Abstract
Aims
We investigated the incidence, risk factors, clinical characteristics, and outcomes of pulmonary embolism (PE) in patients with COVID-19 attending emergency departments (EDs), before hospitalization.
Methods and Results
We retrospectively reviewed all COVID-19 patients diagnosed with PE in 62 Spanish EDs (20% of Spanish EDs, case group) during the first COVID-19 outbreak. COVID-19 patients without PE and non-COVID-19 patients with PE were included as control groups. Adjusted comparisons for baseline characteristics, acute episode characteristics, and outcomes were made between cases and randomly selected controls (1:1 ratio). We identified 368 PE in 74 814 patients with COVID-19 attending EDs (4.92‰). The standardized incidence of PE in the COVID-19 population resulted in 310 per 100 000 person-years, significantly higher than that observed in the non-COVID-19 population [35 per 100 000 person-years; odds ratio (OR) 8.95 for PE in the COVID-19 population, 95% confidence interval (CI) 8.51–9.41]. Several characteristics in COVID-19 patients were independently associated with PE, the strongest being D-dimer >1000 ng/mL, and chest pain (direct association) and chronic heart failure (inverse association). COVID-19 patients with PE differed from non-COVID-19 patients with PE in 16 characteristics, most directly related to COVID-19 infection; remarkably, D-dimer >1000 ng/mL, leg swelling/pain, and PE risk factors were significantly less present. PE in COVID-19 patients affected smaller pulmonary arteries than in non-COVID-19 patients, although right ventricular dysfunction was similar in both groups. In-hospital mortality in cases (16.0%) was similar to COVID-19 patients without PE (16.6%; OR 0.96, 95% CI 0.65–1.42; and 11.4% in a subgroup of COVID-19 patients with PE ruled out by scanner, OR 1.48, 95% CI 0.97–2.27), but higher than in non-COVID-19 patients with PE (6.5%; OR 2.74, 95% CI 1.66–4.51). Adjustment for differences in baseline and acute episode characteristics and sensitivity analysis reported very similar associations.
Conclusions
PE in COVID-19 patients at ED presentation is unusual (about 0.5%), but incidence is approximately ninefold higher than in the general (non-COVID-19) population. Moreover, risk factors and leg symptoms are less frequent, D-dimer increase is lower and emboli involve smaller pulmonary arteries. While PE probably does not increase the mortality of COVID-19 patients, mortality is higher in COVID-19 than in non-COVID-19 patients with PE.
Keywords: Pulmonary embolism, COVID-19, SARS-CoV-2, Incidence, Clinical characteristics, Risk factors, Outcome
Graphical Abstract
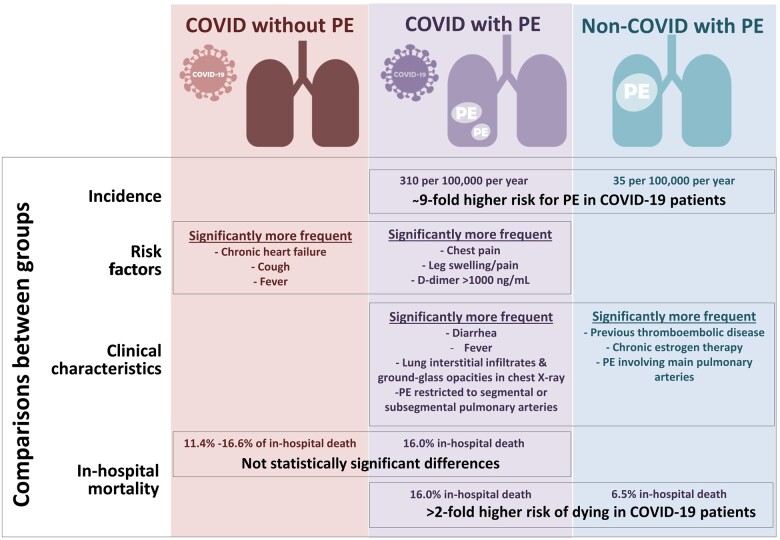
Graphical Abstract.
Summary of the main findings of the study.
Introduction
Infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is mainly characterized by fever and respiratory symptoms, with dyspnoea and lung infiltrates in more severe cases. Many patients also present a pro-coagulant state, which is biochemically detected by increased D-dimer levels and is related to complications and a worse prognosis.1 In this context, a recent Cochrane review identified 16 large reports including 7700 COVID-19 cases of venous thromboembolism, with an estimated weighted mean incidence of 7.4%.2 Nonetheless, many cases of pulmonary embolism (PE) in COVID-19 patients occurred after hospitalization, and as hospitalization itself usually increases complications in bedridden patients with multidrug treatment or in very poor condition, it is difficult to know if SARS-CoV-2 has a pathogenic role in PE development. In addition, irregular initiation of thromboprophylaxis in hospitalized patients during the first wave of the COVID-19 pandemic makes it difficult to interpret the real risk of PE associated with COVID-19. Focus on patients with COVID-19 at emergency department (ED) arrival, before patient hospitalization, could help to answer this question. On the other hand, there are few large series of PE cases carried out in COVID-19 patients describing clinical characteristics and risk factors, or their potential impact on prognosis.2,3 Bearing all these gaps in mind, we designed the current study with the following specific objectives: (i) to determine the relative frequency of PE in patients with COVID-19 coming to the ED and estimate the standardized incidence in the general population; (ii) to uncover the risk factors associated with the development of PE in patients with COVID-19; (iii) to describe whether there is any distinctive clinical characteristic in these patients in comparison with PE observed in non-COVID-19 patients; and (iv) to investigate the outcome of COVID-19 patients presenting with PE.
Methods
Study design and setting
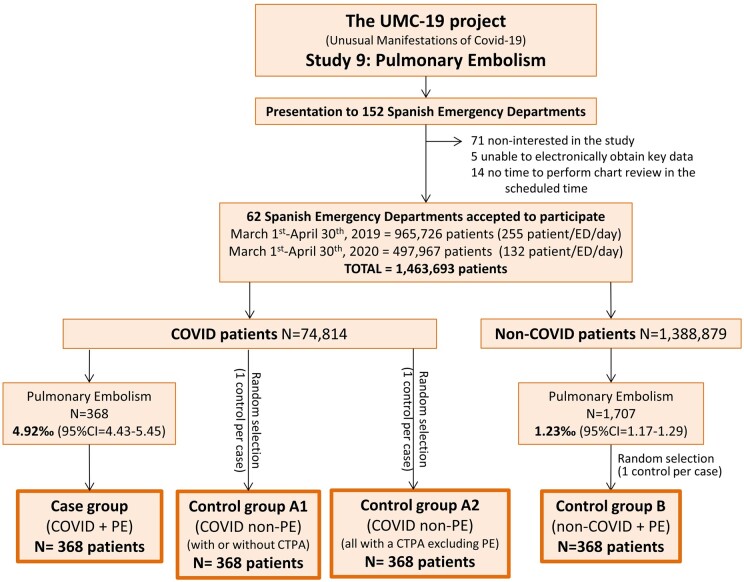
This was a retrospective, case–control, ED-based, multicentre study that reviewed the medical reports of COVID-19 patients attending 62 Spanish EDs (around 20% of public Spanish EDs) who were diagnosed as having objective PE during ED assessment and management, before hospitalization (cases). These 62 EDs have a reference population of 15 094 000 people, which constitutes about 32% of the whole Spanish population (46.9 million). In Spain, the first case of SARS-CoV-2 infection was detected on 31 January 2020 and, accordingly, the definition of the COVID-19 period for patient inclusion in the present study was set from 1 March to 30 April 2020. During this 61-day period, 213 435 cases of COVID-19 were confirmed by the Spanish Ministry of Health.4 The present study forms part of the Unusual Manifestations of Covid-19 (UMC-19) project, which was designed to investigate the potential relationship between COVID-19 and 10 different entities that could be influenced by SARS-CoV-2 infection. Complete details of the UMC-19 project have been published elsewhere.5,6 Study 9 of the UMC-19 project (UMC-19-S9; present study) was designed to specifically investigate PE. Figure 1 resumes the design and patient inclusion flow chart of the UMC-19-S9.
Figure 1.
Study design and inclusion flow chart.
Cases of the UMC-19-S9
The case group was formed by all COVID-19 patients diagnosed with PE during ED stay in March–April 2020. The diagnosis was initially made by radiologists reporting the results of the computed tomography pulmonary angiogram (CTPA) ordered during ED patient care. A second review of CTPA was carried out by radiologists at a local level to confirm the initial diagnosis of PE and to define the location of clots (unilobar or multiple location in lungs; central or peripheral location in vessel lumen) in patients with PE exclusively limited to subsegmental arteries. The diagnosis of COVID-19 was based on SARS-CoV-2 RNA detection in a nasopharyngeal swab by reverse transcriptase polymerase chain reaction (RT-PCR), a clinically compatible clinical picture, or the presence of the typical lung parenchymal infiltrates in chest X-ray or CTPA in patients with some clinical symptoms attributable to COVID-19.
