15 (79) | |
| Non-Hispanic | 33 (48) | 10 (67) | 7 (37) | 12 (80) | 4 (21) | |
| Not reported | 3 (4) | 1 (7) | 1 (5) | 1 (7) | 0 (0) | |
| Hypertensionc | 8 (12) | 1 (7) | 1 (5) | 2 (13) | 4 (21) | .43 |
| Diabetesd | 6 (9) | 1 (7) | 3 (16) | 1 (7) | 1 (5) | .67 |
| BMI ≥30 kg/m2 | 20 (29) | 2 (13) | 7 (37) | 3 (20) | 8 (42) | .21 |
| Prepregnancy BMI, kg/m2, median (IQR) | 27.4 (21.5–30.8) | 25.8 (21.6–29.4) | 28.5 (26.5–32.7) | 21.5 (20.3–29.8) | 29.5 (22.3–32.0) | .06 |
| GA at delivery, completed weeks, median (IQR) | 39 (38–40) | 39 (39–39) | 39 (38–40) | 39 (39–41) | 39 (35–40) | .57 |
| Any labor | 52 (76) | 8 (53) | 16 (84) | 11 (73) | 17 (89) | .07 |
| Neonatal birthweight, g, median (IQR) | 3283 (3005–3590) | 3400 (3060–3590) | 3255 (2920–3340) | 3435 (3260–3730) | 3115 (2615–3590) | .06 |
| GA at positive SARS-CoV-2 test, completed weeks, median (IQR) | 36 (32–39) | NA | 36 (32–39) | NA | 35 (32–39) | .83 |
| COVID-19 disease severitye | .82 | |||||
| Asymptomatic | 16 (42) | NA | 8 (42) | NA | 8 (42) | |
| Mild/moderate | 19 (50) | NA | 9 (47) | NA | 10 (53) | |
| Severe/critical | 3 (8) | NA | 2 (11) | NA | 1 (5) | |
| Time between SARS-CoV-2 symptom onset and delivery, days, median (IQR) | 37 (18–57) | NA | 44 (28–64) | NA | 32 (8–51) | .18 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; GA, gestational age; IQR, interquartile range; NA, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a”SARS-CoV-2 positive” indicates positive test for SARS-CoV-2 by nasopharyngeal real-time polymerase chain reaction during pregnancy.
bSignificant differences between groups were determined using χ 2 test for categorical variables and Kruskal–Wallis test for continuous variables.
c“Hypertension” indicates chronic or pregnancy-associated hypertension.
d“Diabetes” indicates preexisting or gestational diabetes.
eSeverity determinations were made at the time of diagnosis and based on published criteria from the National Institutes of Health.
Placental SARS-CoV-2 Viral Load
We sought to determine SARS-CoV-2 viral load in placental homogenates. SARS-CoV-2 viral load was undetectable (1.0 log10 RNA copies/mL) in all 68 placentas, consistent with our previous findings demonstrating no cases of SARS-CoV-2 RNA in the placenta by RNA in situ hybridization [12]. The same viral load assay was used to detect SARS-CoV-2 in respiratory samples from pregnant study participants and blood from nonpregnant study participants [12, 56].
TMPRSS2 Placental Expression Was Impacted by Fetal Sex and Maternal SARS-CoV-2 Exposure
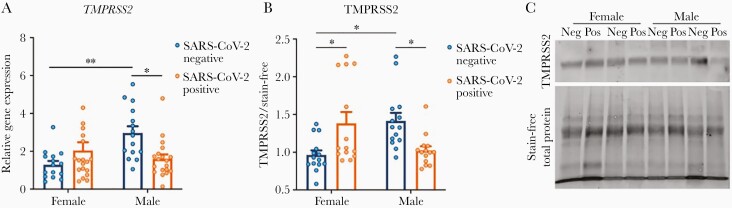
As coexpression of ACE2 and TMPRSS2 is critical for viral entry to host cells [13], we sought to determine whether differences in TMPRSS2 levels were observed in the setting of SARS-CoV-2 exposure or by fetal sex. We found that maternal exposure to SARS-CoV-2 impacted TMPRSS2 placental gene expression in a sexually dimorphic fashion (2-way ANOVA: fetal sex, P = .06; maternal SARS-CoV-2, P = .35; P for interaction = .002; Figure 1A). TMPRSS2 expression was significantly higher in male compared to female placentas of participants negative for SARS-CoV-2 (adjusted P = .006). TMPRSS2 expression was significantly reduced in male placentas of participants positive for SARS-CoV-2 compared to male placentas of SARS-CoV-2–negative controls (adjusted P = .02; Figure 1A). The same pattern was observed at the protein level by Western blot (2-way ANOVA: fetal sex, P = .65; maternal SARS-CoV-2, P = .90; P for interaction = .0002; Figure 1B and 1C). TMPRSS2 protein levels were significantly elevated in male controls compared to female controls (adjusted P = .01). A sexually dimorphic response to maternal SARS-CoV-2 infection was observed, in which TMPRSS2 levels were increased in females (adjusted P = .03) but decreased in males (adjusted P = .04) exposed to maternal SARS-CoV-2 infection, relative to sex-matched controls. Table 3 depicts the results of 2-way ANOVA analyses of qPCR and Western blot results.
