Abstract
Background:
The use of analgesics and sedatives to provide sedation for Patients in Intensive Care Unit (ICU) is inevitable. The present study aimed to determine the effect of sedation protocol using the Richmond Agitation- Sedation Scale on sedation level and amount of pharmacological and non-pharmacological interventions on patients under mechanical ventilation.
Materials and Methods:
This randomized clinical trial was conducted on 79 patients under mechanical ventilation in Zanjan. The patients were recruited using the blocking randomized sampling method. In the experiment group, the sedation was provided hourly, using the Richmond sedation Protocol, during the mechanical ventilation period. The level of sedation and pharmacological and nonpharmacological interventions were compared in the two groups using Fisher exact test.
Results:
Totally, 40 patients in the experiment and 39 patients in the control groups were evaluated. No significant difference was found between the two groups in terms of confounding variables (age, sex, level of consciousness, Acute Physiologic and Chronic Health Evaluation (APACHE) II criterion, underlying disease, and cause of hospitalization). The level of sedation in the experiment group was significantly closer to the ideal score of the Richmond Scale compared to the control group (p < 0.001). The experimental group received significantly more non-pharmacological interventions and fewer pharmacological interventions compared to the control group (P < 0.001).
Conclusions:
Using a sedation protocol could provide better sedation levels in patients under mechanical ventilation, and reduce the use of sedative medications, and consequently, the cost of hospitalization. Further research is suggested.
Keywords: Clinical Trial Protocol, Deep sedation, Ventilators, Mechanical, intensive care units, Iran
Introduction
The Intensive Care Unit (ICU) is one of the most critical and professional wards of a hospital.[1] Mechanical Ventilation (MV) is required in more than 90% of adult patients with critical illness in the intensive care unit.[2] Sedation is often necessary for patients under MV; however, it must be based on individual assessment and patient's needs.[3] It should be noted that using high doses or prolonged sedative medications can cause serious complications in patients.[4] Overdose of sedatives and analgesics can cause serious side effects, such as over-sedation, respiratory depression, hemodynamic instability, and consequences of drug accumulation in the body.[5] Besides, inadequate sedation in some cases can lead to aggressive behaviors towards medical staff, and fighting with the ventilator.[6] Evidence indicates that inappropriate sedation, increases mortality, ventilator-associated pneumonia, ventilator-associated lung injury, and increases treatment costs.[7] The use of sedation protocols reduces medication costs and increases the quality of sedation and analgesia in patients who require long-term sedation.[8]
Nurses play an important role in sedation management because of their continued nursing care and administering sedatives through examining and monitoring patients.[9] Evidence supports that ICUs' nurses do not tend to use pain monitoring tools for patients who are not able to speak and have little knowledge about pain control guidelines, which could negatively affect their performance on pain.[10] Therefore, it is required for nurses to adopt an accurate monitoring method and decision-making framework for the safe administration of sedatives.[9] Evaluating the agitation level of patients using an appropriate protocol, helps nurses to identify the problems causing patients' agitation which have not been relieved by medicine, and to provide better pain relief and comfort for the patient.[11]
There is a large body of evidence about the effect of using sedation protocol on the time of weaning from ventilator support,[3,12] length of stay in the ICU,[3,13,14] length of stay in the hospital,[9] the frequency of self-extubating,[15] the rate of reintubation,[16] and other variables separately. However, there is a contradictory finding in this regard. In some studies, an approved sedation protocol has never been used.[3] On the other hand, the former studies had some limitations such as applying the sedation protocol for a short time (24 to 48 h),[17,18,19] or low interval of assessment (e.g. every 4 or 6 h),[17,18,19] which have been conducted on different participants.[9,13,19] Also, little is known about the frequency of using (every an hour) of Richmond Agitation- Sedation Scale (RASS) for a total period of MV that can affect non-pharmacological procedures (nursing care), consumption of different types of sedatives consumption (pharmacological-interventions), and the cost for patients. Therefore, the current agitation-sedation protocol was proposed by researchers underlying the findings of the other studies. Later on, the study was conducted to determine the effect of sedation protocol using Richmond Agitation- Sedation Scale (RASS) on the level of consciousness, and the amount of pharmacological and nonpharmacological interventions in patients under mechanical ventilation in ICU.
