Abstract
Background
Racial disparities in SARS-CoV-2 prevalence are apparent. Race is a sociocultural construct, necessitating investigation into how sociocultural factors contribute.
Methods
This cross-sectional study linked laboratory data of adult patients between February 29 and May 15, 2020 with socio-demographics variables from the 2018 American Community Survey (ACS). Medical sites included healthcare organizations in Michigan, New York, North Carolina, California, Florida, Pennsylvania, and Washington. Race was treated as a proxy for racism and not biological essentialism. Laboratory data included patient age, sex, race, ethnicity, test result, test location, and residential ZIP code. ACS data included economic and educational variables contributing to an SES Index, population density, proportion Medicaid, and racial composition for corresponding ZIP code. Associations between race/socioeconomic variables and test results were examined using odds ratios (OR).
Results
Of 126 452 patients [mean (SD) age 51.9 (18.4) years; 52 747 (41.7%) men; 68 856 (54.5%) White and 27 805 (22.0%) Black], 18 905 (15.0%) tested positive. Of positive tests, 5238 (SD 27.7%) were White and 7223 (SD 38.2%) were Black. Black race increased the odds of a positive test; this finding was consistent across sites [OR 2.11 (95% CI 1.95–2.29)]. When subset by race, higher SES increased the odds of a positive test for White patients [OR 1.10 (95% CI 1.05–1.16)] but decreased the odds for Black patients [OR 0.92 (95% CI 0.86–0.99)]. Black patients, but not White patients, who tested positive overwhelmingly resided in more densely populated areas.
Conclusions
Black race was associated with SARS-CoV-2 positivity and the relationship between SES and test positivity differed by race, suggesting the impact of socioeconomic status on test positivity is race-specific.
Keywords: race, socioeconomic status, SARS-CoV-2 testing, disparities
Introduction
Impact Statement
Racial disparities in SARS-CoV-2 prevalence are apparent. A collaboration of 8 geographically diverse medical centers investigated how sociodemographic factors contribute by examining laboratory data of 126 452 adult patients from the beginning of the pandemic. Black race increased the odds of a positive test by 2.11 [95% CI 1.95–2.29]. The relationship between socioeconomic status and test positivity differed by race: higher socioeconomic status was protective in Black patients but increased the risk of a positive test in White patients. This suggests the impact of socioeconomic status on SARS-CoV-2 positivity is race-specific, indicating a need to consider how sociodemographic factors create health disparities.
Coronavirus Disease 2019 (COVID-19)-related demands amplified preexisting disparities in health care access, impacting many minority communities, especially Black/African Americans (1). The USA has faced ubiquitous scarcity of diagnostic tests, supplies, and healthcare providers, and anticipated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine shortages and rationing (2). Historical handling of health care resources during prior influenza outbreaks suggests that minorities could be at risk of under-allocation of medical resources during the next phase of this pandemic (3). Testing and vaccination are equally essential tools to mitigate SARS-CoV-2 infections, and both are subject to access barriers. A deeper understanding of SARS-CoV-2 testing disparities during the early days of the pandemic can help anticipate and mitigate access inequality to SARS-CoV-2 vaccines.
Understanding testing disparities can improve response to ongoing testing and pandemic-related health care access challenges. Geographic population factors play a previously understudied role in access to SARS-CoV-2 testing. Rural areas have the lowest rates of testing but high rates of mortality, which are more exaggerated in minority communities (4, 5). In metropolitan cities, the access to SARS-CoV-2 testing has been disproportionately higher in neighborhoods with a high proportion of White residents, despite data demonstrating that predominantly Black and Hispanic populations had higher rates of SARS-CoV-2 positive cases (6, 7).
Previous studies highlighting disparities in SARS-CoV-2 testing have focused on single-site or single-city characteristics (6–11). This study is unique because it identifies and evaluates the factors contributing to SARS-CoV-2 testing inequities across multiple medical institutions in the US, providing a geographically comprehensive perspective. The objective of this study was to understand how minority race and socioeconomic status contributed to rates of SARS-CoV-2 testing and positivity. We evaluated data from early in the pandemic because testing resources were limited and therefore served as the foundation for understanding how race and socioeconomic status impacted access to new and urgently needed medical necessities. By identifying those most vulnerable to inequities in COVID-19 resources, we can implement strategies to better serve these populations (12).
