Abstract
Background
Vaccinations are a cornerstone of preventative medicine in the USA. However, growing concerns regarding facial nerve palsy following vaccination exist.
Objective
This study aims to assess the occurrence of facial palsy as reported by the Vaccine Adverse Event Reporting System (VAERS) database.
Methods
A retrospective analysis of the VAERS database was performed for cases of ‘Facial Palsy’, ‘Bell’s Palsy’, ‘Facial Paralysis’ and ‘Ramsay Hunt Syndrome’ between 2009 and 2018. Subgroup analysis was performed to determine gender, age, history of facial palsy, type of vaccine used, number of days until onset of symptoms and overall facial palsy rate.
Results
Nine hundred and forty-four entries met our inclusion criteria with 961 vaccine administrations resulting in facial paralysis. Facial palsy following vaccinations was evenly distributed across all age cohorts with two peaks between 60 and 74 years old and between 0 and 14 years old. Most patients were female (N = 526, 55.7%) without a reported history of facial palsy (N = 923, 97.8%). In 2009, reported incidence rate was 0.53%, as compared with 0.23% in 2018. The influenza vaccine had the greatest number of cases (N = 166, 17.3%), followed by the varicella (N = 87, 9.1%) and human papillomavirus vaccines (N = 47, 4.9%).
Conclusions
With the SARS-CoV-2 pandemic and recent approvals of the vaccinations, there is growing concern of facial palsy following vaccination. Although it is a known adverse event following vaccination, the likelihood of facial palsy following vaccination is low, with only 0.26% of overall reported cases over a 10-year span.
Keywords: Adverse drug reaction reporting systems, Bell palsy, facial nerve, facial paralysis, iatrogenic disease, vaccination
Key Messages.
Facial palsy is a reported adverse event following vaccination.
From 2009 to 2018, 944 cases of facial palsy were reported to the FDA.
There is a bimodal distribution with a slight female gender preponderance.
The number of cases has decreased from 0.53% to 0.23% in the last 10 years.
Patients should be counselled that while possible, facial palsy is rare.
Background
Acquired facial nerve palsy is an uncommon problem, affecting anywhere between 20 and 32 per 100 000 people per year in the general population (1). However, the deficits caused by injury to the nerve can be devastating, resulting in impairment in essential facial functions, social interactions and psychological well-being. Facial movements such as smiling, blinking, cornea protection, nasal breathing, lip competence and speech may be affected, resulting in diminished quality of life (2). The typical presentation of acquired facial nerve palsy is a rapid onset of partial to complete paralysis of the facial muscles with preservation of facial sensation and reduction in taste and hypersensitivity to sound. Several aetiologies of acquired facial nerve palsy have been described, and can be broadly categorized as idiopathic (Bell’s palsy), infectious (Ramsay Hunt syndrome), neoplastic, traumatic or iatrogenic (3,4).
Acquired facial nerve palsy following vaccination is a growing concern. Despite being one of the most successful medical advances in medical history, parents in the USA have expressed concern that physicians are not well educated on the adverse effects of vaccines or that physicians purposefully withhold information on adverse effects (5,6). According to the Centers for Disease Control and Prevention (CDC), failure to vaccinate resulted in 1282 individual cases of measles in the USA in 2019, the greatest number of cases reported in the USA since 1992 (7). Thus, adequate physician education and counselling is of growing importance (8). Furthermore, consensus on vaccine safety is crucial as the failure to vaccinate puts young infants or those that are immunocompromised at increased risk due to waning community immunity (9).
The proposed mechanisms of idiopathic facial palsy, or Bell’s palsy, are viral infection or autoimmune disease. Bell’s palsy has been associated with influenza vaccines in the past, although no clear association has been demonstrated (10–14). Current research is largely limited to the influenza vaccine, but it has been postulated that other vaccines may lead to facial paralysis through the same cell-mediated autoimmune mechanism (15). Using the CDC and the United States Food and Drug Administration’s (FDA) Vaccine Adverse Event Reporting System (VAERS) database, this study aims to characterize the rate of occurrence of facial palsy reported by patients following vaccination.
