Abstract
Background
The COVID-19 pandemic has resulted in the closure or partial closure of international borders in almost all countries. Here, we investigate the efficacy of imported case detection considering quarantine length and different testing measures for travellers on arrival.
Methods
We examine eight broad border control strategies from utilizing quarantine alone, pre-testing, entry and exit testing, and testing during quarantine. In comparing the efficacy of these strategies, we calculate the probability of detecting travellers who have been infected up to 2 weeks pre-departure according to their estimated incubation and infectious period. We estimate the number of undetected infected travellers permitted entry for these strategies across a prevalence range of 0.1–2% per million travellers.
Results
At 14-day quarantine, on average 2.2% (range: 0.5–8.2%) of imported infections are missed across the strategies, leading to 22 (5–82) imported cases at 0.1% prevalence per million travellers, increasing up to 430 (106–1641) at 2%. The strategy utilizing exit testing results in 3.9% (3.1–4.9%) of imported cases being missed at 7-day quarantine, down to 0.4% (0.3–0.7%) at 21-day quarantine, and the introduction of daily testing, as the most risk averse strategy, reduces the proportion further to 2.5–4.2% at day 7 and 0.1–0.2% at day 21 dependent on the tests used. Rapid antigen testing every 3 days in quarantine leads to 3% being missed at 7 days and 0.7% at 14 days, which is comparable to PCR testing with a 24-hour turnaround.
Conclusions
Mandatory testing, at a minimal of pre-testing and on arrival, is strongly recommended where the length of quarantining should then be determined by the destination country’s level of risk averseness, pandemic preparedness and origin of travellers. Repeated testing during quarantining should also be utilized to mitigate case importation risk and reduce the quarantining duration required.
Keywords: Disease surveillance, COVID-19, SARS-CoV-2, international travel, testing protocols, quarantine
Introduction
At the beginning of the coronavirus disease 2019 (COVID-19) pandemic, travellers formed a high proportion of total incidence,1,2 leading countries to impose travel restrictions, border closures, quarantining of travellers, temperature screening and, when tests became available, testing protocols.3,4 The virus responsible, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can undergo genomic rearrangement and has structural advantages to spread easily between hosts,5 thus has facilitated for ongoing widespread community transmission globally. Long-term and sustainable border control is therefore essential in allowing countries to reap the economic benefits of allowing travel while simultaneously minimizing importation risk and avoiding the application of ineffective intervention procedures. The question of how to reopen safely is especially important as epidemics subside under the combined effect of social distancing, vaccination, and rising population immunity.
The economic and social impacts of COVID-19 on the travel and associated industries have been extensive. An estimated 100–120 million tourism-related jobs are at risk with up to 1.2 trillion USD in lost tourism revenue6 and an estimated decline of at least 27% in global trade was expected7; by October 2020, 43 commercial airlines had declared bankruptcy.8 Additional indirect effects of travel bans in terms of financial loss, inadequate access to support networks, fears of infection and feelings of isolation among migrants9,10 are challenging to quantify, though no less important.
Alongside these factors, as many countries either have passed or will pass peak incidence in 2021,2 or have reached a state of low incidence with localized outbreaks sustained by international importation, increasing pressure is being placed on policymakers to reopen borders. This process of recovery is aided by the approval and ongoing distribution of messenger RNA vaccines from Pfizer-BioNTech and Moderna, which have estimated efficacies of >90%,11,12 among other adenovirus vector vaccines such as those from Oxford-AstraZeneca and Johnson & Johnson, and inactivated virus vaccines.13 However, heterogeneous vaccine rollout speeds,14 the challenge of vaccine hesitancy and low uptake rates,15,16 and the discovery and spread of variants such as the South African strain B.1.35117 against which first-generation vaccines may have reduced efficacy18 complicate the decision of how and when to open borders. Current policies in many countries have thus either continued to ban or at least counsel against non-essential travel, and to impose substantial lengths of quarantining, typically of 10–14 days, but up to three weeks in some jurisdictions19 with testing protocols in place to identify active infections for treatment and isolation.20 As heterogeneity exists between countries in the restrictions imposed on travellers, from varying the length of quarantining, differing in the number of testing events21 and use of polymerase chain reaction (PCR) or rapid antigen tests or both,21 establishing suitable protocols for passengers arriving from locations of differing base prevalence is essential for further outbreak mitigation, especially for countries with limited community transmission.
Shortening, or even removing, quarantine would provide economic benefits if it can be done safely. We have previously developed a mechanistic framework to assess the risk of imported cases evading detection.22 That study did not consider some of the specific practical issues that governments face, however, including how long to set quarantine, whether lower sensitivity but faster rapid antigen tests can be used in place of PCR tests, and how much benefit would accrue from repeat testing. A further difficulty is whether and how to tailor the border policy to the prevalence in the country whence the passengers come. This study therefore quantifies the risk under shorter durations of quarantine, compensated for by additional tests, so that policymakers may determine the options that fall within their risk budget.
Methodology
We consider an assumed volume of travellers who were infected in the fortnight before travel. Traveller  ’s time of infection,
’s time of infection,  (all notation is provided in Table 1), is followed by a duration of infection,
(all notation is provided in Table 1), is followed by a duration of infection,  , composed of an incubation period
, composed of an incubation period  and an infectious period
and an infectious period  (Figure 1). They are assumed to be no longer infectious thereafter. The modelling approach thus aims to calculate the proportion of missed cases that enter the community while still infectious (i.e.
(Figure 1). They are assumed to be no longer infectious thereafter. The modelling approach thus aims to calculate the proportion of missed cases that enter the community while still infectious (i.e.  is later than the end of any quarantine) among these travellers, which, when combined with the number of travellers and the prevalence among them, yields the proportion and number of infected travellers from the source country that reach the receiving country undetected.
is later than the end of any quarantine) among these travellers, which, when combined with the number of travellers and the prevalence among them, yields the proportion and number of infected travellers from the source country that reach the receiving country undetected.
Table 1.
Parameters used within the model
| Variable | Value, range or distribution | Definition |
|---|---|---|