Methods
The institutional review boards of Henry Ford Health System; Montefiore Medical Center; University of California Irvine; University of Florida; University of Michigan; University of North Carolina, Chapel Hill; University of Pittsburgh School of Medicine; and University of Washington reviewed and approved this study. The University of Florida served as the coordinating center and executed data use agreements with each institution. Data were shared with the coordinating center using REDCap.
Laboratory Data
For each institution, we extracted electronic health record (EHR) data for participants who had a SARS-CoV-2 PCR test performed between January 1, 2020 to May 15, 2020, who were aged 18 or older, and had a valid US residential 5-digit ZIP code. Extreme ages indicative of placeholders during emergent cases where age is unknown (e.g., 120 years) were excluded (n = 7). This yielded 151 402 cases. Indeterminant test results (n = 171; 0.1%) were further excluded. Data were limited to the first test a person received; repeaters’ subsequent tests (n = 24 160; 16.0%) were excluded so that an individual only contributed once to the population being evaluated.
Age, sex, race, ethnicity, test result, test location, and residential 5-digit ZIP code were extracted. Race categories included White, Black, Asian, Pacific Islander, American Indian, and Alaska Native, multiracial, other, and unknown. “Other” was a discrete category. Since one of the institutions coded Asian and Pacific Islander together, data from the other institutions were recoded similarly. Test location included emergency department, inpatient, outpatient, and unknown.
Socioeconomic Variables
ZIP code tabulated area (ZCTA)-level data were downloaded from the 2018 American Community Survey (ACS) 5-year estimates 2014–2018 (13). We calculated the percentage of residents on Medicaid (table S2704) and the percentage of Black residents (table B02001) for each ZCTA. Population density was calculated using ACS population estimates (table B01003) and land area estimates from the 2019 Census Bureau’s US Gazetteer Files (7, 14). This variable serves as a proxy for difficulty in physical distancing within crowded communities.
We pulled economic and education variables from the ACS to create a socioeconomic status (SES) index using a principal component analysis (PCA) approach (6, 15). Median household income in the past 12 months (in 2018 inflation-adjusted dollars; table S1901); median gross rent (table B25064); owner-occupied housing value (table B25077); percentage unemployed (table B23025); percentage working class (table C24010); percentage <150% of the poverty line (e.g., an annual salary ≤$26 200 for a 4-person household; table C17002); and education index (derived from table B15002). The education index accounted for the education of those ≥25 years and is a weighted combination of the percentage less than high school, high school completion, and greater than high school educational attainment (15). Higher values indicated higher overall educational attainment within each ZCTA. The first principal component had an eigenvalue >1.0, accounted for 65.59% of the variance observed, and was retained as the SES Index. An SES Index value could not be calculated for the ZCTAs in which at least one variable was missing. This excluded 619 (0.4%) individuals for a final cohort of 126 452 patients.
Population density and SES Index were split into quartiles, where “I” indicates the least and “IV” indicates the highest population density and SES Index, respectively, to facilitate visual representation of the data. ZCTA-level socioeconomic variables were linked to patients’ residential 5-digit ZIP code using the 2018 crosswalk from UDS Mapper (16).
Statistical Analysis
Since multiple patients live in the same ZCTA, this introduces collinear challenges within the data [e.g., high correlations between SES Index and percentage Black (r = −0.55) or percentage Medicaid enrollment (r = −0.83)]. A multivariable, generalized estimating equations (GEE) logit model that adjusts for ZCTA-level clustering of patients offers a robust statistical approach for addressing this collinearity (9). GEE log models were used to investigate the factors associated with a positive SARS-CoV-2 test. An exchangeable covariance structure was chosen because of the likelihood of additional clustered observations (e.g., by medical site). In addition to race and SES Index, models included age, sex, ethnicity, population density, percentage Medicaid, percentage Black, testing location, and medical site. To explore the robustness of findings, analyses were also subset by medical site and by White and Black race. In these additional analyses, medical site and race were removed. Statistical significance was set at α = 0.05 for 2-tailed tests. Analyses were performed using R, v.4.0.3, with the package GEE (17).
