Abstract
Objective
Adolescent depression is increasing during the COVID-19 pandemic, possibly related to dramatic social changes. Individual-level factors that contribute to social functioning, such as temperament and neural reactivity to social feedback, may confer risk for or resilience against depressive symptoms during the pandemic.
Methods
Ninety-three girls (12–17 years) oversampled for high shy/fearful temperament were recruited from a longitudinal study for a follow-up COVID-19 study. During the parent study (2016–2018), participants completed a functional magnetic resonance imaging task eliciting neural activity to performance-related social feedback. Depressive symptoms were assessed during the parent study and COVID-19 follow-up (April–May 2020). In 65 participants with complete data, we examined how interactions between temperament and neural activation to social reward or punishment in a socio-affective brain network predict depressive symptoms during COVID-19.
Results
Depressive symptoms increased during COVID-19. Significant interactions between temperament and caudate, putamen, and insula activation to social reward were found. Girls high in shy/fearful temperament showed negative associations between neural activation to social reward and COVID-19 depressive symptoms, whereas girls lower in shy/fearful temperament showed positive associations.
Conclusions
Girls high in shy/fearful temperament with reduced neural activation to social reward may be less likely to engage socially, which could be detrimental during the pandemic when social interactions are limited. In contrast, girls lower in shy/fearful temperament with heightened neural reactivity to social reward may be highly motivated to engage socially, which could also be detrimental with limited social opportunities. In both cases, improving social connection during the pandemic may attenuate or prevent depressive symptoms.
Keywords: adolescents, COVID-19, depression, neural processes
Introduction
The novel coronavirus disease (COVID-19) pandemic has caused widespread societal disruptions, changing the way people live, work, and interact. Emerging research suggests that pandemic-related social changes could be contributing to increased depressive symptoms in adolescents (Loades et al., 2020; Racine et al., 2020). However, many teens may adjust to these social changes without feeling depressed. In this study, we explore how two factors that may influence an individual’s reactivity to changes in their social environments—neural reactivity to social feedback and shy/fearful temperament—confer risk for or resilience against the development of depression during COVID-19. We focus the study on adolescent girls, given evidence of heightened sensitivity to social evaluation in girls (Rudolph & Conley, 2005). Additionally, sex differences in rates of depression emerge during adolescence; by late adolescence, girls are twice as likely as boys to be diagnosed with depression (Nolen-Hoeksema, 2002). Depression is associated with a host of psychosocial impairments, and has been linked to poorer overall health and higher health care utilization in mid-adolescence (Haarasilta et al., 2004; Keenan-Miller et al., 2007). Pediatricians are often at the front-line for identifying depression in teens and intervening (e.g., by recommending or initiating treatment). Examining factors that contribute to depression in girls during COVID-19 may help pediatricians, as well as clinicians, teachers, and parents, identify girls at risk for depression during the pandemic, and may elucidate potential targets for treatment and intervention.
Peer interactions are of central importance in adolescence, aiding in critical developmental processes, such as identity exploration and formation (Brechwald & Prinstein, 2011). However, the COVID-19 pandemic and resulting social distancing guidelines across most of the United States have drastically changed the social landscape for most teens. For example, most schools have incorporated virtual learning and many extracurricular activities have been canceled, limiting in-person interactions between teens. Many adolescents are restricted to interacting with peers online, and only if they have the means to support this communication (e.g., personal cell-phones or computers). Research on socioemotional outcomes associated with the COVID-19 pandemic suggests that adolescents are feeling lonelier and less connected to their peers (Hinkelman, 2020), and are worried about their peer relationships (Ellis et al., 2020).
Given these significant pandemic-related social changes, risk factors that influence social functioning may be particularly relevant for understanding increases in adolescent depressive symptoms during COVID-19. One such risk factor is neural reactivity to social rewards and threats. Depression in adolescence has been linked to altered reactivity in a socio-affective brain network that includes regions that process salient positive and/or negative social and emotional stimuli (Guyer et al., 2016), such as the striatum, amygdala, insula, ventromedial prefrontal cortex (vmPFC), and subgenual anterior cingulate cortex (sgACC). Reduced activity in regions including the striatum, amygdala, and vmPFC to social rewards (e.g., positive social feedback) and nonsocial rewards (e.g., monetary gains) has been shown to confer risk for depressive symptoms and major depressive disorder (MDD) in adolescence (e.g., Forbes et al., 2006, 2009, 2010; Olino et al., 2014, 2015). Importantly, reduced neural responsivity to rewards also prospectively predicts depressive symptoms in adolescent girls over a period of 1.5–2 years (e.g., Bress et al., 2013; Nelson et al., 2016), suggesting that blunted reward responsivity may be a mechanism supporting the development of depression in high-risk teens. Reduced neural reactivity to rewards could underlie depression-related motivational deficits and blunted emotional reactivity to positive stimuli (Bylsma et al., 2008), contributing to reduced positive affect (Forbes et al., 2009), anhedonia (Keedwell et al., 2005), decreased reward seeking, and increased social withdrawal. This may be especially problematic during the pandemic due to severe social restrictions; youth with a history of reduced reactivity to rewards may be less likely to seek new, creative ways to socialize, contributing to more isolation and depression risk.