Materials and Methods
This single-blind randomized clinical trial (IRCT2017010831824N1) was conducted on eligible head trauma patients in the general ICU in Ayatollah Mousavi Hospital in Zanjan from January to June 2016. This paper is part of the findings of a Master's dissertation of intensive care nursing of which, some were formerly published. According to the results of other studies[11,18] (N = 80) with the power of 0.90 (β: 0.10), α: 0.05, d = 0.08 an attrition rate probability of 10%, a total of 90 participants were estimated and recruited in the study. The standard deviations of the main variable were considered equal to achieve a higher sample size. Totally, 79 patients intervention group (N = 40), the control group (N = 39) were studied, and 11 patients were excluded from the study for different reasons [Figure 1].
Figure 1.
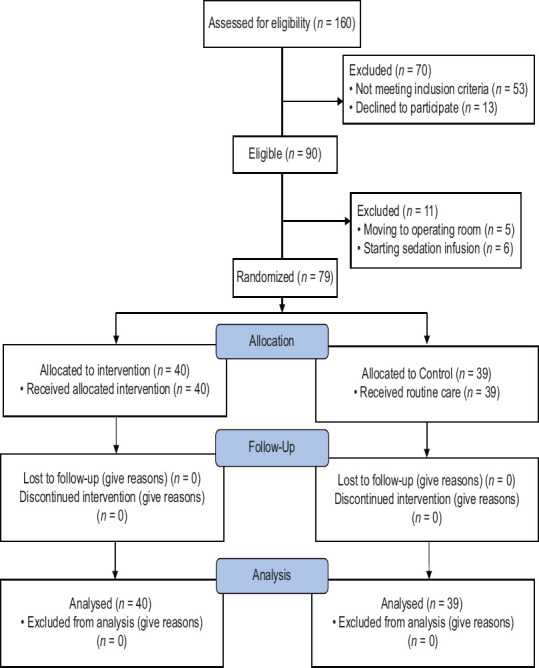
Consort flow diagram of patients' recruitment in the study
Newly admitted patients were selected through simple random sampling according to inclusion criteria. After receiving the informed consent from the patient's companions and their legal guardians, the patients were randomly allocated into two experimental or control groups using the blocking method (nine ten-blocks). According to the inclusion criteria, patients between the ages of 15 and 65, who were admitted to ICU with endotracheal intubation on the first day of hospitalization, mechanically ventilated, being nonaddicted, having nonneurological disorders (according to the hospital report and information from their companions), scoring above -3 on the Richmond Scale, having APACHE II score between 10 and 20, and Glasgow-based level of consciousness between 7 and 13, were included in the study. Exclusion criteria included awakening and withdrawal of endotracheal tubeless than 24 h; changing of the prescribed sedation by the physician, stopping the administration of an analgesic, having surgery, having the consciousness level below 5, and beginning of a continuous infusion of sedative medication.
The Glasgow Coma Scale, Richmond Agitation- Sedation Scale, Acute Physiologic and Chronic Health Evaluation((APACHE II) score Acute Physiologic and Chronic Health Evaluation, pharmacological and non-pharmacological interventions (positioning, physiotherapy, discharge suction, noise reduction, light adjustment, replacement, cover pressure reduction, and environment temperature adjustment) checklists were used for data collection. The Glasgow Coma tool is designed to assess the patient's level of consciousness and responses to stimulators, which is scored between 3 (deep coma) and 15 (full consciousness).). This is one of the standard tools that was revised in 2005.[20,21] The Richmond Agitation- Sedation Scale is one of the recommended[22] and validated scales for measuring the level of sedation in the critical units.[22] This scale measures the level of sedation using a 10-point continuum of -5 to +4 in three levels which in 5 negative scores refer to calm level (-1 = sleepy, -2 = mild relief, -3 = moderate relief, -4 = deep relief, -5 = awake), 0 score refers to normal and calm behavior, and 4 positive score shows the level of agitation (+1 = restless, +2 = agitated, +3 = very agitated, +4 = agitator). Very good reliability has been reported for this tool in different external and internal studies ((α = 79%–95%).[11] The APACHE is a good tool that is used in ICU to accurately predict patient mortality in all patients.[17,18]
The pharmacological intervention checklists included all information about the drug name, frequency, dose, and time of use. The assessed drugs consisted of fentanyl, methadone, morphine, midazolam, thiopental (nesdonal), and haloperidol as sedatives to provide sedation for patients. Nonpharmacological interventions checklist included information about position changing, physiotherapy, suction discharge, noise reduction, light adjustment, adjustment of clothing or sheets, reduction of the ventilator pressure and other medical device accessories, and setting environment temperature. To perform the intervention, the study protocol (using RASS) was developed and validated [Figure 2]. Therefore, the proposed protocol was presented to 10 academic faculty members and experts (including ICU physicians, the head nurse, ICU nurses, and faculty members) of Zanjan University of Medical Sciences, and their comments were incorporated into the protocol.
