Abstract
Perceived relational value describes the extent to which individuals consider themselves to be liked and valued. Given the salience of peer opinions in adolescence, perceived relational value is an important part of adolescents’ developing self-concept. Here, we examined the neural correlates of youth’s perceptions of their relational value in two independent samples (N= 33, Mage= 13.71, SD= 2.71; N= 26, Mage= 15.43, SD= .33). In both studies, peer victimization was associated with lower perceived relational value behaviorally and with altered frontostriatal connectivity when perceiving low relational value during fMRI. Our results suggest that peer victimization may lead youth to become biased about how they will be perceived socially and may disrupt connectivity between brain regions involved in responding to appetitive social stimuli.
Keywords: peer victimization, functional connectivity, adolescence, fMRI
Peer experiences in adolescence can have a profound impact on how people view themselves. Likely, this influence stems from the convergence of two key factors in adolescence. First, compared to other age groups, adolescents are hyper-aware of peer evaluation and peer feedback (M. Gardner & Steinberg, 2005; Somerville, 2013); and, second, compared to adults, adolescents’ self-concepts are still developing and fluid—leading adolescents to often define themselves based on others’ opinions of them, rather than based on their own opinions of themselves (Jankowski et al., 2014; Pfeifer et al., 2009). Critically, negative peer experiences, such as peer victimization, can damage adolescents’ developing self-concepts, and these negative effects can be lasting (Arseneault et al., 2010). Peer victimization is linked to poor self-esteem (Overbeek et al., 2010), negative self-perception (Taylor et al., 2013), and self-blame (Graham & Juvonen, 1998). Thus, given that negative peer experiences in adolescence can have a lasting impact on how adolescents view themselves, it is important to understand the processes by which peer victimization influences adolescents’ self-concept.
Sociometer Theory and Relational Value
Sociometer Theory (Leary, 1999; Leary & Baumeister, 2000) provides a framework for understanding how one component of adolescents’ self-concept—their perceived relational value—could be disrupted by negative peer interactions, such as peer victimization. Perceived relational value is the extent to which individuals consider themselves to have subjective worth (i.e., to be liked and valued), particularly in the eyes of those whom they like and value (Leary, 2005). Sociometer Theory posits that all people possess a ‘sociometer’—an innate, internal monitoring system that detects social acceptance or rejection cues in the environment and provides individuals with information about their relational value to in-group or high-status peers. When individuals experience a negative social interaction, their sociometer detects the rejection and relays this information through a decrease in relational value. Changes in relational value help individuals track the extent to which high-status peers want to have or maintain a relationship with them.
While a single negative social interaction may cause a temporary decrease in relational value, repeatedly experiencing negative social interactions, such as chronic peer rejection, results in overall lower perceived relational value (Leary, 1999). Through repeatedly experiencing negative social feedback, rejected individuals come to believe that they are of lower status, worth, or importance to their group. In subsequent social interactions, they will then anticipate that they are of lower relational value to others, even before receiving feedback. Sociometer Theory further suggests that while the sociometer operates in all social settings, perceptions of one’s relational value are most impacted by individuals whose opinions are valued (Leary, 2005). Specifically, perceived relational value is strongly impacted by rejection from liked and valued others, whereas individuals can “override” the sociometer’s impulse to decrease relational value following social rejection when the feedback comes from unimportant others.
While the sociometer is activated to maintain social homeostasis in individuals across the lifespan, adolescents may have a particularly hyperactive sociometer. Compared to adults, adolescents are hyper-aware of peer evaluation (Westenberg et al., 2004), are hypersensitive to social feedback (Somerville, 2013), and show heightened neural activity in regions of the brain associated with social cognition when anticipating peer evaluation (Gunther Moor et al., 2010; Guyer et al., 2009). Perceived relational value is also particularly likely to be altered by social feedback in adolescents. For example, compared to adults, adolescents are more likely to experience a drop in mood following a negative social evaluation (Sebastian et al., 2010) and to internalize negative peer feedback (Rodman et al., 2017).
Peer Victimization and Encoding Relational Value in the Brain
The hyperactive adolescent sociometer may be even more strongly attuned to social stimuli in peer victimized youth. Social rejection increases attention, processing, and memory of social information (W. L. Gardner et al., 2000), possibly causing the sociometer to become more active. However, no work has specifically examined how peer victimization influences how adolescents encode their relational value at the neural level. The ventral striatum is likely a key region in this process given its role in tracking motivational salience and reward value (Zink et al., 2004) as well as in anticipating social feedback from valued peers (Gunther Moor et al., 2010; Guyer et al., 2009; Kohls et al., 2013; Powers et al., 2013). Notably, adolescence is marked by a peak in dopaminergic signaling, which manifests in hyper-reactivity of the ventral striatum during this period (Galvan, 2010). This adolescent peak in striatal activation correlates with hypersensitivity to social interaction (Galvan, 2013). For example, the ventral striatum tracks valued social groups, peaking in activation to high-value groups in adolescence (Guassi Moreira et al., 2017). Similarly, compared to other age groups, adolescents display greater ventral striatum activity when anticipating and receiving peer feedback (Gunther Moor et al., 2010; Guyer et al., 2009), and when faced with appetitive social cues (Somerville et al., 2011). Thus, while the ventral striatum would likely be involved in perceiving relational value in any age group, the magnitude of this involvement is likely to be particularly heightened in adolescents.
Adolescent hypersensitivity to social stimuli may also result from immature connections between the limbic system and prefrontal cortex. Indeed, while the subcortex—including the ventral striatum—is early maturing, both the prefrontal cortex and connections between the prefrontal cortex and the subcortex are later maturing (Casey et al., 2016; Somerville & Casey, 2010). Potentially, a failure of the prefrontal cortex to downregulate heightened signal from the ventral striatum might further drive adolescents’ sensitivity to appetitive and social stimuli (Galvan, 2010). In support of this hypothesis, adolescents—compared to other age groups—show altered patterns of functional connectivity between the ventral striatum and the ventrolateral prefrontal cortex (vlPFC), medial prefrontal cortex (mPFC), and dorsomedial prefrontal cortex (dmPFC) during various types of social processing, including when making prosocial choices, when being evaluated by peers, and when anticipating social feedback (Jarcho et al., 2015; Somerville et al., 2013; Telzer et al., 2011). Thus, connectivity in frontostriatal circuitry may support perceiving relational value in adolescence.
Current Study
In the current studies, we examined how peer victimization is related to the behavioral and neural correlates of perceiving relational value in adolescents. During an fMRI scan, adolescents completed a social evaluation task designed to probe how they perceive their relational value in real time. Participants first viewed photos of same-aged peers and evaluated if they liked or disliked each peer in order to isolate the peers whom they rated as high value (i.e., liked). Next, participants predicted how each peer would evaluate them in return. According to Sociometer Theory, an individual’s perceived relational value should be impacted most by the feedback of valued (e.g., liked) others (Leary, 2005). Thus, focusing on the peers whom adolescents reported liking, we created two conditions based on whether they predicted the peer would like them in return: high perceived relational value (i.e., “I like you, and I think that you would like me back”) and low perceived relational value (i.e., “I like you, but I think that you would not like me back”). This approach has been used in prior research to reliably simulate prediction of social feedback from liked others (Guyer et al., 2008, 2009).