|

|
Day of infection of individual 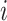 before travelling before travelling |

|
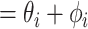
|
Duration of infection of individual 
|

|

|
Incubation period of individual  ; derived from Li et al.41 ; derived from Li et al.41
|

|

|
Duration individual  is infectious; derived from National Centre for Infectious Diseases, Singapore42 is infectious; derived from National Centre for Infectious Diseases, Singapore42
|

|

|
Indicator for individual  being detected being detected |

|

|
Indicator for individual  being asymptomatic being asymptomatic |

|

|
Asymptomatic proportion; derived from the Centre of Disease Control USA43 |

|
Function determined from polynomial regression | Sensitivity of PCR test 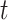 days after the end of the incubation period; derived from Xiao et al.23 days after the end of the incubation period; derived from Xiao et al.23
|

|
Function determined from polynomial regression | Sensitivity of rapid antigen test  days after the end of the incubation period; derived from Pekosz et al.24 days after the end of the incubation period; derived from Pekosz et al.24
|

|

|
Test turn-around time (days), rapid antigen |

|

|
Test turn-around time (days), PCR |

|

|
Prevalence of infection in the source country in the last 2 weeks |

|

|
Duration of quarantine under policy  (days) (days) |
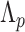
|
 where
where 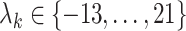
|
Set of test timings (relative to time of travel) under policy  (days) (days) |

|
 Where
Where 
|
Set of indicators of test types (rapid antigen or PCR) under policy  (days) (days) |

|
Derived | Probability an imported case is missed by policy 
|
Figure 1.