Figure 2.
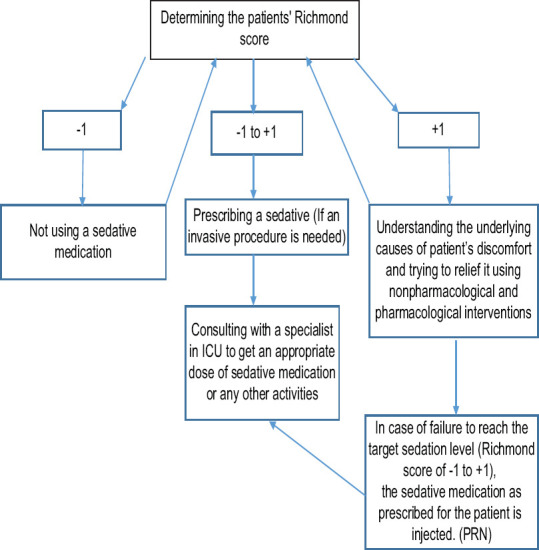
Agitation-Sedation protocol
The application and evaluation of the sedation protocol using the RASS Scale and the infusion of sedative drugs was priory instructed to research assistants individually, on various shifts by the researcher. Following the individual training, each assistant researcher assessed three patients according to the protocol in order to investigate the agreement between the researcher and the research assistants (four people aged 30–40 with more than 3 years of work experience in the ICU). The necessary steps were taken, based on the protocol and the inter-rater agreement coefficient between the researcher and researcher assistants was calculated 0.78.
The research assistants in different shifts were trained on how to use the Richmond Scale, how to assess the patients' level of comfort, how to administer the sedative, and how to use the sedation protocol.
The level of consciousness and sedation of all patients in both the experimental and control groups were measured using the Glasgow Coma Scale and the Richmond Criterion, at the time of entrance into ICU. Information on patients' pharmacological and nonpharmacological interventions, length of stay, duration of mechanical ventilation, and mortality rate were recorded during the intervention (the results reported in another paper).[23] The APACHE II criterion was used to match the severity of the disease and control confounding factors between the experimental and control groups. The whole course of the study, such as the intervention and the clinical status of the patients, was performed under the supervision of the ICU physician. Apart from the sedation process, all medications were identical for both the experimental and the control groups, according to the ICU guidelines. The control group did not receive any interventions based on the proposed sedation protocol, and pain control and sedation procedures were performed as per routine according to the physiological responses of patients, and clinical judgment of nurses, by the injection of the sedative drugs as prescribed by ICU specialist (e.g. intravenous injections of fentanyl, midazolam, thiopental, haloperidol, morphine, and methadone). Pain control and sedation in the experimental group were performed by the research team, according to the developed protocol [Figure 2].
According to the proposed protocol, if the sedation score was between +1 and -1, no special action was needed the sedative medication was only injected if any invasive procedures were necessary. The patient's level of sedation in both groups was assessed every hour while the vital signs of patients were measured and recorded. If the patient's comfort level score was greater or equal to +1, they initially received nonpharmacological interventions (e.g. changing position, reducing endotracheal pressure on the lip or nose, and reducing environmental noise) to provide their comfort. If discomfort was relieved, the patient's comfort level was monitored every hour according to the protocol using RASS. If patients' discomfort continued, the level of agitation was again measured by RASS, and a sedative was administrated as prescribed. The duration of intervention for each patient was equal to the total period under mechanical ventilation. Richmond scores and the amount of pharmacological and nonpharmacological interventions were measured and compared hourly in the morning and evening shifts during the mechanical ventilation period in both the experiment and the control groups.
To avoid measurement bias, the information about the agitation-sedation level was collected and recorded by research assistants, and all data about the pharmacological and nonpharmacological interventions. Kolmogorov-Smirnov test was used to determine the normal distribution of variables, and parametric statistical tests were used to analyze the data due to the normal distribution of variables. Descriptive statistics were used to estimate absolute and relative frequency, mean, and standard deviation (SD), and graphs. Chi-square test (and Fisher's exact test if it was needed) and independent t-test were used to compare underlying and confounding variables of the two groups. To test the research hypotheses, the participants' scores were adjusted for each patient per shift, using Restructure in SPSS version 16, consequently, the Chi-square test (and Fisher's exact test if it was necessary) and independent t-test were used as needed. The significance level was considered as (p < 0.05).
