Abstract
Background
Most pediatric coronavirus disease 2019 (COVID-19) is mild. We assessed nationally severe COVID-19, including pediatric inflammatory multisystem syndrome (PIMS), in hospitalized children.
Methods
An ongoing, prospective, national surveillance was conducted from March 2020 through March 2021, at 20 hospitals treating children <18 years across Israel (~75% of Israeli hospitals).
Results
Overall, 1007 cases (439 outpatients and 568 hospitalized) identified represent 0.35% of pediatric COVID-19 nationwide (n = 291 628). Of hospitalized cases, 464 (82%), 48 (8%), and 56 (10%) had mild, moderate/severe, and PIMS disease, respectively. The mean ± SD age was 5.6 ± 6.4 years. In mild, moderate/severe, and PIMS disease, 55%, 23%, and 4% of patients were <1 year old, respectively. Obesity was reported in 1%, 4%, and 13% of patients, respectively (P < .001). The most common symptom was fever in 67%, 60%, and 100%, respectively, whereas respiratory symptoms were documented in 33%, 41%, and 38% of patients, respectively. Lymphopenia was recorded in 25%, 60%, and 86% of cases, respectively. PIMS diagnosis was mainly serology-based (in 59%). Gastrointestinal symptoms, cardiovascular involvement, rash, and conjunctivitis were noted in 82%, 61%, 57%, and 34% of PIMS episodes, respectively. Elevated C-reactive protein (100%), ferritin, troponin, D-dimer, low albumin, and thrombocytopenia were common in PIMS. Echocardiography revealed pathological findings in 33% of patients. PIMS mainstay treatment included corticosteroids (77%) and intravenous immunoglobulin (53%). No mortality was recorded.
Conclusions
At a national level, pediatric COVID-19 is mild, even in hospitalized cases, with only a third presenting with respiratory involvement. PIMS is rare, but necessitates a high index of suspicion, and with suitable treatment prognosis is favorable.
Keywords: children, coronavirus disease 2019 (COVID-19), multisystem inflammatory syndrome in children (MIS-C), pediatric inflammatory multisystem syndrome (PIMS), SARS-CoV-2
We assessed pediatric COVID-19 in 20/26 Israeli hospitals. Overall, 568 cases (82% mild disease, 8% moderate/severe disease, and 10% PIMS), representing <1% of pediatric SARS-CoV-2 infections nationwide, were identified. Lower respiratory tract infection symptoms were documented in 13% of patients.
In February 2020, the World Health Organization (WHO) designated a new strain of betacoronavirus as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19) [1–4]. SARS-CoV-2 soon spread across the globe, with ~130 million people infected and more than 2 800 000 deaths, as of the end of March 2021 [1].
Epidemiological studies showed that childhood morbidity rates are low compared to their relative proportion in the population [2–7]. Children mainly acquire SARS-CoV-2 infection from their family members [2, 3].
Most pediatric patients have asymptomatic, mild, or moderate disease [5]. Moreover, the literature to date indicates that childhood morbidity tends to be milder compared to adults [2, 3, 8, 9]. COVID-19 can present with gastrointestinal symptoms only [5], and in young infants, it can manifest as nonspecific symptoms, such as fever and decreased appetite [10, 11].
Despite their relative rarity, severe cases, need of pediatric intensive care unit (PICU) admission, and mortality have also been reported in children [4, 12]. Since April 2020, a few studies have described a new febrile pediatric entity involving systemic hyper-inflammation, multi-organ involvement, abdominal pain, sometimes features reminiscent of Kawasaki-like disease, and cardiogenic shock with severe myocardial dysfunction, likely to be caused by a viral infection directly or as a postinfectious phenomenon, designated as pediatric inflammatory multisystem syndrome (PIMS) or multisystem inflammatory syndrome in children (MIS-C) [13–17].
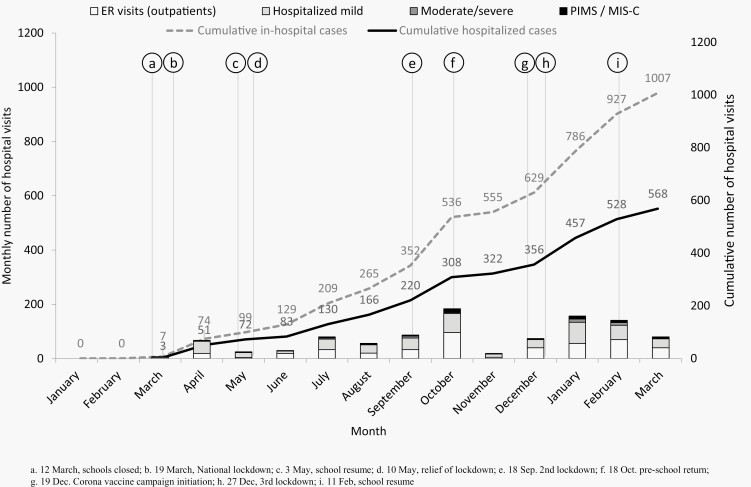
In February 2020, the first COVID-19 patient was diagnosed in Israel. A short while thereafter, in March-May 2020, Israel took drastic social distancing and quarantine (lockdown) measures, which resulted in relatively low morbidity and mortality rates [18, 19]. These measures included closing nearly all flights to Israel in early March, closing schools and universities on March 12, followed by a national lockdown with strict social distancing policy starting March 19. These measures led to a decline in SARS-CoV-2 morbidity and as a result, part of these measures were discontinued in early May. In September 18, a second lockdown was declared, using the same measures as the first lockdown. It was relieved after a month, on October 18. A third lockdown was declared in late December 2020, and it was relieved in early February 2021. Notably, on December 19, 2020, Israel started an adult coronavirus vaccine, with rapid uptake, reaching 60% vaccination rate (2 doses) in adults by late March 2021.
