Abstract
Background
Humoral response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines needs to be evaluated in the fragile population of patients on maintenance haemodialysis (HD).
Methods
We analysed the antibody response to the spike (S) antigen of SARS-CoV-2 before and after each dose of the messenger RNA (mRNA) Comirnaty vaccine (BNT162b2; BioNTech & Pfizer) in patients from a single dialysis centre and detected the presence of neutralizing antibodies (Nabs).
Results
Among the 90 vaccinated HD patients (mean age 69 years, 61% male), 19 (21%) had a history of SARS-CoV-2 infection. A seroconversion with anti-S immunoglobulin G antibodies (Sabs) was documented in 20% of patients after the first dose (early responders) and in 77% after the second dose, while 23% were non-responders. Cardiac disease, cirrhosis and gamma globulin levels were independently predictive of the absence of seroconversion. Nabs were detected in 15.4% of early responders after the first dose and in 84.6% of early responders and 57.9% of late responders after the second dose. Sab titres after the second dose were higher in patients with Nab than without Nab {598 [interquartile range (IQR) 246–882]) versus 134 [IQR 61–390]; P < 0.0001}. All patients with a history of SARS-CoV-2 infection developed both Sabs and Nabs and their titres for Sabs and Nabs were higher than in late responders.
Conclusions
Most HD patients develop a substantial humoral response against SARS-CoV2, with Nabs, following the mRNA vaccine. Whether this immunity persists over time and is able to efficiently protect patients from coronavirus disease 2019 remains to be determined.
Keywords: BNT162 vaccine, COVID-19, haemodialysis, renal dialysis, SARS-CoV-2, vaccine
INTRODUCTION
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in China in December 2019 and was declared as pandemic by the World Health Organization (WHO) in March 2020. Chronic haemodialysis (HD) patients have a 20–30% risk of death in case of COVID-19 [1–3]. The messenger RNA (mRNA)-based Comirnaty vaccine (BNT162b2; BioNTech and Pfizer) was developed and approved in Europe in December 2020 [4]. Given the vulnerability of HD patients to SARS-CoV-2 severe infection, international guidelines call for priority vaccination of HD patients [5, 6]. In a population of in-centre HD patients, we recently documented the favourable safety profile of Comirnaty vaccine administration during the dialysis session regarding bleeding risk [7].
However, since HD patients were broadly excluded from Phase 3 studies evaluating the efficacy and safety of COVID-19 vaccines, the determinants of antibody responses to vaccine remain poorly understood in this specific population. In particular, no data are currently available on the ability of vaccinated patients’ serum to inhibit SARS-CoV-2 replication (seroneutralization ability). HD patients are known to commonly exhibit an impaired immune response against pathogens and vaccines. For example, detectable humoral responses following hepatitis B virus (HBV) vaccine are estimated at 69% in HD patients and at 30–80% following influenza vaccine, which is far lower than in the general population [8, 9]. Unexpectedly, a recent study in Israel showed a detectable seroconversion [circulating anti-spike immunoglobulin G (IgG) antibody (Sab)] with the Comirnaty vaccine in a vast majority of HD patients (96.4%) [10]. This report has been confirmed in other HD patient cohorts [11–13]. Interestingly, older age and lower lymphocyte counts were associated with lower response to vaccine. Concerning the neutralizing ability after the seroconversion, a recent report suggests that about one-third of HD patients develop neutralizing antibodies (Nabs), at low titres, after the first dose of Comirnaty vaccine [14]. Furthermore, another team showed that HD patients are able to generate efficient Tcell immunity by SARS-CoV-2 reactive T cells [15].
Here we present results of the serological response follow-up of HD patients receiving the Comirnaty vaccine in our HD centre. We studied Nabs and Sabs before and following vaccination.