Figure 1.
Sexually dimorphic placental type II transmembrane serine protease (TMPRSS2) expression in the setting of maternal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. A, TMPRSS2 gene expression demonstrates a significant interaction effect of fetal sex and maternal SARS-CoV-2 infection (interaction P = .002). TMPRSS2 expression is elevated in male placentas of SARS-CoV-2–negative mothers compared to female placentas, suggesting higher baseline TMPRSS2 expression in males. In the setting of maternal SARS-CoV-2 infection, TMPRSS2 gene expression is significantly reduced in male placentas, with no significant effect of maternal SARS-CoV-2 infection in females. All expression levels are relative to reference gene YWHAZ. B, TMPRSS2 protein expression by Western blot analysis also demonstrates a significant interaction between fetal sex and maternal SARS-CoV-2 infection (P for interaction < .001). In SARS-CoV-2–negative mothers, TMPRSS2 is elevated in male compared with female placentas. In SARS-CoV-2–positive mothers, TMPRSS2 is significantly reduced in male placentas and increased in female placentas relative to sex-matched controls. *P < .05, **P < .01. Data analyzed by 2-way analysis of variance with Bonferroni post hoc testing. C, Representative Western blots showing TMPRSS2 expression between males and females from pregnancies with and without exposure to maternal SARS-CoV-2 infection. “Neg” and “Pos” designate maternal SARS-CoV-2 status.
Table 3.
Two-Way Analysis of Variance Analyses of Angiotensin-Converting Enzyme 2 and Type II Transmembrane Serine Protease Gene and Protein Expression Results
| Gene or Protein | Maternal SARS-CoV-2 Status | Fetal Sex | Interaction | Bonferroni Female Neg:Pos | Bonferroni Male Neg:Pos | Bonferroni Neg Female:Male | Bonferroni Pos Female:Male |
|---|---|---|---|---|---|---|---|
| Gene | |||||||
| ACE2 | F(1,64) = 0.003, P = .95 | F(1,64) = 3.59, P = .06 | F(1,64) = 0.627, P = .43 | P > .99 | P > .99 | P = .46 | P > .99 |
| TMPRSS2 | F(1,64) = 0.889, P = .35 | F(1,64) = 3.56, P = .06 | F (1,64) = 10.65, P = .002 | P = .63 | P = .025 | P = .006 | P > .99 |
| Protein by Western blot | |||||||
| ACE2 | F(1,52) = 0.006, P = .94 | F(1,52) = 0.0008, P = .98 | F(1,52) = 0.89, P = .35 | P = .93 | P = .89 | P = .90 | P = .92 |
| TMPRSS2 | F(1,52) = 0.02, P = .90 | F(1,52) = 0.21, P = .65 | F (1,52) = 16.57, P = .0002 | P = .027 | P = .044 | P = .014 | P = .081 |
Two-way analysis of variance followed by Bonferroni post hoc analyses was performed to determine significance. All main and interaction effects for genes and proteins of interest are represented for both fetal males and fetal females in addition to all post hoc analyses performed. Statistically significant values are bolded. “Neg” and “Pos” designate maternal SARS-CoV-2 status.
Abbreviations: ACE2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, type II transmembrane serine protease.
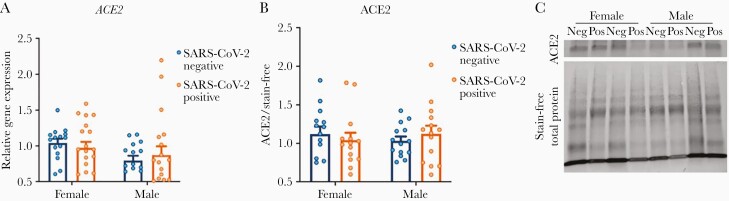
No Differences in ACE2 Levels Were Observed by Fetal Sex or Maternal SARS-CoV-2 Infection
Relative to reference gene YWHAZ, there was no significant difference in placental ACE2 gene expression in placental homogenates by fetal sex or in the presence of maternal SARS-CoV-2 infection (2-way ANOVA: fetal sex, P = .06; SARS-CoV-2 exposure, P = .95; P for interaction = .43; Figure 2A). These findings were confirmed by Western blot, which demonstrated no difference in ACE2 protein levels between groups (2-way ANOVA: fetal sex, P = .98; SARS-CoV-2 exposure, P = .94; P for interaction = .35; Figure 2B and 2C).
Figure 2.