This work aims to describe COVID-19 characteristics in hospitalized Israeli children nationwide, thus representing the most severe pediatric COVID-19 morbidity. This information is critical to enable better appreciation of the impact of various control measures and to prepare for another possible morbidity peaks.
METHODS
This ongoing, prospective surveillance, conducted at 20 hospitals treating children across Israel, was initiated on March 11, 2020. In the current report, we present data spanning between the study initiation date and March 17, 2020.
The Soroka University Medical Center served as the data coordinating center and obtained the hospital Ethics Committee approval with a waiver of informed consent for both local and collaborative data collection. Individual institutions contributing data obtained local ethics committee approval to collect and share de-identified data.
Setting and Study Population
We assessed COVID-19 morbidity in children <18 years treated in the in-hospital setting. We approached all medical centers treating inpatient children in Israel, and 20 out of 26 centers agreed to collaborate and submit weekly demographic and clinical data reports. We compared our number of hospitalizations to the number of pediatric hospitalizations reported by the Israeli Ministry of Health (IMOH) [19], and estimate that our surveillance allowed us a capture of ~60% of all childhood COVID-19 treated at pediatric emergency rooms (ER) or hospitalized.
The geographic distribution of the 20 medical centers reporting COVID-19 pediatric cases included all 6 Israel districts, allowing us to conduct a population-based study. Furthermore, these 20 hospitals included secondary- and tertiary-care hospitals in all areas of Israel.
Currently, the Jewish population accounts for ~80% of the total population in Israel (total population of ~9 000 000 people), with ~20% of the population being Arab Muslims [20, 21]. Notably, within the Jewish population, approximately 15% identify themselves as ultra-orthodox Jews, often living in closed communities [21, 22].
Case Definitions
COVID-19 was defined by a positive polymerase chain reaction (PCR) testing for SARS-CoV-2 of samples obtained from the oral cavity and/or the nasopharynx of a child. Additionally, we included PIMS cases with a positive serologic (or PCR) diagnosis of SARS-CoV-2 infection, performed as approved by the IMOH.
We defined 3 levels of disease severity: (1) mild disease: ranging from asymptomatic infection to symptoms such as fever and/or respiratory symptoms with no dyspnea. These children were hospitalized for various reasons, including social problems, observation, and ruling out serious bacterial infection; (2) moderate or severe disease: including fever, signs of respiratory distress with mild-moderate dyspnea, and children with hemodynamic instability requiring treatment but not defined as shock, for moderate severity, and for severe disease, PICU admission, respiratory failure requiring mechanical ventilation, acute respiratory distress syndrome, shock, and/or multi-organ failure; (3) PIMS: as defined by treating physicians and fulfilling PIMS diagnostic criteria, and included fever, elevated C-reactive protein (CRP) or other inflammatory marker, involvement of at least 2 organs/systems, evidence of SARS CO-V-2 infection or exposure to a COVID-19 patient, and exclusion of other plausible diagnosis (infectious and noninfectious) [23–25].
Data Collection
Data were collected and reported by a pediatric infectious diseases specialist in every medical center participating, using standardized questionnaires. Most patients (or their caregivers) were interviewed by the local study physician (often the treating physician) and, additionally, some data were obtained from the medical records.
The study questionnaires included: age, sex, ethnicity, nasopharyngeal/oral swabs for SARS-CoV-2 PCR obtainment date; recent contact with known COVID-19 patient; background illnesses, including heart disease, chronic lung disease and/or asthma, developmental delay, diabetes, immune compromise, and malignancy; signs and symptoms, including fever, cough, rhinorrhea, respiratory distress, sore throat, weakness, diarrhea, abdominal pain, vomiting, headache, conjunctivitis, nausea, myalgia, rash, loss of smell/taste, chest pain, and irritability; disease outcome data, including hospitalization, disease severity, complications, PICU admission, and mortality; laboratory data, including complete blood count (CBC) parameters, CRP, troponin; nasal swabs for other viral pathogens (co-infection), blood, urine and cerebrospinal fluid cultures performance and results; imaging—chest radiography and computerized tomography (CT) performance and interpretation. Radiographic pneumonia diagnosis was based on the interpretation of the chest X-ray (plain films) by the treating physician.
For PIMS-reported cases, additional data included: method of viral diagnosis (serology, PCR, or both); the presence of all gastrointestinal, cardiovascular, neurologic, renal, and respiratory symptoms; blood levels of ferritin, albumin, D-dimer, and interleukin 6 (IL-6); echocardiography results; and treatment given, including corticosteroids, intravenous immunoglobulin (IV-IG), antibiotics, aspirin, low-molecular-weight heparin, plasmapheresis, remdesivir, and infliximab.
All reports included data regarding date of episode and disease severity. Unfortunately, demographic and clinical data were missing for 23% of mild hospitalized cases, and 47% of mild outpatients (data are full for hospitalized moderate/severe disease and PIMS episodes).