MATERIALS AND METHODS
Study population and design
The study included HD patients who accepted and received the BNT162b2 (Pfizer-BioNTech) vaccination with the recommended dosing interval of 21 days between the first and second doses. All patients included in this study received the first dose on 10 or 11 February and the second dose on 24 or 25 March. Patients with a history of symptomatic COVID-19 documented by polymerase chain reaction (PCR) received only one vaccine dose. Patients with a history of asymptomatic COVID-19 (documented either by rapid serological test in the past 12 months or a positive serology the day of the vaccination) received two doses of vaccine 3 weeks apart. Exclusion criteria were age <18 years and active or recent COVID (in the past 3 months). As part of the care, we monitored serological response to vaccination in this population considered at risk of severe COVID. Serum samples were obtained from all included patients at the beginning of the dialysis session on the day of the vaccination, the day of the second dose and at least 3 weeks after the second dose. For the previously infected symptomatic HD patients, serum samples were obtained at the beginning of the dialysis session on the day of the vaccination and at least 3 and 6 weeks after the single-dose vaccine. The data included in this study were anonymized, approved according to the General Data Protection Regulation and registered at the Health Data Portal and Data Protection Commission of Assistance publique-Hôpitaux de Marseille under the reference X8RF2T. The patients were provided with oral information about this study.
Serological testing
Sera samples were tested for anti-SARS-CoV-2 IgG antibodies directed against the S1 domain of the spike protein of the virus using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Euroimmun, Lübeck, Germany) and quantitative results were expressed in standardized units {binding antibody units (BAUs) per millilitre [16]} as recommended by the manufacturer. For all samples with a semi-quantitative ratio ≥0.7, Nabs against SARS-CoV-2 were detected using a virus neutralization test (VNT100), with four dilutions of each serum (1/20–1/160) as previously described [17, 18].
Seropositive patients were defined by detectable Sabs. We defined as early responders patients who were seropositive after the first vaccine dose, as late responders patients who were seropositive after the second dose and as non-responders patients with an absence of seroconversion after two doses.
Data source/measurement
The following patient baseline characteristics were collected from electronic medical records: age, gender, body mass index (BMI), obesity (BMI >30 kg/m2), previous transplantation and history of or active cancer and classical comorbidities. Their significant usual treatments (corticosteroids and immunosuppressive therapies) were also registered. We collected the routine trimestral blood test monitoring (complete blood count, albuminaemia, gamma globulin level, C-reactive protein, pre-dialysis β2-microglobulinaemia) results from March 2021. Hypogammaglobulinaemia was defined as a gamma globulin level ˂8 g/L assessed by serum protein electrophoresis. Lymphopaenia was defined as a lymphocyte count ˂1.2 × 109/L. Serum albumin level was measured by immunonephelometry. VHB responders were defined by the presence of Hbs antibodies after VHB vaccination.
Statistical analysis
Continuous and categorical variables were presented as median [interquartile range (IQR)] or mean (standard error) and n (%), respectively. We used the Mann–Whitney U-test, chi-squaredtest or Fisher’s exact test to compare differences between groups when appropriate. All tests were two-tailed. Multiple logistic regression analysis was used to determine whether each variable was an independent factor for vaccine response. Covariates of interest for the multivariate logistic regression analysis were selected based on a P-value <0.1 in a univariate analysis. Performance accuracy of the ELISA test to predict effective seroneutralization was assessed using the receiver operating characteristics (ROC) area under the curve (AUC). Cut-off values showing the greatest accuracy were determined using sensitivity or specificity. A P-value <0.05 was considered statistically significant. The data were analysed using Prism software (GraphPad Software, San Diego, CA, USA) and JMP Pro version 14 software (SAS Institute, Cary, NC, USA).
RESULTS
General characteristics of the population
From March to April 2021, 90 HD patients were vaccinated against SARS-CoV-2 in our centre. Among the 19 (21.1%) previously infected HD patients, 11 were symptomatic and 8 were asymptomatic. Baseline characteristics are presented in Table 1. The mean age was 68.9 years (± 13.7) and 54 (60%) patients were male. The mean dialysis vintage was 5.9 years (± 8.7). Seven (7.8%) HD patients were treated with corticosteroid and/or immunosuppressant and 30 (33.3%) had a previous history of organ transplantation and/or a history of or active cancer; 31 (35.2%) patients had hypogammaglobulinaemia and 49 (54.4%) had lymphopaenia.
Table 1.