Results
A total of 126 452 SARS-CoV-2 tests performed on unique patients across 8 healthcare institutions were evaluated, of which 18 905 (15.0%) tested positive. Patient demographics overall and by test result are described in Table 1. In our sample, 54.5% were White, 22.0% were Black, 12.2% were of an unknown race, and the remainder belonged to other racial groups. The mean (standard deviation) age was 51.9 (18.4) years, 41.7% were male, and 8.7% were Hispanic. Patients with a positive test result were characterized by older age, higher proportion of male sex, higher proportion of non-White race, higher proportion of Hispanic ethnicity, and living in a more populated area. The highest population density quartile represents nearly half (47.3%) of positive tests. Black patients made up 38.2% of positive test results but only 22.0% of all tests whereas White patients made up 54.5% of all tests but only 27.7% of positive test results. Hispanic patients accounted for only 8.7% of all tests but 17.4% of positive test results.
Table 1.
Subject demographics of SARS-CoV-2 testing across 8 sites.
| Individuals, no. (%) |
|||
|---|---|---|---|
| Variable | Overall | Negative results | Positive results |
| (n = 126 452) | (n = 107 547) | (n = 18 905) | |
| Age, years | |||
| Mean (SD) | 51.9 (18.4) | 51.3 (18.4) | 55.1 (18.6) |
| Median (IQR) | 52.0 (30.0) | 51.6 (29.2) | 55.3 (28.8) |
| ≥ 65 | 33 910 (26.8) | 27 868 (25.9) | 6042 (32.0) |
| Sex | |||
| Female | 73 624 (58.2) | 63 310 (59.0) | 10 314 (55.0) |
| Male | 52 747 (41.7) | 44 164 (41.0) | 8583 (45.0) |
| Unknown | 81 (0.1) | 73 (0.0) | 8 (0.0) |
| Race | |||
| White | 68 856 (54.5) | 63 618 (59.2) | 5238 (27.7) |
| Black | 27 805 (22.0) | 20 582 (19.1) | 7223 (38.2) |
| Asian/Pacific Islander | 4611 (3.6) | 4009 (3.7) | 602 (3.2) |
| American Indian Alaska Native | 518 (0.4) | 468 (0.4) | 50 (0.3) |
| Multiracial | 843 (0.7) | 594 (0.6) | 249 (1.32) |
| Othera | 8430 (6.7) | 5423 (5.0) | 3007 (15.9) |
| Unknown | 15 389 (12.2) | 12 853 (12.0) | 2536 (13.4) |
| Ethnicity | |||
| Not Hispanic | 100 644 (79.6) | 87 556 (81.4) | 13 088 (69.2) |
| Hispanic | 11 041 (8.7) | 7759 (7.2) | 3282 (17.4) |
| Unknown | 14 767 (11.1) | 12 232 (11.4) | 2535 (13.4) |
| Population density | |||
| Mean (SD) | 7501 (15 601) | 5729 (12 893) | 17 580 (23 727) |
| Median (IQR) | 2349 (5119) | 1963 (4491) | 5136 (25 363) |
| I: (0.128, 499) | 31 614 (25.0) | 29 776 (27.7) | 1838 (9.7) |
| II: (499, 2351) | 31 862 (25.2) | 29 108 (27.1) | 2754 (14.6) |
| III: (2351, 5621) | 31 382 (24.8) | 26 012 (24.2) | 5370 (28.4) |
| IV: (5621, 152 693) | 31 594 (25.0) | 22 651 (21.1) | 8943 (47.3) |
| SES Index | |||
| Mean (SD) | −0.1 (2.1) | 0.0 (2.1) | −0.7 (2.2) |
| Median (IQR) | −0.2 (2.9) | −0.0 (2.9) | −0.7 (3.0) |
| I: (−7.8, −1.5) | 31 835 (25.2) | 24 641 (22.9) | 7194 (38.1) |
| II: (−1.5, −0.2) | 31 442 (24.9) | 27 144 (25.2) | 4298 (22.7) |
| III: (−0.2, 1.4) | 31 668 (25.0) | 27 452 (25.5) | 4216 (22.3) |
| IV: (1.4, 8.4) | 31 507 (24.9) | 28 310 (26.3) | 3197 (16.9) |
| ZCTA % Medicaid | |||
| Mean (SD) | 22.9 (14.1) | 21.5 (13.1) | 31.0 (16.6) |
| Median (IQR) | 19.5 (18.5) | 18.8 (16.8) | 30.2 (27.2) |
| ZCTA % Black | |||
| Mean (SD) | 19.4 (23.8) | 17.1 (22.1) | 32.2 (28.7) |
| Median (IQR) | 10.0 (21.9) | 9.2 (18.4) | 22.8 (40.5) |
| Test location | |||
| Emergency department | 25 533 (20.2) | 18 526 (17.2) | 7007 (37.1) |
| Inpatient | 22 517 (17.8) | 17 715 (16.5) | 4802 (25.4) |
| Outpatient | 77 952 (61.6) | 70 893 (65.9) | 7059 (37.3) |
| Unknown | 450 (0.4) | 413 (0.4) | 37 (0.2) |
Abbreviations: IQR, interquartile range; SES, socioeconomic status; ZCTA, zip code tabulated area.