Statistical Analysis
Descriptive statistical univariate analyses were calculated using SPSS 24. Univariate analysis was performed using 2-tailed χ 2 test, Student t test, or Mann-Whitney U test to assess differences between mild disease, severe/moderate diseases, and PIMS episodes. The threshold of statistical significance was P < .05.
RESULTS
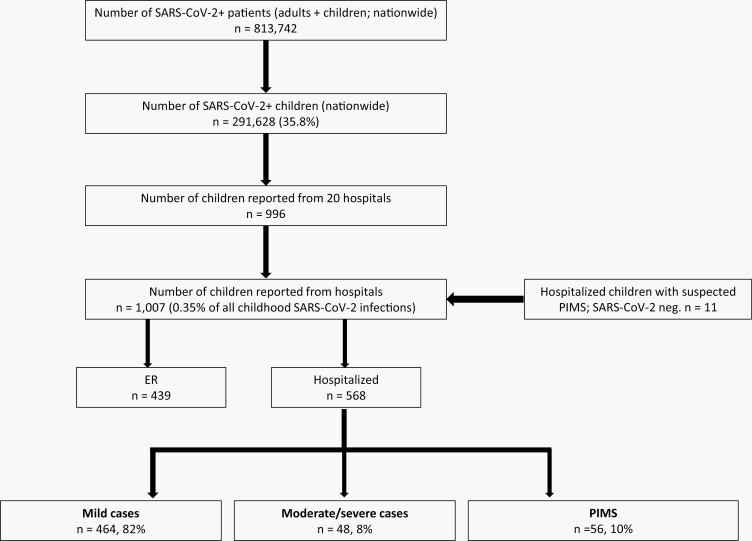
During the study period, 1007 children with SARS-CoV-2 infection, including 11 children with clinical diagnosis of PIMS (without SARS-CoV-2 infection confirmation) were identified in 20 hospitals. Of those 1007 children, 568 (56%) were hospitalized: 82% with mild disease, 8% with moderate/severe COVID-19 (non-PIMS), and 10% with PIMS. Four hundred and thirty-nine children (44%) were discharged from the pediatric ER and followed as outpatients (Figure 1).
Figure 1.
Study population: COVID-19 in children treated in 20 hospitals in Israel, March 11, 2020 through March 15, 2021.
The rates of reports are detailed in Figure 2.
Figure 2.
Dynamics of COVID-19 in children in 20 hospitals in Israel, March 11, 2020 through March 15, 2021.
Magnitude of COVID-19 Morbidity
Nationwide
According to the IMOH data, by March 17, 2021, overall 813 742 SARS-CoV-2–positive patients had been identified; among them, 291 628 (36%) were children [19]. Therefore, the 568 SARS-CoV-2–positive hospitalized children presented here represent 0.2% of the overall pediatric SARS-CoV-2 confirmed morbidity, and the 1007 SARS-CoV-2-positive children treated in pediatric ER or hospitalized represent 0.35% of the overall pediatric SARS-CoV-2 morbidity.
Demographic, Background Illness, and Outcome Characteristics of Pediatric COVID-19–Hospitalized Patients
The mean ± standard deviation (SD) age (years) of the overall study population was 5.6 ± 6.4, and the median age was 1.0 years. Overall, 208 (45%) were children <1 year old. Children with moderate/severe disease and PIMS were older than children with mild disease (Table 1). Overall, 54% of hospitalized patients were males, and 68% were Jewish. Forty-three percent of patients had known contact with a SARS-CoV-2–infected person. Gender distribution was similar in mild diseases and in PIMS, but higher rates of males were noted in moderate/severe disease. Ethnicity distribution was similar when comparing mild, moderate/severe, and PIMS patients.
Table 1.
Demographic, Background Illnesses, and Outcome Characteristics of Pediatric COVID-19–Hospitalized Patients
| Characteristics | Mild—ER (Outpatients)n = 439 (Data, n = 233) | Overall Hospitalized n = 568 (Data, n = 460) | Mild Hospitalized n = 464 (Data, n = 356) | Moderate/Severe, Non-PIMSn = 48 (Data, n = 48) | PIMSn = 56 (Data, n = 56) | P Valuea,b,c |
|---|---|---|---|---|---|---|
| Age | ||||||
| Range | 4 days–17.3 years | 1 day–17.9 years | 1 day–17.9 years | 3 months–17 years | 3 months–18 years | - |
| Mean ± SD (years) | 7.6 ± 6.4 | 5.6 ± 6.4 | 4.6 ± 6.3 | 8.0 ± 6.5 | 9.8 ± 4.5 | <.001a, <.001b, .12c |
| Median (years) | 7.0 | 1.0 | 0.5 | 7.5 | 10.0 | - |
| <1 year, n (%) | 53 (23%) | 208 (45%) | 195 (55%) | 11 (23%) | 2 (4%) | <.001a, <.001b, .006c |
| Gender, n (%) | ||||||