In Study 1, a community sample of youth completed the social evaluation task during fMRI and a questionnaire measure of concurrent peer victimization. The purpose of Study 1 was to conduct an exploratory study to examine how peer victimization is associated with the behavioral and neural processes that underlie how youths perceive their relational value. In Study 2, the same fMRI social evaluation task was administered to a group of adolescent girls recruited from a longitudinal study spanning 2nd−9th grade. Adolescent girls were specifically recruited because prior work suggests that they may be particularly attuned to changes in their relational value (Guyer et al., 2009; Rudolph & Conley, 2005). Indeed, compared to adolescent boys, adolescent girls tend to be more concerned about receiving a negative peer evaluation (Rose & Rudolph, 2006; Rudolph & Conley, 2005) and experience greater reactivity to peer rejection (Stroud et al., 2017). Further, half of the recruited adolescent girls reported experiencing high peer victimization across the previous years of the longitudinal study. Because a history of peer victimization predicts current peer victimization (Hodges & Perry, 1999), this study allowed us to examine the behavioral and neural processes supporting youths’ perceptions of their relational value in a sample with a greater range of concurrent peer victimization experiences. Thus, the goal of Study 2 was to test whether the exploratory results of Study 1 would replicate in a more vulnerable population with greater variability in peer victimization experiences.
In sum, we tested the following hypotheses: (1) In Study 1, we expected that experiencing peer victimization would be associated with lower perceived relational value at the behavioral level. While adults often possess a self-protective bias that buffers against rejection feedback, adolescents are still in the process of developing this bias and tend to be more accurate in their predictions of how peers will view them (Rodman et al., 2017). Indeed, socially rejected adolescents often view themselves accurately, correctly perceiving that others do not like them (Bellmore & Cillessen, 2006). (2) In Study 1, we hypothesized that greater peer victimization would be associated with altered functional connectivity between the ventral striatum and regions of the prefrontal cortex when perceiving low relative to high relational value—a hypothesis based in work highlighting the involvement of prefrontal-striatal connections in social processing in adolescence (Guyer et al., 2009; Jarcho et al., 2015; Telzer et al., 2011). Our goal was to identify regions of the prefrontal cortex that show altered functional connectivity with the ventral striatum when perceiving relational value (3) In Study 2, we hypothesized that the behavioral result from our exploratory Study 1 would replicate, and we tested if the same regions that were functionally connected to the ventral striatum in Study 1 would replicate in Study 2.
Study 1
Method
Participants.
Fifty youth were recruited from the community to participate in an fMRI scan. During recruitment, participants were excluded from study participation for MRI contraindications (e.g., metal implanted in the body), braces, and claustrophobia. Participants were not expressly screened for psychiatric disorders. However, we inquired about medication use, and no participant took psychiatric medication on the day of the scan. Left-handed participants were permitted. 18.18% of participants were left-handed. Of the 50 participants, 3 were excluded due to motion and 14 were excluded because they did not have an adequate number of low perceived relational value trials to model at the neural level (see description below). The final sample included 33 youth (Mage= 13.71, SD= 2.71, 39% male). There was no difference in age (t(48)= .75, p= .46), peer victimization (t(47)= .70, p= .49), sex (X2(1)=.05, p=.83), or race (X2(3)=1.84, p= .61) between the adolescents who were and were not included in the final sample. Participants were 82% White, 12% Mixed Race, 3% Black, and 3% Latina/o. Participants and their guardians provided written assent and consent in accordance with the guidelines set forth by the University’s Institutional Review Board.
Questionnaire measures.
Participants reported on their concurrent peer victimization experiences at the time of the scan by completing a 12-item version of the Social Experiences Questionnaire - Revised (SEQ-R; Rudolph et al., 2014). This measure contains items assessing relational victimization (i.e., being the target of behaviors intended to cause harm through social manipulation or damage to social relationships; 4 items; e.g., “How often does a peer say they won’t like you unless you do what they want you to do?”), overt victimization (i.e., being the target of overt physical and verbal attacks, such as pushing, hitting, or threats; 4 items; e.g., “How often do you get hit by another kid?”), and cyberbullying (e.g., being the target of bullying behavior online; 4 items; e.g., “How often has a peer spread rumors about you online, whether they were true or not?”). Using a 5-point scale (1=Not at All to 5=Very Often), participants indicated how often they experienced each type of victimization. Scores were computed as the mean of the 12 items (α = 0.74).
Relational value task.
Prior to the fMRI scan, participants were told that they would evaluate photographs of other adolescents who had previously participated in the study. Participants then posed for a digital photograph and were informed that their photo would be integrated into the task, so that future participants could also evaluate them. During the scan, participants completed 2 rounds of the task. In the first round, they indicated whether they liked each peer, and in the second round, they indicated whether they thought each peer would like them in return. In each round of the task, participants viewed 72 photos of ethnically diverse, age-matched peer faces. Half of these faces were male. All faces were looking into the camera and smiling. Faces were taken from several databases, including the National Institute of Mental Health Child Emotional Faces Picture Set (NIMH-ChEFS; Egger et al., 2011). On each trial in Round 1, participants were presented with a photo of a peer and asked to indicate whether they liked or disliked that peer. Participants had two response options: “Don’t like” or “Like,” which they specified with one of two button responses. In Round 2, participants viewed the same peers again and were prompted to answer whether they anticipated the peer would like or dislike them in return. Again, the two response options were, “Don’t like” or “Like.” Regardless of handedness, participants used their right hand to respond. Each peer photo was presented once per block in a randomized order. The photo of each peer remained on the screen for 3 seconds. Between each peer photo, a jittered fixation cross appeared (mean jitter length = 2 seconds).
Based on the adolescents’ responses on the task, we generated different trial types. We focused our analyses on trials in which the adolescent rated the peer as “liked” in the first round, and we created two primary conditions: perceived high relational value (Round 1 Like, Round 2 Like; i.e., “I like you, and I think you would like me back”), and perceived low relational value (Round 1 Like, Round 2 Don’t like; i.e., “I like you, but I think that you would not like me back.”). For an illustration, see Figure 1. There were also two other conditions (Round 1 Dislike, Round 2 Like; and Round 1 Dislike, Round 2 Dislike) that were not analyzed in the present study. Because the conditions were determined by the participants’ responses, some participants did not have enough trials to model at the neural level (i.e., ≥ 4 low relational value trials). For this reason, 14 participants were excluded from analyses.
Figure 1.
Task Diagram. Note: participant response was not circled during the task but is shown here for display purposes.