Ethical considerations
The present study was conducted in accordance with the ethical standards as laid down in the Declaration of Helsinki, and the ethical approval was obtained from the Vice Chancellor for Research of the Zanjan University of Medical Sciences on 23.11.2016 (ZUMS.REC.1395.215) and has been registered within the Iranian clinical trial registry. The researcher explained the objectives of the study to the patient's companions and their legal guardians and patients whose legal guardians were willing to participate in the study recruited in the study after receiving the informed consent form of their legal guardians. It was also emphasized that patients' legal guardians can leave the research any time they wish and that this will not have any negative impact on the patient's treatment and nursing care.
Results
Totally 79 traumatic patients, under mechanical ventilation, were assessed in the experiment group (n = 40) and the control group (n = 39). No significant differences were found among demographic data (age, sex), and clinical characteristics (APACHE II score, Glasgow consciousness scale, underlying diseases, and cause of hospitalization) between the two groups. This indicates that the confounding factors have been controlled as much as possible. The restructure in SPSS was used due to the unequal number of ventilation between two groups to achieve the objectives of the study. The comparison of the agitation-sedation level between two groups using an independent t-test showed that the scores of RASS in the experimental group (under the sedation protocol) were significantly within the range of target Richmond scores (scores 0, +1, and -1), during the first 5 days (F10= 1207.50, p < 0.001) and the second 5 days (F10= 1260.45, p < 0.001). These results were repeated for almost all days of intervention (1st to 9th days). The comparison of Richmond scale scores between the experiment and the control groups, during the intervention period (the first 5 days and the second 5 days) are presented in Table 1. The experimental group received significantly more nonpharmacological interventions compared to the control group, during the first 5 days (F13 = 1065.34, p< 0.001) and the second 5 days (F12 = 1177.29, p < 0.001) [Table 2]. The comparison of the pharmacological interventions between the two groups [Table 3] showed that using the number of different sedative drugs in the control group was significantly more than the experimental group. This indicates that sedation protocol can significantly reduce the sedative medication use in the patients under the intervention, during the first 5 days (F15 = 521.10, p < 0.001) and the second 5 days (F13 = 1035.32, p < 0.001).
Table 1.
Comparison of Richmond scale scores between the experiment and the control groups during the intervention period (the first and the second 5 days)
| Richmond score Group |
+ 4n (%) | + 3 n (%) | + 2 n (%) | + 1 n (%) | 0 n (%) | -1 n (%) | -2 n (%) | -3 n (%) | -4n (%) | -5n (%) | Total n (%) | Fisher exact test | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First 5 days | Experiment | 0 (0.00) | 0 (0.00) | 48 (11.00) | 1130 (51.40) | 2253 (60.40) | 343 (31.60) | 9 (2.60) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3785 (48.10) | F 10=1207.50p<0.001 |
| Control | 2 (100.00) | 40 (100.00) | 390 (89.00) | 1068 (48.60) | 1479 (39.60) | 743 (68.40) | 335 (97.40) | 21 (100.0) | 1 (100.00) | 1 (100.00) | 40771 (51.90) | ||
| Total | 2 (100.00) | 40 (100.00) | 438 (100.00) | 2200 (100.00) | 3730 (100.00) | 1086 (100.00) | 344 (100.00) | 21 (100.00) | 1 (100.00) | 1 (100.00) | 7861 (100.00) | ||
| Second 5 days | Experiment | 0 (0.00) | 0 (0.00) | 12 (5.90) | 349 (33.70) | 811 (46.70) | 134 (19.00) | 2 (1.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1308 (33.40) | F 10=1260.45p<0.001 |
| Control | 2 (100.00) | 16 (100.00) | 191 (94.10) | 686 (66.30) | 926 (53.30) | 572 (81.00) | 208 (99.00) | 3 (100.00) | 1 (100.00) | 1 (100.00) | 2606 (66.60) | ||
| Total | 2 (100.00) | 16 (100.00) | 203 (100.00) | 1035 (100.00) | 1737 (100.00) | 706 (100.00) | 120 (100.00) | 3 (100.00) | 1 (100.00) | 1 (100.00) | 3914 (100.00) | ||
Table 2.