| Male | 118 (51%) | 249 (54%) | 186 (52%) | 35 (73%) | 28 (50%) | .008a, .78b, .03c |
| Female | 115 (49%) | 211 (46%) | 170 (48%) | 13 (27%) | 28 (50%) | |
| Ethnicity, n (%) | ||||||
| Jewish | 171 (73%) | 314 (68%) | 251 (71%) | 28 (58%) | 35 (63%) | .10a, .27b, .69c |
| Muslim | 60 (26%) | 135 (29%) | 97 (27%) | 18 (38%) | 20 (36%) | .17a, .20b, 1.0c |
| Unknown/other | 2 (1%) | 11 (2%) | 8 (2%) | 2 (4%) | 1 (2%) | .34a, 1.0b, .59c |
| Contacts, n (%) | ||||||
| Contact with COVID-19 patient | 135 (58%) | 193 (42%) | 159 (45%) | 13 (27%) | 21 (38%) | .03a, .38b, .30c |
| Travel abroad | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
| Background illnesses, n (%) | ||||||
| Overalld | 34 (15%) | 139 (30%) | 107 (30%) | 21 (44%) | 11 (20%) | .07a, .12b, .01c |
| Neurologic | 8 (4%) | 44 (10%) | 37 (10%) | 7 (15%) | 0 (0%) | .46a, .005b, .004c |
| Congenital heart disease | 1 (0.5%) | 17 (4%) | 13 (4%) | 3 (6%) | 1 (2%) | .42a, .70b, .30c |
| Genetic | 3 (1%) | 17 (4%) | 10 (3%) | 7 (15%) | 0 (0%) | .002a, .37b, .04c |
| Chronic lung disease | 7 (3%) | 15 (3%) | 11 (3%) | 4 (8%) | 0 (0%) | .09a, .37b, .04c |
| Obesity | 2 (1%) | 11 (2%) | 2 (0.6%) | 2 (4%) | 7 (13%) | .07a, <.001b, .17c |
| Immunodeficiency | 8 (4%) | 12 (3%) | 8 (3%) | 4 (8%) | 0% (0) | .04a, .61b, .04c |
| Renal failure | 2 (1%) | 8 (2%) | 6 (2%) | 1 (2%) | 1 (2%) | .59a, 1.0b, 1.0c |
| Hematologic | 1 (0.4%( | 9 (2%) | 6 (2%) | 1 (2%) | 2 (4%) | .59a, .30b, 1.0c |
| Prematurity | 3 (1%) | 12 (3%) | 8 (3%) | 4 (8%) | 0 (0%) | .04a, .61b, .04c |
| Malignancy | 1 (0.4%) | 7 (2%) | 5 (1%) | 2 (4%) | 0 (0%) | .20, 1.0b, .21c |
| Diabetes | 1 (0.4%) | 4 (1%) | 3 (1%) | 1 (2%) | 0 (0%) | .40a, 1.0b, .46c |
| Outcome | ||||||
| Intensive care unit | 0 (0%) | 36 (6%) | 0 (0%) | 10 (21%) | 26 (46%) | <.001a, <.001b, .007c |
| Mechanical ventilation | 0 (0%) | 15 (3%) | 0 (0%) | 5 (10%) | 10 (18%) | <.001a, <.001b, .40c |
| Mortality | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1.0 |
Abbreviations: ER, emergency rooms; PIMS, pediatric inflammatory multisystem syndrome.
aMild vs moderate/severe; bmild vs PIMS; cmoderate/severe vs PIMS; dOverall background illnesses included all detailed diagnoses in the table (with a possibility of more than 1 background illness per child).
Background illness was reported in 30% of patients, with prematurity, immunodeficiency, genetic, neurologic, and chronic lung disease most common in moderate/severe patients, and obesity significantly more common in PIMS patients.
Mechanical ventilation and PICU admission were more common in PIMS than in mild and moderate/severe diseases. No mortality was recorded among the entire study population.
The demographic, background illness, and outcome characteristics of pediatric COVID-19 outpatients (ER visits) are detailed in Table 1.
Clinical Characteristics of Pediatric COVID-19
Overall Hospitalized Study Population
The most common symptoms at presentation were fever (71%), cough (21%), and respiratory distress (15%). Notably, 7% of all patients were asymptomatic (hospitalized for various reasons, including social problems, trauma, and for observation) (Table 2).
Table 2.