Baseline characteristics
| Patient characteristics | Values |
|---|---|
| Age (years), mean (SD) | 68.9 (13.7) |
| Male, n (%) | 54 (60) |
| BMI (kg/m2), mean (SD) | 25.8 (5.4) |
| DN and/or nephroangioslcerosis, n (%) | 25 (27.7) |
| Glomerulonephritis, n (%) | 34 (37.8) |
| Autosomal dominant polycystic kidney disease, n (%) | 1 (1.1) |
| Tubulo-interstitial nephropathy, n (%) | 18 (20) |
| Indeterminate nephropathy, n (%) | 12 (13.3) |
| Blood group, n (%) | |
| O | 39 (44.3) |
| A | 34 (38.2) |
| B | 13 (14.6) |
| AB, missing value (n = 2) | 2 (2.2) |
| Dialysis vintage (years), mean (SD) | 5.9 (8.7) |
| Active on transplant list, n (%) | 3 (3.3) |
| On steroids and/or immunosuppressants, n (%) | 7 (7.8) |
| Diabetes, n (%) | 38 (42.2) |
| Chronic respiratory disease, n (%) | 30 (33.3) |
| Arterial hypertension, n (%) | 77 (85.6) |
| Ischaemic/rhythmic cardiac disease, n (%) | 44 (48.9) |
| Stroke, n (%) | 18 (20) |
| Peripheral artery disease, n (%) | 26 (28.9) |
| Cirrhosis, n (%) | 4 (4.4) |
| History or active cancer/transplantation, n (%) | 30 (33.3) |
| History of COVID-19, n (%) | 19 (20.1) |
| Symptomatic, n/N | 11/19 |
| Asymptomatic, n/N | 8/19 |
| Albuminaemia (g/L), mean (SD) | 35.3 (4.3) |
| Gamma globulin (g/L), mean (SD) | 9.4 (3.2) |
| Hypogammaglobulinaemia (<8 g/L), n (%) | 31 (35.2) |
| Lymphocyte count (×109/L), mean (SD) | 1.3 (0.8) |
| Lymphopaenia, n (%) | 49 (54.4) |
| Pre-dialysis β2-microglobulinaemia (mg/L), mean (SD) | 27.2 (6.5) |
| Pre-dialysis β2-microglobulinaemia >30 mg/L, n (%) | 30 (33.7) |
| C-reactive protein (mg/L), mean (SD) | 11.6 (14.9) |
| HBV vaccine responders, n/N (%) | 34/51 (66.7) |
Analysis of patients free of history of COVID-19 SARS-CoV-2 seropositivity
Before the first vaccine dose, none of the HD patients had detectable Sabs. After the first vaccine dose, seroconversion was observed in 13/65 (20%) HD patients (early responder group). Three weeks after the second dose (late responder group), seroconversion was observed in 54/70 (77.1%) patients, while among those tested, 16/70 (22.8%) were non-responders (Figure 1).
FIGURE 1:
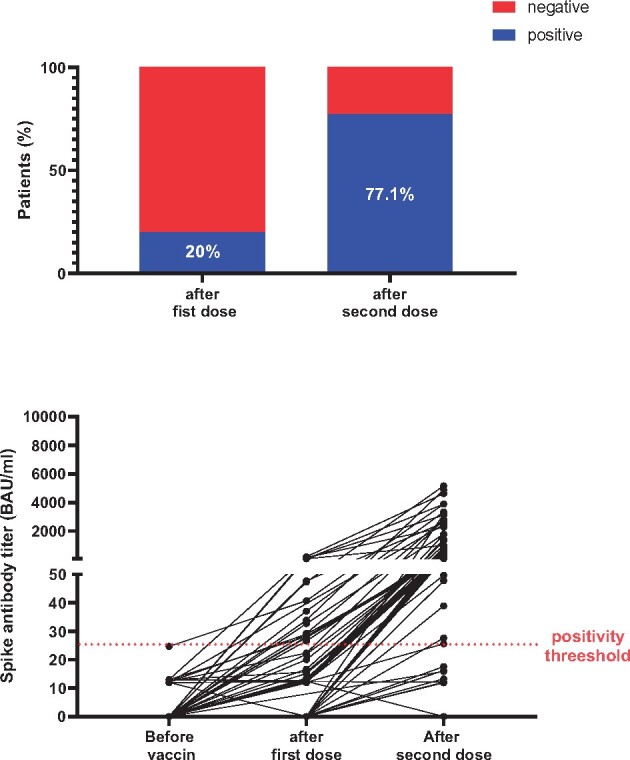
Humoral response to COVID-19 vaccination in patients without a history of COVID-19.