Controls of the UMC-19-S9
We defined three control groups. One group was formed by COVID-19 patients without PE attending the ED during the same period of cases. Selection was randomly performed by the inclusion of a COVID-19 patient seen immediately before or after each case included by the centre (case: control ratio of 1:1). Patients included in this group (control group A1) had no clinical evidence of PE, and only 11% of them had a CTPA ruling out PE. In contrast, the second group (control group A2) was formed by COVID-19 patients in whom PE had been excluded by CTPA in all cases (case: control ratio of 1:1). The third group (control group B) was made up of non-COVID-19 patients with a diagnosis of PE attending the ED during the same period as the cases (1 March to 30 April 2020) as well as from 1 March to 30 April 2019, just 1 year before the COVID-19 pandemic. This control group was formed by selecting one non-COVID-19 patient with PE for every case detected by the centre (case:control ratio of 1:1), which was randomly selected from the complete list of non-COVID-19 patients diagnosed with PE during these two periods.
Independent variables
We collected 21 variables related to baseline characteristics and 38 variables related to the acute episode. Drugs used in the ED to treat COVID-19 and anticoagulants were specifically recorded. In patients with PE (cases and control group B), we also recorded the main findings of the CTPA: type of pulmonary artery where thrombi were located (main pulmonary arteries, lobar arteries, segmental or subsegmental arteries); location of clots in lungs (unilobar or multilobar) and in vessel lumen (central or peripheral) in patients with subsegmental PE; number of lungs affected by PE (uni- or bilateral); and the presence of indirect data of right ventricular dysfunction (RVD). In addition, we also recorded data of deep vein thrombosis (DVT) in legs if this was explored, either by complete compression Doppler ultrasound or by computed tomography (CT) during lung examination by CTPA. For patients in whom echocardiography was performed during ED stay, we also recorded the presence of RVD.
Outcomes
We defined three different outcomes: (i) the need to be admitted to an intensive care unit (ICU); (ii) prolonged hospitalization (longer than 7 days); and (iii) all-cause in-hospital mortality. We also specifically investigated causes of death in patients with PE, which were divided into cardiovascular, non-cardiovascular or unknown according to the Academic Research Consortium-2 (ARC-2) consensus.7
Statistical analysis
The relative frequency of PE in COVID-19 and non-COVID-19 patients coming to the ED was expressed as cases per thousand (‰) with 95% confidence intervals (CI). Standardized incidences (cases per 100,000 person per year) were calculated based on the catchment area of the 62 EDs involved in the study. To estimate COVID-19 and non-COVID-19 populations in each ED catchment area, we used the Spanish provincial SARS-CoV-2 seroprevalences determined between 27 April 27 and 11 May 2020.8 Discrete variables were expressed as absolute values and percentages, and continuous variables as median and interquartile range. Differences between case and control groups were assessed by the chi-square test (or Fisher exact test if needed) for qualitative variables, with previous dichotomization of the continuous variables using clinically meaningful cut-offs or values around the median. Adjusted odds ratios (OR) with 95% CI were calculated in a multivariable model including all the variables with statistically significant differences between groups found in the univariable analysis. Discriminative capacity for PE identification in COVID-19 patients of the model containing all the independent significant variables was estimated using the c-statistic. Beta coefficients obtained in the multivariate logistic regression were used to assign a unique score to every patient. The same adjustment strategy was used for estimations of outcomes, with quantitative variables entered as continuous variables. As the number of events was not expected to be larger, adjustment was performed twice, once using baseline variables and again using acute episode variables. To manage missing values in the variables included in the adjustment, we used a multiple imputation technique provided by SPSS to generate five new datasets without missing data. We also ran a sensitivity analysis of all comparisons by including only COVID-19 patients of the case group and control groups A1 and A2 with microbiological confirmation of SARS-CoV-2 infection by RT-PCR. Statistical significance was accepted if P-value was <0.05, 95% CI of the OR excluded the value 1 or if 95% CI of c-statistic excluded the value 0.5. The analyses were performed with the SPSS (v.24) statistical software package (IBM, Armonk, NY, USA).
Ethics
The UMC-19 project was approved with reference number HCB/2020/0534 by the Ethical Committee of the Hospital Clínic of Barcelona (Spain) that acted as the central ethics committee. The UMC-19-S9 was carried out in strict compliance with the Declaration of Helsinki principles.
Results
Frequency and standardized incidence of PE
A total of 74 814 patients with COVID-19 attended the 62 Spanish EDs participating in the UMC-19-S5 (Figure 1) during the 61-day study period. In 368 of these patients, PE was diagnosed in the ED (frequency 4.92‰, 95% CI 4.43–5.45) and constituted the case group. A second review of CTPA confirmed all PEs, although five subsegmental PEs were reclassified as segmental. Control group A1 was formed by 368 randomly selected COVID-19 patients without PE attending the ED during the same period, while control group A2 was formed by 368 COVID-19 randomly selected patients in whom PE was excluded by CTPA. COVID-19 infection was confirmed by RT-PCR in 271 cases, in 271 control group A1 patients (73.6% in both groups, P = 1.00) and in 275 control group A2 patients (74.7%, P = 0.74).
On the other hand, 1 388 879 non-COVID-19 patients were seen during the 122-day period (423 153 during the 61 days in the 2020 COVID-19 period, and 965 726 during the 61 days in the 2019 pre-COVID-19 period). Of these, 1707 were diagnosed with PE (717 in the COVID-19 period, and 990 in the pre-COVID-19 period). Accordingly, the overall relative frequency was 1.23‰ (95% CI 1.17–1.29) with a COVID-19 period relative frequency of 1.69‰ (95% CI 1.57–1.82) and a pre-COVID-19 period relative frequency of 1.02‰ (95% CI 0.96–1.09). A sample of 368 of these patients randomly selected constituted control group B (165 corresponding to the pre-COVID-19 period and 205 to the COVID-19 period—89% having a negative SARS-CoV-2 RT-PCR). After the second CTPA review, all PEs were confirmed, and one was reclassified from the subsegmental to the segmental category.
The overall standardized incidences of PE were 310.1 per 100 000 COVID-19 individuals per year (95% CI 297.3–323.3) and 34.7 per 100 000 non-COVID-19 individuals per year (95% CI 33.8–35.7; with partial standard incidences of 28.5 in the COVID-19 period and 41.3 in the pre-COVID-19 period). Accordingly, the OR for PE in COVID-19 patients compared to non-COVID-19 patients was of 8.95 (95% CI 8.51–9.41) (OR 7.53 compared to the COVID-19 period, 95% CI 7.17–7.91; OR 10.91 compared to the pre-COVID-19 period, 95% CI 10.67–11.49).
Risk factors for PE in COVID-19 patients
The median age of COVID-19 patients with PE (cases) was 66 years; 58% were males, and 33% had at least one risk factor for PE. Other common comorbidities were hypertension (49%), dyslipidemia (36%) and diabetes mellitus (18%) (Table 1). The most frequent symptoms were dyspnoea (72%), cough (48%) and fever (45%), while chest pain was referred by 30% of cases, leg swelling or pain by 14%, syncope by 4% and hemoptisis by 2% (Table 2). The abnormalities in vitals at ED arrival most often found were room air pulsioxymetry <95% (46%) and respiratory rate >20 b.p.m. (45%). More than half of the cases had D-dimer levels >1000 ng/mL (83%), C-reactive protein >5 mg/dL (64%) and lactate dehydrogenase >300 U/L (53%). Abnormalities in lung parenchyma were very frequently found on chest X-ray, while cardiomegaly and pleural effusion were only present in 8% of cases each. The frequency of the remaining findings related to the acute episode is shown in Table 2.
Table 1.