Blunted neural reactivity to positive social feedback could be a particularly potent risk factor for depression during COVID for adolescent girls with a history of shy/fearful temperament. Shy, fearful, and behaviorally inhibited (BI) temperaments in childhood have been linked to higher rates of depression in later adolescence (Gladstone & Parker, 2006), particularly for girls (Cyranowski et al., 2000). The link between shy temperament and depression may be mediated by high levels of social anxiety (Gladstone & Parker, 2006) and avoidance of potentially positive social interactions (Silk et al., 2012). Some studies have also found enduring alterations in neural reward processing in adolescents with a history of childhood BI, including heightened striatal activation to reward anticipation (e.g., Guyer et al., 2006) and reduced striatal activation to social and nonsocial reward feedback (e.g., Helfinstein et al., 2011; Guyer et al., 2014). Blunted neural reactivity to rewards in girls high in shy/fearful temperament could support increased social withdrawal, which may be detrimental during the pandemic, conferring risk for depression. On the other hand, for girls lower in shy temperament, blunted neural reactivity to social feedback could be more protective during the pandemic. These girls may not miss socializing as much as their peers with higher neural reactivity and are at lower risk for depression relative to girls with histories of high shy/fearful temperament.
Depressive symptoms and MDD in youth have also been associated with heightened neural reactivity to social threats, such as fearful faces and social rejection, in neural regions including the amygdala, sgACC, and insula (e.g., Monk et al., 2008; Silk et al., 2014). Longitudinally, heightened neural activation to social exclusion has been shown to predict increases in depressive symptoms during adolescence (Masten et al., 2011). Increased neural activity to threats could support heightened emotional reactivity to negative stimuli in depression (Bylsma et al., 2008). Adolescents with heightened neural reactivity to social punishment may be particularly sensitive to rejection from peers (Masten et al., 2009). Although opportunities for in-person social rejection are limited during the COVID-19 pandemic, negative social interactions in the home or experienced virtually may increase risk for depression during COVID-19 in adolescents with heightened reactivity to social punishment. Alternatively, neural activity to social threat may not be as potent a predictor of depressive symptoms during the pandemic, due to reduced frequency of peer rejection and victimization.
Adolescents with a history of shy, fearful temperament and heightened neural reactivity to social punishment may be at particular risk for depressive symptoms during the pandemic, as research suggests that shy, socially anxious youth experience more rejection from peers in daily life (Kingery et al., 2010). Shy, inhibited temperament in infancy and childhood has also been associated with enduring alterations in threat-related neural activity, including amygdala hyperreactivity (Caouette & Guyer, 2014). However, amygdala hyperreactivity to threat in adolescents high in BI in early childhood may be related to attentional constraints of the task (Pérez-Edgar et al., 2007), novelty (Schwartz et al., 2003), and/or unpredictability (Jarcho et al., 2016). Moreover, during passive viewing of threatening faces, Pérez-Edgar et al. (2007) found that adolescents high in childhood BI actually showed amygdala deactivation.
Guided by this prior research and the social context of the COVID-19 pandemic, this study examined how preexisting patterns of neural activity to social reward and threat in a socio-affective brain network are associated with depressive symptoms during the pandemic in mid-adolescent girls differing in early adolescent temperament. We focused on brain regions within this network that have been linked to adolescent depression and that activate to rewards (i.e., caudate, putamen, nucleus accumbens, insula, amygdala, and vmPFC; Silverman et al., 2015), as well as threats (i.e., amygdala, insula, and sgACC; e.g., Bolling et al., 2011). Aligning with previous research, we hypothesized that girls with a history of shy/fearful temperament and blunted neural activity to positive social feedback (i.e., social reward), as well as heightened neural activity to negative social feedback (i.e., social punishment), would report the highest levels of depressive symptoms during COVID-19.