Angiotensin-converting enzyme 2 (ACE2) expression does not vary by fetal sex or maternal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) status. A, ACE2 gene expression by fetal sex and maternal SARS-CoV-2 infection, relative to female negative controls, demonstrating no significant differences between groups. All expression levels are relative to reference gene YWHAZ. B, No significant differences were observed in ACE2 protein expression by Western blot analysis. Data analyzed by 2-way analysis of variance with Bonferroni post hoc testing. C, Representative Western blots showing ACE2 expression between males and females from pregnancies with and without exposure to maternal SARS-CoV-2 infection.
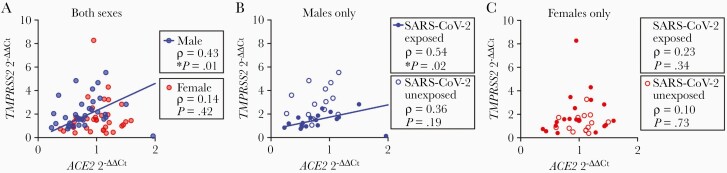
TMPRSS2 Was Significantly Correlated With ACE2 Expression in Male Placentas Exposed to Maternal SARS-CoV-2
We next sought to identify whether ACE2 and TMPRSS2 gene expression levels were significantly correlated within the same placenta in a sex-dependent fashion. Placental TMPRSS2 expression was significantly correlated with ACE2 expression in males (Spearman ρ = 0.42, P = .01) but not females (ρ = 0.14, P = .42), as demonstrated in Figure 3A. This relationship was driven by a significant correlation in male placentas exposed to SARS-CoV-2 (ρ = 0.54, P = .02; Figure 3B) whereas SARS-CoV-2–unexposed male placentas had no significant correlation between ACE2 and TMPRSS2 gene expression (Figure 3B). Placental TMPRSS2 and ACE2 expression levels were not significantly correlated in either female cases or controls (Figure 3C).
Figure 3.
Correlation of ACE2 and TMPRSS2 expression within the same placenta. A, TMPRSS2 is significantly correlated with ACE2 in fetal male placentas (ρ = 0.43, P = .01, n = 34) but not female placentas (ρ = 0.14, P = .42, n = 34). B, In fetal male placentas, TMPRSS2 was significantly correlated with ACE2 in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–exposed pregnancies (ρ = 0.54, P = .02, n = 19) but not controls (ρ = 0.36, P = .19, n = 15). C, There was no significant correlation between ACE2 and TMPRSS2 expression in fetal female placentas in either cases (ρ = 0.23, P = .34, n = 19) or controls (ρ = 0.10, P = .73, n = 15). All ΔΔCt values for TMPRSS2 and ACE2 expression are normalized to female negative controls.
DISCUSSION
Here we describe for the first time baseline sex differences in TMPRSS2 gene and protein expression in the human placenta, and sexually dimorphic placental expression of TMPRSS2 gene and protein in the setting of maternal SARS-CoV-2 infection, even in the absence of placental infection. In females, maternal SARS-CoV-2 was associated with higher placental TMPRSS2 expression, whereas in males, maternal SARS-CoV-2 was associated with reduced TMPRSS2 expression. The consistent expression of TMPRSS2 transcript and protein in third trimester placentas using qRT-PCR and Western blotting suggest that standard immunohistochemical methods demonstrating weak or no expression of TMPRSS2 in the placenta [12, 15] may lack the sensitivity to reliably detect TMPRSS2, which is expressed at a lower level than ACE2. We demonstrate no changes in placental ACE2 gene or protein expression in the setting of fetal sex nor maternal SARS-CoV-2 infection. Maternal SARS-CoV-2 infection is associated with increased correlation between ACE2 and TMPRSS2 transcript levels in male placentas only. The impact of these sex-specific placental changes in canonical cell entry mediators for SARS-CoV-2 is not yet known, given the relative rarity of placental infection and in utero transmission of SARS-CoV-2 and lack of reported sex-disaggregated data in these areas [2–4]. However, multiple studies have identified higher expression of ACE2 and TMPRSS2 in the respiratory tract of individuals with clinical risk factors for severe COVID-19 disease, including male sex [45, 57–59], which may suggest that ACE2 and TMPRSS2 expression levels are linked to COVID-19 disease susceptibility and/or severity. Recent direct evidence points to a strong association between higher ratio of TMPRSS2 to ACE2 expression in nasopharyngeal samples and severe COVID-19 disease [60]. Reporting the sex differences described here represents the first essential step toward a more nuanced understanding of how fetal sex may influence vulnerability or resilience to placental and fetal infection with SARS-CoV-2. The finding of sex differences in TMPRSS2 expression may have implications for understanding sex differences in COVID-19 symptomatology [61–63] and for elucidating sex differences in diseases for which TMPRSS2 is implicated in the pathogenesis, such as H1N1 influenza and prostate adenocarcinoma [64, 65].