For our behavioral analyses, we calculated a perceived relational value score for each participant by subtracting the number of trials on which a participant perceived low relational value from the number of trials on which the participant perceived high relational value. Positive scores reflect a perception of higher relational value whereas negative scores reflect a perception of lower relational value.
fMRI data acquisition.
Scanning was performed on a 3T Siemens Trio MRI Scanner. Structural scans included a T2*weighted, matched-bandwidth, high-resolution, anatomical scan (TR = 4s; TE = 64 ms; FOV = 230; matrix = 192 × 192; slice thickness = 3 mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 1.9 s; TE = 2.3 ms; FOV = 230; matrix = 256 × 256; sagittal plane; slice thickness = 1 mm; 192 slices. In Round 2 of the task (from which we report our neural results), 185 T2*weighted echo-planar images (EPIs) were collected [slice thickness = 3 mm; 38 slices; TR = 2 s; TE = 25 ms; matrix = 92 × 92; FOV = 230 mm; voxel size = 2.5 × 2.5 × 3 mm3]. The orientation for the T2 and functional scans was oblique axial to optimize coverage area and reduce signal dropout.
Data preprocessing and analysis.
We conducted analyses using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). To correct for head motion, images were spatially realigned, and images with more than 2.5 mm of maximum slice-to-slice absolute movement were censored. There was very low motion across participants. Only 0.0039% of trials were censored. There was no correlation between task condition and censoring. However, trials that were censored were more likely to occur when the jittered fixation cross appeared. 45.83% of all trials censored due to motion occurring during the jitter. Realigned functional data were co-registered to the MPRAGE, which was then segmented into cerebrospinal fluid, gray matter, and white matter. After segmentation, a normalization transformation matrix was applied to the functional and T2 structural images. This process places each participant’s data within the standard stereotactic space set forth by the Montreal Neurological Institute. Normalized functional data were smoothed with an 8 mm Gaussian kernel, full-width-at-half maximum, to optimize signal-to-noise ratio. High-pass temporal filtering (128s) was applied to remove low-frequency drift in the time series. A restricted maximum likelihood algorithm was used to estimate serial autocorrelations with an autoregressive model order of 1.
Because we were interested in neural responses when perceiving relational value, all neural analyses were conducted using fMRI data from Round 2 of the task. Behavioral data from Round 1 were used to generate our conditions of interest, but no neural data from Round 1 are reported here. At the individual level, a fixed-effects analysis was modeled as an event-related design, with two conditions of interest, perceived high relational value and perceived low relational value, both of which were based on the trials in which participants indicated that they liked the peer in the first round. Trials in which participants disliked the peer from the first round were modeled as a separate regressor of no interest. The jittered inter-trial intervals were treated as null events that were not explicitly modeled and constituted an implicit baseline. The individual subject contrasts were submitted to random effects group-level analyses. At the group level, our primary contrast of interest was perceiving low > high relational value. Low relational value was defined by neural response in the second round of the task to faces that the participant rated “like” in the first round, but “dislike” in the second round. In contrast, high relational value was defined by the neural response in the second round of the task to faces the participant rated “like” in the first round and “like” in the second round.
Given that Study 1 was exploratory and given that prior research has found that prefrontal connectivity with the ventral striatum is often implicated in processing salient social stimuli (Jarcho et al., 2015; Somerville et al., 2011; Telzer et al., 2011), we conducted generalized psychophysiological interaction (PPI) analyses with the bilateral ventral striatum (VS) as the seed region. The automated gPPI toolbox in SPM (gPPI; McLaren et al., 2012) was used to (1) extract the deconvolved times series from the bilateral VS region of interest (ROI) for each participant to create the physiological variables; (2) convolve each trial type with the canonical HRF, creating the psychological regressor; and (3) multiply the time series from the psychological regressors with the physiological variable to create the PPI interaction. The bilateral VS was defined by combining the left and right VS in the AAL atlas in the WFU Pick Atlas (Maldjian et al., 2003; Tzourio-Mazoyer et al., 2002). A coverage check confirmed that all Study 1 participants had complete coverage of the VS without drop out.
To explore how neural activation and neural connectivity varied with peer victimization, we conducted whole-brain regression analyses in which we regressed peer victimization onto the contrast perceived low > perceived high relational value. Because girls are more rejection-sensitive and more concerned with peer evaluation than boys (Rose & Rudolph, 2006) and because younger children may be less attuned to social evaluation than older children and adolescents (Gunther Moor et al., 2010), all analyses controlled for age and biological sex. To correct for multiple comparisons, the spatial autocorrelation function (acf) option was used in AFNI’s 3dFWHMx to estimate intrinsic smoothness. The group-level residuals were entered into 3dFWHMx along with the brain mask. Because real fMRI data does not have a true Gaussian-shaped ACF, 3dFWHMx estimates smoothness by fitting the ACF to a mixed model that is Gaussian plus mono-exponential. 3dClustSim was used to estimate the probability of false positives using 2-sided thresholding and the NN1 option using the corrected approach recommended by Eklund et al. (2016). Cluster size corrections for multiple comparisons at α < 0.05 over the whole-brain were achieved at a voxel-wise threshold of p < 0.001. Results of the simulation yielded a voxel-wise threshold of p < .001 combined with a minimum cluster size of 50 voxels for the main effect, 49 voxels for the regression of peer victimization, and 37 voxels for the regression of peer victimization on VS functional connectivity, corresponding to p < .05, family-wise error corrected. For each of these analyses, respectively, the group level acf values were: 0.474359, 5.98076, 33.2616; 0.491425, 5.86802, 30.0572; 0.362809, 5.20718, 16.4077.
Results
Peer victimization and perceived relational value.