Baseline characteristics of COVID-19 patients with pulmonary embolism compared with COVID-19 patients without pulmonary embolism (control groups A1 and A2) and non-COVID-19 patients with pulmonary embolism (control group B)
| Cases (COVID-19 PE) N = 368 n (%) | Control group A1 (COVID-19 non-PE) N = 368 n (%) | P-valuea | Control group A2 (COVID-19 non-PE) N = 368 n (%) | P-valueb | Control group B (non-COVID-19 + PE) N = 368 n (%) | P-valuec | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age (years) [median (IQR)] | 66(55–78) | 66(52–77) | 0.51 | 64(53–75) | 0.13 | 71(58–80) | 0.01 |
| Age <60 years | 139(37.8) | 148(40.2) | 0.50 | 140(38.0) | 0.28 | 107(29.1) | 0.01 |
| Male sex | 215(58.4) | 199(54.1) | 0.23 | 205(55.7) | 0.46 | 180(48.9) | 0.01 |
| Risk factors for PE | |||||||
| Obesity (clinically estimated) | 62(16.8) | 59(16.0) | 0.77 | 82(22.3) | 0.06 | 75(20.4) | 0.22 |
| Active cancer | 42(11.4) | 37(10.1) | 0.55 | 45(12.2) | 0.73 | 99(26.9) | <0.001 |
| Recent immobilization (last month) | 34(9.2) | 16(4.3) | 0.008 | 28(7.6) | 0.43 | 41(11.1) | 0.39 |
| Previous thromboembolic disease | 13(3.5) | 17(4.6) | 0.46 | 26(7.1) | 0.032 | 58(15.8) | <0.001 |
| On chronic oestrogen therapy | 3(0.8) | 5(1.4) | 0.48 | 15(4.1) | 0.004 | 21(5.7) | <0.001 |
| Other comorbidities | |||||||
| Hypertension | 181(49.2) | 168(45.7) | 0.34 | 181(49.2) | 1.00 | 196(53.3) | 0.27 |
| Dyslipidaemia | 133(36.1) | 127(34.5) | 0.64 | 142(38.6) | 0.49 | 133(36.1) | 1.00 |
| Diabetes mellitus | 66(17.9) | 69(18.8) | 0.78 | 85(23.1) | 0.08 | 62(16.8) | 0.70 |
| Asthma | 30(8.2) | 27(7.3) | 0.68 | 35(9.5) | 0.52 | 10(2.7) | 0.001 |
| Dementia | 27(7.3) | 33(9.0) | 0.42 | 17(4.6) | 0.12 | 28(7.6) | 0.89 |
| COPD | 25(6.8) | 33(9.0) | 0.27 | 38(10.3) | 0.09 | 42(11.4) | 0.03 |
| Active smoker | 24(6.5) | 25(6.8) | 0.88 | 39(10.6) | 0.047 | 60(16.3) | <0.001 |
| Cerebrovascular disease | 24(6.5) | 25(6.8) | 0.88 | 19(5.2) | 0.43 | 22(6.0) | 0.76 |
| Coronary artery disease | 23(6.3) | 29(7.9) | 0.39 | 36(9.8) | 0.08 | 18(4.9) | 0.42 |
| Chronic kidney disease | 18(4.9) | 24(6.5) | 0.34 | 32(8.7) | 0.040 | 21(5.7) | 0.62 |
| Immunosuppressed | 13(3.5) | 17(4.6) | 0.46 | 34(9.2) | 0.002 | 29(7.9) | 0.01 |
| Peripheral artery disease | 11(3.0) | 20(5.4) | 0.10 | 17(4.6) | 0.25 | 23(6.3) | 0.04 |
| Chronic heart failure | 9(2.4) | 31(8.4) | <0.001 | 25(6.8) | 0.005 | 24(6.5) | 0.008 |
| Chronic liver disease | 9(2.4) | 15(4.1) | 0.21 | 8(2.2) | 0.81 | 8(2.2) | 0.81 |
Bold values denote statistically significant differences (P < 0.05).
Comparison between cases and control group A1.
Comparison between cases and control group A2.
Comparison between cases and control group B.
COPD, chronic obstructive pulmonary disease; IQR, interquartile range; PE, pulmonary embolism.
Table 2.
Characteristics of the acute episode of COVID-19 patients with pulmonary embolism compared with COVID-19 patients without pulmonary embolism (control groups A1 and A2) and non-COVID-19 patients with pulmonary embolism (control group B)
| Cases (COVID-19 + PE) N = 368 n (%) | Control group A1 (COVID-19 non-PE) N = 368 n (%) | P-valuea | Control group A2 (COVID-19 non-PE) N = 368 n (%) | P-valueb | Control group B (non-COVID-19 + PE) N = 368 n (%) | P-valuec | |
|---|---|---|---|---|---|---|---|
| Symptoms at ED arrival | |||||||
| Duration of symptoms (days) [median (IQR)] | 7(3–12) | 7(3–10) | 0.18 | 7(4–10) | 0.72 | 3(1–7) | <0.001 |
| Duration of symptoms >7 days | 156(42.4) | 128(34.8) | 0.03 | 148(40.5) | 0.61 | 64(17.4) | <0.001 |
| Dyspnoea | 263(71.5) | 201(54.6) | <0.001 | 224(60.9) | 0.002 | 251(68.2) | 0.34 |
| Cough | 176(47.8) | 211(57.3) | 0.01 | 240(65.2) | <0.001 | 47(12.8) | <0.001 |
| Fever (>38°C) | 165(44.8) | 219(59.5) | <0.001 | 222(60.3) | <0.001 | 20(5.4) | <0.001 |
| Chest pain | 111(30.2) | 44(12.0) | <0.001 | 64(17.4) | <0.001 | 132(35.9) | 0.10 |
| Diarrhoea | 60(16.3) | 62(16.8) | 0.84 | 78(21.2) | 0.09 | 7(1.9) | <0.001 |
| Leg swelling or pain | 53(14.4) | 14(3.8) | <0.001 | 20(5.4) | <0.001 | 128(34.8) | <0.001 |
| Expectoration | 38(10.3) | 54(14.7) | 0.08 | 55(14.9) | 0.06 | 28(7.6) | 0.20 |
| Confusion | 30(8.2) | 27(7.3) | 0.68 | 15(4.1) | 0.021 | 20(5.4) | 0.14 |
| Vomiting | 24(6.5) | 24(6.5) | 1.00 | 22(6.0) | 0.761 | 8(2.2) | 0.004 |
| Dysgeusia | 21(5.7) | 28(7.6) | 0.30 | 31(8.4) | 0.15 | 2(0.5) | <0.001 |
| Anosmia | 20(5.4) | 23(6.3) | 0.64 | 35(9.5) | 0.035 | 1(0.3) | <0.001 |
| Abdominal pain | 20(5.4) | 18(4.9) | 0.74 | 17(4.6) | 0.61 | 23(6.3) | 0.64 |
| Rhinorrhea | 17(4.6) | 24(6.5) | 0.26 | 14(3.8) | 0.58 | 6(1.6) | 0.02 |
| Syncope | 15(4.1) | 15(4.1) | 1.00 | 14(3.8) | 0.85 | 48(13.0) | <0.001 |
| Hemoptysis | 8(2.2) | 2(0.5) | 0.06 | 5(1.4) | 0.40 | 8(2.2) | 1.00 |
| Signs at ED arrival | |||||||
| Room air pulsioxymetry <95% (368/362/368/366) | 168(45.7) | 132(36.5) | 0.01 | 188(51.4) | 0.12 | 153(41.6) | 0.27 |
| Respiratory rate > 20 b.p.m.. (363/351/355/328) | 162(44.6) | 112(31.9) | <0.001 | 133(40.5) | 0.28 | 92(25.9) | <0.001 |
| Heart rate >100 b.p.m. (368/359/368/365) | 123(33.4) | 80(22.3) | 0.001 | 106(29.0) | 0.20 | 132(35.9) | 0.49 |
| Temperature >38°C (367/360/367/362) | 72(19.6) | 83(23.1) | 0.26 | 92(25.4) | 0.09 | 28(7.6) | <0.001 |
| SBP <100 mmHg (367/357/368/362) | 28(7.6) | 22(6.2) | 0.44 | 31(8.6) | 0.64 | 22(6.0) | 0.37 |
| Laboratory findings (N/N/N/N)d | |||||||
| D-dimer (ng/mL) [median (IQR)] (338/274/345/286) | 4,894(1,678–10,810) | 655(360–1,280) | <0.001 | 1,100(500–3,740) | <0.001 | 4,841(1,872–10,000) | <0.001 |
| D-dimer >1000 ng/mL | 281(83.1) | 96(35.0) | <0.001 | 183(53.0) | <0.001 | 264(92.3) | 0.001 |
| C-reactive protein >5 mg/dL (350/318/356/260) | 223(63.7) | 177(55.7) | 0.03 | 228(64.0) | 0.93 | 105(40.4) | <0.001 |
| Lactate dehydrogenase >300 U/L (296/260/311/137) | 156(52.7) | 109(41.9) | <0.001 | 169(54.3) | 0.69 | 52(38.0) | 0.004 |
| NT-proBNP >500 pg/mL (88/50/54/94) | 43(48.9) | 20(40.0) | 0.32 | 29(53.7) | 0.575 | 58(61.7) | 0.08 |
| Lymphocyte count <1 × 103 cells/μL (365/336/366/344) | 141(40.8) | 131(41.1) | 0.93 | 196(53.6) | 0.254 | 79(23.0) | <0.001 |
| Leukocyte count >10 × 103 cells/μL (346/319/288/344) | 142(38.9) | 60(17.9) | <0.001 | 84(22.8) | <0.001 | 134(36.7) | 0.54 |
| Increased troponin (>99th percentile) (331/296/246/305) | 95(36.5) | 30(22.4) | <0.004 | 39(15.9) | <0.001 | 116(46.8) | 0.02 |
| Aspartate animo transferase >40 U/L (302/275/307/173) | 106(35.1) | 89(32.4) | 0.49 | 115(37.5) | 0.55 | 32(18.5) | <0.001 |
| Platelets >300 × 103 elements/μL (361/333/349/364) | 110(30.5) | 46(13.8) | <0.001 | 81(23.2) | 0.029 | 63(17.3) | <0.001 |
| Creatinine >1.3 mg/dL (360/334/341/362) | 64(17.8) | 47(14.1) | 0.18 | 125(36.7) | <0.001 | 59(16.3) | 0.60 |
| Haemoglobin <120 g/L (363/336/365/363) | 62(17.1) | 59(17.6) | 0.87 | 55(15.1) | 0.46 | 96(26.4) | 0.002 |
| Bilirubin >1 mg/dL (282/239/287/164) | 44(15.6) | 26(10.9) | 0.12 | 79(27.5) | 0.001 | 20(12.2) | 0.32 |
| Chest X-ray (N/N/N/N)d | |||||||
| Ground-glass lung opacities (358/357/357/294) | 234(65.4) | 205(57.4) | 0.03 | 275(75.8) | 0.007 | 41(13.9) | <0.001 |
| Interstitial lung infiltrates (bilateral) (358/357/363/294) | 175(48.9) | 161(45.1) | 0.31 | 147(41.2) | 0.025 | 21(7.1) | <0.001 |
| Cardiomegaly (358/357/360/294) | 28(7.8) | 35(9.8) | 0.35 | 55(15.3) | 0.005 | 56(19.0) | <0.001 |
| Pleura effusion (358/357/359/294) | 28(7.8) | 14(3.9) | 0.03 | 25(7.0) | 0.56 | 45(15.3) | 0.003 |
| ECG (N/N/N/N)d | |||||||
| Atrial fibrillation (310/233/70/287) | 16 (5.2) | 20 (8.6) | 0.11 | 7 (10.0) | 0.13 | 17 (5.9) | 0.68 |
Bold values denote statistically significant differences (P < 0.05).