Schematic of model processes. Travellers have been infected in a uniform distribution from 0 to 13 days ago, and are undergoing an incubation, infectious or post-infectious period when they are no longer infectious. Passengers may be screened with a PCR or rapid antigen test before boarding (pre-test) and on arrival (entry). Upon arrival, they may be quarantined in a secured facility where they are unable to interact with members of the local community and receive PCR or rapid antigen tests. Rapid antigen tests are assumed to be processed within the same day (0DT; 0-day turnaround) whereas PCR tests can be processed within the same day (0DT), the next day (1DT) or two days (2DT)
Two tests were considered for the screening process for travellers. The polymerase chain reaction (PCR) test sensitivity profile was taken from data from Xiao et al.,23 and the rapid antigen test profile from Pekosz et al.24 To infer test sensitivity across time inclusive of the incubation period, we used polynomial regression assuming that both tests could not detect infection 5 days pre-illness (symptom or no symptom) onset, and 30 post (Supplementary Figure 1a). The test sensitivity  days after the end of the incubation period is denoted
days after the end of the incubation period is denoted  and
and  for PCR and rapid antigen tests, respectively. Rapid antigen tests typically develop within 20 minutes,25 whereas PCR tests, depending on laboratory processing times and administrative speed, can require up to a 2-day turnaround (2DT)26 for the individual to receive a result. We denote the turnaround times to be
for PCR and rapid antigen tests, respectively. Rapid antigen tests typically develop within 20 minutes,25 whereas PCR tests, depending on laboratory processing times and administrative speed, can require up to a 2-day turnaround (2DT)26 for the individual to receive a result. We denote the turnaround times to be  and
and  . For all PCR tests, we assume
. For all PCR tests, we assume  ,
,  or
or 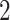 , to represent typical testing backlogs, which may vary between different countries of origin.
, to represent typical testing backlogs, which may vary between different countries of origin.
Upon arrival (day 0), entry testing may be conducted; the results are released after a test processing delay. The number of days of quarantine ( for policy scenario
for policy scenario  ) ranges from
) ranges from  (no quarantine) to
(no quarantine) to  days post-arrival, during which individuals may be tested one or more times in
days post-arrival, during which individuals may be tested one or more times in  . Policy
. Policy  is determined through the length of quarantine,
is determined through the length of quarantine,  , and the timings
, and the timings 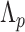 and test types
and test types  (both sets of length
(both sets of length  ) imposed.
) imposed.
Asymptomatic individuals are identified through testing alone, testing positive with probability,
 |
(1) |
Symptomatic individuals are assumed to be identified if they develop symptoms during quarantine, or if they test positive prior to symptom onset, and are identified with probability,
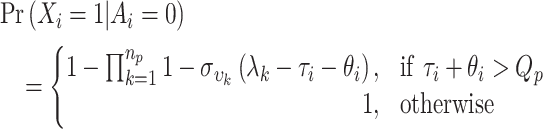 |
(2) |
This is under the assumption that quarantine is closely monitored and that those with onset during quarantine will be investigated and their infection status is identified. The probability of identifying a case is thus,
 |
(3) |
The balance of probability is almost then the probability of missing a case, the target of inquiry,  . However, an additional factor is required to account for cases when, for longer quarantine periods, the traveller is no longer infectious by the end of quarantine. Thus,
. However, an additional factor is required to account for cases when, for longer quarantine periods, the traveller is no longer infectious by the end of quarantine. Thus,  .We investigated different families of screening strategies for travellers, examining (S1) quarantine alone, (S2) pre-test and entry test with quarantine, (S3) pre-test, entry test, quarantine and testing on exit, (S4) pre-test, entry test, quarantine and daily testing until exit, (S5) pre-test, entry test, quarantine and tests conducted two days apart, (S6) pre-test, entry test, quarantine and tests conducted three days apart, (S7) pre-test, entry test, quarantine with antigen tests every three days during and PCR on exit, and (S8) pre-test, entry test, quarantine and the alternate test on exit (if PCR on pre-test and entry, rapid antigen tests are conducted thereafter). In all, we consider 29 unique strategy permutations. Through these scenarios, we explore the impact of longer and shorter durations of quarantine, of layering pre-departure and on-arrival tests on top of quarantine, of requiring an exit test before leaving quarantine, and of adding tests during quarantine at various frequencies.