Methods
The VAERS database is a nationwide public health data collection system used to gather information regarding reports of vaccine-related adverse events. VAERS is co-managed by the CDC and the FDA following the enactment of the National Childhood Vaccine Injury Act (NCVIA) of 1986, which states that health care providers are required by law to report vaccine-related adverse events (16,17). A retrospective analysis of the VAERS database was performed for all reported cases reported between 2009 and 2018. According to New Jersey Medical School institutional review board (IRB) policies, IRB approval and informed consent were not required due to the deidentified nature of the VAERS database.
Reports provided to the VAERS database are publicly available on their webpage (vaers.hhs.org). Data were downloaded in .xlsx format from 2009 to 2018, totalling 358 769 entries. Each entry was represented by a unique VAERS ID, and signified an individual event reported to the database. Cases with duplicate ID’s were removed from our dataset. Cases that included multiple patients were excluded from the analysis to allow for specific analysis of each vaccine administration. All cases including the terms ‘Facial Palsy’, ‘Bell’s Palsy’, ‘Facial Paralysis’ and ‘Ramsay Hunt Syndrome’ were included for analysis. Because adverse events in the VAERS database have been pre-labelled according to the information provided during the submission, categorization of these cases according to the guidelines presented by the Brighton Collaboration Bell’s Palsy Working Group is not applicable (18). Subgroup analysis was performed to determine gender, age, history of facial palsy, type of vaccine used, number of days until onset of symptoms and overall facial palsy rate. Ages were grouped into 15-year cohorts.
Results
A total of 944 cases of facial paralysis out of 358 769 (0.26%) total adverse events were reported to the VAERS database between 2009 and 2018. The ages of the total cohort were well distributed across most of the age cohorts, with two peaks, one with 178 patients between 0 and 14 years and the other with 189 patients between 60 and 74 years. There was one outlier beyond 90-years-old (Table 1). Most patients reported were female (N = 526, 55.72%) (Table 1). The majority of cases did not have a reported history of facial palsy (N = 923, 97.78%), while only 21 cases (2.22%) reported a history of prior facial palsy. Of all cases, 580 patients (61.44%) developed symptoms within 7 days of vaccination with 300 patients (31.78%) developing symptoms greater than 7 days from vaccination. Duration from vaccination was unknown in 64 patients (6.78%).
Table 1.
Demographics for 944 patients reporting facial palsy following vaccination in the VAERS database (2009–18)
| Characteristic | Frequencya | Percentage (%)a |
|---|---|---|
| Age, years | ||
| 0–14 | 178 | 18.86 |
| 15–29 | 143 | 15.15 |
| 30–44 | 172 | 18.22 |
| 45–59 | 164 | 17.37 |
| 60–74 | 189 | 20.02 |
| 75–89 | 25 | 2.65 |
| ≥90 | 1 | 0.11 |
| Unknown | 72 | 7.63 |
| Gender | ||
| Male | 391 | 41.42 |
| Female | 526 | 55.72 |
| Unknown | 27 | 2.86 |
| History of facial palsy | ||
| No | 923 | 97.78 |
| Yes | 21 | 2.22 |
| Days after vaccination | ||
| Less than 7 days | 580 | 61.44 |
| Greater than 7 days | 300 | 31.78 |
| Unknown | 64 | 6.78 |
a944 total reported cases.
