Dear Editor,
Global application of vaccination is ongoing against SARS-CoV-2 (COVID-19) pandemic. There are increasing reports regarding the activation of herpes zoster (HZ) following SARS-CoV-2 mRNA vaccines (Pfizer-BNT162b2 mRNA and Moderna mRNA-1273).1–5 The Oxford/AstraZeneca vaccine, AZD1222 (ChAdOx1 nCoV-19) which uses a live modified chimpanzee adenovirus, has been approved and used in many European and Asian countries. According to the multinational placebo-controlled clinical trial conducted in Brazil, South Africa and the UK, no event of HZ followed by AZ vaccination was reported.6 Before this manuscript was written, there has been no report regarding the phenomenon of about HZ following injection of AZD1222 vaccine.
We wish to report three cases from Taiwan.
Case 1: A 71-year-old healthy man, denied systemic disease, received his first injection of Moderna COVID-19 vaccine. Two days later, grouped erythematous papules and vesicles appeared on his left flank with itching and pain (Figure 1). HZ involving left T8 dermatome was diagnosed. He received oral acyclovir for 1 week, and all vesicles become dry without obvious complication.
Figure 1.
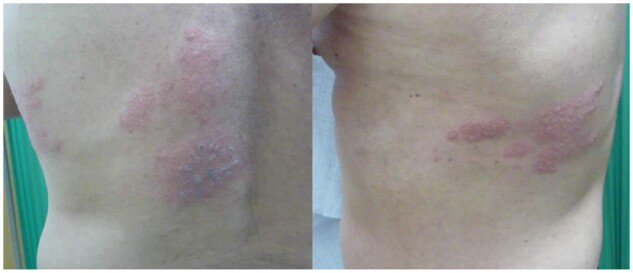
This 71-year-old man had painful itchy grouped vesicles over left T8 dermatome area since 2 days after receiving the first dose of COVID-19 vaccine.
Case 2 and 3: Another 2 healthy men (46- and 42-years-old) developed pain and itch over ipsilateral flank at 2 days and 7 days following receiving their first dose of AZD1222 vaccine. HZ was diagnosed later when the typical clinical presentation of HZ as grouped vesicles was present over left T11 and right T10 dermatomes, respectively. They received oral acyclovir for 1 week, and all vesicles become dry without post-herpetic neuralgia.
HZ does not often appear after the administration of other kinds of vaccinations. But we believed that there might be a link between COVID-19 vaccine and HZ emergence. One of the reasons is the short delay of onset after vaccination. The other reason is that these three patients were immunocompetent.
The mechanism is currently unknown about how the COVID-19 vaccination induces HZ. Reactivation of varicella zoster virus (VZV) is a failure of cell mediated immune response in maintaining control of the latent virus. In the clinical trials and previous laboratory studies, patients who received AZD1222 (live virus), BNT162b2 (mRNA) and Moderna mRNA-1273 vaccine do not exhibit symptoms suggesting immunosuppression. It is possible that the vaccine causes certain immunomodulation that allows VZV to awaken from latency. Activation of VZV in COVID-19 vaccine patients may be also related to physical, emotional stress and to vaccine-induced alteration of immunity.
It is worth mentioning that all of reported HZ cases following COVID-19 vaccination (including our three cases) were mild and resolved after proper antiviral treatment. We advise that awareness be raised regarding the recognition of HZ emergence following COVID-19 vaccination. We also recommend that steps be taken to address post-herpetic complication by administrating antiviral treatment early.
To our knowledge, there are increasing case reports about development of HZ following mRNA-based COVID-19 vaccine, and this is the first cases of HZ following adenovirus-based vaccine. It invites the urgent need for more studies to investigate the particular mechanism behind this phenomenon and identify the risk factors.
Conflict of interest. All authors have no potential conflict of interest.
References
- 1. Rodríguez-Jiménez P, Chicharro P, Cabrera L-M, Seguí M, Morales-Caballero Á, Llamas-Velasco M, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep 2021; 12:58–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee C, Cotter D, Basa J, Greenberg HL. Post-COVID‐19 vaccine related shingles cases seen at the Las Vegas dermatology clinic and sent to us via social media. J Cosmetic Dermatol 2021; 20:1960–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines 1975; 24:1639–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eid E, Abdullah L, Kurban M, Abbas O. Herpes Zoster emergence following mRNA COVID‐19 Vaccine. J Med Virol 2021; 93:5231–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA Covid-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford, England) 1975; 24:1639–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]