.We investigated different families of screening strategies for travellers, examining (S1) quarantine alone, (S2) pre-test and entry test with quarantine, (S3) pre-test, entry test, quarantine and testing on exit, (S4) pre-test, entry test, quarantine and daily testing until exit, (S5) pre-test, entry test, quarantine and tests conducted two days apart, (S6) pre-test, entry test, quarantine and tests conducted three days apart, (S7) pre-test, entry test, quarantine with antigen tests every three days during and PCR on exit, and (S8) pre-test, entry test, quarantine and the alternate test on exit (if PCR on pre-test and entry, rapid antigen tests are conducted thereafter). In all, we consider 29 unique strategy permutations. Through these scenarios, we explore the impact of longer and shorter durations of quarantine, of layering pre-departure and on-arrival tests on top of quarantine, of requiring an exit test before leaving quarantine, and of adding tests during quarantine at various frequencies.
All probabilities are derived through Monte Carlo simulation with 14 000 000 replications. We vary the prevalence of infection in the last 2 weeks among travellers,  , over the range 0.1–2%. The proportion of all travellers from the source country who are infected and yet missed by border policy
, over the range 0.1–2%. The proportion of all travellers from the source country who are infected and yet missed by border policy  is thus
is thus  .
.
Results
Abridged results of the eight strategy families (S1–S8) are presented in Table 2 with the full tables displaying other combinations of testing in the Supplementary Information. Of travellers infected in the fortnight prior to travel for all individuals, 3.3% are modelled to arrive having completely cleared their infection (Supplementary Figure 1b). This group is characterized by short incubation and infectious periods and being infected early relative to the date of travel. The others are either infectious or incubating by the time of travel.
Table 2.
Number of missed cases at a prevalence of 0.1, 1 and 2% for a million travellers
| ID | Policy description | Proportion of cases missed (%) by quarantine length: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0d | 3d | 5d | 7d | 10d | 14d | 18d | 21d | |||
| S1 | Quarantine only | – | 47.9 | 37.8 | 29.6 | 19.2 | 8.2 | 2.8 | 1.4 | |
| S2c | PCR [2DT] | Entry test | – | 17.5 | 14.9 | 11.7 | 9.2 | 6.6 | 2.8 | 1.3 |
| S2d | Rapid antigen | 16.9 | 16.0 | 14.3 | 10.7 | 8.1 | 6.2 | 2.8 | 1.3 | |
| S3c | PCR [2DT] | Entry and exit test | – | 17.4 | 8.0 | 4.9 | 2.6 | 1.5 | 0.7 | 0.4 |
| S3d | Rapid antigen | 16.9 | 8.6 | 5.5 | 3.6 | 2.7 | 2.8 | 1.4 | 0.7 | |
| S4c | PCR [2DT] | Pre-test, entry, and daily test | – | 17.6 | 7.2 | 4.2 | 2.0 | 0.8 | 0.4 | 0.2 |
| S4d | Rapid antigen | 16.9 | 7.2 | 4.3 | 2.6 | 1.4 | 0.6 | 0.3 | 0.2 | |
| S5c | PCR [2DT] | Pre-test, entry, and test every 2 days | – | 17.5 | 8.0 | 4.5 | 2.1 | 0.9 | 0.4 | 0.2 |
| S5d | Rapid antigen | 16.9 | 7.8 | 4.6 | 2.8 | 1.4 | 0.7 | 0.3 | 0.2 | |
| S6c | PCR [2DT] | Pre-test, entry, and test every 3 days | – | 17.5 | 8.0 | 4.9 | 2.2 | 0.9 | 0.4 | 0.2 |
| S6d | Rapid antigen | 16.9 | 8.6 | 5.5 | 3.0 | 1.5 | 0.7 | 0.3 | 0.2 | |
| S7c | PCR [2DT] | Pre-test, entry, rapid antigen test every 3 days, PCR [2DT] exit test | – | 17.4 | 8.0 | 4.9 | 2.1 | 0.8 | 0.4 | 0.2 |
| S7d | Rapid antigen | Pre-test, entry, rapid antigen test every 3 days, PCR [0DT] exit test | 16.9 | 8.2 | 5.1 | 2.9 | 1.5 | 0.7 | 0.3 | 0.2 |
| S8c | PCR [2DT] | Pre-test, entry, and rapid antigen exit test | – | 8.2 | 5.3 | 3.7 | 3.1 | 3.0 | 1.5 | 0.7 |
| S8d | Rapid antigen | Pre-test, entry, and PCR [2DT] exit test | 16.9 | 12.1 | 8.0 | 4.8 | 2.6 | 1.5 | 0.7 | 0.4 |
The proportion of cases missed across different quarantine durations is presented at 0, 3, 5, 7, 10, 14, 18 and 21 days. The eight strategies are described under policy description where PCR 2DT (subset c) and rapid antigen (subset d) are displayed for comparison. Thus, PCR tests in this table have an assumed 2d turnaround (i.e.  ) except for S7d where
) except for S7d where  to be comparable to rapid antigen processing times during quarantine
to be comparable to rapid antigen processing times during quarantine
The first test administered has the greatest effect in reducing risk: with pre-departure testing the proportion of cases reaching the destination falls to 32.8% if a PCR test is conducted 2d before arrival, falling to 27.0% and 20.8% if the test is done on the day before or day of flight—assuming results can be obtained before flying. A rapid antigen test immediately before flying would likewise filter the infected arrivals down to 24.4% of the number without any test (Table 2 and Supplementary Table 2). However, pre-departure testing alone permits the entry of 3000–11 000 infected travellers per million at a prevalence of 2% in the last two weeks at the source country, which may not be sufficiently risk averse for countries with a low domestic prevalence or with large inbound travel volumes.