Comparison of nonpharmacological interventions between the experiment and the control group during the intervention period (the first and the second 5 days)
| Type of non-pharmacological action Group |
Changing Positionn (%) | Physiotherapy n (%) | Suction n (%) | Reduce ambient noisen (%) | Adjust the lightsn (%) | Replacement of cloth or beddingn (%) | Reducing the pressure of the connections n (%) | Set ambient temperaturen (%) | No actionn (%) | Totaln (%) | Fisher exact test | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First 5 days | Experiment | 327 (90.50) | 129 (81.10) | 401 (46.30) | 55 (100.00) | 37 (100.00) | 123 (63.10) | 128 (100.00) | 50 (100.00) | 2817 (41.40) | 3785 (48.10) | F 13=1065.34p<0.001 |
| Control | 33 (9.50) | 30 (18.90) | 292 (53.70) | 0 (0.00) | 0 (0.00) | 72 (36.90) | 0 (0.00) | 0 (0.00) | 3980 (58.60) | 40771 (51.90) | ||
| Total | 360 (100.00) | 159 (100.00) | 693 (100.00) | 55 (100.00) | 37 (100.00) | 195 (100.00) | 128 (100.00) | 50 (100.00) | 6797 (100.00) | 7861 (100.00) | ||
| Second 5 days | Experiment | 103 (78.00) | 54 (58.10) | 136 (37.60) | 4 (100.00) | 11 (100.00) | 44 (43.10) | 34 (100.00) | 17 (94.40) | 890 (28.00) | 1308 (33.40) | F 12=1177.29p<0.001 |
| Control | 36 (22.00) | 39 (41.90) | 166 (62.40) | 0 (0.00) | 0 (0.00) | 58 (56.90) | 0 (0.00) | 1 (5.60) | 2294 (72.00) | 2606 (66.60) | ||
| Total | 327 (90.50) | 129 (81.10) | 401 (46.30) | 55 (100.00) | 37 (100.00) | 123 (63.10) | 128 (100.00) | 50 (100.00 | 2817 (41.40) | 3785 (48.10) | ||
Table 3.
Comparison of pharmacological intervention (sedative medication) between the experiment and the control group during the intervention period (the first and the second 5 days
| Type of pharmacological action Group |
Fentanyln (%) | Methadonen (%) | Morphinen (%) | Midazolamn (%) | Haloperidoln (%) | Thiopentaln (%) | Without any drugn (%) | Totaln (%) | Fisher exact test | |
|---|---|---|---|---|---|---|---|---|---|---|
| First 5 days | Experiment | 179 ( 41.80 ) | 46 ( 43.40) | 0 ( 0.00) | 419 ( 28.80) | 11 ( 45.80) | 9 ( 27.30) | 3103 ( 50.40) | 3785 ( 48.10) | F 15=521.10p<0.001 |
| Control | 246( 58.20) | 60 ( 56.60) | 6 ( 100.00) | 627 ( 71.20) | 13 ( 54.20) | 16 ( 72.70) | 3057 ( 49.60) | 40771 ( 51.90) | ||
| Total | 425 (100.00) | 106 (100.00) | 6 (100.00) | 1046 (100.00) | 24 (100.00) | 25 (100.00) | 6160 (100.00) | 7861 (100.00) | ||
| Second 5 days | Experiment | 55 (27.10) | 12 (24.00) | 0 (0.00) | 117 (14.50) | 0 (0.00) | 0 (0.00) | 1118 (35.90) | 1308 (33.40) | F 13=1035.32p<0.001 |
| Control | 149 (72.90) | 38 (76.00) | 4 (100.00) | 366 (85.50) | 7 (100.00) | 10 (100.00) | 1998 (61.40) | 2606 (66.60) | ||
| Total | 204 (100.00) | 50 (100.00) | 4 (100.00) | 43 (100.00) | 7 (100.00) | 10 (100.00) | 3116 (100.00) | 3914 (100.00) | ||
Discussion
The purpose of this study was to determine the effect of sedation protocol using the Richmond Agitation-Sedation scale on the level of sedation and the amount of pharmacological and nonpharmacological interventions in patients under mechanical ventilation in the intensive care unit. The results showed that using the sedation protocol can provide better sedation and analgesia for patients under mechanical ventilation along with using fewer sedative medications and more nonpharmacological interventions. The frequency of the ideal range of the Richmond scale score also was significantly more in the experiment group – under the sedation protocol – compared to the control group. The above-mentioned finding is in line with the results of some studies.[11,17,18,19] However, Bucknall et al.[24] concluded in their research that using sedation protocol by nurses does not contribute much to the sedation level of patients. They also reported that their different findings might be due to some nursing policies in Australia, which made nurses better perceive their key role in assessing patients' pain and sedation levels without using a sedation protocol, to provide patients' well-being and comfort. Therefore, as Australian nurses appropriately assess sedation level as a routine job, using the protocol does not affect the sedation level of their patients.
The amount of medication taken in the experimental group was significantly less than the control group, most of the days. The results of the current study are consistent with the study of Mirzaei et al.[9] who reported in terms of sufentanil sedation, although the sedation scale used and the type of participants in the mentioned study was different from the current one. They used the Riker criterion for sedation in patients under coronary artery bypass graft surgery, and only the amount of sufentanil was evaluated. Whereas, in the present study, the Richmond scale was used for sedation evaluation in traumatic patients, and the amount of fentanyl, methadone, morphine, midazolam, thiopental (Nesdonal), and haloperidol was assessed in both the experimental and the control groups.