Clinical and Treatment Characteristics of Pediatric Mild Disease, Moderate/Severe COVID-19– and PIMS–Hospitalized Patients
| Characteristics | Mild—ER (Outpatients)n = 439 (Data, n = 233) | Overall Hospitalized n = 568 (Data, n = 460) | Mild Hospitalized n = 464 (Data, n = 356) | Moderate/Severe, Non-PIMSn = 48 (Data, n = 48) | PIMS n = 56 (Data, n = 56) | P Valuea,b,c |
|---|---|---|---|---|---|---|
| Clinical presentation, n (%) | ||||||
| Fever | 138 (59%) | 325 (71%) | 240 (67%) | 29 (60%) | 56 (100%) | .33a, <.001b, <.001c |
| Upper respiratory tract infection (URI) | 69 (30%) | 100 (22%) | 88 (25%) | 3 (6%) | 9 (16%) | .003a, .18b, .14c |
| Lower respiratory tract infection (LRI) | 6 (3%) | 59 (13%) | 30 (8%) | 17 (35%) | 12 (21%) | <.001a,.007b, .13c |
| Cough | 64 (27%) | 95 (21%) | 73 (21%) | 13 (27%) | 9 (16%) | .35a, .59b, .23c |
| Respiratory distress | 25 (11%) | 71 (15%) | 39 (11%) | 16 (33%) | 16 (29%) | <.001a, .001b, .67c |
| Runny nose | 32 (14%) | 48 (10%) | 41 (12%) | 4 (8%) | 3)5%) | .63a, .24b, .70c |
| Sore throat | 20 (9%) | 26 (6%) | 15 (4%) | 1 (2%) | 10 (18%) | .71a, <.001b, .01c |
| Chest pain | 9 (4%) | 19 (4%) | 9 (3%) | 2 (4%) | 8 (14%) | .63a, <.001b, .10c |
| Diarrhea | 19 (9%) | 48 (10%) | 26 (7%) | 3 (6%) | 19 (34%) | 1.0a, <.001b, <.001c |
| Vomiting | 26 (12%) | 56 (12%) | 26 (7%) | 9 (19%) | 21 (38%) | .02a, <.001b, .05c |
| Nausea | 5 (2%) | 23 (5%) | 8 (2%) | 3 (6%) | 12 (21%) | .13a, <.001b, .05c |
| Abdominal pain | 17 (8%) | 55 (12%) | 18 (5%) | 8 (17%) | 29 (52% | .007a, <.001b, <.001c |
| Rash | 5 (2%) | 44 (10%) | 10 (3%) | 2 (4%) | 32 (57%) | .64a, <.001b, <.001c |
| Conjunctivitis | 0 (0%) | 22 (5%) | 3 (1%) | 0 (0%) | 19 (34%) | 1.0a, <.001b, <.001c |
| Seizures/encephalopathy | 4 (2%) | 13 (3%) | 8 (2%) | 2 (4%) | 3 (5%) | .34a, .18b, 1.0c |
| Irritability/apathy | 1 (0.4%) | 25 (5%) | 17 (5%) | 0 (0%) | 8 (14%) | .24a, .01b, .007c |
| Headache | 18 (8%) | 42 (9%) | 20 (6%) | 7 (15%) | 15 (27%) | .03a, <.001b, .15c |
| Loss of smell/taste | 6 (3%) | 17 (4%) | 5 (1%) | 11 (23%) | 1 (2%) | <.001a, .59b, .001c |
| Bell’s palsy | 3 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1.0a, 1.0b, 1.0c |
| Weakness | 15 (6%) | 54 (12%) | 22 (6%) | 10 (21%) | 22 (39%) | .009a, <.001b, .06c |
| Dizziness | 3 (1%) | 12 (3%) | 6 (2%) | 1 (2%) | 5 (9%) | 1.0a, .01b, .21c |
| Myalgia | 5 (2%) | 17 (4%) | 8 (2%) | 3 (6%) | 6 (11%) | .13a, .006b, .50c |
| Asymptomatic | 31 (13%) | 31 (7%) | 31 (9%) | - | - | - |
| Chest radiography, n (%) | ||||||
| Performed | 22 (9%) | 113 (25%) | 61 (17%) | 25 (52%) | 27 (48%) | <.001a, <.001b, .84c |
| Pneumonia (per radiography) | 3 (1%) | 35 (8%) | 15 (4%) | 12 (25%) | 8 (14%) | <.001a, .007b, .21c |
| Evidence for bacterial infectiond | 5 (2%) | 30 (7%) | 28 (8%) | 2 (4%) | - | .56a, –, – |
| Evidence for other viral infectione | 0 (0%) | 13 (3%) | 8 (2%) | 5 (10%) | - | .01a, –, – |
| Treatment, n (%) | ||||||
| Antibiotics given | 9 (4%) | 181 (39%) | 131 (37%) | 23 (48%) | 27 (48%) | .16a, .11b, 1.0c |
| Laboratory | ||||||
| Lymphocytes; mean ± SD | 3.4 ± 2.4 | 3.3 ± 2.5 | 3.9 ± 2.5 | 2.1 ± 1.4 | 1.1 ± 1.0 | <.001a, <.001b, <.001c |
| Lymphopenia (≤2000), n (%) | 42% (30/71) | 37% (147/398) | 25% (75/302) | 60% (24/40) | 86% (48) | <.001a, <.001b, .008c |
| ANC/LYM ratio; mean ± SD | - | 5.7 ± 6.4 | 1.7 ± 2.2 | 4.2 ± 3.5 | 17.3 ± 32.4 | <.001a, <.001b, <.001c |
| ANC/LYM ratio ≥3.5, n (%) | - | 87/371 (23%) | 28/274 (10%) | 17/48 (35%) | 42/49 (86%) | <.001a, <.001b, <.001c |
| PLT; %mean ± SD | 298 ± 111 | 306 ± 137 | 325 ± 128 | 269 ± 139 | 179 ± 136 | .008a, <.001b, .008c |
| Thrombocytopenia, n (%) | 2/67 (3%) | 43 (9%) | 12/300 (4%) | 10/42 (24%) | 21 (38%) | <.001a, <.001b, .19c |
| CRP (mg/dL); mean ± SD | 1.6 ± 2.0 | 4.5 ± 7.6 | 1.8 ± 3.1 | 4.4 ± 4.8 | 19.2 ± 8.9 | <.001,a <.001b, <.001c |
| CRP >6 mg/dL | 4/62 (6%) | 89/376 (24%) | 23/279 (8%) | 24% (10/41) | 100% (56) | .004a, <.001b, <.001c |
| Elevated troponin (>25, n (%) | - | 15/68 (22%) | 0/18 (0%) | 2/151 (3%) | 13/35 (37%) | .20a, .002b, .18c |
Abbreviations: ANC/LYM, absolute neutrophil count/lymphocyte; CRP, C-reactive protein; ER, emergency rooms; PIMS, pediatric inflammatory multisystem syndrome; PLT, platelet; SD, standard deviation.