Other was a distinct racial category.
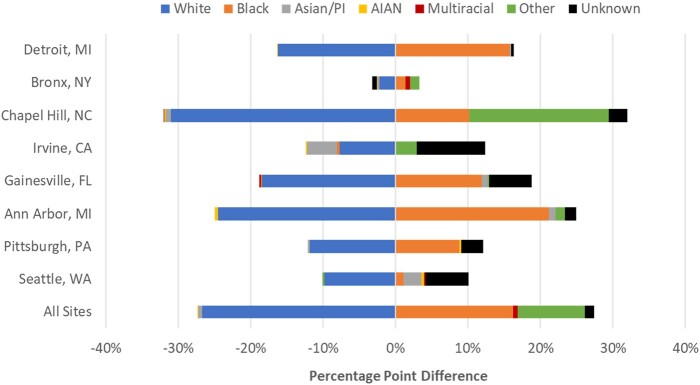
Treating the overall sample racial proportion as the expected racial composition of positive tests, Fig. 1 shows the percentage difference between expected and actual racial composition of positive tests. Analysis at the individual site level revealed an identical trend in which White patients consistently and disproportionately tested negative more frequently than expected. Despite variability in non-White races, Black race was the most consistently pronounced non-White race that tested positive more frequently than expected.
Fig. 1.
Percentage point difference between expected and actual racial composition of positive tests shows that SARS-CoV-2 positivity is disproportionately higher in non-White patients.
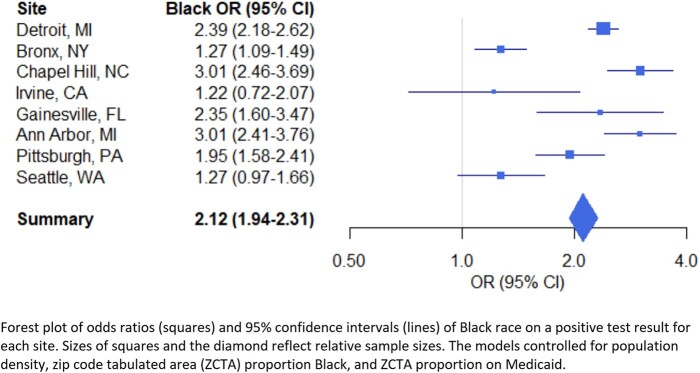
Increasing age; male sex; both Hispanic and unknown ethnicity were significantly associated with odds of getting a positive SARS-CoV-2 test (Table 2). Obtaining a test in the emergency department increased the odds of a positive result [odds ratio, OR, 1.31 (95% confidence interval, CI, 1.17–1.47)] whereas obtaining a test in the outpatient setting decreased the odds [OR 0.72 (95% CI 0.64–0.80)]. All non-White racial categories, except for American Indian Alaskan Native, increased the odds of a positive test. Multiracial [OR 2.32 (95% CI 1.90–2.80)] and Black [OR 2.11 (95% CI 1.95–2.29)] race were the 2 categories that increased the odds of a positive test the most. Black race was consistently associated with increased odds of a positive regardless of geographical location, although the 2 west-coast sites did not reach statistical significance (Fig. 2).