The addition of entry testing to pre-departure testing displays diminishing marginal returns, but would reduce missed infected travellers to 15.2% (for PCR with 0d delay in processing time pre-departure and on arrival) or 16.9% (for rapid antigen pre-departure and on arrival). A short quarantine period of 3d to allow results for an on-arrival PCR test would reduce the missed fraction to 18.0% (coupled with PCR 2d before flying), with a corresponding 3d quarantine to 16.0% (with rapid antigen 0d delay).
Quarantining alone (S1) would lead to the proportion of missed cases being 29.6% at 7 days, 8.2% at 14 days and 1.4% at 21 days. Coupled with PCR 2d pre-departure, these proportions would fall to 11.7, 6.6 and 1.03%, respectively. They would fall further—to 4.9, 1.5 and 0.4%—with an additional on-arrival PCR test. For very long quarantine periods longer than 14d, there is almost no additional benefits from including pre-testing and entry testing, though for a highly risk averse stance, long quarantine serves to remove the uncertainty of improper pre-testing and entry testing practices.
For shorter quarantine periods, however, substantial reductions in risk occur when pre-testing and entry testing are included: there is a dramatic reduction from 47.9% missed under 3d of quarantine alone to 15.0–17.5% across the different tests considered (S2); the equivalent for 5d is from 37.8 to 13.8–14.9%. At a high prevalence of 2%, this represents ~ 9600 versus ~ 3000–3500 missed cases per million travellers for three-day quarantining, and ~ 7500 versus 2800–3000 for five-day quarantining. The introduction of an exit test (S3) causes a further reduction of 6.4–8.4% at day 5 of quarantining, down to 0.7–1.0% at day 21 of quarantining, and the introduction of daily testing (S4), as the most risk averse strategy, reduces the proportion further to 4.2–7.2% at day 5 and 0.1–0.2% at day 21. For such high frequencies of testing, however, the benefits from day 14 onwards marks a modest 0.1–0.5% reduction at day 21, when only individuals with a combined long incubation period (>21 days) and relatively recent infection time are not detected.
Rapid PCR processing times can have a substantial benefit, creating larger differences in the number captured at the time of test result notification than repeatedly testing. For example, at 3-day quarantining with pre-testing, entry and exit testing, the proportion of missed cases for PCR with same day results would be 8.0% (S3a); it would be 10.2 or 17.4% with 1–2d turnaround times.
Notably, although rapid antigen testing has a less sensitive profile across time since infection than same-day PCR (Supplementary Figure 1a), its use generally outperforms scenarios in which PCR results take 1 or 2d to come through, necessitating an earlier pre-departure test—i.e. leaving a longer exposed period prior to flying in which infections cannot be detected—or an earlier test to end quarantine. At 7 days of quarantining, the average proportion of cases missed using rapid antigen across all explored strategies is 0.046, which is comparable to PCR with no delay at 0.043, slightly outperforming PCR with a 1-day and 2-day turnaround at 0.048 and 0.049. If used repeatedly throughout a week of quarantine, rapid antigen tests would lead to only 2.6% of cases being missed (S4d), comparable to 2.5% for PCR with same day results. Thus, the rapid turnaround of rapid antigen makes it a practical option, if it is not cost prohibitive, as it allows pre-departure and exit quarantine tests to be spaced more effectively to detect people with recent infections and long incubation periods, respectively.
Reducing the frequency of testing to every two days has a slight increase in the proportion of cases being missed across all test types; the lowest impact is observed for same-day PCR and rapid antigen (S5a, S5d). For two-day repeat testing during 5d of quarantine, these two test types have a difference of 0.3% in missed cases compared to daily testing strategies. For three-day repeat testing, there is a slight degradation of 0.8 and 1.2%, respectively. Daily testing therefore may not be necessary unless very high prevalence exists among travellers or very low risk tolerance is desired.
The choice of a quarantine and testing scenario is dependent on the risk tolerance or budget for each country (Figures 2 and 3 and Supplementary Figures 2–4). For a risk budget of 100 infected cases per million travellers, a policy of pre-testing, entry and exit testing (S3) would be affordable for a prevalence of up to 0.2% in the source country, requiring 6–7d of quarantine across all tests. Above this prevalence, quarantine would have to be extended to 10–17d for a source country prevalence of 0.5%, and 17d for 1.0%. Beyond this prevalence, it is not possible to stay within this budget without reducing the number of travellers for all test types.
Figure 2.