aMild vs moderate/severe; bmild vs PIMS; cmoderate/severe vs PIMS; devidence for bacterial infection: 15 urinary tract infection episodes (10 with Escherichia coli, 3 with Enterococcus spp., 2 with Klebsiella spp.), 9 periodontal, parapharyngeal, and skin and soft tissue infections (2 in the moderate/severe group), 2 positive sputum cultures treated as pneumonia, 2 central line infections, and 2 Salmonella gastroenteritis cases; eevidence for viral infection, other than SARS-CoV-2: 8 episodes in the mild disease group (3 human metapneumovirus, 4 rhinovirus (3 as dual infections), 1 adenovirus, 1 enterovirus, 1 Epstein-Barr virus, 1 parainfluenza), and 5 episodes in the moderate/severe group (2 rhinovirus, 1 adenovirus, 1 herpes simplex virus encephalitis, 1 enterovirus meningitis).
Upper respiratory tract infection (URI) was reported in 22% of hospitalized patients, with higher rate in mild disease. In contrast, lower respiratory tract infection (LRI) signs and symptoms were reported in 13% of cases, with highest rate (35%) in moderate/severe disease.
Chest radiography was more commonly performed in PIMS and moderate/severe patients than in mild cases (52% and 48% vs 17%, P < .001). The rates of radiographic pneumonia were 4%, 25%, and 14% for mild, moderate/severe, and PIMS, respectively.
Other Symptoms
Rates of gastrointestinal symptoms, including diarrhea, vomiting, nausea, and abdominal pain, as well as rates of rash, conjunctivitis, irritability/apathy, myalgia, and weakness, were higher in PIMS episodes than in other groups. In contrast, rates of loss of smell/taste were higher in moderate/severe episodes. The clinical characteristics of pediatric COVID-19 outpatients (ER visits) are detailed in Table 2.
Evidence for Bacterial or Viral (Non-SARS-CoV-2) Infection
Evidence for bacterial infection was recorded in 8% and 4% of mild and moderate/severe disease patients, respectively (none for PIMS, where “other infectious etiology” is an exclusion criterion).
Evidence for viral infection, other than SARS-CoV-2, included 8 episodes in the mild disease group and 5 episodes in the moderate/severe group.
Laboratory Characteristics
Lymphopenia, higher rate of neutrophils/lymphocytes >3.5, and thrombocytopenia were most common in PIMS. Elevated CRP and elevated troponin were mostly found in PIMS patients, while their rates were low in the other groups (Table 2).
PIMS
PIMS was diagnosed by serologic tests, indicating past infection, in 57% of cases. Nevertheless, 23% of PIMS were diagnosed only through positive SARS-CoV-2 PCR test, and in 11 cases (20%), diagnosis was clinical without a viral test support (based on clinical presentation criteria and exposure to COVID-19 patient) (Table 3).
Table 3.
Clinical and Treatment Characteristics of PIMS Patients
| Characteristics, n (%) | PIMS, n = 56 |
|---|---|
| Diagnosis of SARS-CoV-2 | |
| Serology only | 26 (46%) |
| Serology + PCR | 6 (11%) |
| PCR only | 13 (23%) |
| No viral confirmation (contact with patient) | 11 (20%) |
| Clinical presentation | |
| Fever | 56 (100%) |
| Gastrointestinal symptoms | 46 (82%) |
| Cardiovascular | 34 (61%) |
| Rash | 32 (57%) |
| Respiratory | 22 (39%) |
| Conjunctivitis | 19 (34%) |
| Acute kidney injury | 11 (20%) |
| Neurologic | 11 (20%) |
| Laboratory | |
| Lymphopenia <2000 | 48 (86%) |
| ANC/LYM ratio ≥3.5 | 42/49 (86%) |
| Thrombocytopenia | 21 (38%) |
| CRP >6 mg/dL | 56 (100%) |
| Elevated ferritin | 32/35 (91%) |
| Elevated troponin (>25) | 13/35 (37%) |
| Albumin <3.5 | 27/42 (64%) |
| D-dimer checked; elevated | 38/47 (81%) |
| Elevated IL-6 | 3/8 (38%) |
| Echocardiography | |
| Pathologic/done | 32/40 (57%) |
| Treatment | |
| Steroids | 46 (82%) |
| IV-IG | 32 (57%) |
| Steroids and IV-IG | 28 (50%) |
| Antibiotics | 27 (48%) |
| Aspirin | 23 (41%) |
| Low-molecular-weight heparin | 14 (25%) |
| Plasmapheresis | 1 (2%) |
| Remdesivir | 1 (2%) |
| Infliximab | 1 (2%) |
Abbreviations: ANC/LYM, absolute neutrophil count/lymphocyte; IL-6, interleukin 6; IV-IG, intravenous immunoglobulin; PCR, polymerase chain reaction; PIMS, pediatric inflammatory multisystem syndrome PLT, platelet; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Fever, gastrointestinal symptoms, cardiovascular involvement, and rash were noted in mot PIMS episodes. Respiratory presentation was recorded only in a minority of patients (39%). Lymphopenia and elevated CRP were observed in most of the cases, while thrombocytopenia was noted in 38% of patients. Other common laboratory findings included elevated levels of ferritin, troponin, and D-dimer, as well as low albumin levels.