Table 2.
Odds of SARS-CoV-2 positivity at all sites by race.
| Variable | Overall | Black | White |
|---|---|---|---|
| n = 126 452 | n = 27 805 | n = 68 856 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age, years | 1.01 (1.01–1.01)* | 1.01 (1.01–1.01)* | 1.01 (1.00–1.01)* |
| Malea | 1.14 (1.10–1.19)* | 1.03 (0.96–1.10) | 1.15 (1.08–1.23)* |
| Raceb | |||
| Black | 2.11 (1.95–2.29)* | ||
| Asian/Pacific Islander | 1.69 (1.48–1.93)* | ||
| American Indian Alaskan Native | 1.27 (0.90–1.80) | ||
| Multiracial | 2.32 (1.91–2.83)* | ||
| Other | 1.72 (1.48–1.99)* | ||
| Unknown | 1.75 (1.61–1.90)* | ||
| Ethnicityc | |||
| Hispanic | 1.51 (1.33–1.72)* | 0.96 (0.74–1.24) | 2.02 (1.67–2.46)* |
| Unknown | 1.22 (1.13–1.32)* | 1.11 (0.95–1.30) | 1.35 (1.15–1.58)* |
| SES Index | 1.04 (1.00–1.08)* | 0.92 (0.86–0.99)* | 1.10 (1.05–1.16)* |
| Test locationd | |||
| Emergency department | 1.31 (1.17–1.47)* | 1.17 (1.02–1.35)* | 1.10 (0.98–1.24) |
| Outpatient | 0.72 (0.64–0.80)* | 0.56 (0.48–0.65)* | 0.78 (0.71–0.86)* |
The models controlled for population density, zip code tabulated area (ZCTA) proportion Black, ZCTA proportion on Medicaid, and site.
Indicates statistical significance;
Reference: Female;
Reference: White;
Reference: Not Hispanic;
Reference: Inpatient.
Fig. 2.
Black race is consistently associated with increased odds of a positive test regardless of geographical location.
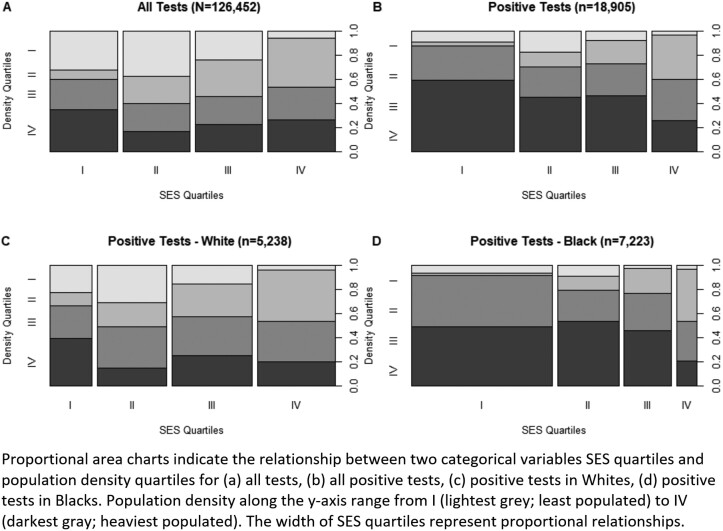
In the White patients-only subset, male sex, Hispanic ethnicity, and unknown ethnicity increased odds of a positive test but this was not observed in the Black-patients-only subset (Table 2). SES Index was a significant predictor for both Black and White patients, but in opposite directions. For White patients, a higher SES increased the odds of a positive test [OR 1.10 (95% CI 1.05–1.16)] but for Black patients, SES decreased the odds of a positive test [OR 0.92 (95% CI 0.86–0.99)]. This relationship is also graphically represented in Fig. 3, where the lowest SES quartile is the smallest share of White positive tests but the largest share of Black positive tests. Black patients testing positive are also more likely to reside in a ZCTA with higher population density than White patients are. Most Black patients testing positive (Fig. 3, D) resided in the 2 densest quartiles whereas there was greater variability in White patients testing positive (Fig. 3, C).
Fig. 3.