The results of a study by Yousefi et al.[3] did not show any significant difference in the mean consumption of midazolam and morphine after the intervention,[3] which is not consistent with the results of our study. Another study by Abdar et al.[17] reported a significant decrease in the amount of midazolam and morphine in the experimental group under sedation protocol compared to the control group,[17] which is consistent with the findings of the current study. However, the mentioned study was different from the current one in terms of the participants (all patients admitted to the ICU were included), and the type of sedatives that were assessed (midazolam and morphine only). Similar findings were reported by Robinson et al.[25] about the reduction of using propofol in the protocol group compared to the nonprotocol group. However, Robinson et al.[25] only assessed opioids and propofol, whereas the present study included a wide range of sedative drugs (e.g. fentanyl, methadone, morphine, midazolam, thiopental (Nesdonal), and haloperidol).
Rafiei et al.[19] reported a nonsignificant decrease in the use of morphine and midazolam. These results are in line with the results of the present study, although Rafiei et al.[19] examined addicted participants and evaluated the agitationsedation level with 4-h intervals versus hourly assessment in the current study. Weisbrodt et al.[7] found no significant difference in the amount of fentanyl and midazolam sedatives administered in patients under mechanical ventilation, which is inconsistent with the results of the present study. The patients under study and the type of drug administration (continuous infusion) might be the possible reasons for the different results of the two studies. Payen et al.[26] reported that patients under mechanical ventilation received between 40% and 50% extra sedative medications, and using the sedation protocol slightly reduced the rate of administrating midazolam, propofol, fentanyl, sufentanil, emifentanil, and methadone during 6 days, in the intensive care unit. However, the results of the present study showed a significant decrease during 10 days of hospitalization in ICU. This slight difference in the results could be because of a longer length of stay in ICU, the nature of the patients studied, and the intervals of sedation level assessment. Payen et al. [26] excluded patients with a head injury from their study, whereas in the present study head trauma patients were included, which might be the reason for longer staying in the ICU, and consequently, a greater difference in the amount of sedative administration.
To the best of our knowledge, this is the first study that has been conducted to determine the effect of applying a sedation protocol to improve the sedation level in patients under mechanical ventilation, on an hourly basis, for the total duration of ventilation and evaluation of wide range of sedative medications. The results provide a good basis for improving sedation administration and preventing agitation and receiving unnecessary sedatives in patients under mechanical ventilation. This study is strengthened with the evaluation of patients in the total duration of mechanical ventilation. Similar interventions were conducted only for 24 to 48 h, with every 2, 3, or 4 h,[11,13,18] whereas in the current study, agitation –sedation level was measured hourly, for 10 days. The results showed that the rate of nonpharmacological interventions performed in the intervention group was significantly higher than in the control group. Skrobik et al.[27] also concluded in their study that using combinational systematic management protocols for pain control, sedation, and treatment of delirium using nonpharmacological actions, and individual-based interventions are associated with better clinical outcomes in patients (e.g. better pain relief, less mortality rate, shorter mechanical ventilation and hospitalization period). No other relevant article was found about the effect of sedation protocol on the amount of nonpharmacological interventions, although some articles were retrieved on the effect of nonpharmacological interventions (like the interventions taken in this article) on reducing the pain of patients in the ICU[28] increasing sleep quality,[29] and reducing delirium.[30] All mentioned studies concluded that the use of nonpharmacological measures can be safe, low-cost, and effective in managing the quality of sleep and reducing pain and delirium in ICU patients.
The current study had some limitations. First, as this pilot study was only conducted on traumatic patients in a general ICU in Zanjan, it is not possible to generalize the result of this study to all patients and ICUs. Second, it was impossible to follow up on the recovery situation of patients because of the time limitation of the intervention period for 10 days. The effect of confounding factors was adjusted using randomization, matching medications, and using APACHE scale for the severity of the disease in designing and conducting the study. As the number of days for intervention in patients under mechanical ventilation was unequal, the restructuring was used in SPSS statistical software to solve the problem. Finally, there was the possibility of measurement bias, which was controlled by blinding the study's research assistants.