Comparison between cases and control group A1.
Comparison between cases and control group A2.
Comparison between cases and control group B.
(N/N/N/N) indicates the number of patients for whom these data were available in case/control A1/control A2/control B groups; when this is not indicated, all patients of the four groups had data (no missing values).
ED, emergency department; IQR, interquartile range; SBP, systolic blood pressure; NT-proBNP, N-terminal pro B-type natriuretic peptide; ECG, electrocardiogram; PE, pulmonary embolism.
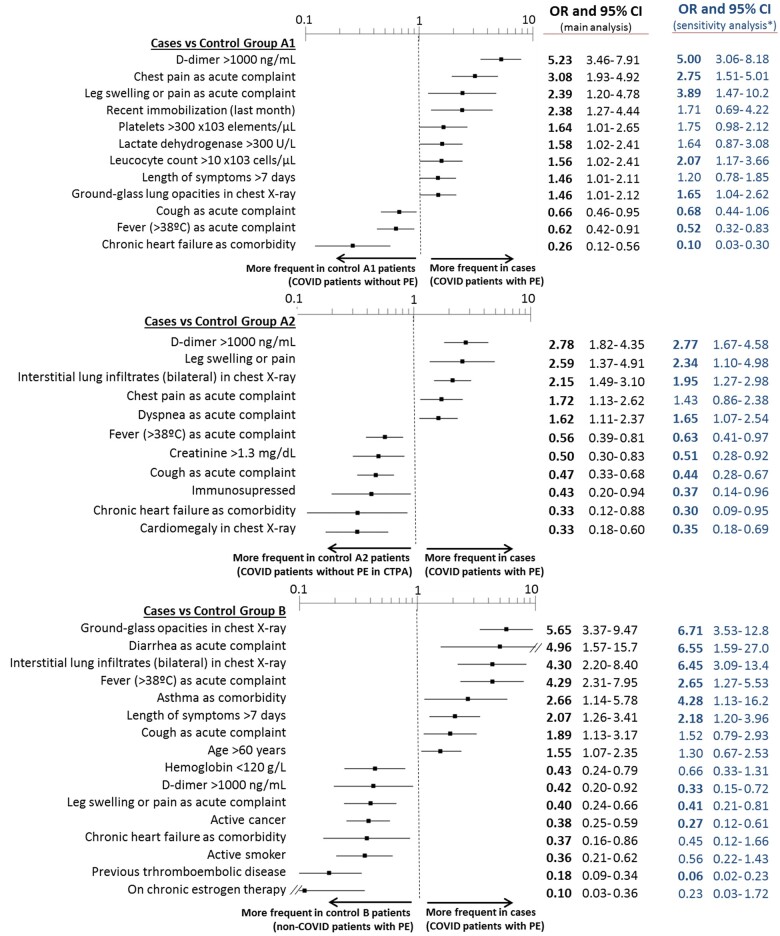
Cases differed in 19 (with respect to control group A1) and 22 (with respect to control group A2) out of the 48 baseline and acute episode characteristics (Tables 1 and 2). Of these characteristics, 12 and 11, respectively, were independently associated with PE in COVID-19 patients in the adjusted models (Figure 2), with c-statistics for PE prediction in COVID-19 patients of 0.84 (0.80–0.87) and 0.77 (0.73–0.81), respectively. Remarkably, six of these independent characteristics were common in both comparisons (with control groups A1 and A2), with D-dimer levels >1000 ng/mL, chest pain, and leg swelling or pain showing direct associations, and cough, fever, and chronic heart failure showing inverse associations. Although there were no differences in the proportion of cases and control groups A1 or A2 having atrial fibrillation, atrial fibrillation was more frequently present in patients with than without chronic heart failure (28.2% vs. 6.3%, P < 0.001). Very similar results were found in the sensitivity analysis restricted to the 271 cases and 271 and 275 patients of control groups A1 and A2, respectively, with microbiological confirmation of SARS-CoV-2 infection (Figure 2).
Figure 2.
Baseline and acute episode characteristics found on multivariate analysis to be independently associated with COVID-19 patients with pulmonary embolism (cases) with respect to COVID-19 patients without pulmonary embolism (control groups A1 and A2, upper and middle panels) and non-COVID-19 patients with pulmonary embolism (control group B, lower panel). *Sensitivity analysis is presented only by blue figures (no graphs) and was run by using only patients with SARS-CoV-2 infection microbiologically confirmed in the case group (n = 271, 73.6%), control group A1 (n = 271, 73.6%), and control group A2 (n = 275, 74.7%). CI, confidence interval; OR, odds ratio.
Distinctive clinical characteristics of PE in COVID-19 patients
Cases differed from non-COVID-19 patients with PE in 35 out of the 48 clinical characteristics (Tables 1 and 2). Many of the differences were mainly directly related to COVID-19 infection. After adjustment, 16 characteristics remained statistically significant, the strongest being ground-glass opacities and bilateral interstitial infiltrates on chest X-ray (OR 5.65, 95% CI 3.37–9.47 and OR 4.30, 95% CI 2.20–8.40, respectively), diarrhoea and fever as clinical complaints (OR 4.96, 95% CI 1.57–15.7 and OR 4.29, 95% CI 2.31–7.95, respectively), and previous thromboembolic disease and chronic oestrogen therapy (OR 0.18, 95% CI 0.09–0.34 and OR 0.10, 95% CI 0.03–0.36, respectively). Remarkably, D-dimer levels >1000 ng/mL, leg swelling/pain, and active cancer were also significantly less present in cases (Figure 2).
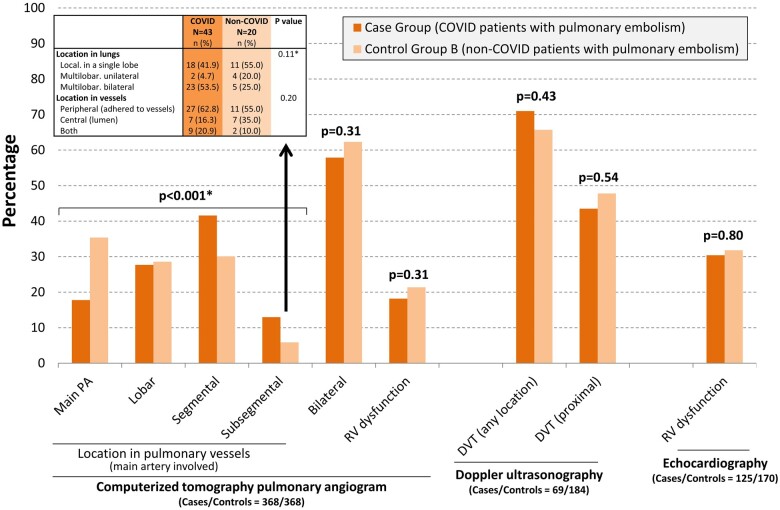
In patients with PE, the main pulmonary arteries were more frequently involved in non-COVID-19 patients, while thrombi were more frequently restricted to segmental or subsegmental arteries in COVID-19 patients (Figure 3). Nonetheless, no significant differences were found in lung or vessel clot location in subsegmental PE of COVID-19 and non-COVID-19 patients. RVD was similar in COVID-19 and non-COVID-19 patients, and leg DVT (wherever or proximal) was also similar in both groups.