TMPRSS2, which is highly expressed in prostate and lung tissue, among other sites, enables cellular infection with SARS-CoV-2 through priming of the spike protein to facilitate binding the ACE2 receptor [66, 67]. The strong regulation of TMPRSS2 by androgens has inspired the hypothesis that the male predominance in COVID-19 disease might be explained in part by increased TMPRSS2 levels [33, 36]. A large-scale cohort study investigating TMPRSS2 variants in an Italian population with high COVID-19 mortality rates, and male-to-female case ratio of 1.75, identified increased population frequency of a rare allele associated with increased TMPRSS2 expression [44]. Based on observational data that men on androgen-deprivation therapy for cancer risk reduction are at lower risk for acquiring SARS-CoV-2 [68], both androgen-deprivation therapy and agents blocking TMPRSS2 activity have been considered as therapeutic options for SARS-CoV-2 [24, 67].
Consistent with evidence in support of higher TMPRSS2 expression in other male tissues [43, 44], we identified higher TMPRSS2 levels in placentas of male compared to female offspring. Whether the observed higher baseline levels of TMPRSS2 in male placenta observed in our study represent increased vulnerability to placental infection is intriguing, but cannot be assessed in a cohort of this size, given the rarity of placental infection and vertical transmission. The identification of a sexually dimorphic response to maternal SARS-CoV-2 infection in placental TMPRSS2 levels at both the gene and protein levels represents an important first step to understanding whether and how fetal sex impacts the risk for transplacental transmission. This observation is particularly important given the known increased vulnerability of the male fetus to prenatal insults [69–71]. Whether lower TMPRSS2 levels in the presence of maternal SARS-CoV-2 infection reflect a protective compensatory process in the male placenta, potentially triggered by activation of the maternal innate immune system in response to SARS-CoV-2 infection, warrants further study. Increased TMPRSS2 expression is associated with enhanced viral replication and disease pathogenicity in feline coronavirus and H1N1 viral models, in which TMPRSS2 plays a similar role [72, 73], and TMPRSS2 gene expression data in humans suggest that lower TMPRSS2 levels in nasal and lung epithelium are linked to decreased SARS-CoV-2 susceptibility [74]. Recent data from the CDC demonstrate slightly lower rates of vertical transmission in male neonates compared to female, with females accounting for 50.8% of SARS-CoV-2 diagnosed at birth, males accounting for 47.5% of cases, and 1.7% of SARS-CoV-2 diagnosed perinatally occurring in neonates with unknown/not recorded sex at birth (overall n = 59) [75]. Given the small number of reported cases of perinatal transmission, future large cohorts reporting on vertical and perinatal transmission should do so in a sex-disaggregated fashion to further our understanding of the potential for sex-specific vulnerability to or protection from SARS-CoV-2 transplacental transmission.
We identified no significant effect of fetal sex or maternal SARS-CoV-2 infection on placental ACE2 gene or protein expression. As part of the renin-angiotensin-aldosterone system (RAAS) that regulates fluid homeostasis, the ACE2 enzyme is present in various cell types throughout the body, including the lung and respiratory tract [76]. Sex steroids—androgens and estrogens—influence the RAAS system [77, 78], and sex-specific differences in ACE2 expression render males more vulnerable to renal and cardiovascular disease, with higher levels considered more detrimental [79–81]. Evidence suggests that sex steroids can influence ACE2 expression in the respiratory tract and that higher ACE2 levels may be causally related to increased COVID-19 severity [42, 82–85]. However, studies investigating ACE2 expression in respiratory tract tissues by sex have not consistently shown higher expression in males, highlighting that levels of ACE2 may not fully explain the observed male bias in severe COVID-19 disease [44, 85–88]. While our data and several other studies demonstrate robust expression of ACE2 in the human placenta that may vary by gestational age [12, 15, 17–19, 89, 90], few studies have evaluated sex-specific placental ACE2 expression, and these have reported conflicting results [91, 92]. We are not aware of any studies to date that have evaluated sex differences in placental ACE2 expression in the setting of maternal SARS-CoV-2 infection. A study of maternal decidual explant cultures reported that secreted ACE2 mRNA was significantly increased in 48-hour explant cultures from mothers carrying a female fetus [91], while evaluations of rat placenta have found reduced ACE2 expression in the setting of maternal protein restriction and dexamethasone administration [93, 94], but no sex differences in this regard. Our data suggest that ACE2 expression in placental tissue does not differ significantly by offspring sex or in the setting of maternal SARS-CoV-2 infection. Based on this finding, differences in placental ACE2 levels likely do not represent a source of sex-specific vulnerability to placental infection or transplacental transmission.