In the early responder group, median Sab titres increased significantly after the second dose [3121 BAU/mL (IQR 2028–4255)] compared with the first dose [47.3 BAU/mL (IQR 33.2–59.8)] (P = 0.0002), denoting a clear booster effect. In the late responder group, the median Sab titres were significantly lower [381.4 BAU/mL (IQR 102.5–750.4)] compared with the early responder group [3121 BAU/mL (IQR 2028–4255)] (P < 0.0001).
Nabs
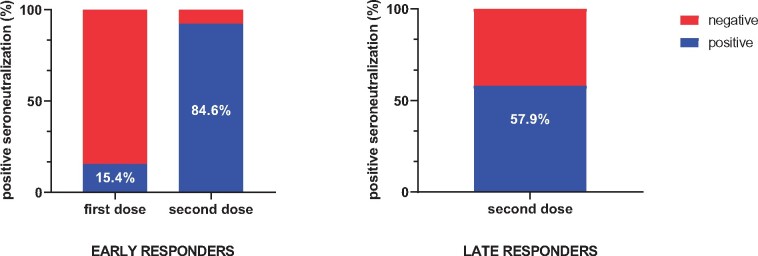
Nabs were detected in 15.4% of the early responder group after the first dose and increased to 84.6% after the second dose.
In the late responder group, Nabs were detected in 57.9% of patients after the second dose (Figure 2). In this group, median Sab titres were higher in patients with Nabs [597.8 BAU/mL (IQR 246.5–881.8)] compared with patients without Nabs [134.4 BAU/mL (IQR 61.5–389.6)] (P = 0.01). In the overall responder group, Nabs could be detected in 37/55 (67.3%) after the second vaccine dose.
FIGURE 2:
Detectable Nabs in seroconverted patients.
Sab titres were higher in patients with detectable Nabs compared with patients without Nabs [840.2 BAU/mL (IQR 406–2796.7) versus 111 (54.6–309)] (P < 0.0001) after the second vaccine dose. The AUC of the ROC curve of Sab titres for detectable Nabs was 94% [95% confidence interval (CI) 76–96] (P < 0.0001). The highest accuracy was observed for a Sab titre of 513.8 BAU/mL, with 73% (57–84.6) sensitivity, 94.4% (74.2–99.7) specificity and a likelihood ratio of 13.14 (Figure 3).
FIGURE 3:
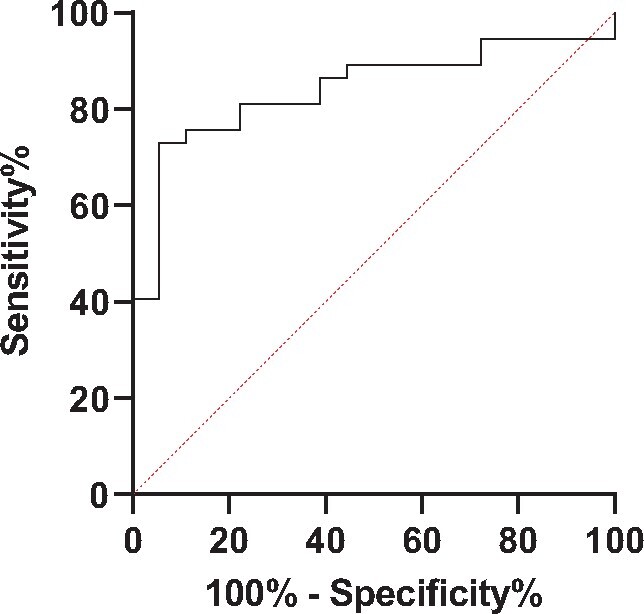
Diagnostic accuracy of circulating Sabs to predict detectable Nabs.
Factors associated with vaccine response
Non-responders were more likely to be treated with corticosteroid and/or immunosuppressants to have chronic respiratory disease, ischaemic/rhythmic cardiac disease, cirrhosis or a previous transplantation and/or history of or active cancer compared with responders (Table 2). Gammaglobulin levels were significantly lower in non-responders [7.3 g/L (± 0.8)] compared with responders [early responder group, 11.1 g/L (± 0.8); late responder group, 9.1 g/L (± 0.5)] (P = 0.006). Standard binary logistic regression to predict vaccine response shows that ischaemic/rhythmic cardiac disease, cirrhosis and gammaglobulin levels were independently associated with the absence of seropositivity (Table 3).