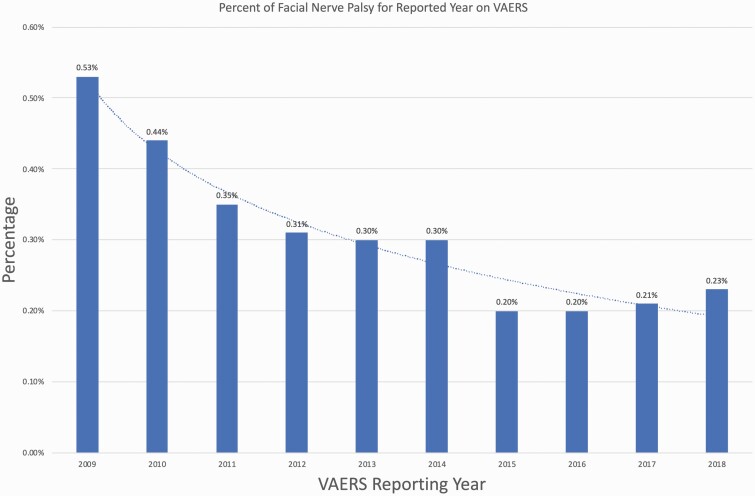
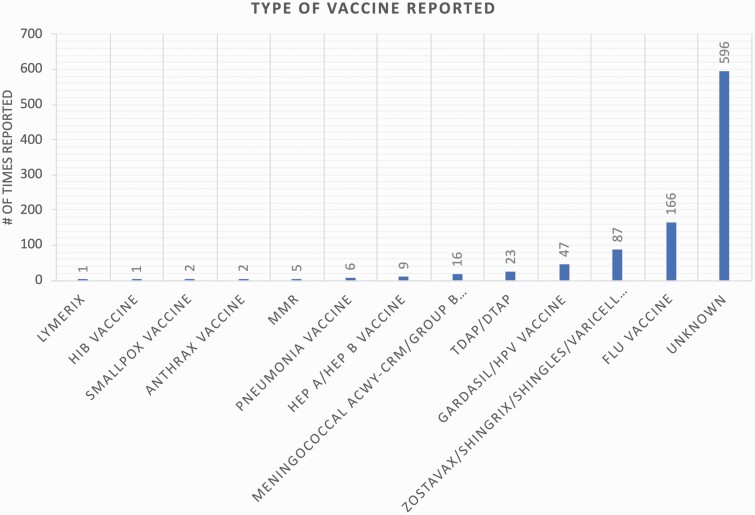
In 2009, 178 cases reporting facial palsy symptoms were reported out of 32 780 total cases (0.53%), compared with 2018 where 113 cases out of 49 168 total cases were reported (0.23%). Thus, an overall downward trend in the percent of reported facial palsy was observed in this 9-year period compared with total adverse events reported in the VAERS database (Fig. 1). For the 944 cases of facial paralysis, a total of 961 vaccines were administered that subsequently had an association with facial paralysis, reflecting that 17 cases occurred following the administration of multiple vaccines. Most entries did not list the type of vaccine used (N = 596, 61.95%). Of the known types of vaccines administered prior to symptom onset, the flu vaccine was most frequently reported (N = 166, 17.27%), followed by the varicella vaccine (N = 87, 9.05%) and the human papillomavirus vaccine (N = 47, 4.89%) (Fig. 2). The exact formulation of flu vaccine administered for these adverse events is unknown.
Figure 1.
Percent of VAERS cases that involved facial nerve palsy as a fraction of all adverse events reported to the database for each year (2009–18).
Figure 2.
Number of cases of facial palsy following vaccine administration as reported in the VAERS database (2009–18). DTaP, diphtheria, tetanus, pertussis; HEP A, hepatitis A virus; HEP B, hepatitis B virus; HiB, Haemophilus influenza type B; HPV, human papillomavirus; MMR, measles, mumps, rubella; Tdap, adult tetanus, diphtheria, pertussis.
Conclusions
Facial nerve palsy following vaccination is a very rare phenomenon, representing a total of 944 cases (0.26%) out of 358 769 reported adverse events. A meta-analysis of all safety data available from 28 completed clinical trials in patients receiving the influenza vaccine determined that the incidence rate of facial paresis and/or facial nerve (cranial nerve VII) paralysis per 100 000 person-years was 55.6 (19). In comparison, the annual incidence rate of Bell’s palsy, the most common aetiology of facial paralysis, is estimated in the literature to be between 13 and 53 cases per 100 000 person-years (4,18). Thus, its occurrence in our analysis of the VAERS database is significantly smaller than that in the literature, reflecting the rarity of facial paralysis following vaccination.