We found that ACE2 and TMPRSS2 transcript levels are highly correlated in male placentas in the setting of maternal SARS-CoV-2 infection, but not in uninfected male pregnancies nor in female placentas regardless of maternal infection status. Expression profiling of ACE2 and TMPRSS2 in human tissues has shown a strong positive correlation across multiple cell lines and tissue types [59, 95], with authors postulating that any intervention or mechanism targeting one gene may affect expression of the other. ACE2 and TMPRSS2 tend to be co-regulated by factors such as obesity, diabetes, viral infections, and androgens [95]. TMPRSS2 is known to be regulated by androgen/androgen receptor signaling in prostate cancer [96], and a recent study identified positive correlations between androgen receptor and ACE2 expression in multiple tissues [37]. Recent evidence suggests that the innate immune response may impact ACE2 and TMPRSS2 expression through interferon signaling pathways [72, 97, 98], which play an important role in the host viral immune response to SARS-CoV-2 infection [99–101], and which may also demonstrate sex-specific patterns of activation [102]. Our group has recently identified sexually dimorphic patterning of interferon-stimulated genes in the setting of maternal exposure to SARS-CoV-2 [103]. Further investigation of the interplay between fetal sex, the maternal and placental innate immune response to SARS-CoV-2 infection, and placental ACE2/TMPRSS2 expression may yield important insights into placental susceptibility to infection and vertical transmission of SARS-CoV-2.
Our histopathological data from term placentas demonstrating low expression of TMPRSS2 relative to ACE2 and physically distant expression of these 2 cell entry mediators [12, 15] suggest that the increased correlation of ACE2 and TMPRSS2 expression observed here in male SARS-CoV-2–exposed placentas may not represent increased co-localization of ACE2 and TMPRSS2. Both negligible co-transcription of placental ACE2 and TMPRSS2 across gestation by nearly all placental cells [17] and the relatively low expression of TMPRSS 2 relative to ACE2 in full-term placenta [12] have been cited as potential mechanisms protecting against placental SARS-CoV-2 infection. It remains unclear, however, whether enhanced synchronicity in ACE2/TMPRSS2 expression patterns represents an increased placental vulnerability to infection.
Strengths of our study include a relatively large sample size of pregnancies with SARS-CoV-2 infection, including 58% with symptomatic illness and 8% with severe or critical disease. Another strength of this cohort is the identification and inclusion of a contemporaneously enrolled control population. To control for heterogeneity and regional differences in the placenta, we used 4 biological replicates with equal representation of fetal and maternal surfaces. Our study is limited by the timing of SARS-CoV-2 infection in pregnancy, which largely occurred in the third trimester. Although it is possible that women identified as SARS-CoV-2 negative at the time of delivery may have had resolved, asymptomatic SARS-CoV-2 infection earlier in pregnancy, the timing of enrollment of the included controls early in the pandemic from April to June 2020 makes this possibility less likely (the first wave in greater Boston area began in mid-March) [104]. While the misclassification after asymptomatic resolved infection in the control group remains a theoretical possibility, an unidentified exposure in the negative control group would likely have biased our results to the null hypothesis (diminishing differences between groups). The effects of first- or second-trimester infection on ACE2 and TMPRSS2 expression should be evaluated, as infection earlier in pregnancy could be associated with increased risk of transplacental transmission. The impact of sex differences in placental patterns of ACE2 and TMPRSS2 expression will need to be evaluated in large cohorts with the ability to link biological data with disease outcomes of interest such as transplacental transmission.
These data demonstrate striking sex differences in placental patterns of SARS-CoV-2 cell entry mediators in the setting of maternal SARS-CoV-2 infection. Moreover, the baseline sex differences in placental TMPRSS2, and the sexually dimorphic response of TMPRSS2 to maternal infection even in the absence of placental infection, suggest a potential role for TMPRSS2 in the placenta beyond SARS-CoV-2 entry that may be affected by fetal sex or maternal exposures. The findings presented here demonstrate the importance of reporting sex-disaggregated placental and neonatal outcomes data in the setting of maternal SARS-CoV-2 infection. These data also elucidate potential placental defenses against viral infection with SARS-CoV-2, including altered TMPRSS2 expression in the presence of maternal SARS-CoV-2 infection.
Notes
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant numbers 1R01HD100022-01, 3R01HD100022-02S1, and 3R01HD100022-02S2 to A. G. E. and 1K12HD103096-01 to L. L. S.); the National Institute on Mental Health (grant number F32MH116604 to E. A. B.); and the National Heart, Lung, and Blood Institute (grant numbers K08HL1469630-02 and 3K08HL146963-02S1 to K. J. G.). A. G. E. is also supported by the Claflin Award from the Massachusetts General Hospital Executive Committee on Research.
Supplement sponsorship. This supplement is sponsored by the Harvard University Center for AIDS Research.
Potential conflicts of interest. K. J. G. has received consulting fees from Illumina, BillionToOne, and Aetion. D. J. R. receives author royalties from Cambridge University Press. D. P. owns stock in Vertex Pharmaceuticals, Illumina, Gilead Sciences, BioNano Genomics, Biogen, Bluebird Bio, ImmunoGen, Pfizer, and Bristol-Myers Squibb. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 41st Annual Pregnancy Meeting of the Society for Maternal-Fetal Medicine, Virtual, 30 January 2021. Abstract 915.
References
- 1. Centers for Disease Control and Prevention. COVID-19 cases, deaths, and trends in the US.2020. https://covid.cdc.gov/covid-data-tracker/?CDC_AA_refVal=https% 3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2 Fcases-updates%2Fcases-in-us.html#pregnant-population. Accessed 11 July 2021.