Table 2.
Baseline characteristics of patients without a COVID-19 history according to seronversion status after COVID-19 vaccination
| Variable | Early responder group | Late responder group | Non-responder group | P-value |
|---|---|---|---|---|
| Age (years), mean (SD) | 64.4 (3.6) | 70 (2.1) | 73.5 (3.2) | 0.17 |
| Male, n (%) | 7 (53.9) | 24 (63.2) | 9 (56.3) | 0.8 |
| BMI (kg/m2), mean (SD) | 27.8 (1.6) | 26 (1) | 24 (1.4) | 0.2 |
| DN and/or nephroangioslcerosis, n (%) | 4 (30.8) | 9 (23.7) | 5(31.3) | 0.5 |
| Glomerulonephritis, n (%) | 5 (38.5) | 16 (42.1) | 7 (43.8) | |
| Autosomal dominant polycystic kidney disease, n (%) | 0 | 0 | 0 | |
| Tubulo-interstitial nephropathy, n (%) | 3 (23.1) | 9 (23.7) | 3 (18.8) | |
| Indeterminate nephropathy, n (%) | 1 (7.7) | 4 (10.5) | 1 (6.3) | |
| Blood group, n (%) | 0.6 | |||
| O | 6 (46.2) | 19 (51.4) | 9 (56.3) | |
| A | 6 (46.2) | 11 (29.7) | 7 (43.8) | |
| B | 1 (7.7) | 6 (16.2) | 0 | |
| AB, missing value (n = 2) | 0 | 1 (2.7) | 0 | |
| Dialysis vintage (years), mean (SD) | 6 (2.6) | 6.7 (1.5) | 4.8 (2.4) | 0.8 |
| On steroids and/or immunosuppressants, n (%) | 0 | 3 (7.9) | 4 (25) | 0.07 |
| Diabetes, n (%) | 5 (38.5) | 19 (50) | 5 (31.3) | 0.4 |
| Chronic respiratory disease, n (%) | 3 (23.1) | 18 (47.4) | 2 (12.5) | 0.03 |
| Arterial hypertension, n (%) | 11 (84.6) | 33 (86.8) | 13 (81.3) | 0.9 |
| Ischaemic cardiac disease, n (%) | 3 (23.1) | 14 (36.8) | 11 (68.8) | 0.03 |
| Rhythmic cardiac disease, n (%) | 2 (15.4) | 7 (18.4) | 6 (37.5) | 0.2 |
| Stroke, n (%) | 3 (23.1) | 8 (21.1) | 4 (25) | 0.9 |
| Peripheral artery disease, n (%) | 2 (15.4) | 12 (31.6) | 5 (31.3) | 0.5 |
| Cirrhosis, n (%) | 0 | 1(2.6) | 3 (18.8) | 0.04 |
| History of or active cancer/transplantation, n (%) | 3 (23.1) | 10 (26.3) | 10 (62.5) | 0.02 |
| Albumin (g/L), mean (SD) | 36.1 (1.1) | 36 (0.7) | 34 (1.1) | 0.2 |
| Gamma globulin (g/L), mean (SD) | 11.1 (0.8) | 9.1 (0.5) | 7.3 (0.8) | 0.006 |
| Lymphocyte count (×109/L), mean (SD) | 1.2 (0.2) | 1.2 (0.1) | 1.5 (0.2) | 0.6 |
| Pre-dialysis β2-microglobulinaemia (mg/L), mean (SD) | 28.2 (1.7) | 28 (1) | 27 (1.6) | 0.9 |
| C-reactive protein (mg/L), mean (SD) | 10.3 (4.4) | 13.9 (2.6) | 9.6 (4) | 0.6 |
| HBV vaccine responders, n (%) | 5 (38.5) | 14 (36.8) | 4 (25) | 0.7 |
Table 3.