Conclusion
Most patients in ICU experience restlessness due to ICU conditions and device connections. Pain and sedation control in these patients are important for nurses and other members of the medical team. As nurses face more sedation problems and due to their continued presence in ICUs and involvement with the patient's care, compared to other team members, they can play an essential role in using the sedation protocol for ICU patients. The results of the present study showed that the use of the sedation protocol using Richmond's Agitation- Sedation Scale by nurses improves patient comfort and could result in using more nonpharmacological care, and fewer sedatives. Using this proposed protocol in the ICUs, and conducting further research to achieve a better understanding in this regard is recommended.
Financial support and sponsorship
Research Vice-Chancellor of Zanjan University of Medical Sciences
Conflicts of interest
Nothing to declare.
Acknowledgements
The researchers would like to express their gratitude and appreciation to the director of the Nursing Office of Ayatollah Mousavi Hospital in Zanjan, the head nurse and the ICU nurses, who co-operated in this research, as well as the participants and their families who have devotedly helped us to implement this study (Grant Number: A-11-147-6).
References
- 1.Marino PL. Marino's the ICU Book. Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 2.Yazdannik AR, Salmani F, Irajpour AR, Abasi S. Effect of the nurse-directed weaning readiness assessment on the duration of mechanical ventilation: A randomized clinical trial. Qom Univ Med Sci J. 2013;7:89–94. (In persian) [Google Scholar]
- 3.Yousefi H, Toghyani F, Yazdannik AR, Fazel K. Effect of using Richmond Agitation Sedation Scale on duration of mechanical ventilation, type and dosage of sedation on hospitalized patients in intensive care units. Iran J Nurs Midwifery Res. 2015;20:700–4. doi: 10.4103/1735-9066.170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuthill JA, Jarvie L, McGovern C, Shaw M. The effects of sedation cessation within the first four hours of intensive care unit admission in mechanically ventilated critically ill patients–A quality improvement study. EClinicalMedicine. 2020;26:100486. doi: 10.1016/j.eclinm.2020.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi Z, Yang S, Qu J, Li M, Zheng J, Huang R, et al. Effects of nurse-led sedation protocols on mechanically ventilated intensive care adults: A systematic review and meta-analysis. Aust Crit Care. 2020 doi: 10.1016/j.aucc.2020.07.013. doi: 10.1016/j.aucc. 2020.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Haenggi M, Ypparila-Wolters H, Hauser K, Caviezel C, Takala J, Korhonen I, et al. Intra-and inter-individual variation of BIS-index® and Entropy® during controlled sedation with midazolam/remifentanil and dexmedetomidine/remifentanil in healthy volunteers: An interventional study. Critical Care. 2009;13:R20. doi: 10.1186/cc7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisbrodt L, McKinley S, Marshall AP, Cole L, Seppelt IM, Delaney A. Daily interruption of sedation in patients receiving mechanical ventilation. Am J Crit Care. 2011;20:e90–8. doi: 10.4037/ajcc2011415. [DOI] [PubMed] [Google Scholar]
- 8.Altawalbeh SM, Saul MI, Seybert AL, Thorpe JM, Kane-Gill SL. Intensive care unit drug costs in the context of total hospital drug expenditures with suggestions for targeted cost containment efforts. J Crit Care. 2018;44:77–81. doi: 10.1016/j.jcrc.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Mirzaei M, Kalhori RP, Moradi G, Khatoni A, Rezaei M. The effect of Riker sedation-agitation scale on clinical outcome of patients under coronary artery bypass graft surgery. Iran J Crit Care Nurs. 2014;6:217–22. [Google Scholar]
- 10.Rose L, Smith O, Gélinas C, Haslam L, Dale C, Luk E, et al. Critical care nurses' pain assessment and management practices: A survey in Canada. Am J Crit Care. 2012;21:251–9. doi: 10.4037/ajcc2012611. [DOI] [PubMed] [Google Scholar]
- 11.Ebrahimi TE, Keykha AA, Abbaszadeh A, Rafiei H, Enayati H, Khodadadi HBM. The effect of the sedation protocol on the level of consciousness in ventilator-dependent trauma patients hospitalized in Intensive Care Unit(ICU) Med Surg Nurs J. 2015;4(1):e88119. (In Persian) [Google Scholar]
- 12.Borkowska M, Labeau S, Schepens T, Vandijck D, Van de Vyver K, Christiaens D, et al. Nurses' sedation practices during weaning of adults from mechanical ventilation in an intensive care unit. Am J Crit Care. 2018;27:32–42. doi: 10.4037/ajcc2018959. [DOI] [PubMed] [Google Scholar]