Figure 3.
Imaging findings in patients with pulmonary embolism, comparing those with (cases) and without (control group B) COVID-19. *Comparison between cases and control group B was performed by chi-square test for trend. DVT, deep vein thrombosis; PA, pulmonary artery; RV, right ventricle.
Treatments started at the emergency department
Cases received some drugs related to COVID-19 more frequently than control group A1 patients, but at a similar frequency to that of control group A2 patients (Table 3). Low-molecular-weight heparin was used in 97% of patients with PE (89% at high dosage). Anticoagulant therapy was used equally in COVID-19 and in non-COVID-19 patients with PE (Table 3). We observed a peak of PE diagnosis in COVID-19 patients during late March and early April 2020 (coinciding with the highest peak of COVID-19 diagnosis during the first pandemic wave in Spain4), but no differences were observed in the use of anticoagulant therapy along the study period (Figure 4).
Table 3.
Specific treatments initiated in the emergency department
| Cases (COVID-19 + PE) N = 368 n (%) | Control group A1 (COVID-19 non-PE) N = 368 n (%) | P-valuea | Control group A2 (COVID-19 non-PE) N = 368 n (%) | P-valueb | Control group B (non-COVID-19 + PE) N = 368 n (%) | P-valuec | |
|---|---|---|---|---|---|---|---|
| Drugs related to COVID-19 | |||||||
| Hydroxycloroquine | 274(74.5) | 240(65.2) | 0.006 | 283(77.1) | 0.401 | – | – |
| Antibiotics (other than azithromicyn) | 248(67.4) | 201(54.6) | <0.001 | 241(65.7) | 0.620 | – | – |
| Azithromicyn | 229(62.2) | 214(58.2) | 0.259 | 240(65.4) | 0.372 | – | – |
| Corticosteroids | 136(37.0) | 88(23.9) | <0.001 | 159(43.6) | 0.068 | – | – |
| Lopinavir-ritonavir | 123(33.4) | 110(29.9) | 0.303 | 106(28.9) | 0.184 | – | – |
| Tocilizumab | 70(19.0) | 25(6.8) | <0.001 | 70(19.1) | 0.986 | – | – |
| Remdesivir | 5(1.4) | 2(0.5) | 0.451 | 7(1.9) | 0.554 | – | – |
| Baricitinib | 4(1.1) | 1(0.3) | 0.373 | 3(0.8) | 1.000 | – | – |
| Anakinra | 4(1.1) | 1(0.3) | 0.373 | 2(0.5) | 0.686 | – | – |
| Anticoagulation | |||||||
| Heparin (LMWH) | <0.001* | <0.001* | 0.334* | ||||
| High doses | 329(89.4) | 30(8.2) | 53(14.4) | 342(92.9) | |||
| Intermediate doses | 11(3.0) | 39(10.6) | 34(9.2) | 4(1.1) | |||
| Low doses | 17(4.6) | 127(35.5) | 223(60.6) | 9(2.4) | |||
| No anticoagulation | 11(3.0) | 172(46.7) | 58(15.8) | 13(3.5) |
Bold values denote statistically significant differences (P < 0.05).
Comparison between cases and control group A1.
Comparison between cases and control group A2.
Comparison between cases and control group B.
P-values calculated with chi-square for trend.
LMWH, low-molecular-weight heparin; PE, pulmonary embolism.
Figure 4.
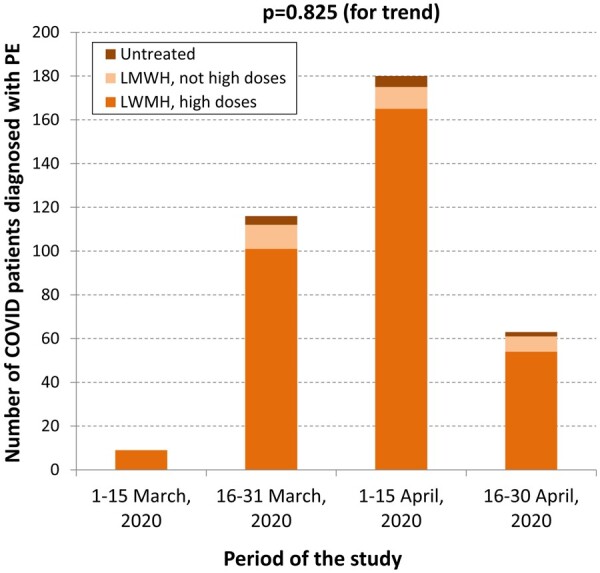
Number of COVID-19 patients diagnosed with pulmonary embolism along the study period, and anticoagulation regimen provided in the emergency department after the diagnosis of pulmonary embolism. LMWH, low-molecular-weight heparin; PE, pulmonary embolism.
Outcomes
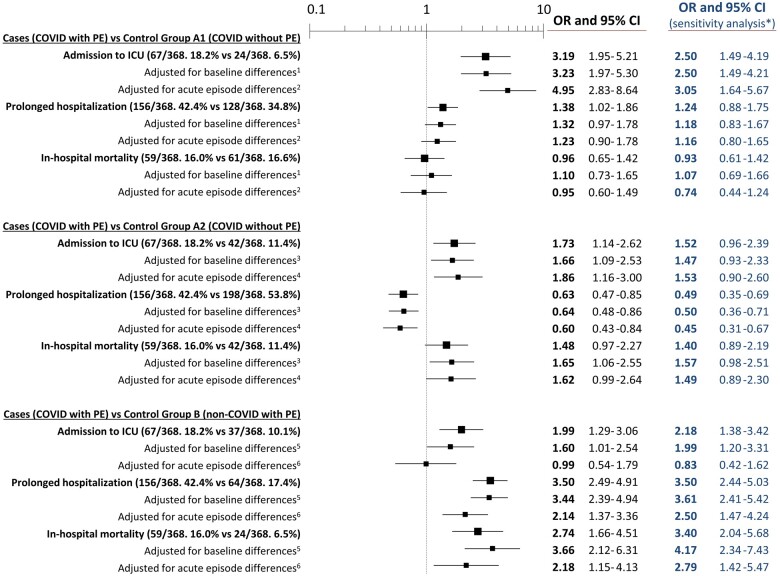
ICU admission was significantly higher in cases than in the control group A1 patients (18.0% vs. 6.5%; OR 3.19, 95% CI 1.95–5.21) and control group A2 patients (18.0% vs. 11.4%; OR 1.73, 95% CI 1.14–2.62) and these differences remained significant after adjustments (Figure 5). Conversely, we did not find statistically significant differences in mortality between cases and control group A1 patients (16.0% vs. 16.6%; OR 0.96, 95% CI 0.65–1.42) and control group A2 patients (16.0% vs. 11.4%; OR 1.48, 95% CI 0.97–2.27). Adjusted models did not change these estimations, except for comparison with control group A2 patients adjusted for baseline characteristics (OR 1.65, 95% CI 1.05–2.55).
Figure 5.
Outcomes of patients with COVID-19 and pulmonary embolism compared with COVID-19 patients without pulmonary embolism (control groups A1 and A2) and non-COVID-19 patients with pulmonary embolism (control group B). *Sensitivity analysis is presented only by blue figures (no graphs) and was run using only patients with SARS-CoV-2 infection microbiologically confirmed in the case group (n = 271, 73.6%), control group A1 (n = 271, 73.6%) and control group A2 (n = 275, 74.7%). 1Adjusted for recent immobilization and chronic heart failure. 2Adjusted for dyspnoea, cough, fever, chest pain and leg swelling/pain as clinical complaints, lactate dehydrogenase, leucocytes, platelets, D-dimer, and ground-glass lung opacities on chest X-ray (missing values were replaced using multiple imputation). 3Adjusted for immunosuppressed and chronic heart failure. 4Adjusted by dyspnoea, cough, fever, chest pain, leg swelling/pain as clinical complaints, creatinine, D-dimer, and interstitial lung infiltrates and cardiomegaly on chest X-ray (missing values were replaced using multiple imputation). 5Adjusted for age, active cancer, recent immobilization, chronic oestrogen therapy, asthma, active smoker, and chronic heart failure. 6Adjusted for dyspnoea, cough, fever, diarrhoea and leg swelling/pain as clinical complaints, haemoglobin, D-dimer, and lung interstitial bilateral infiltrates and ground-glass opacities on chest X-ray (missing values were replaced using multiple imputation). CI, confidence interval; ICU, intensive care unit; OR, odds ratio; PE, pulmonary embolism.