Standard binary logistic regression to predict responder group (early responder versus late responder versus non-responders)
| Variable | Coefficient estimation (95% CI) | P-value |
|---|---|---|
| On steroids and/or immunosuppressants | −1.3 (−3.3 to −0.6) | 0.17 |
| History of or active cancer/transplantation | −0.9 (−2.2–0.2) | 0.12 |
| Chronic respiratory disease | −0.22 (−1.4–0.9) | 0.69 |
| Ischaemic/rhythmic cardiac disease | −1.34 (−2.5 to −0.2) | 0.017 |
| Cirrhosis | −2.3 (−5.5–0.2) | 0.08 |
| Gamma globulin | 0.23 (0.04–0.4) | 0.016 |
Analysis of patients with a history of COVID-19 seropositivity and seroneutralizing antibodies
Among the 19 previously infected HD patients, 11 (57.9%) had persistent Sab seropositivity before the first vaccine dose. They were younger, tended to have a shorter time between SARS-CoV-2 infection and the vaccine day [120.5 days (IQR 87.5–239) versus 275 (169.5–317.8); P = 0.061] and a higher pre-dialysis β2-microglobulin level than patients in whom Sabs were undetectable at the time of the first vaccine dose (Table 4). Six weeks after vaccination, all the patients had detectable Sabs and Nabs.
Table 4.
Baseline characteristics of patients with COVID-19 history
| Characteristics | Persistent seroconversion | No persistent seroconversion | P-value |
|---|---|---|---|
| (n = 11) | (n = 8) | ||
| Age (years), median (IQR) | 57.8 (45.7–73.6) | 72.1 (68.3–78.6) | 0.03 |
| Male, n (%) | 8 (72.7) | 4 (50) | 0.3 |
| BMI (kg/m2), median (IQR) | 27.3 (23.3–31.1) | 25.5 (20.7–37.5) | 0.26 |
| DN and/or nephroangioslcerosis, n (%) | 3 (27.3) | 1 (12.5) | 0.16 |
| Glomerulonephritis, n (%) | 1 (9.1) | 5 (62.5) | |
| Autosomal dominant polycystic kidney disease, n (%) | 1 (9.1) | 0 | |
| Tubulo-interstitial nephropathy, n (%) | 2 (18.2) | 1 (12.5) | |
| Indeterminate nephropathy, n (%) | 4 (36.4) | 1 (12.5) | |
| Blood group, n (%) | 0.4 | ||
| O | 2 (20) | 2 (25) | |
| A | 5 (50) | 2 (25) | |
| B | 2 (20) | 4 (50) | |
| AB, missing value (n = 2) |
1 (10) |
0 | |
| Dialysis vintage (years), median (IQR) | 3 (1.3–8.6) | 3 (1.3–5.8) | 0.5 |
| On steroids and/or immunosuppressants, n (%) | 0 | 0 | – |
| Diabetes, n (%) | 7 (63.6) | 5 (62.5) | 0.9 |
| Chronic respiratory disease, n (%) | 4(36.4) | 2 (25) | |
| Arterial hypertension, n (%) | 11 (100) | 5 (62.5) | 0.03 |
| Ischaemic/rhythmic cardiac disease, n (%) | 3 (27.3) | 4 (50) | 0.4 |
| Stroke, n (%) | 1 (9.1) | 1 (12.5) | 0.8 |
| Peripheral artery disease, n (%) | 3 (27.3) | 1 (12.5) | 0.4 |
| Cirrhosis, n (%) | 0 | 0 | – |
| History of or active cancer/transplantation, n (%) | 8 (72.7) | 6 (75) | 0.9 |
| Gamma globulin (g/L), median (IQR) | 11.9 (10–12.8) | 9.2 (6.1–11.4) | 0.07 |
| Lymphocyte count (×109/L), median (IQR) | 1.4 (0.9–1.7) | 1.1 (0.6–1.3) | 0.2 |
| Pre-dialysis β2-microglobulinaemia (mg/L), median (IQR) | 27.5 (22.7–32.1) | 22 (13.2–25.9) | 0.04 |
| Albumin (g/L), median (IQR) | 35.8 (32.3–39.1) | 34.7 (32.3–37.2) | 0.9 |
| CRP (mg/L), median (IQR) | 3.6 (1.7–18.9) | 3.1 (1.4–16.9) | 0.8 |
| History of symptomatic COVID, n (%) | 5 (45.5) | 3 (37.5) | 0.7 |
| Delay between COVID-19 diagnosis and vaccine (days), median (IQR) | 120.5 (87.5–239) | 275 (169.5–317.7) | 0.06 |
| HBV vaccine responders, n (%) | 6/6 (100) | 3/5 (60) | 0.18 |
Immune response comparison in HD patients with or without previous SARS-CoV-2 infection
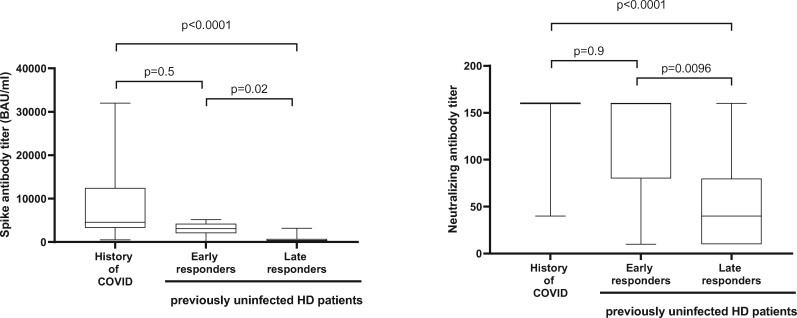
We observed a significant difference for both Sab and Nab titres, respectively, between previously infected HD patients [median level 4548 BAU/mL (IQR 3308–12 449) and 160 dilution level (IQR 160–160)] compared with previously uninfected late responder HD patients [median level 381.4 BAU/mL (IQR 112.5–750.4) and 40 dilution level (IQR 10–80)] (Figure 4). Conversely, no significant difference for both Sab and Nab titres was observed in previously infected HD patients compared with previously uninfected early responder HD patients [median level 3121 BAU/mL (IQR 2028–4255) and 160 dilution level (IQR 80–160)] (Figure 4).