Echocardiography was performed in 40 patients, with pathological findings identified in 32, including pericardial effusion (17 cases), coronary dilatation or hyperemia (10 cases), and reduced cardiac function/contraction (5 cases). The mainstay of treatment in PIMS included corticosteroids (82%) and IV-IG (57%).
DISCUSSION
We report nationwide the characteristics and clinical course of hospitalized children with COVID-19 treated in Israel between March 11 and March 17, 2021. The study population comprises most (but not all) of the pediatric cases treated in hospitals nationwide, allowing real appreciation of the presentation and prognosis of the most severe pediatric COVID-19 cases.
Our main findings include low disease rate (<1%) in terms of the proportion of children treated in hospitals of all children diagnosed with SARS-CoV-2 infection nationally. Furthermore, although the children in our study were all treated in hospitals, thus representing the most severe pediatric cases, >80% of the children had a mild disease, a third of the patients had no fever, and most patients presented without respiratory symptoms.
The finding of a low rate of respiratory symptoms among pediatric COVID-19 cases, and especially of LRI symptoms among mild, moderate/severe, and PIMS patients is somewhat surprising. Specifically, it stands in contrast to the findings in other studies reporting COVID-19 in adults, where LRI was a common presentation [26–28]. Nevertheless, our findings are similar to those found in a French study assessing severe childhood COVID-19 [25]. Therefore, these findings suggest that most SARS-CoV-2 infections in children are mild, and that among the most severe cases (ie, hospitalized patients), they can be divided generally into 3 groups. The first contains children with mild disease who are hospitalized for other, non-COVID-19 directly related, reasons. This is evident by the high proportion of young (often febrile) infants in this group, suggesting they were often hospitalized for a sepsis workup and exclusion of serious bacterial infections, rather than for direct COVID-19 symptoms. Furthermore, 9% of the patients in this group were asymptomatic. Notably, during the first outbreak peak (March through April), according to the IMOH policy, SARS-CoV-2–positive individuals had to be hospitalized, even if asymptomatic. During that period, 11 asymptomatic children were hospitalized. Therefore, the rate of asymptomatic patients in the overall pediatric population is probably higher. The second group of moderate/severe disease was characterized by high rates of non-COVID-19–related morbidity, including (among others) seizures in children with previous neurological conditions, appendicitis and duodenal rupture, exacerbation in diabetes mellitus, diagnosis of myelo/lymphoproliferative diseases, herpes encephalitis, bacterial infections, and hereditary spherocytosis. Thus, in the 2 groups of non-PIMS, the most severe cases of COVID-19 were often hospitalized for their comorbidity rather than for the direct impact of SARS-CoV-2 infection. The third group comprises patients with PIMS and represents a unique, rare, postinfectious phenomenon, which requires high level of suspicion from treating physicians for its recognition [23, 25]. Of note, obesity was overrepresented in our series of children who had PIMS, as it was in other study findings [23] as well as in studies assessing obesity in severe COVID-19 in adults [28–30].
Laboratory findings are also inconsistent among hospitalized children with COVID-19, with lymphopenia, thrombocytopenia, highly elevated CRP, and elevated troponin being the most common findings in distinguishing mild, moderate/severe, and PIMS cases.
These findings suggest that it is almost impossible to assess the SARS-CoV-2 infection rate (ie, deciding upon a sampling policy) based only on clinical signs and symptoms. This presents a major challenge for infection control efforts and suggests that a low threshold for SARS-CoV-2 testing in the inpatient setting is required. Therefore, early SARS-CoV-2 detection and sampling policy on a population level should probably be based on a combination of signs and symptoms, known disease geographic clusters, and high-risk group screening.
Our findings reaffirm that children comprise a small fraction of severe COVID-19 cases, and that their symptoms are often mild. However, a few pediatric cases may progress to severe disease, and initial atypical presentations may delay the diagnosis of COVID-19, leading to unfavorable outcomes [8]. While severe diseases were uncommon in our study, some of their aspects are notable. First, all moderate/severe and PIMS cases were in children ≥3 months of age. This finding differs from early reports of severe COVID-19 where severity was associated with very young age [3, 4, 12]. Nevertheless, in recent reports describing hyper-inflammation, Kawasaki-like disease, MIS-C, and PIMS, most children were older than 5 years of age. [15, 23, 25] Second, PIMS patients often had gastrointestinal and myocardial involvement along with signs of hyper-inflammation with a few respiratory symptoms. Laboratory findings upon hospital admission associated with PIMS presentation were lymphopenia, thrombocytopenia, neutrophilia, and elevated CRP, troponin, ferritin, and D-dimer levels. Third, all children recovered completely. These findings suggest that early recognition of severe COVID-19 is essential for an optimal outcome. Specifically, for PIMS, early recognition and treatment with IV-IG and steroids are probably associated with a more favorable clinical course [31]. Notably, antibiotics were used in more than a third of all cases (in each of the 3 groups), probably reflecting either high rate of febrile infant receiving antibiotic treatment until exclusion of serious bacterial infection, or older children with severe disease/PIMS, receiving antibiotics due to their “toxic” appearance.