On the other hand, in-hospital mortality of cases was higher than in control group B patients (6.5%; OR 2.74, 95% CI 1.66–4.51), and this increased mortality remained statistically significant after adjustment for differences in baseline (OR 3.66, 95% CI 2.12–6.31) and acute episode (OR 2.18, 95% CI 1.15–4.13) characteristics. The causes of death of patients with PE could be classified in all but six patients (unknown in five COVID-19 and one non-COVID-19) and did not differ between COVID-19 and non-COVID-19 patients, being cardiovascular in 4 (7.4%) and 3 (13.0%), and non-cardiovascular in 50 (92.6%) and 20 (87.0%), respectively (P = 0.42). Admission to an ICU and prolonged hospitalization were also increased in cases with respect to control group B patients, although statistical differences in the former disappeared after adjustment for differences in the acute episode characteristics (Figure 5). All outcome associations were very similar in the sensitivity analysis and in the principal analysis (Figure 5).
Discussion
The UMC-19-S9 reports four main findings needing to be highlighted, summarized in Graphical Abstract. First, PE is relatively uncommon as a manifestation of SARS-CoV-2 infection before patient hospitalization (0.5%); however, when we consider standardized incidence per year, this is almost 9-fold greater in the COVID-19 than in the non-COVID-19 population. Second, D-dimer levels >1000 ng/mL and chest pain as a clinical complaint were the strongest risk factors directly associated with PE development in COVID-19 patients. Third, COVID-19 patients with PE differed from non-COVID-19 patients with PE mainly in the clinical characteristics directly related to COVID-19 infection, although, remarkably, they had fewer risk factors for PE, complained less frequently of leg swelling/pain, exhibited a more discrete rise in D-dimers, and thrombi affected smaller pulmonary arteries. Fourth, the mortality was high (one in six patients died during hospitalization), being more than double the number observed in the general population (non-COVID-19) with PE. Conversely, no statistical differences were found in in-hospital mortality of COVID-19 patients with and without PE; therefore, PE should not be considered as a risk factor for death in patients with COVID-19.
Our estimation of the standardized incidence of PE of 35 per 100 000 person-years in the general (non-COVID-19) population (41 considering only the pre-COVID-19 period, when fear of COVID-19 contagion in EDs was not present) is close to that reported in previous studies: 69 in the USA in the period from 1966 to 1990 (with a progressive decline over time being noticed),9 50 in Norway in the period from 1995 to 2001,10 and 38 in Canada in the period from 2002 to 2012 (with a stable incidence over time),11 which makes our estimation quite reliable. Therefore, the incidence of PE of 310 per 100 000 person-years found in COVID-19 patients strongly suggests that SARS-CoV-2 is associated with a marked and significant increment in the risk of PE and could have a direct potential pathogenic role in the development of PE. Nonetheless, our results must be interpreted with caution, as they could be biased by the particular circumstances of the first pandemic wave, including population lockdown, the high number of CTPA ordered in COVID-19 patients (making the diagnosis of PE easier and more frequent) and the fact that non-COVID-19 patients with small PEs could have been less diagnosed as they stayed at home (due to fear of contagion in EDs) or were less frequently explored with CTPA if they complained of non-specific symptoms. However, as difference in standardized incidences in both populations was very large (OR 8.95), and as the OR for PE in COVID-19 patients was also very high when compared with PE diagnosed in non-COVID-19 patients during the pre-COVID-19 period (OR 10.91), and lastly, as nearly half of PE in COVID-19 patients involved the main or lobar pulmonary arteries (only 12% were very small PEs, affecting exclusively subsegmental arteries), we believe that there really is an increase in the incidence of PE in patients with COVID-19.
Our comparison between COVID-19 patients with and without PE was performed before hospitalization and, accordingly, it did not take into account the secondary effects of being bedridden for a long time and/or intubation that can adversely affect hospitalized patients and favour thromboembolic disease. In this scenario of ED, COVID-19 patients complaining of chest pain and having D-dimer levels over 1000 ng/mL were at increased risk of having PE. D-dimers are a classical biomarker of PE in the general population, although cut-off adjustments must be made in a number of situations, the most relevant being patient age.12 Similarly, it is possible that a specific D-dimer cut-off value for COVID-19 patients is needed, as non-specific mild increments of D-dimer are usually observed in such a population.1 On the other hand, the inverse association of PE with chronic heart failure found in the present study is striking, and contrasts with previously reported data identifying chronic heart failure as a risk factor for PE.13 We can hypothesize two potential explanations for our finding: the competing risk of death or the higher presence of atrial fibrillation in chronic heart failure patients (in our series, 28.2% compared to 6.3% in the rest of patients; P < 0.001). Atrial fibrillation usually associates a high use of oral anticoagulants and this could have been, in fact, the factor related to the apparent protective effect of chronic heart failure for development of PE in COVID-19 patients.
It was of note that leg swelling/pain and risk factors for PE were significantly less present in COVID-19 than in non-COVID-19 patients with PE, despite DVT and proximal DVT being diagnosed in a similar percentage of patients. This suggests that some PE could develop in situ in lungs, favoured by a highly inflammatory involvement and, in fact, in situ immunothrombosis has been proposed to play a role in the pathophysiology of COVID-19-associated PE.14 In this sense, alveolar injury and the inflammatory storm present during COVID-19 pneumonia along with disruption of the thrombo-protective state of the pulmonary vascular endothelial cells might contribute to the formation of deep small vessel thrombi. We made a second review of CTPA for adjudication of morphological vascular findings, with special focus on lumen location of thrombi placed in small pulmonary arteries, in order to figure this hypothesis out. Nonetheless, our study does not provide definitive data on this regard: while anatomical involvement of smaller pulmonary arteries would support the hypothesis of a local (lung) process in PE developed in COVID-19 patients, no differences were found in clot location in subsegmental PEs. Finally, it is remarkable that involvement of smaller pulmonary arteries (segmental or subsegmental) does not translate in a different severity of PE with respect to the general population, as we found a similar proportion of patients with PE presenting RVD.
When outcomes were analysed, the need for ICU care was clearly increased in COVID-19 patients with PE compared to COVID-19 patients without PE, in agreement with a previous study by Fauvel et al.3 in 24 French hospitals. Conversely, our results were more inconclusive for in-hospital mortality. Overall, we did not find significant increments in mortality, in line with the results reported by Fauvel et al.3 However, while the mortality was similar when compared with COVID-19 patients in whom PE was mainly excluded on the basis of clinical findings, it showed a trend to increase when compared with COVID-19 patients in whom PE was excluded using CTPA in all cases, and even achieved statistical significance after adjustment for baseline characteristics. It is difficult to decide which of these two control groups of COVID-19 patients without PE is better for making comparisons since while the former could include some patients having PE in whom PE was not diagnosed during ED management, the latter could include less sick patients in whom lung scans were routinely performed to detect parenchymal lung involvement by COVID-19. Accordingly, caution is recommended when interpreting the impact of PE on mortality in COVID-19 patients, as it is difficult to isolate the adverse effects (death, in this case) of COVID-19 (essentially lung involvement due to inflammation and hyperimmune reaction) from the adverse effects of PE. On the other hand, the mortality was higher in COVID-19 than in non-COVID-19 patients with PE, but the causes of death were not significantly different between the two groups. Although part or all of this increase could be related to severe COVID-19 (with lung parenchymal involvement and secondary respiratory failure), the true role of PE in determining poor outcomes in COVID-19 patients remains to be elucidated.
Limitations
This study has several limitations. First, PE was only detected if CT was performed in the ED. However, during the COVID-19 pandemic, emergency physicians had a very low threshold for ordering chest imaging. Second, undiagnosed PE might have been present, although radiologists reviewed all the imaging studies, making this possibility very unlikely. Third, we did not quantify the number of CTPA performed in COVID-19 patients; therefore, the proportion of CTPA positivity in unknown. However, a recent analysis has shown that, once PE is suspected in the ED and a CTPA is ordered, acute PE is diagnosed in the same proportion of COVID-19 and non-COVID-19 patients.15 Fourth, we did not adjust the incidence of PE in COVID-19 by patient-related or disease-related factors that could have accounted for the increased risk of PE diagnosis with respect to non-COVID-19 patients. In this sense, non-COVID-19 patients with small size PE and/or mild symptoms could have avoided the ED visit for health care due to fear of SARS-CoV-2 contagion as has been reported for patients with acute coronary syndromes and acute cardiovascular diseases in general.16,17 However, the almost 9-fold excess of the standardized incidence in PE in COVID-19 with respect to the non-COVID-19 population makes unlikely that adjustments could even correct for such a huge difference. Fifth, estimations of standardized incidences did not take into account PE developed or discovered once the patient was already hospitalized. However, this possibility would further increase the differences in the incidence of PE between COVID-19 and non-COVID-19 patients. Sixth, in many cases the diagnosis of COVID-19 was based on clinical and/or radiological findings, with no microbiological confirmation due to shortage of diagnostic tests experienced in Spain during the first pandemic wave,18 and a few of patients with PE included in the COVID-19 period could have been misclassified with respect to their COVID-19 status. This limitation was managed by performing a sensitivity analysis limited to patients with confirmed SARS-CoV-2 infection, which showed very similar results as the main analysis. Seventh, as a retrospective study, although the case record form was standardized, there was no monitoring of data collection methods and diagnosis, and outcomes were locally adjudicated. Eighth, as treatments provided during hospitalization were not recorded, the impact of inappropriate management on outcomes was not assessed in the present study. Finally, the UMC-19-S9 was performed during the first wave of the COVID-19 pandemic. As the mortality has shifted lower and new variants of the SARS-CoV-2 have emerged, we do not know if our reported findings will endure in the subsequent waves.