FIGURE 4:
Immune response in participants with or without previous SARS-CoV-2 infection. In each box-and-whisker plot, the horizontal line represents the median, the top and bottom of the box the IQR and the whiskers the minimum and maximum values.
DISCUSSION
In this study we report the step-by-step humoral response analysis following COVID-19 vaccination with the Comirnaty vaccine in HD patients. To our knowledge, we are the first to described the efficacy of COVID-19 vaccine in HD patients with both Sab and Nab titres [10]. Grupper et al. [10] reported good efficacy in dialysis patients, about 96%, comparable to the results of the pivotal trial. However, in their cohort, Sabs were lower in HD patients than in healthy controls.
In our HD patient’s cohort, the overall immunogenicity of the Comirnaty vaccine 3 weeks after the last dose is 100% and 77.2% in those with and without a history of SARS-CoV-2 infection, respectively. We provide the first data related to the evolution of humoral response after the first and second vaccine doses according to COVID-19 history in HD patients and showed that only 20% of HD patients developed an IgG antibody response to the spike antigen after the first dose, but this increased to 77% after the second dose. This serologic profile allows us to conclude the immunogenicity of the vaccination scheme with two doses in COVID-19-naïve HD patients. Immunogenicity was lower in the present cohort than in the recent findings reported by Grupper et al. [10] in Israel, which could be due in part to the absence of knowledge on previous SARS-CoV-2 infection in their cohort and/or to the older age and higher burden of comorbidities in patients from our centre. The overall humoral response of our patients is close to that reported in a recent study in which previous COVID-19 disease was documented before the vaccination [13].
Interestingly, only three-fourths of the seropositive patients had detectable Nabs after two vaccine doses. Whether seropositive patients whose sera display no effective seroneutralization ability are efficiently protected against COVID-19 or remain at high risk and could benefit from a third dose of vaccine remains to be elucidated.
Indeed, our study highlighted some factors that can predict a lower response to vaccination. First, treatment with gamma globulin was associated with a lower response to vaccination, as reported in transplant recipients [19]. Second, ischaemic/rhythmic cardiac disease and cirrhosis seem also to be associated with lower response. Unexpectedly, other factors like corticosteroids, immunosuppressants and age were not independently associated with a lower response but should be interpreted with caution given the small size of the study.
It has been recently reported that the humoral response against SARS-CoV-2 in the general population with a history of SARS-CoV-2 infection is greater than the response in previously uninfected patients who have received a second dose [20]. Our study shows that the early responder HD patients have similar Sab and Nab titres after the second dose compared with previously infected HD patients. Furthermore, the late responders have significantly lower Sab and Nab titres after the second dose compared with the early responders and previously infected HD patients. These findings provide evidence that in previously uninfected patients, a third dose seems necessary in 18% of our HD patient cohort. It is unclear how the Nab titres protect against COVID-19 disease and influence the ability of the host to transmit the virus. Accordingly, it remains to be determined if the late responders without Nabs need a third vaccine dose.