Number of missed cases per 1 million travellers at a prevalence of 0–2% with thresholds of having less than 10 and 100 missed cases according to the interventions described where for Panel a) S2a: PCR 2DT with pre-testing and entry testing at quarantine levels of 3 days (red), 7 days (orange), 10 days (green), 14 days (blue) and 21 days (purple) is used, b) S2d: rapid antigen pre-testing and entry testing, c) S3a: PCR 2DT pre-testing, entry testing and exit testing upon quarantine completion, d) S3d: rapid antigen pre-testing, entry testing and exit testing upon quarantine completion, e) S6a: PCR 2DT pre-testing, entry testing, and tests every 3 days whilst in quarantine, and f) S6d: rapid antigen pre-testing, entry testing, and tests every 3 days whilst in quarantine
Figure 3.

Minimum quarantining period required in scenarios S2c, S3c, S4c, S5c, S6c, S7c and S8c, focusing on the use of PCR with a 2-day delay in processing time, according to differing risk tolerances in the number of missed cases entering the local community across a prevalence of 0.1–2% among travellers. Missed infected individuals have not been identified by any testing measures or have infectious time remaining despite quarantining measures
For countries with a highly risk averse stance that may utilize daily testing (S4), and a risk budget of 10 cases per million travellers, a longer quarantine is needed for even lower prevalence source countries (12–14d for 0.1%), and no policy is safe at this level for a prevalence above 0.7% (Figure 3). With testing every 2d or 3d during quarantine (S5, S6), marginal increases in quarantining period (~1–2 days) are observed. Within strategies, minor differences are also generally observed between different test types (~2 days) where PCR with 0d processing delay is the optimal test choice (Figure 3 and Supplementary Figures 2–4). In scenarios with only exit testing (S3, S8), differences up to 5 days are observed between PCR and rapid antigen, highlighting the importance of repeated testing events with the use of rapid antigen testing.
Overall, current schemes with testing on arrival and on quarantine exit will only be able to achieve such case importation numbers with a prevalence among travellers below 0.4% (Figure 2c and d). Without quarantine exit testing, a very low prevalence of less than 0.1% is required, highlighting the necessity of testing in quarantine (Figure 2a and b). The addition of more PCR or rapid antigen tests every three days gives greater flexibility in the risk budget, dependent on the quarantine period, but is still unable to almost negate the risk of case importation (Figure 2e and f). As daily testing may be unfeasible for the majority of countries and additionally be cost-ineffective, the appropriate risk tolerance at each entry point will be determined by a country’s ability to trace and contain missed cases.
Discussion
Modern economies are reliant on the rapid flow of goods, services and individuals: in 2019 over 4 billion passengers were carried domestically and internationally.27 The increasing interdependence of economies and reliance on cross border travel has placed considerable pressure on policymakers to maintain open borders, or reopen borders promptly, but concurrent pressure is felt to avoid case importation. The economic case for open borders is bolstered by predictions that the global economy will not recover until 2022 at the earliest, with an estimated decline of 4.5% in real global GDP and 9.4% increase in unemployment in 2020.28 Multiple countries have, therefore, emerged from lockdown or eased restrictions, only to implement new control measures, including those in Europe,29 USA30 and China,31 in part due to the reintroduction of cases through importation. Sustainable long-term airport screening strategies are therefore required.
The first intervention in a policy has the most dramatic effect in identifying and filtering out infections; the earliest we considered is pre-departure testing. Further advantages for the receiving country of testing in the source country, beyond reducing the risk of missing cases among travellers, is that positive cases can be denied entry, and the duty of care passed back to the country in which the case was, presumably, infected. It may also reduce the risk of travel-acquired infection among other passengers who would otherwise be exposed during the voyage.20 Relying on pre-departure testing may be risky, however, without assurances of testing and laboratory protocols in the country of origin. Concerns of adherence to laboratory protocols and regulations, and lower accuracy in testing results, should additionally be considered.32 Some of these issues may be addressed by rapid tests conducted at the departure airport, which could be administered under conditions specified by the destination country, as with some security regulations. In situations where that is not possible, and accreditation of the source country’s laboratories are in doubt, on-arrival testing, as a supplement or replacement, is similarly effective but may require quarantine both to obtain results and allow for symptom onset for at least some of the infected travellers.
Long quarantine presents challenges, however. With the travel industry under considerable economic stress, the unattractiveness and cost of 14 to 21 days spent under quarantine in a dedicated facility or repurposed hotel may deter travel, or delay it until the pandemic abates. The simulations presented here show that shorter quarantine need not be less safe, if coupled with sufficiently frequent testing. The performance of rapid antigen testing in isolation suggests it should not be used to replace PCR unless PCR turnaround times take 1–2d for results, or are excessively costly. However, the differences between the two test types narrows when they are part of a package of measures including quarantine and repeat testing, supporting previous findings of the importance of frequency and turnaround time in COVID-19 screening.33 In this case, practicalities would trump the small difference in test accuracies. One such practicality is the ability to time the test optimally. If PCR results are not obtainable on the same day, then pre-departure testing would need to be done several days in advance, increasing the risk of an infection prior to travel being missed, and exit testing would similarly be earlier in the incubation period and less likely to yield a true positive.
Even for long quarantine with entrance and exit testing, there remains a risk, albeit small, that an infected traveller may fail to be identified, due to the long tail in the incubation period distribution. Whatever border policy is enacted, therefore, must be part of a broader suite of societal control measures to mop up any spillover to the general community. The risk tolerance of the country’s policy makers is therefore critical in determining how much travel to allow and what measures travellers should undergo. The model presented herein provides a calculus for this. Logically, a risk tolerance expressed as missed infections per million travellers should lead to differentiated policies for different risk strata of the source country. This tailored approach is already in place in some locations and forms the rationale for travel bubbles. In Singapore, for example, at the time of writing, low-risk travellers—from New Zealand or China for instance—require a PCR test on arrival, while higher risk travellers (e.g. from the EU) must undergo 14d of quarantine and highest risk travellers (UK and South Africa) 21d of quarantine, with additional testing during quarantine. As vaccination becomes more widespread, vaccine status should become an additional factor in the risk calculation, discounting the risk of the traveller relative to the overall point prevalence in the source country, and potentially allowing quarantine to be shortened or circumvented.