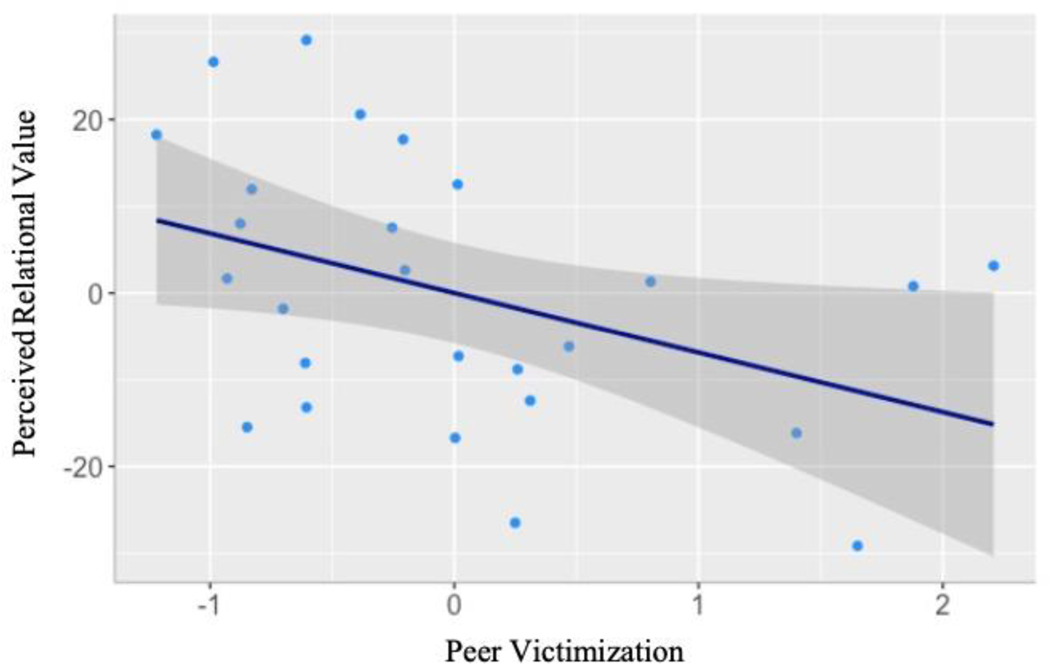
Perceived relational value was calculated as the number of trials on which the participant perceived that a peer whom they liked would not like them in return subtracted from the number of trials on which the participant perceived that a peer that they liked would like them in return (see Table 1 for descriptives). First, we examined the association between concurrent peer victimization and perceived relational value at the behavioral level. We regressed perceived relational value onto peer victimization, controlling for age and sex. Peer victimization was negatively associated with perceived relational value (B= −13.05, SE= 6.02, β= −.38, p= .04), such that as peer victimization increased, perceived relational value decreased. In other words, adolescents experiencing more concurrent peer victimization were more likely to predict that peers that they liked would not like them in return (see Figure 2).
Table 1.
Descriptive statistics of concurrent peer victimization and perceived relational value in Study 1 and Study 2
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| Study 1 | |||
| Perceived Relational Value | 12.70 | 12.41 | −15 – 39 |
| Peer Victimization | 1.45 | 0.36 | 1 – 2.42 |
| Study 2 | |||
| Perceived Relational Value | 17.15 | 16.15 | −17 – 49 |
| Peer Victimization | 1.92 | 0.95 | 1 – 4.25 |
Figure 2.
Partial regression plot depicting the association between peer victimization and perceived relational value rated behaviorally in Study 1, controlling for age and sex. Navy line is the line of best fit. Grey boundary represents the 95% confidence interval.
Neural activation when perceiving relational value.
Main effects.
We conducted a whole brain analysis to examine neural activation when perceiving low > high relational value controlling for age and sex. For this contrast, there were two clusters of significant activation in the precentral gyrus and the cerebellum (see Table 2). There were no clusters of significant activation when perceiving high > low relational value. Next, we conducted a whole brain analysis to examine functional connectivity with the VS when perceiving low > high relational value. No suprathreshold clusters were identified for perceived low > high relational value or vice versa.
Table 2.
Main effect of neural activation for the perceived low > high relational value contrast, controlling for age and sex, in Study 1
| Region | x | y | z | k | t |
|---|---|---|---|---|---|
| Precentral Gyrus | 48 | −19 | 58 | 138 | 6.00 |
| Left Cerebellum | −18 | −49 | −26 | 84 | 5.50 |
Note. L and R refer to left and right hemispheres; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; x, y, and z refer to the MNI coordinates. Corrected cluster size: 50 contiguous voxels. All results are p < .001, corrected.
Whole brain regressions with peer victimization.
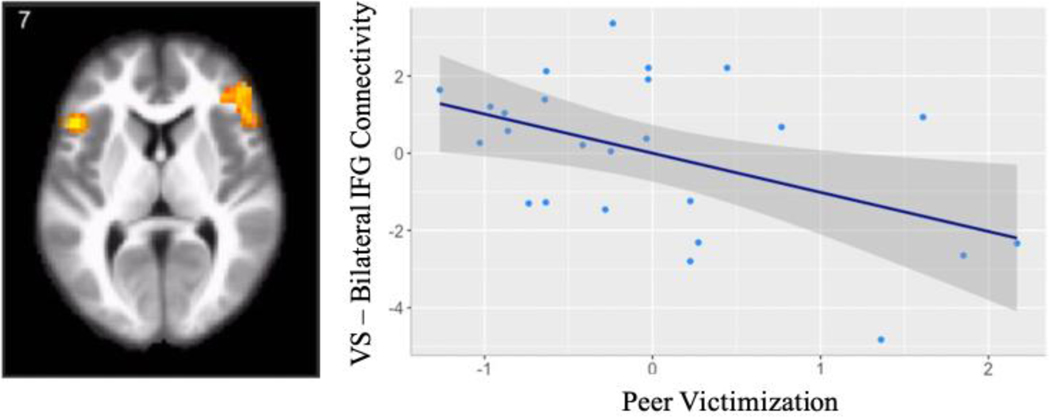
Controlling for age and sex, we regressed peer victimization onto neural activation when perceiving low > high relational value. Adolescents reporting greater peer victimization showed greater activation in the calcarine gyrus (see Table 3). No other suprathreshold activations correlated with peer victimization. Next, we regressed peer victimization onto functional connectivity with the bilateral VS seed for perceived low > high relational value, controlling for age and sex. When perceiving low relative to high relational value, youth reporting greater peer victimization showed reduced connectivity between the VS and bilateral inferior frontal gyrus (IFG), right putamen, and the medial prefrontal cortex (mPFC) as well as greater functional connectivity between the VS and the left inferior occipital gyrus (see Table 3). Given our a priori hypotheses about frontostriatal functional connectivity, for descriptive purposes, we extracted parameter estimates of VS-IFG and VS-mPFC functional connectivity and plotted them with peer victimization (see Figure 3). As shown in the scatterplot, with increasing peer victimization, youth showed reduced frontostriatal functional connectivity when perceiving low relational value. Because our neural contrast represents low > high relational value, this finding can also be interpreted in the context of greater connectivity when perceiving high relational value, as indexed by connectivity values below 0 on the y-axis.
Table 3.
Concurrent victimization regressed onto activation and functional connectivity for the perceived low > high relational value contrast, controlling for age and sex, in Study 1
| Region | x | y | z | k | t |
|---|---|---|---|---|---|
| Activation | |||||
| Calcarine Gyrus | 18 | −76 | 19 | 95 | 4.42 |
| Connectivity with ventral striatum seed region | |||||
| L IFG | −42 | 35 | 7 | 95 | −5.15 |
| R IFG | 36 | 44 | −8 | 399 | −4.97 |
| R Putamen | 30 | 5 | −2 | 60 | −4.97 |
| L mPFC* | −9 | 35 | −5 | 59 | −5.23 |
| R mPFC* | 6 | 41 | −5 | 59 | −4.07 |
| L Inferior Occipital Gyrus | −27 | −91 | −2 | 44 | 4.95 |
Note. L and R refer to left and right hemispheres; IFG = inferior frontal gyrus; mPFC= medial prefrontal cortex; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster, with negative values indicating negative correlation with victimization and positive scores indicating positive correlation with victimization; x, y, and z refer to the MNI coordinates. Corrected cluster size: 49 contiguous voxels (activation) and 37 contiguous voxels (connectivity). All results are p < .001, corrected.
denotes part of the same cluster.