Mutsch et al. performed a matched case–control study with a case-series analysis looking at whether 773 known Bell’s palsy patients also received the intranasal influenza vaccine (13). Based on their analysis, the relative risk of Bell’s palsy was estimated to be 19 times the risk identified in control patients, with the greatest risk being 31–60 days following vaccination. From these data, it was proposed that the mechanism of injury was not from direct toxic effect of the vaccine but rather an autoimmune process or from reactivation of a latent herpes simplex virus isoform 1 (HSV-1) or varicella zoster virus (VZV) laying dormant in the seventh nerve ganglion (20). However, it is unknown if the influenza vaccine directly contributes to the reactivation. Other studies have proposed that the intranasal administration of the influenza vaccine lends itself to facial nerve compression contributing to the increased occurrence of Bell’s palsy in patients receiving the vaccine (21). This hypothesis does not explain the development of facial nerve palsy in patients receiving the VZV, Gardasil or diphtheria/tetanus/pertussis (DTaP/Tdap) vaccines, all of which are administered intramuscularly or subcutaneously. Based on our findings, these vaccines had the next highest rates of facial nerve palsy with 87 (9.05%), 47 (4.89%) and 23 (2.39%) reported cases, respectively (Fig. 2). Thus, more research is needed in order to establish whether a causal relationship between intranasal delivery and facial nerve palsy exists.
While facial nerve palsy is a known adverse effect of vaccination, the implications can have significant effects on quality of life and function for patients. The most alarming symptom experienced by patients is paresis, with up to three quarters of patients believing the paresis resulted from a stroke (22). Patients may also experience otalgia, aural fullness, retroauricular pain and the symptoms may impair speech and eating (23). It is comforting then that the percentage of cases reporting facial nerve palsy following vaccine administration, related or unrelated to the vaccine itself, has decreased. Based on our analysis of the VAERS database, since 2009–18, there has been an overall downward trend of the yearly percentage rates, with 0.53% of cases (178 out of 32 780 reported cases) reported in 2009 down to 0.23% (113 out of 49 168 reported cases) of all cases in 2018 (Fig. 1). Further, based on data from the CDC, the total number of influenza vaccine doses distributed from 2009 to 2018 has increased from 110.9 million doses in the 2008–09 season to 155.3 million doses in the 2017–18 season (24). Thus, while an exact approximation of the number of facial paresis cases per million doses cannot be determined, the decrease in the total number of cases with the concomitant rise in vaccine distribution during the study period suggests that the cases of facial paresis following vaccine administration may be trending downwards. As the most frequently administered vaccine, with 174.5 million doses distributed in the 2019–20 influenza season, it is unsurprising that the influenza vaccine had the highest number of cases reported, with a total of 166 (17.27%) reported cases (24). Further, age and gender did not seem to play a large role in determining whether or not a patient would experience palsy (Table 1).
The downward trend and the relatively low frequency of facial nerve palsy provide comfort that this feared complication is only becoming more infrequent. Further, review of the literature on these cases indicates that these symptoms are often transient and can resolve with administration of corticosteroids with or without antiviral therapy (21,25). With the SARS-CoV-2 (COVID-19) pandemic and the expedited FDA approval of the Pfizer-BioNTech and Moderna vaccines in December 2020, there is growing concern regarding facial nerve palsy after vaccination (26–28). Facial palsy was a reported symptom of COVID infection as reported by Codeluppi et al., witnessed in 21% of patients in Emergency Departments in Italy, an incidence that is significantly higher than in years prior (29). Similar cases were reported by Lima et al. and Brisca et al. (30,31). The incidence of Bell’s palsy following COVID vaccinations, reported as four cases following the Pfizer-BioNTech vaccine and three cases following the Moderna vaccine, has also contributed to fears regarding facial paralysis following COVID-19 vaccination. This concern was addressed by the American Academy of Otolaryngology-Head and Neck Surgery’s statement on Bell’s Palsy following COVID vaccination who reported that the incidence of facial paralysis after vaccination is not significantly higher than the background rate of facial paralysis in the general population (32). However, reports of adverse events will continue to be forwarded to the VAERS database and a subsequent analysis after widespread distribution of the vaccines should be performed.