- 2. Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol 2021; 224:35–53.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woodworth KR, Olsen EO, Neelam V, et al. CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team; COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT) . Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mullins E, Hudak ML, Banerjee J, et al. Pregnancy and neonatal outcomes of COVID-19: co-reporting of common outcomes from PAN-COVID and AAP SONPM registries. Ultrasound Obstet Gynecol 2021; 57:573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fenizia C, Biasin M, Cetin I, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun 2020; 11:5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baud D, Greub G, Favre G, et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020; 323:2198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020; 11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Algarroba GN, Rekawek P, Vahanian SA, et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020; 223:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest 2020; 130:4947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patanè L, Morotti D, Giunta MR, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019—positive mothers and neonates at birth. Am J Obst Gynecol MFM 2020; 2:100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sisman J, Jaleel MA, Moreno W, et al. Intrauterine transmission of SARS-COV-2 infection in a preterm infant. Pediatr Infect Dis J 2020; 39:e265–7. [DOI] [PubMed] [Google Scholar]
- 12. Edlow AG, Li JZ, Collier AY, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open 2020; 3:e2030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 2020; 251:228–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol 2020; 33:2092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gengler C, Dubruc E, Favre G, Greub G, de Leval L, Baud D. SARS-CoV-2 ACE-receptor detection in the placenta throughout pregnancy. Clin Microbiol Infect 2021; 27:489–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pique-Regi R, Romero R, Tarca AL, et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife 2020; 9:e58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li M, Chen L, Zhang J, Xiong C, Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One 2020; 15:e0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen W, Yuan P, Yang M, et al. SARS-CoV-2 entry factors: ACE2 and TMPRSS2 are expressed in peri-implantation embryos and the maternal–fetal interface. Engineering 2020; 6:1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kreis N-N, Ritter A, Louwen F, Yuan J. A message from the human placenta: structural and immunomodulatory defense against SARS-CoV-2. Cells 2020; 9:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open 2020; 10:e040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020; 11:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pradhan A, Olsson PE. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ 2020; 11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol 2020; 20:442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhopal SS, Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet 2020; 396:532–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godfred-Cato S, Bryant B, Leung J, et al. California MIS-C Response Team . COVID-19-associated multisystem inflammatory syndrome in children—United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolescent Health 2020; 4:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention COVID-19 Response Team. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feldstein LR, Rose EB, Horwitz SM, et al. Overcoming COVID-19 Investigators; CDC COVID-19 Response Team . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yonker LM, Neilan AM, Bartsch Y, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr 2020; 227:45–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Majdic G. Could sex/gender differences in ACE2 expression in the lungs contribute to the large gender disparity in the morbidity and mortality of patients infected with the SARS-CoV-2 virus? Front Cell Infect Microbiol 2020; 10:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mjaess G, Karam A, Aoun F, Albisinni S, Roumeguère T. COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol 2020; 30:484–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H, Sun X, VonCannon JL, Kon ND, Ferrario CM, Groban L. Estrogen receptors are linked to angiotensin-converting enzyme 2 (ACE2), ADAM metallopeptidase domain 17 (ADAM-17), and transmembrane protease serine 2 (TMPRSS2) expression in the human atrium: insights into COVID-19 [manuscript published online ahead of print 3 February 2021]. Hypertens Res 2021. doi: 10.1038/s41440-021-00626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viveiros A, Rasmuson J, Vu J, et al. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am J Physiol Heart Circ Physiol 2021; 320:H296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strope JD, PharmD CHC, Figg WD. TMPRSS2: potential biomarker for COVID-19 outcomes. J Clin Pharmacol 2020; 60:801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei X, Xiao Y-T, Wang J, et al. Sex differences in severity and mortality among patients with COVID-19: evidence from pooled literature analysis and insights from integrated bioinformatic analysis. arXiv [Preprint]. Posted online 30 March 2020. http://arxiv.org/abs/2003.13547. Accessed 11 July 2021.