With repeat testing, concerns have emerged on the occurrence of substantial numbers of false positives with rapid antigen testing,34,35 though this problem surfaces for PCR as well, especially among convalescent patients in whom fragments of the virus may still be present in the pharynx. If assuming a rate of 0.5–2% false-positive testing, with three tests, 20–78 travellers per 1000 uninfected travellers will require further evaluation and may be put into isolation. This increases to 39–149 for seven tests (Supplementary Table 4). False-positive outcomes have reduced since the beginning of the global pandemic with improved sampling and lower contamination rates36 but can be further mitigated through examining symptom profiles or previous potential exposure to COVID-19 patients and using PCR testing to confirm the diagnosis. Individuals who are correctly identified as being negative would then be assumed to re-enter the quarantining process until clearance, though this may not be possible for false positives at pre-departure testing.
Further considerations for policymakers are the costs of managing and maintaining quarantining measures, and the time required for the appropriate training of staff, which can rapidly escalate with multiple entry points. Such costs may divert resources away from other health and social programs; therefore, border screening measures should form part of a wider COVID-19 control strategy budget. The number of travellers a destination country can accept should also account for not only the quarantining capacity available but the acceptable deemed risk of imported cases entering the community. As countries may also be accepting travellers from multiple destinations, heterogeneous prevalence estimates will require risk banding and differing quarantine or testing practices for different groups, including those who may be travelling very regularly for work purposes. Those arriving from high-risk countries of origin should be expected to undergo substantial quarantining periods of at least 14 days upwards whereas those at low risk may require shorter periods of 3 days or less. All, however, should receive repeated testing events including a test pre-arrival, on arrival and on quarantine exit, where applicable.
There are a number of limitations in this study. This study focused on the number of missed cases, excluding only those who completed their entire infection as not being missed. This is primarily due to the complexities regarding onward infection for travellers on arrival. The risk imposed on the community primarily depends on the infectivity profile of individuals during their infectious period and their behaviour. Where community wide interventions are in place and social activities are limited, spread may be limited to close contacts within households or with appropriate infection control in hotel type accommodation, no spread may occur. The relationship of viral load and infectivity over time is also complex as the detection of viral RNA by PCR may not equate to infectiousness, and transmission capacity after the first week of illness has not been fully documented.37 The severity of infectiousness appears to be dependent on symptom profile: most cases, approximately 80%, are mild where no pneumonia manifestations are observed. From all infections, an estimated 17–20% are asymptomatic completely,38 and may be limited in their ability to transmit to others. The remaining proportion, which are paucisymptomatic, displaying mild symptoms such as fever, mild cough and malaise, rather than severe pneumonia, may contribute to the easy and rapid spread of SARS-CoV-237 with sufficiently high viral loads alongside being challenging to detect without testing in quarantine.
Other limitations include limited information regarding test sensitivity in pre-symptomatic individuals and in those with particularly long incubation periods, assumptions on quarantine compliance in countries without institutional quarantine measures in place,39 and potentially changing prevalence rates across time although dramatic changes across two weeks periods are unlikely to occur. Heterogeneous prevalence rates may exist, however, within populations in countries according to social determinants and vaccine uptake, which were not accounted for. Utilizing the upper band of prevalence estimation for travellers may be appropriate, which should be revised as seroprevalence and case data continue to be collected. Lastly, the emergence of different variants, which may be able to spread more easily,40 can complicate border control and require further flexibility in the risk profiling of travellers. This suggests that even among the lowest risk countries, a minimal level of COVID-19 screening is prudent, where policymakers should rapidly respond with reports on the emergence of a new variant at a location. With the dissemination of information regarding variants, the development of new test kits with differing efficacies over infection time, or identification of dramatic changes in the distributions utilized, more analyses will be required.
These limitations notwithstanding, border control has been demonstrated in countries that implemented it early and consistently to be very effective in cutting the risk of spread into their communities, while other countries were experiencing massive outbreaks. As we move into the next phase of the pandemic, it will be necessary to relax the strictness of border control. We hope that through judicious use of testing, this need not increase risk.
Supplementary Material
Acknowledgements
We would like to thank Caitlin Asjes for her helpful comments on the manuscript.
Contributor Information
Joel R Koo, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Jue Tao Lim, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Minah Park, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Haoyang Sun, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Yinxiaohe Sun, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Zitong Zeng, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Sharon Esi Duoduwa Quaye, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Hannah E Clapham, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Hwee Lin Wee, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Alex R Cook, Saw Swee Hock School of Public Health, National University of Singapore and National University Health System.
Funding
The work was supported by funding from Singapore’s National Medical Research Council through grants COVID19RF-004 and NMRC/CG/C026/2017_NUHS. Additional funding was received through an Independent Research Grant from Becton Dickinson.
Contributions
B.L.D. carried out the modelling analyses and wrote the first draft. J.R.K., J.T.L. and H.S. assisted in the analyses. Y.S., Z.Z., E.S.D.Q., H.E.C. and H.L.W. reviewed the manuscript and provided comments. A.R.C. assisted in the interpretation of the results and in the writing of the manuscript.
Conflict of interest: Additional funding was received through an Independent Research Grant from Becton Dickinson. The funder had no role in the design, analyses, or outcome of this study.
References