Despite the promising results of this study, the nature of the VAERS database does not allow us to establish causality between vaccine administration and facial nerve palsy. This is in part due to the inherent limitations of the VAERS database itself, which provides limited and unverified information regarding adverse event data. The CDC provided the following statement on limitations regarding the VAERS database: ‘Vaccine providers are encouraged to report any clinically significant health problem following vaccination to VAERS, whether or not they believe the vaccine was the cause. Reports may include incomplete, inaccurate, coincidental and unverified information. The number of reports alone cannot be interpreted or used to reach conclusions about the existence, severity, frequency, or rates of problems associated with vaccines’ (33). Although the reporting is mandated by law, at the time of the analysis, key data may have been unavailable due to the lack of input by the user. Examples of such, which were reflected in our study, included demographic information, resolution of symptoms, accurate past medical histories, exact formulation of vaccine administered, serious versus non-serious status of adverse events and reporter type. For this reason, it is difficult to draw definitive conclusions regarding specific vaccines and facial palsy. Despite this, the VAERS database successfully provides a broad overview of facial nerve palsy following vaccine administration, providing groundwork for further study on this crucial topic.
Declaration
Funding: no sources of funding to report.
Ethical approval: this study was exempt from review of the Institutional Review Board of Rutgers New Jersey Medical School.
Conflict of interest: none.
Data availability
The data underlying this article are available in the article.
References
- 1. Peitersen E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol 2002; 122(7): 4–30. [PubMed] [Google Scholar]
- 2. Kleiss IJ, Hohman MH, Susarla SM, Marres HA, Hadlock TA. Health-related quality of life in 794 patients with a peripheral facial palsy using the Fa CE Scale: a retrospective cohort study. Clin Otolaryngol 2015; 40(6): 651–6. [DOI] [PubMed] [Google Scholar]
- 3. Wilbourn AJ. Iatrogenic nerve injuries. Neurol Clin 1998; 16(1): 55–82. [DOI] [PubMed] [Google Scholar]
- 4. Bleicher JN, Hamiel S, Gengler JS, Antimarino J. A survey of facial paralysis: etiology and incidence. Ear Nose Throat J 1996; 75(6): 355–8. [PubMed] [Google Scholar]
- 5. Glanz JM, Wagner NM, Narwaney KJet al. A mixed methods study of parental vaccine decision making and parent-provider trust. Acad Pediatr 2013; 13(5): 481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sutter RW, Maher C. Mass vaccination campaigns for polio eradication: an essential strategy for success. In: Plotkin SA (ed). Mass Vaccination: Global Aspects—Progress and Obstacles. Berlin, Heidelberg: Springer, 2006, pp. 195–220. [DOI] [PubMed] [Google Scholar]
- 7. Cases M. Outbreaks. Centers for Disease Control and Prevention (CDC), 2019, p. 7. [Google Scholar]
- 8. Spencer JP, Trondsen Pawlowski RH, Thomas S. Vaccine adverse events: separating myth from reality. Am Fam Physician 2017; 95(12): 786–94. [PubMed] [Google Scholar]
- 9. Atwell JE, Van Otterloo J, Zipprich Jet al. Nonmedical vaccine exemptions and pertussis in California, 2010. Pediatrics 2013; 132(4): 624–30. [DOI] [PubMed] [Google Scholar]
- 10. Chou CH, Liou WP, Hu KIet al. Bell’s palsy associated with influenza vaccination: two case reports. Vaccine 2007; 25(15): 2839–41. [DOI] [PubMed] [Google Scholar]
- 11. Baxter R, Toback SL, Sifakis Fet al. A postmarketing evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in adults 18–49 years of age. Vaccine 2012; 30(20): 3053–60. [DOI] [PubMed] [Google Scholar]
- 12. Izurieta HS, Haber P, Wise RPet al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA 2005; 294(21): 2720–5. [DOI] [PubMed] [Google Scholar]