Proportional area charts illustrate that the relationship between population density quartiles and socioeconomic status (SES) quartiles differs by race for positive SARS-CoV-2 tests.
Discussion
Health disparities noted in past influenza outbreaks have mirrored racial inequalities seen during the COVID-19 pandemic in the US (18). In contrast to widely available influenza testing, however, SARS-CoV-2 testing was markedly scarce in the early pandemic and continues to be a limited resource. Thus, this pandemic presents new access challenges and requires intentional navigation of preexisting barriers to effectively mitigate harm to vulnerable minority communities.
Our study showed that SARS-CoV-2 positivity was disproportionately higher in non-White patients, underscoring a growing body of evidence that non-White race was a risk factor for contraction of SARS-CoV-2 (8, 19). Our study corroborated this inequality across representative regions of the US, strengthening the evidence that racial disparity in SARS-CoV-2 positivity is a national crisis. Black race was associated with increased odds of a positive test regardless of geographical location.
While racial disparities in SARS-CoV-2 positivity in large urban areas may be unsurprising given the well-established ecology of disadvantage in these cities (20), some of the starkest increases seen in positivity odds were observed in mid-size college towns like Ann Arbor, Chapel Hill, and Gainesville. This could be explained by historic socioeconomic disparities within these mid-sized university communities reflected by income and educational achievement gaps (21).
We also showed that in the early stage of the pandemic 2 groups were more likely to test positive: (a) low SES Black patients and (b) high-SES White patients in densely populated communities. Black individuals living in low SES neighborhoods bore a disproportionate positivity rate, consistent with other studies showing association of population density as a risk factor for infection in minority populations (7). In a Massachusetts study, racial segregation of Non-Hispanic Black/African Americans and Hispanic populations was associated with increased COVID-19 incidence rate, as was overcrowding (defined by more than one person per room) (22). The role of non-White patients as essential workers during the pandemic required increased use of public transportation and a limited ability to control exposure from the public. Conversely, our data show that Black patients who live in high-SES neighborhoods were protected from higher rates of SARS-CoV-2 positivity.
We found that SARS-CoV-2 positivity actually increased for high-SES White patients. One explanation for the counterintuitive risk of positivity in socioeconomically privileged White patients could be a sense of unconcern due to perceptions of insulation from harm (23). A poll of 8000 California voters in the Sacramento region during the first months of the pandemic found that almost one-third of White respondents never feared for their physical safety during California’s initial lockdown order, more than 3 times greater than the number of Black respondents in the same area. The same poll showed that 39% of White individuals saw decreases to income during this time, while 62% of Black respondents reported income loss (24). Apart from the highest SES category, White race predicted lower SARS-CoV-2 positivity. This could be explained by several factors. Many high-earning White patients were able to work from home with pay, utilize essential workers for their food and resource needs through mobile shopping and food delivery services, with living quarters that enabled effective isolation; whereas Black workers may have been dismissed from their work or not paid for the work they missed if they tested positive, which may have incentivized them to delay testing (25, 26).
We demonstrate how race and SES impacted SARS-CoV-2 positivity and provide an opportunity to alleviate the disproportional burden of disease on minority communities with lower SES. Interestingly, a large cohort study of nearly 6 million patients from the Veterans’ Affairs found that while Black patients were more likely to be tested than Hispanic or White patients and Black and Hispanic patients were more likely to test positive, 30-day mortality did not differ by race. This study suggests that the more equitable health care access provided to veterans could offset socioeconomic factors leading to the higher test positivity rate (27). In another study of individual ZCTAs in Miami-Dade County, FL, SARS-CoV-2 test positivity was correlated with economic disadvantage but not race, arguing that economic privilege can help offset other social risks for contracting the SARS-CoV-2 infection (28). The emerging work around race, economics, and COVID-19 underscores the complexity of these factors and the need for equitable economic opportunities.