Conclusions
The UMC-19-S9 demonstrates that PE in COVID-19 patients is unusual (about 0.5%) at ED presentation, but standardized incidence is about 9-fold higher than expected in the general (non-COVID-19) population. PE by itself probably does not increase in-hospital mortality of the COVID-19 population presenting to the ED. As risk factors and leg symptoms are less frequent, and the increase in D-dimer is lower than in non-COVID-19 patients developing PE, a red flag should be raised by any treating physicians assessing COVID-19 patients in order to detect PE in these patients, and to promptly start specific anticoagulant treatment, as recent data show that this is a safe treatment that should be provided to COVID-19 patients with PE.19
Contribution of authors
All the authors discussed the idea and design of study and provided patients. Data analysis and first draft writing was done by OM. All the authors have read this draft and provided insight for the final version. OM is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to publication of the article.
Funding
The present work was performed without any direct or indirect financial support.
Conflict of interest: none declared.
Data availability
Acces to data will be addressed to the main author and will have evaluated and decided by the stering committe.
Appendix
The SIESTA network is formed by the following researchers and centres (all from Spain):
Steering committee:
Òscar Miró, Sònia Jiménez (Hospital Clínic, Barcelona), Juan González del Castillo, Francisco Javier Martín-Sánchez (Hospital Clínico San Carlos, Madrid), Pere Llorens (Hospital General de Alicante), Guillermo Burillo-Putze (Hospital Universitario de Canarias, Tenerife), Alfonso Martín (Hospital Universitario Severo Ochoa de Leganés, Madrid), Pascual Piñera Salmerón (Hospital General Universitario Reina Sofía, Murcia), E. Jorge García Lamberechts (Hospital Clínico San Carlos, Madrid), Javier Jacob (Hospital Universitario de Bellvitge, L’Hospitalet de Llobregat, Barcelona), and Aitor Alquézar-Arbé (Hospital de la Santa Creu i Sant Pau, Barcelona).
Participating centres:
Hospital Universitario Doctor Peset Aleixandre de Valencia: María Luisa López Grima, Ma Ángeles Juan Gómez.
Hospital Universitario y Politécnico La Fe de Valencia: Javier Millán, Leticia Serrano Lázaro.
Hospital Universitario General de Alicante: Joan Gil, José Manuel Ramos.
Hospital Clínico Universitario de Valencia: José Noceda.
Hospital Arnau de Vilanova de Valencia: María José Cano Cano, Rosa Sorando Serra.
Hospital Francesc de Borja de Gandía, Valencia: María José Fortuny Bayarri, Francisco José Salvador Suárez.
Hospital General Universitario de Elche, Alicante: Matilde González Tejera.
Hospital Marina Baixa de Villajoyosa de Alicante: Ana María Caballero Mateos, Sonia Alonso Sanchez.
Hospital Virgen de los Lirios, Alcoy Alicante: Napoleón Meléndez, Patricia Borrás Albero.
Hospital Universitario Vinalopó de Elche (Alicante): Adelaida Mateo Arenas, Tamara Martín Casquero.
Hospital Universitario de Torrevieja de Alicante: Irene Ruiz Minano, Jemoma Jolina Tope Love.
Hospital LluisAlcanys de Xativa: Carles Pérez García, Pilar Sánchez Amador.
Hospital Universitario de La Ribera de Valencia: José Vicente Brasó Aznar, José Luis Ruiz López.
Hospital de la Vega Baja Orihuela de Alicante: María Carmen Ponce.
Hospital Universitario Sant Joan Alicante: Elena Díaz Fernández.
Hospital General de Requena de Valencia: Maribel Marzo Lambíes, Eva Robles Montesinos.
Hospital de Lliria de Valencia: Ana Peiró Gómez, Elena Gonzalo Bellver.
Hospital de la Santa Creu i Sant Pau (Barcelona): Elena Avellana Pardina, Mar Soler Ferrer, Laura Moragues Escalona.
Hospital Clinic (Barcelona): Carlos Cardozo.
Hospital Universitari de Bellvitge de Hospitalet de Llobregat (Barcelona): Ferran Llopis-Roca, Carles Ferré-Losa.
Hospital UniversitariGermansTrias i Pujol de Badalona (Barcelona): Anna Sales Montufo, Pepe Ferrer Arbaizar.
Hospital de Terrassa (Barcelona): Josep Tost.
Hospital del Mar (Barcelona): Isabel Cirera Lorenzo, Silvia Mínguez Masó.
Hospital Universitari Joan XXIII (Tarragona): Ruth Gaya Tur.
Hospital Universitari de Girona Dr. Josep Trueta (Girona): MariaAdroher Muñoz, Ester Soy Ferrer.
Hospital Universitari de Vic (Barcelona): Lluís LLauger García.
Hospital de Sant Pau i Santa Tecla (Tarragona): Enrique Martín Mojarro, Brigitte Silvana Alarcón Jiménez.
Clinica Sagrada Familia (Barcelona): Arturo Huerta.
Hospital Clínico San Carlos (Madrid): Marcos Fragiel.
Hospital Universitario La Paz (Madrid): Paloma Romero Gallego Acho, Francisco Marqués González, Susana Martinez Álvarez.
Hospital Universitario de la Princesa (Madrid): Guillermo Fernández Jiménez.
Hospital Universitario Severo Ochoa de Leganés (Madrid): María Cruz Yagüe, Dolores Corbacho Loarte.
Hospital Universitario Rey Juan Carlos (Madrid): Belén Rodríguez Miranda, María José Venegas De ĹHotellerie.
Hospital Universitario del Henares (Madrid): Carmen Puerta Castellano, Catalina Mocanu.
Hospital Universitario de Fuenlabrada (Madrid): María Jesús Dominguez, Cristina Latorre Marco.
Hospital Universitario Infanta Cristina de Parla (Madrid): Alicia Fuente Gaforio, Beatriz Honrado Galán.
Hospital Comarcal El Escorial (Madrid): Sara Gayoso Martín, Frida Vallejo Somohano.
Clínica Universidad Navarra de Madrid: Raquel Piñero Panadero, María García-Uría.
Hospital Universitario de Salamanca: Francisco Diego Robledo, Manuel Angel Palomero Martín.
Complejo Asistencial Universitario de León: Susana García Escudero, Mercedes Matias Flecha.
Hospital Universitario de Burgos: María Pilar López Díez.
Hospital Universitario Rio Hortega (Valladolid): Monserrat Alvarez Rabanal, Silvia FernandezCalderon, Ramiro Alonso del Busto.
Complejo Asistencial de Soria: Enrique del Hoyo Pelaez, Laura Tejada de los Santos.
Hospital Universitario Regional de Málaga: Lorena Muñoz González, Infantes Ramos Rafael.
Hospital Universitario Juan Ramón Jiménez: Esther Maldonado Perez, Verónica Rodríguez Martin.
Hospital Costa del Sol de Marbella: Carmen Agüera Urbano, Elisa Delgado Padial.
Hospital Valle de los Pedroches de Pozoblanco (Córdoba): Jorge Pedraza García.
Hospital Virgen del Rocío de Sevilla: Amparo Fernández de Simón Almela.
Complejo Hospitalario Universitario de A Coruña: Ricardo Calvo López.
Hospital Universitario Lucus Augusti Lugo: Juan José López Díaz.
Complejo Hospitalario Universitario de Vigo. Hospital Álvaro Cunqueiro: María Teresa Maza Vera, Raquel Rodríguez Calveiro.
Hospital Universitario General de Albacete: Francisco Javier Lucas-Galan, Francisco Javier Lucas-Imbernón.
Hospital Virgen de la Luz (Cuenca): Félix González Martínez, Diana Moya Olmeda.
Hospital Nuestra Señora del Prado de Talavera de la Reina (Toledo): Ricardo Juárez.
Hospital Universitario de Canarias (Tenerife): Marcos ExpositoRodriguez, José Francisco Fernández Rodríguez.
Hospital Universitario de Gran Canaria Dr. Negrín: José Pavón Monzo, Nayra Cabrera González.
Hospital Universitario Central Asturias: Pablo Herrero Puente, Beatriz María Martínez Bautista.
Hospital Universitario de Cabueñes (Gijón): Ana Patricia Niembro Valdés, María del Rosario Carrió Hevia.
Hospital Clínico Universitario Virgen de la Arrixaca: Nuria Tomas García.
