Learning points for clinicians
The vaccination of ChAdOx1 nCoV-19 (AstraZeneca) may trigger the expression of antiplatelet antibodies, further resulting in thrombocytopenia and thrombotic events. We present a case of COVID-19 vaccine-associated immune thrombosis and thrombocytopenia. She experienced coexisting cerebral venous sinus thrombosis and pulmonary artery thromboembolism after receiving the first dose of ChAdOx1 nCoV-19 vaccine. The early diagnosis of these associated complications and optimal management can improve clinical outcomes.
Introduction
Coronavirus disease 2019 (COVID-19) is still an ongoing pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, the administration of vaccines against SARS-CoV-2 has resulted in reports of adverse events after vaccination in worldwide. Several cases of thrombotic events and thrombocytopenia developed after receiving the first dose of ChAdOx1 nCoV-19 (AstraZeneca) vaccine.1,2 Including cerebral venous sinus thrombosis, pulmonary embolus, and deep vein thrombosis have been reported recently.1–3 The reaction is mediated by platelet-activating antibodies against platelet factor 4 (PF4), which clinically mimics autoimmune heparin-induced thrombocytopenia.
Case report
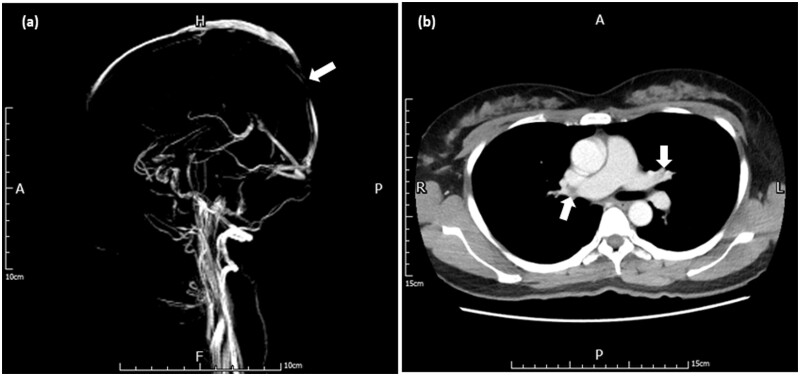
A 41-year-old healthy woman was admitted to our hospital due to fever and headache for 1 week after the first vaccination with ChAdOx1 nCoV-19. In addition, she complained of pain and swelling erythema on bilateral palms, which was considered the erythromelalgia related to COVID-19 vaccination. She has no medical history of systemic diseases, autoimmune disorders, or current medication. The polymerase chain reaction test for SARS-CoV-2 was negative. Laboratory investigations showed thrombocytopenia (platelets 36 [normal 150–400] × 109/l), D-Dimer > 10 000 (normal ≤ 500) ng/ml, fibrinogen 213 (normal 200–400) mg/dl, international normalized ratio 1.08 (normal 0.9–1.1) and positive anti-PF4 antibodies 113.17 (normal ≤ 40) ng/ml. The brain magnetic resonance imaging showed focal T1 intermediate-signal-intensity and T2 hyper-signal-intensity lesion within the posterior superior sagittal sinus, corresponding to the filling defect on the magnetic resonance venography (MRV) images (Figure 1a). The diagnosis of cerebral venous sinus thrombosis was then made based on these findings. Chest computed tomography (CT) showed evident pulmonary emboli at the left pulmonary artery and segmental branches (Figure 1b), which confirmed the diagnosis of pulmonary artery embolism. Medical treatments including intravenous immunoglobulin (1 g per kg for 2 days), direct oral anticoagulants and steroid were administered.4 We regularly monitored her clinical condition and followed up the laboratory investigations during hospitalization.
Figure 1.
(a) Brain magnetic resonance venography (MRV) revealed filling defect within the posterior superior sagittal sinus. (b) Chest computed tomography (CT) showed pulmonary emboli at left pulmonary artery and segmental branches.
Discussion
Thrombocytopenia and thrombotic complications at unusual sites may develop around 1–2 weeks after the first vaccine dose of ChAdOx1 nCov-19. The incidence is still not well-known but it appears to be extremely rare.1 Clinicians should be aware that a syndrome similar to autoimmune heparin-induced thrombocytopenia may occur in very few persons after exposure to the ChAdOx1 nCoV-19 vaccines. However, these vaccinated patients did not receive any heparin to explain the subsequent occurrence of thrombosis and thrombocytopenia. In all cases reported to date, this syndrome of thrombocytopenia and venous thrombosis appears to be triggered by receipt of the first dose of the vaccine.1–3 The pathomechanism is presumably the formation of antibodies against PF4, causing platelet consumption with thrombocytopenia and thrombus formation.3–5 Whether these anti-PF4 autoantibodies induced by the strong inflammatory stimulus of vaccination or caused by the vaccine that cross-react with PF4 and platelets requires further investigation.1–3 Administration of high-dose intravenous immunoglobulin (1 g/kg daily for 2 days) or dexamethasone (40 mg days for 4 days) may be useful to interrupt the prothrombotic mechanism.4 Use of direct oral anticoagulants is also suggested. In these patients, platelet transfusions should not be transfused in the absence of bleeding. Anticoagulation with unfractionated heparin or low molecular weight heparin should be avoided.3–5
In conclusion, COVID-19 vaccine-associated immune thrombosis and thrombocytopenia is a phenomenon with devastating effects for otherwise healthy young adults and requires a thorough risk–benefit analysis. Clinicians should be aware of these associated complications, the early recognition and well-timed treatment can improve clinical outcomes.
Conflict of interest. The authors declare that they have no conflict of interest.
References
- 1. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021; 384:2092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384:2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384:2124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franchini M, Liumbruno GM, Pezzo M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): diagnostic and therapeutic recommendations for a new syndrome. Eur J Haematol 2021; 107:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after "COVID-19 vaccine AstraZeneca" exposure. J Clin Med 2021; 10:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]