Our study is strengthened by the aggregation of several urban and rural testing sites throughout the USA, providing a robust sample size and increased generalizability to the US population. A noted limitation of our study is the use of EHR-documented race, which cannot distinguish between self-reported or provider-reported race. Health care providers may incorrectly assume the race of patients, which may skew racial data (29). There was also an insufficient number of American Indian or Alaskan Natives (AIAN) to power the findings of our study and draw conclusions for this group. The limited number of AIANs is most likely due to the availability of the healthcare services for this population from the Indian Health Service. Additionally, we excluded patients who were tested but did not have a ZCTA included in their tested sample. This may have excluded unhoused persons: a highly vulnerable population and one with an overrepresentation of Black individuals (30). Although our dataset does not include medical comorbidity, one study suggests that medical comorbidities play a lesser role than sociocultural factors in test positivity (8). Last, this manuscript included only the first test performed on each patient, which resulted in excluding 16% of the testing. Further analysis will be necessary to understand the racial and socioeconomic differences in retesting practices.
Established barriers to health care access in minority communities, such as limited health insurance, low SES, and limited ability to access local testing have continued to play a role during the COVID-19 pandemic and may contribute to the testing disparities shown in our data. Without treatment or widespread vaccination to protect populations, the World Health Organization’s containment strategy at the beginning of the COVID-19 pandemic was to isolate, test, and trace (31). With the rapid development of vaccines, testing will still play a critical role in the cycle of containment. Access to all elements of the pandemic control response will remain a challenge to disadvantaged minority populations unless policy and health care leaders take decisive action against the lines of geographic, economic, and social segregation separating the USA.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Nonstandard Abbreviations: SARS-CoV-2 severe acute respiratory syndrome coronavirus 2 COVID-19 coronavirus disease 2019 EHR electronic health record ZCTA ZIP code tabulated area ACS American Community Survey PCA principal component analysis SES socioeconomic status GEE generalized estimating equations OR odds ratio AIAN American Indian, or Alaskan natives
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
C.J. Hsiao and A.G.M. Patel had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: C.J. Hsiao, H.O. Fasanya, M. Stoffel, S.G. Beal, G.N. Winston-McPherson, D.N. Greene, A.G.M. Patel.
Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: C.J. Hsiao, H.O. Fasanya, M. Stoffel, A.G.M. Patel. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: C.J. Hsiao, A.G.M. Patel. Obtaining funding: N/A. Administrative, technical, or material support: N/A.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest: Employment or Leadership: D.N. Greene, The Journal of Applied Laboratory Medicine, AACC. Consultant or Advisory Role: None declared. Stock Ownership: None declared. Honoraria: None declared. Research Funding: Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427, and by the National Institute of Child Health and Health Development of the National Institutes of Health under award number F30HD097935 (C.J. Hsiao). Expert Testimony: None declared. Patents: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
REFERENCES
- 1.Webb Hooper M, Nápoles AM, Pérez-Stable EJ.. COVID-19 and racial/ethnic disparities. JAMA 2020;323:2466–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, et al. Fair allocation of scarce medical resources in the time of COVID-19. N Engl J Med 2020;382:2049–55. [DOI] [PubMed] [Google Scholar]
- 3.Grohskopf LA, Liburd LC, Redfield RR.. Addressing influenza vaccination disparities during the COVID-19 pandemic. JAMA 2020;324:1029–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souch JM, Cossman JS.. A commentary on rural-urban disparities in COVID-19 testing rates per 100,000 and risk factors. J Rural Health 2020;37:188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng KJG, Sun Y, Monnat SM.. COVID-19 death rates are higher in rural counties with larger shares of Blacks and Hispanics. J Rural Health 2020;36:602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman-Cribbin W, Tuminello S, Flores RM, Taioli E.. Disparities in COVID-19 testing and positivity in New York City. Am J Prev Med 2020;59:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vahidy FS, Nicolas JC, Meeks JR, Khan O, Pan A, Jones SL, et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open 2020;10:e039849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu T, Mack JA, Salvatore M, Prabhu Sankar S, Valley TS, Singh K, et al. Characteristics associated with racial/ethnic disparities in COVID-19 outcomes in an academic health care system. JAMA Netw Open 2020;3: e2025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muñoz-Price LS, Nattinger AB, Rivera F, Hanson R, Gmehlin CG, Perez A, et al. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Netw Open 2020;3:e2021892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golestaneh L, Neugarten J, Fisher M, Billett HH, Gil MR, Johns T, et al. The association of race and COVID-19 mortality. EClinicalMedicine 2020;25:100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HN, Lan KF, Nkyekyer E, Neme S, Pierre-Louis M, Chew L, Duber HC.. Assessment of disparities in COVID-19 testing and infection across language groups in Seattle, Washington. JAMA Netw Open 2020;3:e2021213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler SE, Hasskamp JH, Peck Palmer OM.. Recognizing laboratory medicine's collaborative role in identifying and eliminating health disparities. J Appl Lab Med 2021;6:274–84. [DOI] [PubMed] [Google Scholar]
- 13.American Community Survey. 2018. American Community Survey 5-year estimates. US Census Bureau. https://data.census.gov/cedsci/ (Accessed June 2021).