Hospital General Universitario Reina Sofía de Murcia: Ines García Rosa, María Encarnación Sánchez Canovas.
Hospital San Pedro de Logroño: Noemí Ruiz de Lobera.
Hospital Clínico Universitario Lozano Blesa: José María Ferreras Amez, Belen Arribas Entrala
References
- 1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC.. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020;324:782–793. [DOI] [PubMed] [Google Scholar]
- 2. Pellicori P, Doolub G, Wong CM, Lee KS, Mangion K, Ahmad M, Berry C, Squire I, Lambiase PD, Lyon A, McConnachie A, Taylor RS, Cleland JG.. COVID-19 and its cardiovascular effects: a systematic review of prevalence studies. Cochrane Database Syst Rev 2021;3:CD013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N, Douair A, Barbin E, Fraix A, Bouchot O, Benmansour O, Godeau G, Mecheri Y, Lebourdon R, Yvorel C, Massin M, Leblon T, Chabbi C, Cugney E, Benabou L, Aubry M, Chan C, Boufoula I, Barnaud C, Bothorel L, Duceau B, Sutter W, Waldmann V, Bonnet G, Cohen A, Pezel T, Critical Covid-19 France Investigators. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020;41:3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ministry of Health. Spanish Government. COVID-19 Geographical Distribution. (https://cnecovid.isciii.es/covid19/#niveles-de-gravedad (accessed 1 June 2020).
- 5. Miró O, González Del Castillo J.. Collaboration among Spanish emergency departments to promote research: on the creation of the SIESTA (Spanish Investigators in Emergency Situations TeAm) network and the coordination of the UMC-19 (Unusual Manifestations of COVID-19) macroproject. Emergencias 2020;32:269–277. [PubMed] [Google Scholar]
- 6. Gil-Rodrigo A, Miró Ò, Piñera P, Burillo-Putze G, Jiménez S, Martín A, Martín-Sánchez FJ, Jacob J, Guardiola JM, García-Lamberechts EJ, Espinosa B, Martín Mojarro E, González Tejera M, Serrano L, Agüera C, Soy E, Llauger L, Juan MÁ, Palau A, Del Arco C, Rodríguez Miranda B, Maza Vera MT, Martín Quirós A, Tejada de Los Santos L, Ruiz de Lobera N, Iglesias Vela M, Torres Garate R, Alquézar-Arbé A, González Del Castillo J, Llorens P;en representación de la red de investigación SIESTA. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emergencias 2020;32:233–241. [PubMed] [Google Scholar]
- 7. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, Fearon WF, Taggart D, Kappetein AP, Krucoff MW, Vranckx P, Windecker S, Cutlip D, Serruys PW; Academic Research Consortium. Standardized end point definitions for coronary intervention trials. The Academic Research Consortium-2 consensus document. Circulation 2018;137:2635–2650. [DOI] [PubMed] [Google Scholar]
- 8. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, Sanmartín JL, Fernández-García A, Cruz I, Fernández de Larrea N, Molina M, Rodríguez-Cabrera F, Martín M, Merino-Amador P, León Paniagua J, Muñoz-Montalvo JF, Blanco F, Yotti R, Blanco F, Gutiérrez Fernández R, Martín M, Mezcua Navarro S, Molina M, Muñoz-Montalvo JF, Salinero Hernández M, Sanmartín JL, Cuenca-Estrella M, Yotti R, León Paniagua J, Fernández de Larrea N, Fernández-Navarro P, Pastor-Barriuso R, Pérez-Gómez B, Pollán M, Avellón A, Fedele G, Fernández-García A, Oteo Iglesias J, Pérez Olmeda MT, Cruz I, Fernandez Martinez ME, Rodríguez-Cabrera FD, Hernán MA, Padrones Fernández S, Rumbao Aguirre JM, Navarro Marí JM, Palop Borrás B, Pérez Jiménez AB, Rodríguez-Iglesias M, Calvo Gascón AM, Lou Alcaine ML, Donate Suárez I, Suárez Álvarez O, Rodríguez Pérez M, Cases Sanchís M, Villafáfila Gomila CJ, Carbo Saladrigas L, Hurtado Fernández A, Oliver A, Castro Feliciano E, González Quintana MN, Barrasa Fernández JM, Hernández Betancor MA, Hernández Febles M, Martín Martín L, López López L-M, Ugarte Miota T, De Benito Población I, Celada Pérez MS, Vallés Fernández MN, Maté Enríquez T, Villa Arranz M, Domínguez-Gil González M, Fernández-Natal I, Megías Lobón G, Muñoz Bellido JL, Ciruela P, Mas I Casals A, Doladé Botías M, Marcos Maeso MA, Pérez del Campo D, Félix de Castro A, Limón Ramírez R, Elías Retamosa MF, Rubio González M, Blanco Lobeiras MS, Fuentes Losada A, Aguilera A, Bou G, Caro Y, Marauri N, Soria Blanco LM, del Cura González I, Hernández Pascual M, Alonso Fernández R, Merino-Amador P, Cabrera Castro N, Tomás Lizcano A, Ramírez Almagro C, Segovia Hernández M, Ascunce Elizaga N, Ederra Sanz M, Ezpeleta Baquedano C, Bustinduy Bascaran A, Iglesias Tamayo S, Elorduy Otazua L, Benarroch Benarroch R, Lopera Flores J, Vázquez de la Villa A; ENE-COVID Study Group. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020;396:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ. 3rd,. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998;158:585–593. [DOI] [PubMed] [Google Scholar]
- 10. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J.. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 2007;5:692–699. [DOI] [PubMed] [Google Scholar]
- 11. Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS.. Secular trends in incidence and mortality of acute venous thromboembolism: the AB-VTE population-based study. Am J Med 2016;129:879.e19-25. [DOI] [PubMed] [Google Scholar]
- 12. Righini M, Van Es J, Den Exter PL, Roy PM, Verschuren F, Ghuysen A, Rutschmann OT, Sanchez O, Jaffrelot M, Trinh-Duc A, Le Gall C, Moustafa F, Principe A, Van Houten AA, Ten Wolde M, Douma RA, Hazelaar G, Erkens PM, Van Kralingen KW, Grootenboers MJ, Durian MF, Cheung YW, Meyer G, Bounameaux H, Huisman MV, Kamphuisen PW, Le Gal G.. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 2014;311:1117–1124. [DOI] [PubMed] [Google Scholar]
- 13. Sørensen HT, Horvath-Puho E, Lash TL, Christiansen CF, Pesavento R, Pedersen L, Baron JA, Prandoni P.. Heart disease may be a risk factor for pulmonary embolism without peripheral deep venous thrombosis. Circulation 2011;124:1435–1441. [DOI] [PubMed] [Google Scholar]
- 14. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D.. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freund Y, Drogrey M, Miró Ò, Marra A, Féral-Pierssens AL, Penaloza A, Hernandez BAL, Beaune S, Gorlicki J, Vaittinada Ayar P, Truchot J, Pena B, Aguirre A, Fémy F, Javaud N, Chauvin A, Chouihed T, Montassier E, Claret PG, Occelli C, Roussel M, Brigant F, Ellouze S, Le Borgne P, Laribi S, Simon T, Lucidarme O, Cachanado M, Bloom B; IMPROVING EMERGENCY CARE FHU Collaborators. Association between pulmonary embolism and COVID-19 in ED patients undergoing CTPA: the PEPCOV international retrospective study. Acad Emerg Med 2020;27:811–820. [DOI] [PubMed] [Google Scholar]
- 16. Kiss P, Carcel C, Hockham C, Peters SAE.. The impact of the COVID-19 pandemic on the care and management of patients with acute cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes 2021;7:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pascual Calleja I, Álvarez Velasco R, Almendarez Lacayo M, Arboine Aguirre L, Avanzas Fernández P, Moris de la Tassa C.. Impact of the COVID-19 pandemic on acute myocardial infarction care times. Emergencias 2020;32:440–442. [PubMed] [Google Scholar]
- 18. Alquézar-Arbé A, Piñera P, Jacob J, Martín A, Jiménez S, Llorens P, Martín-Sánchez FJ, Burillo-Putze G, García-Lamberechts EJ, González Del Castillo J, Rizzi M, Agudo Villa T, Haro A, Martín Díaz N, Miró Ò.. Impact of the COVID-19 pandemic on hospital emergency departments: results of a survey of departments in 2020—the Spanish ENCOVUR study. Emergencias 2020;32:320–331. [PubMed] [Google Scholar]
- 19. Rosovsky RP, Grodzin C, Channick R, Davis GA, Giri JS, Horowitz J, Kabrhel C, Lookstein R, Merli G, Morris TA, Rivera-Lebron B, Tapson V, Todoran TM, Weinberg AS, Rosenfield K; PERT Consortium. Diagnosis and treatment of pulmonary embolism during the coronavirus disease 2019 pandemic: a position paper from the National PERT Consortium. Chest 2020;158:2590–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Acces to data will be addressed to the main author and will have evaluated and decided by the stering committe.