- 13. Mutsch M, Zhou W, Rhodes Pet al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med 2004; 350(9): 896–903. [DOI] [PubMed] [Google Scholar]
- 14. Zhou W, Pool V, DeStefano Fet al. ; VAERS Working Group. A potential signal of Bell’s palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS)—United States, 1991–2001. Pharmacoepidemiol Drug Saf 2004; 13(8): 505–10. [DOI] [PubMed] [Google Scholar]
- 15. Greco A, Gallo A, Fusconi Met al. Bell’s palsy and autoimmunity. Autoimmun Rev 2012; 12(2): 323–8. [DOI] [PubMed] [Google Scholar]
- 16. Chen RT, Rastogi SC, Mullen JRet al. The Vaccine Adverse Event Reporting System (VAERS). Vaccine 1994; 12(6): 542–50. [DOI] [PubMed] [Google Scholar]
- 17. Singleton JA, Lloyd JC, Mootrey GT, Salive ME, Chen RT. An overview of the vaccine adverse event reporting system (VAERS) as a surveillance system. VAERS Working Group. Vaccine 1999; 17(22): 2908–17. [DOI] [PubMed] [Google Scholar]
- 18. Rath B, Gidudu JF, Anyoti Het al. ; Brighton Collaboration Bell’s Palsy Working Group. Facial nerve palsy including Bell’s palsy: case definitions and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine 2017; 35(15): 1972–83. [DOI] [PubMed] [Google Scholar]
- 19. Vaughn DW, Seifert H, Hepburn Aet al. Safety of AS03-adjuvanted inactivated split virion A(H1N1)pdm09 and H5N1 influenza virus vaccines administered to adults: pooled analysis of 28 clinical trials. Hum Vaccin Immunother 2014; 10(10): 2942–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Couch RB. Nasal vaccination, Escherichia coli enterotoxin, and Bell’s palsy. N Engl J Med 2004; 350(9): 860–1. [DOI] [PubMed] [Google Scholar]
- 21. Lewis DJ, Huo Z, Barnett Set al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One 2009; 4(9): e6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilden DH. Bell’s palsy. N Engl J Med 2004; 351(13): 1323–31. [DOI] [PubMed] [Google Scholar]
- 23. Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol 2008; 265(7): 743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prevention CfDCa. Historical Reference of Seasonal Influenza Vaccine Doses Distributed. 2020. https://www.cdc.gov/flu/prevent/vaccine-supply-historical.htm (accessed on 5 May 2021).
- 25. Gocko X, Poulteau S, Beyens MN, Bertholon P, Pozzetto B. Case report: Recurrent peripheral facial paralysis following two influenza vaccinations in 2009 and 2016. Vaccine 2019; 37(35): 4864–6. [DOI] [PubMed] [Google Scholar]
- 26. Ledford H, Cyranoski D, Van Noorden R. The UK has approved a COVID vaccine—here’s what scientists now want to know. Nature 2020; 588(7837): 205–6. [DOI] [PubMed] [Google Scholar]
- 27. Mahase E. Covid-19: Moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ 2020; 371. [Google Scholar]
- 28. Cohen J. Vaccine Designers Take First Shots at COVID-19. Washington, DC: American Association for the Advancement of Science, 2020. [DOI] [PubMed] [Google Scholar]
- 29. Codeluppi L, Venturelli F, Rossi Jet al. Facial palsy during the COVID-19 pandemic. Brain Behav 2021; 11(1): e01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lima MA, Silva MTT, Soares CNet al.. Peripheral facial nerve palsy associated with COVID-19. J Neurovirol 2020; 26: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brisca G, Garbarino F, Carta Set al. Increased childhood peripheral facial palsy in the emergency department during COVID-19 pandemic. Pediatr Emerg Care 2020; 36(10): e595–6. [DOI] [PubMed] [Google Scholar]
- 32. Surgery AAoO-HaN. AAO-HNS Statement on Bell’s Palsy Related to Approved COVID-19 Vaccines. 2020. https://www.entnet.org/content/aao-hns-statement-bell%E2%80%99s-palsy-related-approved-covid-19-vaccines (accessed on 28 December 2020).
- 33. Prevention CfDCa. About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/controller/datarequest/D8 (accessed on 6 February 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.