- 14.United States Census Bureau. Gazetteer files: 2019. ZIP Code Tabulated Areas. https://www.census.gov/geographies/reference-files/time-series/geo/gazetteer-files.html (Accessed September 2020).
- 15.Yost K, Perkins C, Cohen R, Morris C, Wright W.. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Cause Control 2001;12:703–11. [DOI] [PubMed] [Google Scholar]
- 16.UDS Mapper. ZIP code to ZCTA crosswalk. https://udsmapper.org/zip-code-to-zcta-crosswalk/ (Accessed September 2020).
- 17.Carey VL, Moler C. Generalized estimation equation solver. R package version 4.13-20. https://CRAN.R-project.org/package=gee (Accessed June 2021).
- 18.Sloan C, Chandrasekhar R, Mitchel E, Schaffner W, Lindegren ML.. Socioeconomic disparities and influenza hospitalizations, Tennessee, USA. Emerg Infect Dis 2015;21:1602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin-Miller LA, Artiga S, Sullivan S. COVID-19 racial disparities in testing, infection, hospitalization, and death: analysis of epic patient data. https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-racial-disparities-testing-infection-hospitalization-death-analysis-epic-patient-data/ (Accessed January 2021).
- 20.Benitez JCC, Yelowitz A.. Racial and ethnic disparities in COVID-19: evidence from six large cities. J Econ Race Policy 2020;3:243–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reardon SF, Shores K, Kalogrides D. The geography of racial/ethnic test score gaps. Stanford Center for Education Policy Analysis. No. CEPA Working Paper, The American Journal of Sociology 2019;16–0.
- 22.Hu T, Yue H, Wang C, She B, Ye X, Liu R, et al. Racial segregation, testing site access, and COVID-19 incidence rate in Massachusetts, USA. Int J Environ Res Public Health 2020;17: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nino M, Harris C, Drawve G, Fitzpatrick KM.. Race and ethnicity, gender, and age on perceived threats and fear of COVID-19: Evidence from two national data sources. SSM Popul Health 2021;13:100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt EA, Dela Cruz Y, Williams S, Newham J, Schneider J, Nixon A.. 2020. The COVID-19 resilience poll. Valley Vision. https://www.valleyvision.org/resources/the-covid-19-resilience-poll/ (Accessed June 2021).
- 25.Khazanchi R, Beiter ER, Gondi S, Beckman AL, Bilinski A, Ganguli I.. County-level association of social vulnerability with COVID-19 cases and deaths in the USA. J Gen Intern Med 2020;35:2784–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The plight of essential workers during the COVID-19 pandemic. Lancet 2020;395:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: a nationwide cohort study. PloS Med 2020;9:e1003379. doi: ARTN e1003379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palacio A, Tamariz L.. Social determinants of health mediate COVID-19 disparities in South Florida. J Gene Intern Med 2020;36:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel AGM, Hsiao CJ, Fasanya HO, Stoffel M, Lyon ME.. Accuracy in measuring racial disparities during the COVID-19 pandemic needs improvement. J Appl Lab Med 2021;6:310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Health, homelessness, and racial disparities. https://nhchc.org/wp-content/uploads/2019/08/health-homelessness-and-racial-disparities.pdf (Accessed January 2021).
- 31.Lacina L. WHO coronavirus briefing: Isolation, testing and tracing comprise the ‘backbone’ of response. https://www.weforum.org/agenda/2020/03/testing-tracing-backbone-who-coronavirus-wednesdays-briefing/ (Accessed January 2020).