Figure 3.
(A) Partial regression plot demonstrating the relationship between peer victimization and VS – IFG connectivity, controlling for age and biological sex, in Study 1. (B) Partial regression plot demonstrating the relationship between peer victimization and VS – mPFC connectivity, controlling for age and sex, in Study 1. As peer victimization increased, adolescents showed reduced frontostriatal connectivity when perceiving low relative to high relational value, controlling for age and sex. For descriptive purposes, parameter estimates were extracted from the IFG and mPFC clusters, representing functional connectivity with the VS, and the association with peer victimization was plotted.
Study 1 Discussion
In Study 1, adolescents reporting greater peer victimization demonstrated a bias towards perceiving low relational value at the behavioral level. That is, they were more likely to predict that peers that they liked would not like them in return. At the neural level, we demonstrated that greater peer victimization was associated with reduced functional connectivity between the VS and the bilateral IFG, and between the VS and the bilateral mPFC when perceiving low > high relational value. Notably, functional connectivity between the VS and IFG and between the VS and mPFC have been implicated in self-regulation in the face of appetitive cues (Behan et al., 2015; Guassi Moreira & Telzer, 2018; Qu et al., 2015). In this context, our results suggest that peer victimized youth may be processing the appetitive cue of perceiving high relational value differently from non peer victimized youth.
Next, we wanted to attempt to replicate these results in a second, independent study. Given our focus on peer victimization, it is important to note that the sample in Study 1 was a community sample of youth for whom peer victimization scores were relatively low. Thus, we conducted a second study in which we sought to replicate these findings in a sample of youth with more variability in their peer victimization experiences. Because past peer victimization predicts current peer victimization (Paul & Cillessen, 2003), we recruited a group of adolescents with well-characterized histories of either high or low chronic peer victimization. We focused specifically on adolescent girls because girls tend to be more sensitive to changes in their perceived relational value. Compared to adolescent boys, adolescent girls are more interpersonally and rejection sensitive (Rudolph, 2002), are more concerned with receiving a positive evaluation from peers (Rudolph & Conley, 2005), and are generally more relationally-oriented (Cross & Madson, 1997)—all of which might underlie greater sensitivity to perceiving low relative to high relational value in girls. In Study 2, we hypothesized that greater peer victimization would correlate with reduced perceived relational value behaviorally. At the neural level, we hypothesized that greater peer victimization would correlate with reduced functional connectivity between the VS and IFG and between the VS and mPFC when perceiving low relative to high relational value.
Study 2
Methods
44 adolescent girls participated in Study 2, all of whom were recruited from a larger longitudinal study that followed youth from the 2nd - 9th grades. (For more details on the longitudinal study, see Rudolph et al., 2014). During recruitment, exclusion criteria were the same as in Study 1. 6 adolescent girls were excluded from the present analyses due to noncompliance on the fMRI task (e.g., not responding, pressing the incorrect buttons), leaving 38 adolescent girls in the sample. Adolescent girls who had previously reported chronic victimization (N= 21) as well as adolescent girls who reported little or no victimization (N= 17) comprised this group. 12 participants were excluded because they did not have enough (≥ 4 trials) low relational value trials to model at the neural level, resulting in 26 participants (Mage= 15.43, SD= 0.32) in the final sample. This included 17 victimized and 9 non-victimized adolescents. There was no difference in age (t(36)= −.07, p= 0.94), race (X2(3)=1.05, p= .79), or proportion of victims (X2(1)= 2.24, p=.13) between the broader sample of 38 and the final sample of 26. Participants were 65% White, 27% Black, 4% Latina, and 4% Asian. The same informed consent/assent procedures and exclusion criteria as Study 1 were applied. No participants took psychiatric medication on the day of the scan. 15.38% of Study 2 participants were left-handed.
Questionnaire measures.
Concurrent peer victimization was assessed using the same measure as Study 1 (SEQ-R; Rudolph et al., 2014). Participants completed this measure (α= 0.93) at the time of the scan. Adolescents in Study 2 had significantly higher victimization scores than Study 1 participants (t(57)= 2.61, p= .01). Study 2 participants also had a broader range of victimization than Study 1 participants (see Table 1).
Relational value task.
Participants completed a two-round social evaluation task that was almost identical to the task employed in Study 1. The only differences between this task and the one used in Study 1 were that participants viewed 60 photos (half male) rather than 72, and participants had four response options during the task: “Dislike a lot,” “Dislike a little,” “Like a little,” and “Like a lot.” “Dislike a lot” and “Dislike a little” were binned into a “Don’t like” category, while “Like a little” and “Like a lot” were binned into a “Like” category in order to create low and high relational value scores for the behavioral analyses and neural contrasts.
fMRI data acquisition and analysis.
All fMRI data acquisition, preprocessing, and first level models were conducted in the same way as Study 1. We conducted psychophysiological interaction (PPI) analyses with the automated gPPI toolbox in SPM (gPPI; McLaren et al., 2012), using the bilateral VS as the seed region. The striatum ROI was the same as that used in Study 1. All participants had complete coverage of the VS, except one participant with minor dropout but the majority of the ROI covered. 0.0017% of trials were censored. There was no correlation between task condition and censoring, but censored trials were more likely to occur during the jitter. (71% occurred during the jitter).
Given our specific a priori hypothesis based on results from Study 1 that greater peer victimization would be associated with reduced functional connectivity between the VS and IFG and between the VS and mPFC when perceiving low relational value, we focused our PPI analyses on the IFG and mPFC. We conducted a small volume correction to examine functional connectivity between the bilateral VS and the mPFC and between the bilateral VS and IFG. We performed Monte Carlo simulations using 3dClustSim in the AFNI software package (Ward, 2000); updated April 2016). An anatomical mask of the IFG and the mPFC were used for small volume correction. The bilateral IFG mask was defined anatomically by combining the bilateral pars orbitalis and bilateral pars triangularis regions in the AAL atlas in the WFU Pick Atlas. The bilateral mPFC mask was defined as the mPFC ROI used in Mills et al. (2014)—a region of the medial frontal cortex anterior to the cingulate cortex, extending medially through BA11. Results of the simulation for the regression with peer victimization on functional connectivity between the VS yielded a voxel-wise threshold of p < .05 combined with a minimum cluster size of 33 voxels for the IFG, and 24 voxels for the mPFC, corresponding to p < .05, family-wise error corrected. For each of these analyses, respectively, the acf values were: 0.428608, 4.72261, 4.13971; 0.993997, 6.13838, 3.00155.
Results
Peer victimization and perceived relational value.
We calculated a perceived relational value score for each participant in the same way as Study 1 (see Table 1 for descriptives). Replicating Study 1, concurrent peer victimization was negatively associated with perceived relational value when controlling for age (B= −6.87, SE= 3.16, β= −.40, p= .04; see Figure 4).