- 13.Babamohamadi H, Tangestani F, Soleimani M, Abbasi Dorche S. Effect of a sedation guideline on the duration of mechanical ventilation and length of stay in intensive care unit. Koomesh. 2017;19(2):380–90. (In Persian) [Google Scholar]
- 14.Aitken LM, Bucknall T, Kent B, Mitchell M, Burmeister E, Keogh SJ. Protocol-directed sedation versus non-protocol-directed sedation in mechanically ventilated intensive care adults and children. Cochrane Database Syst Rev. 2018;11:CD009771. doi: 10.1002/14651858.CD009771.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): A randomised controlled trial. Lancet. 2008;371:126–34. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 16.Nantsupawat N, Nantsupawat T, Limsuwat C, Sutamtewagul G, Nugent K. Factors associated with reintubation in patients with chronic obstructive pulmonary disease. Qual Manag Health Care. 2015;24:200–6. doi: 10.1097/QMH.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 17.Abdar ME, Rafiei H, Abbaszade A, Hosseinrezaei H, Abdar ZE, Delaram M, et al. Effects of nurses' practice of a sedation protocol on sedation and consciousness levels of patients on mechanical ventilation. Iran J Nurs Midwifery Res. 2013;18:391–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Akbar Keykha A, Abbaszadeh A, Enayati H, Borhani F, Rafiei H, Hoseini BMK. Applying the instruction of pain control and sedation of the patients hospitalized in intensive care unit. Iran J Crit Care Nurs. 2014;6:243–50. [Google Scholar]
- 19.Rafiei H, Ahmadinejad M, Amiri M, Abdar ME. Effect of nursing implemented sedation and pain protocol on the level of sedation, pain and amount of sedative and analgesic drugs use among opium addicted critically ill patients. Asian J Nurs Educ Res. 2013;3:37–41. [Google Scholar]
- 20.Khan M, O'Keeffe T, Jehan F, Kulvatunyou N, Kattaa A, Gries L, et al. The impact of Glasgow Coma Scale–age prognosis score on geriatric traumatic brain injury outcomes. J Surg Res. 2017;216:109–14. doi: 10.1016/j.jss.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Jennett B. Development of Glasgow coma and outcome scales. Nepal J Neurosci. 2005;2:24–8. [Google Scholar]
- 22.Carlson KK. AACN Advanced Critical Care Nursing-E-Book Version to be sold via e-commerce site. Elsevier Health Sciences. 2008 [Google Scholar]
- 23.Taran Z, Namadian M, Faghihzadeh S, Naghibi T. The effect of sedation protocol using Richmond Agitation-Sedation Scale (RASS) on some clinical outcomes of mechanically ventilated patients in intensive care units: A randomized clinical trial. J Caring Sci. 2019;8:199–206. doi: 10.15171/jcs.2019.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucknall TK, Manias E, Presneill JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian intensive care unit. Crit Care Med. 2008;36:1444–50. doi: 10.1097/CCM.0b013e318168f82d. [DOI] [PubMed] [Google Scholar]
- 25.Robinson BR, Mueller EW, Henson K, Branson RD, Barsoum S, Tsuei BJ. An analgesia–delirium–sedation protocol for critically ill trauma patients reduces ventilator days and hospital length of stay. J Trauma Acute Care Surg. 2008;65:517–26. doi: 10.1097/TA.0b013e318181b8f6. [DOI] [PubMed] [Google Scholar]
- 26.Payen J-F, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou J-L, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill PatientsA prospective multicenter patient-based study. Anesthesiology. 2007;106:687–95. doi: 10.1097/01.anes.0000264747.09017.da. [DOI] [PubMed] [Google Scholar]
- 27.Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi DK, Kavanagh BP. Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111:451–63. doi: 10.1213/ANE.0b013e3181d7e1b8. [DOI] [PubMed] [Google Scholar]
- 28.Gelinas C, Arbour C, Michaud C, Robar L, Côté J. Patients and ICU nurses' perspectives of non-pharmacological interventions for pain management. Nurs Crit Care. 2013;18:307–18. doi: 10.1111/j.1478-5153.2012.00531.x. [DOI] [PubMed] [Google Scholar]
- 29.Foreman B, Westwood AJ, Claassen J, Bazil CW. Sleep in the neurological intensive care unit: Feasibility of quantifying sleep after melatonin supplementation with environmental light and noise reduction. J Clin Neurophysiol. 2015;32:66–74. doi: 10.1097/WNP.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 30.Bannon L, McGaughey J, Verghis R, Clarke M, McAuley DF, Blackwood B. The effectiveness of non-pharmacological interventions in reducing the incidence and duration of delirium in critically ill patients: A systematic review and meta-analysis. Intensive Care Med. 2019;45:1–12. doi: 10.1007/s00134-018-5452-x. [DOI] [PubMed] [Google Scholar]