- 1. Menkir TF, Chin T, Hay JA et al. Estimating internationally imported cases during the early COVID-19 pandemic. Nat Commun 2021; 12:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Russell TW, Wu JT, Clifford S et al. Effect of internationally imported cases on internal spread of COVID-19: a mathematical modelling study. Lancet Public Health 2021; 6:e12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilmore B, Ndejjo R, Tchetchia A et al. Community engagement for COVID-19 prevention and control: a rapid evidence synthesis. BMJ Glob Health 2020; 5:e003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanièce Delaunay C, Saeed S, Nguyen QD. Evaluation of testing frequency and sampling for severe acute respiratory syndrome coronavirus 2 surveillance strategies in long-term care facilities. J Am Med Dir Assoc 2020; 21:1574–1576.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elrashdy F, Redwan EM, Uversky VN. Why COVID-19 transmission is more efficient and aggressive than viral transmission in previous coronavirus epidemics? Biomolecules 2020; 10:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. United Nations World Tourism Organisation . Secretary-General’s Policy Brief on Tourism and COVID-19. https://www.unwto.org/tourism-and-covid-19-unprecedented-economic-impacts (10 March 2021, date last accessed).
- 7. United Nations Committee for the Coordination of Statistical Activities . How COVID-19 is changing the world: a statistical perspective.
- 8. Cirium . Weekly COVID-19 aviation update. https://www.cirium.com/thoughtcloud/cirium-weekly-covid-19-updates/.
- 9. Mækelæ MJ, Reggev N, Dutra N et al. Perceived efficacy of COVID-19 restrictions, reactions and their impact on mental health during the early phase of the outbreak in six countries. R Soc Open Sci 2020; 7:200644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brooks SK, Webster RK, Smith LE et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. The Lancet 2020; 395:912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baden LR, El Sahly HM, Essink B et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention . Different COVID-19 Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html. [PubMed]
- 14. John Hopkins Coronavirus Resource Center . International Vaccine Efforts. https://coronavirus.jhu.edu/vaccines/international.
- 15. Razai MS, Osama T, McKechnie DGJ, Majeed A. Covid-19 vaccine hesitancy among ethnic minority groups. BMJ 2021; 372:n513. doi: 10.1136/bmj.n513. [DOI] [PubMed] [Google Scholar]
- 16. Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health 2021; 6:E210–E221. doi: 10.1016/S2468-2667(21)00012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mwenda M, Saasa N, Sinyange N et al. Detection of B.1.351 SARS-CoV-2 variant strain — Zambia, December 2020. MMWR Morb Mortal Wkly Rep 2021; 70:280–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen J. South Africa suspends use of AstraZeneca’s COVID-19 vaccine after it fails to clearly stop virus variant. Science 2021. doi: 10.1126/science.abg9559. [DOI] [Google Scholar]
- 19. The Government of the Hong Kong Special Administrative Region . Quarantine for Inbound Travellers. https://www.coronavirus.gov.hk/eng/inbound-travel-faq.html.
- 20. Centers for Disease Control and Prevention . Testing and International Air Travel. https://www.cdc.gov/coronavirus/2019-ncov/travelers/testing-air-travel.html.
- 21. Gov.uk . Foreign Travel Advice. https://www.gov.uk/foreign-travel-advice.
- 22. Dickens BL, Koo JR, Lim JT et al. Strategies at points of entry to reduce importation risk of COVID-19 cases and reopen travel. J Travel Med 2020; 27:taaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin Infect Dis 2020; 71:2249–2251. doi: 10.1093/cid/ciaa460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pekosz A, Parvu V, Li M et al. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin Infect Dis 2021; ciaa1706. doi: 10.1093/cid/ciaa1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Food and Drug Administration, United States . Coronavirus (COVID-19) Update: FDA Authorizes Antigen Test as First Over-the-Counter Fully At-Home Diagnostic Test for COVID-19. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-antigen-test-first-over-counter-fully-home-diagnostic.
- 26. Organisation for Economic Co-operation and Development . Testing for COVID-19: How to best use the various tests? http://www.oecd.org/coronavirus/policy-responses/testing-for-covid-19-how-to-best-use-the-various-tests-c76df201/.
- 27. International Civil Aviation Organisation . The World of Air Transport in 2019. https://www.icao.int/annual-report-2019/Pages/the-world-of-air-transport-in-2019.aspx.
- 28. Organisation for Economic Co-operation and Development . The territorial impact of COVID-19: Managing the crisis across levels of government. http://www.oecd.org/coronavirus/policy-responses/the-territorial-impact-of-covid-19-managing-the-crisis-across-levels-of-government-d3e314e1/.
- 29. Kupferschmidt K. Europe is locking down a second time. But what is its long-term plan? Science 2020. doi: 10.1126/science.abf5429. [DOI] [Google Scholar]
- 30. New York Times . New York City Schools to Shut Down Again as Coronavirus Cases Rise. https://www.nytimes.com/2020/11/18/nyregion/nyc-schools-covid.html.
- 31. Reuters . Chinese city of Langfang goes into lockdown amid new COVID-19 threat. https://www.reuters.com/article/us-health-coronavirus-china-idUSKBN29H07C.
- 32. Allam M, Cai S, Ganesh S et al. COVID-19 diagnostics, tools, and prevention. Diagnostics (Basel) 2020; 10:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larremore DB, Wilder B, Lester E et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv 2021; 7:eabd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. American Society for Microbiology . SARS-CoV-2 Testing: Sensitivity Is Not the Whole Story. https://asm.org/Articles/2020/November/SARS-CoV-2-Testing-Sensitivity-Is-Not-the-Whole-St.
- 35. Armstrong S. Covid-19: Tests on students are highly inaccurate, early findings show. BMJ 2020; m4941. doi: 10.1136/bmj.m4941. [DOI] [PubMed] [Google Scholar]
- 36. Surkova E, Nikolayevskyy V, Drobniewski F. False-positive COVID-19 results: hidden problems and costs. Lancet Respir Med 2020; 8:1167–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020; 371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 38. Pollock AM, Lancaster J. Asymptomatic transmission of COVID-19. BMJ 2020; 371:m4851. doi: 10.1136/bmj.m4851. [DOI] [Google Scholar]
- 39. Dickens BL, Koo JR, Wilder-Smith A, Cook AR. Institutional, not home-based, isolation could contain the COVID-19 outbreak. The Lancet 2020; 395:1541–1542. doi: 10.1016/S0140-6736(20)31016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Centers for Disease Control and Prevention . Variants of the Virus that Causes COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html.
- 41. Li Q, Guan X, Wu P et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 2020; 382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. National Centre for Infectious Diseases, Singapore & Academy of Medicine, Singapore . Position Statement from the National Centre for Infectious Diseases and the Chapter of Infectious Disease Physicians, Academy of Medicine, Singapore −23 May 2020. https://www.ams.edu.sg/view-pdf.aspx?file=media%5C5556_fi_331.pdf&ofile=Period+of+Infectivity+Position+Statement+(final)+23–5-20+(logos).pdf.
- 43. Centers for Disease Control and Prevention . COVID-19 Pandemic Planning Scenarios, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