Figure 4.
Partial regression plot of the relationship between peer victimization and perceived relational value rated behaviorally in Study 2, controlling for age. Navy line is the line of best fit. Grey boundary represents the 95% confidence interval.
Neural connectivity when perceiving relational value.
Next, given our a priori hypotheses based on the results from Study 1, we examined how functional connectivity between the bilateral VS and the bilateral IFG and mPFC varied with peer victimization, controlling for age. Similar to Study 1, adolescents experiencing more peer victimization showed reduced functional connectivity between the VS and the bilateral IFG when perceiving low relative to high relational value (see Table 4 and Figure 5). One participant had a connectivity value that was an outlier. This participant was excluded, but this exclusion did not influence the results. Notably, the clusters of activation in this independent sample are localized to the same regions and nearly identical coordinates as the IFG clusters identified in Study 1. However, diverging from the result found in Study 1, there was no correlation between peer victimization and VS-mPFC connectivity.
Table 4.
Concurrent peer victimization regressed on functional connectivity for the perceived low > high relational value contrast, controlling for age, in Study 2
| Region | x | y | z | k | t |
|---|---|---|---|---|---|
| L IFG | −51 | 20 | 4 | 39 | −2.78 |
| R IFG | 48 | 29 | 10 | 86 | −2.55 |
Note. L and R refer to left and right hemispheres; IFG = inferior frontal gyrus; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; x, y, and z refer to the MNI coordinates. Corrected cluster size: 33 contiguous voxels for the IFG, 24 for the mPFC.
Figure 5.
Partial regression plot demonstrating the relationship between peer victimization and VS – IFG connectivity, controlling for age, in Study 2. As concurrent peer victimization increased, Study 2 participants showed reduced functional connectivity between the ventral striatum and the bilateral inferior frontal gyrus (IFG) when perceiving low relative to high relational value, controlling for age. For descriptive purposes, parameter estimates were extracted from the IFG clusters, representing functional connectivity with the VS, and the association with peer victimization was plotted.
Discussion
Perceived relational value describes the extent to which individuals consider themselves to be liked and valued by important others (Leary, 2005). In the two present studies, we explored how peer victimization is associated with adolescents’ perceptions of their relational value at both the behavioral and neural level. Behaviorally, across both studies, we found that greater concurrent peer victimization was associated with a bias toward perceiving low relational value. Although victimization scores were low in Study 1, the association between higher concurrent peer victimization and lower perceived relational value held in an independent sample of girls reporting higher victimization, suggesting that peer victimization—even if it is not severe or experienced over a long period—can shape an adolescent’s perception of his or her relational value. This is important given that experiencing some level of peer victimization is very common, with over 49% of youth in elementary, middle, and high schools reporting being bullied at school at least once in the past month (Bradshaw et al., 2007). Given that we found robust associations even among a sample with lower overall levels of peer victimization, it is possible that many youths may experience changes in their perceived relational value as a result of peer victimization. Further, this occurs at a time when youth are rapidly integrating information provided by their peers into their self-concepts (Pfeifer et al., 2009), and when youth have not yet fully developed a self-protective bias to buffer against negative peer evaluations (Rodman et al., 2017).
At the neural level, we examined how neural activation and functional connectivity varied with peer victimization as youth perceived their relational value to unknown peers. In Study 1, we found that, as peer victimization increased, youth showed reduced functional connectivity between the VS and IFG and between the VS and mPFC when perceiving low relative to high relational value. In Study 2, we similarly found that, as peer victimization increased, there was reduced VS-IFG functional connectivity when perceiving low relative to high relational value. Our neural contrast of low > high relational value can also be interpreted as greater connectivity when perceiving high relative to low relational value, as indexed by connectivity values below 0 on the y-axis. In line with our finding in Study 1—that greater victimization correlated with greater connectivity between the VS and mPFC when perceiving high relative to low relational value—previous work shows that adolescents show heightened co-activation and functional connectivity between the VS and mPFC when anticipating positive peer feedback (Gunther Moor et al., 2010; Guyer et al., 2009; Jarcho et al., 2015), and this signal is stronger in adolescents than in adults (Gunther Moor et al., 2010). We did not find this result in Study 2, but it is possible that we were underpowered to detect this connectivity in Study 2’s smaller sample size. With respect to our finding in both studies—that greater peer victimization is associated with greater functional connectivity between the VS and IFG when perceiving high relative to low relational value—functional connections between the VS and IFG are often implicated in regulatory control in the face of appetitive cues. Indeed, adolescents show increased connectivity between the striatum and IFG when anticipating high relative to low stakes (Insel et al., 2017). Greater positive functional connectivity is generally considered indicative of a failure of the IFG to downregulate the striatum when a cue is particularly appetitive or salient (Behan et al., 2015; Jollans et al., 2016). It is possible that, for victimized youth, perceiving high relational value, a particularly high social stake, is so salient or novel that the IFG cannot constrain VS activation. In this context, our results could suggest that there may be something particularly appetitive about perceiving high relational value for a victimized youth. Notably, many victimized youth seek out social interactions, despite victimization (Adler & Adler, 1995). Perhaps, for victimized youth, the heightened reactivity of the VS, and the failure of the IFG to constrain it, helps propel continued interest in social interaction despite a behavioral bias that peers will not like them.
Contributions, Limitations, and Future Directions
Although a few neuroimaging studies have examined the neural processes that underlie how adolescents anticipate and predict social feedback (Gunther Moor et al., 2010; Guyer et al., 2008, 2009; Jarcho et al., 2015), this study examines how concurrent peer victimization relates to adolescents’ neural encoding of relational value. A major strength of this study was our ability to ask this question in two independent samples: a mixed-sex sample of healthy youth with relatively low peer victimization scores, and in a sample of adolescent girls experiencing a broader range and greater severity of concurrent peer victimization. Our study was strengthened by replicating the behavioral results and largely replicating the neural results in both samples.
Further, our task was deliberately naturalistic in that participants were never explicitly provided feedback about what the other peers thought of them in return. This was done in order to tap into perceptions of relational value. According to Sociometer Theory, negative or positive social interactions cause individuals to feel temporarily better or worse about themselves. This change in emotional state provides individuals with key information about their relational value to others. While change in emotional state decreases in response to a direct, negative social interaction, an individual’s perception of his or her relational value develops over repeated social interactions and manifests before a social interaction even occurs (Leary, 2005). By asking youth to predict if a same-aged peer that they liked would like them in return, we were able to evaluate how adolescents think peers will feel about them in the absence of direct social feedback. This is important because, in the real world, adolescents are rarely provided with direct feedback about who likes or dislikes them. Instead, they often must predict how others will feel about them based on past experiences, implicit cues, or other non-explicit forms of feedback, as they did in our task. Thus, our study tests neural processes underlying adolescents’ perceptions of their relational value, distinguishing this work from studies that have pinpointed the neural systems that support adolescents’ anticipation of direct social feedback (Gunther Moor et al., 2010; Guyer et al., 2008, 2009).