One important implication of our study is that with increasing use of SARS-CoV-2 vaccines, currently targeting the adult population [32], future vaccination strategies should include a vaccine for children aiming at interruption of transmission, in contrast to the adult vaccines which aim is to prevent severe disease. It is also possible that with increased adult vaccination rates, a shift toward infection in young population, including children, will be noted with an increase in severe COVID-19 in children. Indeed, In Israel, where vaccine uptake among adults was the highest worldwide during December 2020 through March 2021, a campaign for vaccinating children ≥16 years old was initiated in February 2021, and the IMOH is considering future vaccination of younger children.
Our study has several limitations: First, although this is a nationwide surveillance, we were unable to retrieve data from several hospitals treating pediatric population in Israel. Additionally, some data were missing in certain cases.
In conclusion, pediatric SARS-CoV-2 infections in the in-hospital setting among Israeli children were characterized by a low disease rate, suggesting that most infected children have a mild disease, often without respiratory symptoms. Although uncommon, physicians should maintain a high index of suspicion for severe COVID-19 cases, including hyper-inflammation and myocardial involvement. Lymphopenia, thrombocytopenia, neutrophilia, and elevated CRP, troponin, ferritin, and D-dimer levels are more frequent in severe and especially PIMS cases.
Notes
Financial support. None.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Coronavirus disease (COVID-19) pandemic. Accessed April 8, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr 2020; 174:882–9. [DOI] [PubMed] [Google Scholar]
- 3. Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J 2020; 39:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC COVID-19 Response Team. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020; 145:e20200702. [DOI] [PubMed] [Google Scholar]
- 6. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020; 109:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 2020; 382:2302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi SH, Kim HW, Kang JM, Kim DH, Cho EY. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. 2020; 63:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020; 382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paret M, Lighter J, Pellett Madan R, Raabe VN, Shust GF, Ratner AJ. SARS-CoV-2 infection (COVID-19) in febrile infants without respiratory distress. Clin Infect Dis. 2020; 71:2243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feld L, Belfer J, Kabra R, et al. A case series of the 2019 novel coronavirus (SARS-CoV-2) in three febrile infants in New York. Pediatrics. 2020; 146:e20201056. [DOI] [PubMed] [Google Scholar]
- 12. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020; 174:868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paediatric Intensive Care Society (PICS) Statement: increased number of reported cases of novel presentation of multi-system inflammatory disease. 2020. Accessed June 9, 2020. https://picsociety.uk/wp-content/uploads/2020/04/PICS-statement-re-novel-KD-C19-presentation-v2-27042020.pdf
- 15. Centers for Disease Control and Prevention. Health Alert Network (HAN): multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Accessed June 9, 2020. https://emergency.cdc.gov/han/2020/han00432.asp
- 16. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020; 395:1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Centre for Disease Prevention and Control. Paediatric Inflammatory Multisystem Syndrome and SARS-CoV-2 Infection in Children – 15 May 2020. Stockholm: ECDC; 2020. Accessed June 9, 2020. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-risk-assessment-paediatric-inflammatory-multisystem-syndrome-15-May-2020.pdf [Google Scholar]
- 18. Eilam S, Hatuel-Radoshitzky M. The Corona Crisis and the International System: A Comparative Overview. The Institute for National Security Studies. 2020. Accessed June 9, 2020. https://www.inss.org.il/publication/coronavirus-and-the-international-system/ [Google Scholar]
- 19. State of Israel. Ministry of Health. The novel coronavirus. Accessed March 17, 2021. https://govextra.gov.il/ministry-of-health/corona/corona-virus/?gclid=EAIaIQobChMI7vinoaPW6QIVkOvtCh2A7ADREAAYASAAEgIV0fD_BwE
- 20. State of Israel. Central Bureau of Statistics. Table 2.3. Population, by population group, religion, sex and age. Accessed June 9, 2020. https://www.cbs.gov.il/he/publications/doclib/2019/2.shnatonpopulation/st02_03.pdf
- 21. Cahaner L, Malach G, Choshen M. Press Release: Statistical Report on Ultra-Orthodox Society in Israel. 2017. The Israel Democracy Institute. Accessed June 9, 2020. https://en.idi.org.il/media/10441/statistical-report-on-ultra-orthodox-society-in-israel-2017.pdf [Google Scholar]
- 22. Malach G, Cahaner L. Press Release: 2018 Statistical Report on Ultra-Orthodox Society in Israel. 2018. The Israel Democracy Institute. Accessed June 9, 2020. https://en.idi.org.il/articles/25385 [Google Scholar]
- 23. Bautista-Rodriguez C, Sanchez-de-Toledo J, Clark BC, et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. 2021; 147:e2020024554. [DOI] [PubMed] [Google Scholar]
- 24. Bailey LC, Razzaghi H, Burrows EK, et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr 2021; 175:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ouldali N, Yang DD, Madhi F, et al. Factors associated with severe SARS-CoV-2 infection. Pediatrics. 2021; 147:e2020023432. [DOI] [PubMed] [Google Scholar]
- 26. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 27. Del Rio C, Malani PN. 2019 Novel coronavirus – important information for clinicians. JAMA. 2020; 323:1039–40. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention. COVID-19 in children and teens. Information for parents and caregivers about COVID-19 in children and teens. Accessed April 11, 2021. https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/children/symptoms.html
- 29. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020; 28:1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med 2020; 173:773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ouldali N, Toubiana J, Antona D, et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021; 325:855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021; 69:1657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]