Our study allows for improvement of the vaccination strategy in HD patients. Serological surveys of COVID-19-naïve HD patients may provide rational third vaccine dose prescription by identifying non-responders after two doses. In HD patients with a history of symptomatic COVID-19, we show 100% seropositivity after a single dose, as observed in the general population. Whether patients with a history of asymptomatic COVID need two doses remains to be determined.
CONCLUSION
HD patients develop a substantial humoral immune response following mRNA vaccination. Protection afforded by SARS-CoV-2 seropositivity, the kinetics of antibodies and a serological basis usable to establish correlates of protection remain to be clarified in this high-risk population.
AUTHORS’ CONTRIBUTIONS
T.R., M.G., G.L., L.N., L.S., J.F., P.M.C.B., T.F. and X.L. designed the study, collected data and wrote the paper. N.J.C. and P.B. helped write the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest regarding this study.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author
REFERENCES
- 1.Lano G, Braconnier A, Bataille S. et al. Risk factors for severity of COVID-19 in chronic dialysis patients from a multicentre French cohort. Clin Kidney J 2020; 13: 878–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jager KJ, Kramer A, Chesnaye NC. et al. Results from the ERA-EDTA registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 2020; 98: 1540–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilbrands LB, Duivenvoorden R, Vart P. et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant 2020; 35: 1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ERA-EDTA Council, ERACODA Working Group.Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 2021; 36: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis A, Baigent C, Ikizler TA. et al. The urgent need to vaccinate dialysis patients against severe acute respiratory syndrome coronavirus 2: a call to action. Kidney Int 2021; 99: 791–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke C, Prendecki M, Dhutia A. et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol 2020; 31: 1969–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giot M, Robert T, Brunet P. et al. Vaccination against COVID-19 in a haemodialysis centre: what is the risk of bleeding complications? Clin Kidney J 2021; 14: 1701–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Udomkarnjananun S, Takkavatakarn K, Praditpornsilpa K. et al. Hepatitis B virus vaccine immune response and mortality in dialysis patients: a meta-analysis. J Nephrol 2020; 33: 343–354 [DOI] [PubMed] [Google Scholar]
- 9.Mastalerz-Migas A, Gwiazda E, Brydak LB.. Effectiveness of influenza vaccine in patients on hemodialysis—a review. Med Sci Monit 2013; 19: 1013–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupper A, Sharon N, Finn T. et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol 2021; 16: 1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attias P, Sakhi H, Rieu P. et al. Antibody response to BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int 2021; 99: 1490–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berar Yanay N, Freiman S, Shapira M. et al. Experience with SARS-CoV-2 BNT162b2 mRNA vaccine in Q1 dialysis patients. Kidney Int 2021; 99: 1496–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billany RE, Selvaskandan H, Adenwalla SF. et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients on haemodialysis: a call to arms. Kidney Int 2021; 99: 1492–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torreggiani M, Blanchi S, Fois A. et al. Neutralizing SARS-CoV-2 antibody response in dialysis patients after the first dose of the BNT162b2 mRNA COVID-19 vaccine: the war is far from being won. Kidney Int 2021; 99: 1494–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anft M, Blazquez-Navarro A, Paniskaki K. et al. SARS-CoV-2-reactive cellular and humoral immunity in hemodialysis population. Kidney Int 2021; 99: 1489–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristiansen PA, Page M, Bernasconi V. et al. WHO international standard for anti-SARS-CoV-2 immunoglobulin. Lancet 2021; 397: 1347–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallian P, Pastorino B, Morel P. et al. Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors. Antiviral Res 2020; 181: 104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurtop E, Villarroel PMS, Pastorino B. et al. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol J 2018; 15: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyarsky BJ, Werbel WA, Avery RK. et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021; 325: 1784–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anichini G, Terrosi C, Gandolfo C. et al. SARS-CoV-2 antibody response in persons with past natural infection. N Engl J Med 2021; 385: 90–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author