However, this study is not without limitations. While our task design could be considered a strength, it has some weaknesses. Recent work has found that youth have less of a self-protective bias in the face of social rejection than adults (Rodman et al., 2017). Thus, we did not anticipate that some youth would not predict low relational value on enough trials to model their behavior. Interestingly, in our samples it was somewhat normative for youth to predict that they would be liked back by those that that they liked (e.g., perceive high relational value). This meant that several participants in both Study 1 and Study 2 did not have enough trials to analyze in our neuroimaging analyses, which led to a smaller overall sample size in both sets of neuroimaging analyses—another limitation of the study. This also means that our samples were restricted to youth with overall lower perceptions of their relational value. For this reason, our findings do not generalize to youth with very high perceptions of their relational value, as they were excluded from the sample. Another key limitation is that this work is correlational, limiting the inferences that we can make. However, because perceived relational value has to do specifically with how individuals view their own social value to other high value peers, it develops over time and is challenging to experimentally manipulate. Finally, while the racial and ethnic makeup of the Study 1 and Study 2 samples generally matched the diversity of the community in which the research took place, the participants in both studies were predominantly White. Future studies should ensure a more diverse sample.
Conclusions
Our work may have some implications for intervention. Lonely individuals and peer victimized youth are actually more attuned to social cues than individuals who are socially included (W. L. Gardner et al., 2005; Telzer et al., 2019). Yet, given that peer victimization is generally stable over time (Hodges & Perry, 1999), peer victimized youth may have difficulty translating their greater knowledge of social information into social connection. Our results suggest that peer victimized youth possess a bias that others will not like them in return, and that this could be part of what leads victimization to be so stable. Alternatively, peer victimized youth often do seek out opportunities for re-inclusion, but they may not have the social skills to connect appropriately, leading them to maintain their victim status. In this way, our results might suggest that social skills training could be helpful to peer victimized youth, by helping them put their greater knowledge of social information to use in effective relationship-building. Moreover, our neural results suggest that victimized youth may find high relational value particularly salient, given that when they do perceive high relational value they show a pattern of relatively greater VS-IFG functional connectivity previously implicated in difficulty regulating in the face of a salient appetitive cue (Behan et al., 2015; Weafer et al., 2019). Notably, many interventions for victimized youth focus on how to respond to negative peer behavior, but this result could suggest the value of structuring interventions to help peer victimized youth optimize positive peer signals—of which they may already be hyper-aware.
Footnotes
This research was supported by a University of Illinois Research Board Award to K.D.R and E.H.T., a National Institute of Mental Health Grant (MH68444) awarded to K.D.R., a National Institute of Mental Health Grant (MH105655) awarded to K.D.R. and E.H.T., a National Science Foundation grant (1459719) to E.H.T., and a National Institutes of Health grant (R01DA039923) to E.H.T.
References
- Adler PA, & Adler P. (1995). Dynamics of inclusion and exclusion in preadolescent cliques. Social Psychology Quarterly, 58(3), 145–162. [Google Scholar]
- Arseneault L, Bowes L, & Shakoor S. (2010). Bullying victimization in youths and mental health problems: Much ado about nothing? Psychological Medicine, 40(5), 717–729. 10.1017/S0033291709991383 [DOI] [PubMed] [Google Scholar]
- Behan B, Stone A, & Garavan H. (2015). Right prefrontal and ventral striatum interactions underlying impulsive choice and impulsive responding. Human Brain Mapping, 36(1), 187–198. 10.1002/hbm.22621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmore AD, & Cillessen AHN (2006). Reciprocal influences of victimization, perceived social preference, and self-concept in adolescence. Self and Identity, 5(3), 209–229. 10.1080/15298860600636647 [DOI] [Google Scholar]
- Bradshaw CP, Sawyer AL, & O’Brennan LM (2007). Bullying and peer victimization at school: Perceptual differences between students and school staff. School Psychology Review, 36(3), 361–382. [Google Scholar]
- Casey BJ, Galván A, & Somerville LH (2016). Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Developmental Cognitive Neuroscience, 17, 128–130. 10.1016/j.dcn.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SE, & Madson L. (1997). Models of the self: Self-construals and gender. Psychological Bulletin, 122(1), 5–37. 10.1037/0033-2909.122.1.5 [DOI] [PubMed] [Google Scholar]
- Egger H, Pine DS, Nelson E, Leibenluft E, Ernst M, Towbin KE, & Angold A. (2011). The NIMH Child Emotional Faces Picture Set (NIMH‐ChEFS): A new set of children’s facial emotion stimuli. Int J Methods Psychiatr Res, 20(3), 145–156. 10.1002/mpr.343.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences, 113(28), 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience, 4(6), 1–9. 10.3389/neuro.09.006.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. (2013). The Teenage Brain: Sensitivity to Rewards. Current Directions in Psychological Science, 22(2), 88–93. 10.1177/0963721413480859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M, & Steinberg L. (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Developmental Psychology, 41(4), 625–635. 10.1037/0012-1649.41.4.625 [DOI] [PubMed] [Google Scholar]
- Gardner WL, Pickett CL, & Brewer MB (2000). Social exclusion and selective memory: How the need to belong influences memory for social events. Personality and Social Psychology Bulletin, 26(4), 486–496. [Google Scholar]
- Gardner WL, Pickett CL, Jefferis V, & Knowles M. (2005). On the outside looking in: Loneliness and social monitoring. Personality and Social Psychology Bulletin, 31(11), 1549–1560. 10.1177/0146167205277208 [DOI] [PubMed] [Google Scholar]
- Graham S, & Juvonen J. (1998). Self-blame and peer victimization in middle school: an attributional analysis. Developmental Psychology, 34(3), 587–599. 10.1037/0012-1649.34.3.587 [DOI] [PubMed] [Google Scholar]
- Moreira Guassi, Joao F, Bavel J. J. Van, & Telzer EH (2017). The Neural Development of ‘Us and Them.’ Social Cognitive and Affective Neuroscience, September 2016, 184–196. 10.1093/scan/nsw134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira Guassi, João F, & Telzer EH (2018). Mother still knows best: Maternal influence uniquely modulates adolescent reward sensitivity during risk taking. Developmental Science, 21(1). 10.1111/desc.12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor B, van Leijenhorst L, Rombouts SA, Crone EA, & Van der Molen MW (2010). Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience, 5(6), 461–482. 10.1080/17470910903526155 [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJR, Leibenluft E, Fox NA, Ernst M, Pine DS, & Nelson EE (2008). Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry, 65(11), 1303–1312. 10.1001/archpsyc.65.11.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, & Nelson EE (2009). Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development, 80(4), 1000–1015. 10.1111/j.1467-8624.2009.01313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges EVE, & Perry DG (1999). Personal and interpersonal antecedents and consequences of victimization by peers. Journal of Personality and Social Psychology, 76(4), 677–685. 10.1037/0022-3514.76.4.677 [DOI] [PubMed] [Google Scholar]