- 38. Wang Y, Wang Y, Luo W, et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci 2020; 17:1522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fowler PC, Naluai ÅT, Oscarsson M, et al. Differential expression of angiotensin-converting enzyme 2 in nasal tissue of patients with chronic rhinosinusitis with nasal polyps. medRxiv [Preprint]. Posted online 3 February 2021. doi: 10.1101/2021.02.01.21250623. [DOI] [Google Scholar]
- 40. Song H, Seddighzadeh B, Cooperberg MR, Huang FW. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol 2020; 78:296–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salah HM, Mehta JL. Hypothesis: sex-related differences in ACE2 activity may contribute to higher mortality in men versus women with COVID-19. J Cardiovasc Pharmacol Ther 2021; 26:114–8. [DOI] [PubMed] [Google Scholar]
- 42. Kalidhindi RSR, Borkar NA, Ambhore NS, Pabelick CM, Prakash YS, Sathish V. Sex steroids skew ACE2 expression in human airway: a contributing factor to sex differences in COVID-19? Am J Physiol Lung Cell Mol Physiol 2020; 319:L843–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Okwan-Duodu D, Lim EC, You S, Engman DM. TMPRSS2 activity may mediate sex differences in COVID-19 severity. Signal Transduct Target Ther 2021; 6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY) 2020; 12:10087–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cai G, Bossé Y, Xiao F, Kheradmand F, Amos CI. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med 2020; 201:1557–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Acheampong DO, Barffour IK, Boye A, Aninagyei E, Ocansey S, Morna MT. Male predisposition to severe COVID-19: review of evidence and potential therapeutic prospects. Biomed Pharmacother 2020; 131:110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shook LL, Shui JE, Boatin AA, et al. Rapid establishment of a COVID-19 perinatal biorepository: early lessons from the first 100 women enrolled. BMC Med Res Methodol 2020; 20:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. American Academy of Pediatrics. FAQs: management of infants born to mothers with suspected or confirmed COVID-19.2020. https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/faqs-management-of-infants-born-to-covid-19-mothers/. Accessed 11 July 2021.
- 49. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. 2020. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed 11 July 2021.
- 51. Meller M, Vadachkoria S, Luthy DA, Williams MA. Evaluation of housekeeping genes in placental comparative expression studies. Placenta 2005; 26:601–7. [DOI] [PubMed] [Google Scholar]
- 52. Murthi P, Fitzpatrick E, Borg AJ, Donath S, Brennecke SP, Kalionis B. GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction. Placenta 2008; 29:798–801. [DOI] [PubMed] [Google Scholar]
- 53. Li Y, Lu H, Ji Y, Wu S, Yang Y. Identification of genes for normalization of real-time RT-PCR data in placental tissues from intrahepatic cholestasis of pregnancy. Placenta 2016; 48:133–5. [DOI] [PubMed] [Google Scholar]
- 54. Centers for Disease Control and Prevention. Research use only 2019-novel coronavirus (2019-nCoV) real-time RT-PCR primers and probes.2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html. Accessed 11 July 2021.
- 55. Goldfarb IT, Clapp MA, Soffer MD, et al. Prevalence and severity of coronavirus disease 2019 (COVID-19) illness in symptomatic pregnant and postpartum women stratified by Hispanic ethnicity. Obstet Gynecol 2020; 136:300–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fajnzylber J, Regan J, Coxen K, et al. Massachusetts Consortium for Pathogen Readiness . SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020; 11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saheb Sharif-Askari N, Saheb Sharif-Askari F, Alabed M, et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev 2020; 18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 2020; 323:2427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Piva F, Sabanovic B, Cecati M, Giulietti M. Expression and co-expression analyses of TMPRSS2, a key element in COVID-19. Eur J Clin Microbiol Infect Dis 2021; 40:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rossi ÁD, de Araújo JLF, de Almeida TB, et al. COVID-19 UFRJ Workgroup . Association between ACE2 and TMPRSS2 nasopharyngeal expression and COVID-19 respiratory distress. Sci Rep 2021; 11:9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kumar A, Faiq MA, Pareek V, et al. Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients. Med Hypotheses 2020; 144:110271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Suárez-Fariñas M, Tokuyama M, Wei G, et al. Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS-CoV-2-related disease. Gastroenterology 2021; 160:287–301.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maleki Dana P, Sadoughi F, Hallajzadeh J, et al. An insight into the sex differences in COVID-19 patients: what are the possible causes? Prehosp Disaster Med 2020; 35:438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hatesuer B, Bertram S, Mehnert N, et al. TMPRSS2 is essential for influenza H1N1 virus pathogenesis in mice. PLoS Pathog 2013; 9:e1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ko CJ, Hsu TW, Wu SR, et al. Inhibition of TMPRSS2 by HAI-2 reduces prostate cancer cell invasion and metastasis. Oncogene 2020; 39:5950–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lucas JM, Heinlein C, Kim T, et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov 2014; 4:1310–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov 2020; 10:779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol 2020; 31:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aghai ZH, Goudar SS, Patel A, et al. Gender variations in neonatal and early infant mortality in India and Pakistan: a secondary analysis from the Global Network Maternal Newborn Health Registry. Reprod Health 2020; 17:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pongou R. Why is infant mortality higher in boys than in girls? A new hypothesis based on preconception environment and evidence from a large sample of twins. Demography 2013; 50:421–44. [DOI] [PubMed] [Google Scholar]
- 71. Tyson JE, Parikh NA, Langer J, Green C, Higgins RD; National Institute of Child Health and Human Development Neonatal Research Network . Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med 2008; 358:1672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mettelman RC, O’Brien A, Whittaker GR, Baker SC. Generating and evaluating type I interferon receptor-deficient and feline TMPRSS2-expressing cells for propagating serotype I feline infectious peritonitis virus. Virology 2019; 537:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cheng Z, Zhou J, To KK-W, et al. Identification of TMPRSS2 as a susceptibility gene for severe 2009 pandemic A(H1N1) influenza and A(H7N9) influenza. J Infect Dis 2015; 212:1214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Abbasi AZ, Kiyani DA, Hamid SM, Saalim M, Fahim A, Jalal N. Spiking dependence of SARS-CoV-2 pathogenicity on TMPRSS2. J Med Virol 2021; 93:4205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Galang R. Co-lead, pregnancy and infant linked outcomes team (PILOT) epidemiology studies task force, COVID-19 response. Atlanta, GA: Centers for Disease Control and Prevention, 2021. [Google Scholar]
- 76. Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol 2006; 6:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reckelhoff Jane F. Gender differences in the regulation of blood pressure. Hypertension 2001; 37:1199–208. [DOI] [PubMed] [Google Scholar]
- 78. McGuire BB, Watson RW, Pérez-Barriocanal F, Fitzpatrick JM, Docherty NG. Gender differences in the renin-angiotensin and nitric oxide systems: relevance in the normal and diseased kidney. Kidney Blood Press Res 2007; 30:67–80. [DOI] [PubMed] [Google Scholar]
- 79. Clotet-Freixas S, Soler MJ, Palau V, et al. Sex dimorphism in ANGII-mediated crosstalk between ACE2 and ACE in diabetic nephropathy. Lab Invest 2018; 98:1237–49. [DOI] [PubMed] [Google Scholar]
- 80. Liu J, Ji H, Zheng W, et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ 2010; 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dalpiaz PL, Lamas AZ, Caliman IF, et al. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One 2015; 10:e0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol 2020; 92:726–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J 2020; 41:1810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Foresta C, Rocca MS, Di Nisio A. Gender susceptibility to COVID-19: a review of the putative role of sex hormones and X chromosome. J Endocrinol Invest 2021; 44:951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baratchian M, McManus JM, Berk M, et al. Sex, androgens and regulation of pulmonary AR, TMPRSS2 and ACE2. bioRxiv [Preprint]. Posted online 14 October 2020. doi: 10.1101/2020.04.21.051201. [DOI] [Google Scholar]
- 86. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 2020; 9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Han T, Kang J, Li G, Ge J, Gu J. Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann Transl Med 2020; 8:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xie X, Xudong X, Chen J, et al. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci 2006; 78:2166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pavličev M, Wagner GP, Chavan AR, et al. Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res 2017; 27:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bloise E, Zhang J, Nakpu J, et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am J Obstet Gynecol 2021; 224:298.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang Y, Pringle KG, Sykes SD, et al. Fetal sex affects expression of renin-angiotensin system components in term human decidua. Endocrinology 2012; 153:462–8. [DOI] [PubMed] [Google Scholar]
- 92. South AM, Shaltout HA, Washburn LK, Hendricks AS, Diz DI, Chappell MC. Fetal programming and the angiotensin-(1–7) axis: a review of the experimental and clinical data. Clin Sci 2019; 133:55–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gao H, Yallampalli U, Yallampalli C. Maternal protein restriction reduces expression of angiotensin I-converting enzyme 2 in rat placental labyrinth zone in late pregnancy. Biol Reprod 2012; 86:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ghadhanfar E, Alsalem A, Al-Kandari S, Naser J, Babiker F, Al-Bader M. The role of ACE2, angiotensin-(1-7) and Mas1 receptor axis in glucocorticoid-induced intrauterine growth restriction. Reprod Biol Endocrinol 2017; 15:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gkogkou E, Barnasas G, Vougas K, Trougakos IP. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol 2020; 36:101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chakravarty D, Nair SS, Hammouda N, et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol 2020; 3:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Su S, Jiang S. A suspicious role of interferon in the pathogenesis of SARS-CoV-2 by enhancing expression of ACE2. Signal Transduct Target Ther 2020; 5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sajuthi SP, DeFord P, Li Y, et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun 2020; 11:5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lowery SA, Sariol A, Perlman S. Innate immune and inflammatory responses to SARS-CoV-2: implications for COVID-19 [manuscript published online ahead of print 17 May 2021]. Cell Host Microbe 2021. doi: 10.1016/j.chom.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Galani IE, Rovina N, Lampropoulou V, et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol 2021; 22:32–40. [DOI] [PubMed] [Google Scholar]
- 101. Oladunni FS, Park JG, Pino PA, et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun 2020; 11:6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38. [DOI] [PubMed] [Google Scholar]
- 103. Bordt EA, Shook LL, Atyeo C, et al. Sexually dimorphic placental responses to maternal SARS-CoV-2 infection. bioRxiv [Preprint]. Posted online 29 March 2021. doi:10.1101/2021.03.29.437516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Massachusetts Department of Public Health. COVID-19 response reporting.https://www.mass.gov/info-details/covid-19-response-reporting. Accessed 11 July 2021.