- Insel C, Kastman EK, Glenn CR, & Somerville LH (2017). Development of corticostriatal connectivity constrains goal-directed behavior during adolescence. Nature Communications, 8(1). 10.1038/s41467-017-01369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski KF, Moore WE, Merchant JS, Kahn LE, & Pfeifer JH (2014). But do you think I’m cool?: Developmental differences in striatal recruitment during direct and reflected social self-evaluations. Developmental Cognitive Neuroscience, 8, 40–54. 10.1016/j.dcn.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Romer AL, Shechner T, Galvan A, Guyer AE, Leibenluft E, Pine DS, & Nelson EE (2015). Forgetting the best when predicting the worst: Preliminary observations on neural circuit function in adolescent social anxiety. Developmental Cognitive Neuroscience, 13, 21–31. 10.1016/j.dcn.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollans L, Zhipeng C, Icke I, Greene C, Kelly C, Banaschewski T, & Whelan R. (2016). Ventral Striatum Connectivity During Reward Anticipation in Adolescent Smokers. Developmental Neuropsychology, 41(1–2), 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ, Troiani V, Price E, Faja S, Herrington JD, & Schultz RT (2013). The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia, 51(11), 2062–2069. 10.1016/j.neuropsychologia.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary MR (1999). Making Sense of Self-Esteem. Current Directions in Psychollogical Science, 8(1), 32–35. [Google Scholar]
- Leary MR (2005). Sociometer theory and the pursuit of relational value: Getting to the root of self-esteem. European Review of Social Psychology, 16(1), 75–111. 10.1080/10463280540000007 [DOI] [Google Scholar]
- Leary MR, & Baumeister RF (2000). The nature and function of self-esteem: Sociometer theory. Advances in Experimental Social Psychology, 32, 1–62. 10.1016/s0065-2601(00)80003-9 [DOI] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Lalonde F, Clasen LS, Giedd JN, & Blakemore SJ (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience, 9(1), 123–131. 10.1093/scan/nss113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek G, Zeevalkink H, Vermulst A, & Scholte RHJ (2010). Peer victimization, self-esteem, and ego resilience types in adolescents: A prospective analysis of person-context interactions. Social Development, 19(2), 270–284. 10.1111/j.1467-9507.2008.00535.x [DOI] [Google Scholar]
- Paul JJ, & Cillessen AHN (2003). Perception and attitudes toward bullying in middle school youth: A developmental examination across the bully/victim. Journal of Applied School Psychology, 192(2), 25–43. 10.1300/J008v19n02 [DOI] [Google Scholar]
- Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, & Lieberman MD (2009). Neural correlates of direct and reflected self-appraisals in adolescents and adults: When social perspective-taking informs self-perception. Child Development, 80(4), 1016–1038. 10.1111/j.1467-8624.2009.01314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KE, Somerville LH, Kelley WM, & Heatherton TF (2013). Rejection sensitivity polarizes striatal-medial prefrontal activity when anticipating social feedback. Journal of Cognitive Neuroscience, 25(11), 1887–1895. 10.1162/jocn_a_00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Galvan A, Fuligni AJ, Lieberman MD, & Telzer EH (2015). Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience, 35(32), 11308–11314. 10.1523/JNEUROSCI.1553-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman AM, Powers KE, & Somerville LH (2017). Development of self-protective biases in response to social evaluative feedback. Proceedings of the National Academy of Sciences of the United States of America, 114(50), 13158–13163. 10.1073/pnas.1712398114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, & Rudolph KD (2006). A review of sex differences in peer relationship processes: Potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin, 132(1), 98–131. 10.1037/0033-2909.132.1.98.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD (2002). Gender differences in emotional responses to interpersonal stress during adolescence. Journal of Adolescent Health, 30(4 SUPPL. 1), 3–13. 10.1016/S1054-139X(01)00383-4 [DOI] [PubMed] [Google Scholar]
- Rudolph KD, & Conley CS (2005). The socioemotionol costs and benefits of social-evaluative concerns: Do girls care too much? Journal of Personality, 73(1), 115–138. 10.1111/j.1467-6494.2004.00306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Lansford JE, Agoston AM, Sugimura N, Schwartz D, Dodge KA, Pettit GS, & Bates JE (2014). Peer victimization and social alienation: Predicting deviant peer affiliation in middle school. Child Development, 85(1), 124–139. 10.1111/cdev.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Viding E, Williams KD, & Blakemore SJ (2010). Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition, 72(1), 134–145. 10.1016/j.bandc.2009.06.008 [DOI] [PubMed] [Google Scholar]
- Somerville LH (2013). The Teenage Brain: Sensitivity to Social Evaluation. Current Directions in Psychological Science, 22(2), 121–127. 10.1177/0963721413476512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, & Casey BJ (2010). Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology, 20(2), 236–241. 10.1016/j.conb.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, & Casey BJ (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–2134. 10.1162/jocn.2010.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, & Casey BJ (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science, 24(8), 1554–1562. 10.1177/0956797613475633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, D’Angelo CM, Brush B, & Lloyd-Richardson EE (2017). Sex differences in biological response to peer rejection and performance challenge across development: A pilot study. Physiology and Behavior, 169, 224–233. 10.1016/j.physbeh.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Taylor KA, Sullivan TN, & Kliewer W. (2013). A longitudinal path analysis of peer victimization, threat appraisals to the self, and aggression, anxiety, and depression among urban African American adolescents. Journal of Youth and Adolescence, 42(2), 178–189. 10.1007/s10964-012-9821-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fowler CH, Davis MM, & Rudolph KD (2019). Hungry for inclusion: Exposure to peer victimization and heightened social monitoring in adolescent girls. Development and Psychopathology, 1–14. 10.1017/s0954579419001433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Masten CL, Berkman ET, Lieberman MD, & Fuligni AJ (2011). Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. NeuroImage, 58(1), 242–249. 10.1016/j.neuroimage.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, & Joliot M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Ward BD (2000). Simultaneous inference for fMRI data (pp. 1–16). [Google Scholar]
- Weafer J, Crane NA, Gorka SM, Phan KL, & de Wit H. (2019). Neural correlates of inhibition and reward are negatively associated. NeuroImage, 196(February), 188–194. 10.1016/j.neuroimage.2019.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberg PM, Drewes MJ, Goedhart AW, Siebelink BM, & Treffers PDA (2004). A developmental analysis of self-reported fears in late childhood through mid-adolescence: Social-evaluative fears on the rise? Journal of Child Psychology and Psychiatry and Allied Disciplines, 45(3), 481–495. 10.1111/j.1469-7610.2004.00239.x [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-skurski ME, Chappelow JC, & Berns GS (2004). Human striatal responses to monetary reward depend on saliency. Neuron, 42, 509–517. [DOI] [PubMed] [Google Scholar]