Methods
Participants and Procedures
Participants were recruited from the larger Pittsburgh area from a three-year longitudinal study of 129 girls oversampled for high temperamental risk (HTR) for social anxiety and depression. Two-thirds of the sample were classified as high in shy or fearful temperament (HTR), with the remaining one-third in the normal range of shy or fearful temperament (low temperamental risk [LTR]); this design was chosen to enrich variability in threat and reward responsivity, as well as symptomology. Participants were between ages 11 and 13 at enrollment (baseline) and were excluded if they met DSM-5 diagnostic criteria at the baseline assessment for any current or past anxiety disorder (except social phobia), MDD, or autism spectrum disorder, or reported acute suicidality or psychosis.
All participants who provided written informed parental consent and youth assent for future contact from the parent study (N = 113) were considered eligible for the present COVID-19 follow-up study; there were no additional eligibility criteria. Participants were sent information about the COVID-19 study about 20 days following the start of state-wide stay-at-home orders in the state of Pennsylvania (PA), where the majority of participants resided. All schools in PA were under state-issued closure at this time. Adolescents who had moved outside of PA (N = 2) were under similar state-wide stay-at-home orders in their respective locations. These stay-at-home orders urged residents to only leave the house to perform essential activities such as work, buying groceries, or exercising outside. In accordance with the University’s institutional review board, youth and their parents provided written informed assent and consent, respectively, online to indicate their agreement to participate in the COVID-19 study.
Of 113 eligible participants, 93 girls ages 12 – 17 years (34 LTR, 59 HTR; Mage= 15.02, SD = 1.21) consented to the COVID-19 study in late April to early May 2020; one participant was ineligible due to no longer being under stay-at-home orders, one participant declined because they did not want to discuss COVID-related issues, and 18 did not respond to recruitment emails or phone-calls. Included participants did not differ from girls who were eligible but did not consent for the study in age or depressive symptoms at baseline (ps > .30). However, the majority (85%) of eligible participants who did not consent to the COVID-19 study had been classified as HTR. Because girls were originally recruited for the parent study between 2016 and 2018, time between the baseline assessment and COVID-19 follow-up study varied (range = 1.4–4.4 years; mean = 2.8 years). At the time of assessment for the COVID-19 study, no participants had tested positive for COVID-19 or had a parent or sibling test positive for COVID-19. Many participants were able to maintain contact with their friends during stay-at-home orders. Over half of the participants (67.7%) reported that they communicated with their friends every day or almost every day; 23.7% communicated with their friends several times a week, 5.4% communicated with their friends about once a week, and 3.2% communicated with their friends less often than once a week. Almost all participants (97.8%) reported communicating with friends by texting, and 82.8% used videocalls.
Sixty-five girls who consented for the COVID-19 study had usable functional magnetic resonance imaging (fMRI) data, thus were included in the full analyses for this study (27 LTR, 38 HTR; Mage=15.06; SD = 1.20). Reasons for missing fMRI data included excess movement in the scanner (N = 14), scanner error or missing brain data (N = 3), incidental finding that impeded analysis (N = 1), missing behavioral data (N = 1), and failure to complete the scan (N = 9). Twenty-five participants (16 HTR and 9 LTR) had a current DSM-5 diagnosis at their most recent clinical interview prior to the start of the COVID-19 study.1 Nineteen girls (34% HTR and 22% LTR) had at least one current anxiety diagnosis (i.e., specific phobia, social anxiety disorder, separation anxiety disorder, and/or generalized anxiety disorder) and one participant had a current MDD diagnosis. Clinical and demographic information can be found in Table I.
Table I.
Descriptive Information and Task Performance by Temperament Group
| HTR (N = 38) | LTR (N = 27) | |
|---|---|---|
| Demographic characteristics | ||
| Age (years)—M (SD) | 15.15 (1.11) | 14.92 (1.34) |
| Race/Ethnicity—N (%) | ||
| White, non-Hispanic | 29 (76.3%) | 18 (66.7%) |
| Black or African American, non-Hispanic | 5 (13.2%) | 5 (18.5%) |
| Asian | 0 (0.0%) | 1 (3.7%) |
| Biracial | 3 (7.9%) | 3 (11.1%) |
| Other | 1 (2.6%) | 0 (0.0%) |
| Hispanic or Latinx | 1 (2.6%) | 3 (11.1%) |
| Total annual incomea,b | ||
| Median | 9.00 | 6.59 |
| Mean | 7.68 | 7.00 |
| Clinical characteristics | ||
| Depressive symptoms—M (SD) | ||
| Baseline | 9.37 (7.28) | 8.15 (7.14) |
| COVID-19 | 14.45 (9.62) | 11.00 (8.30) |
| Most recent current diagnosisa—N (%) | ||
| Any anxiety disorder | 13 (34.2%) | 6 (22.2%) |
| Specific phobia | 4 (10.5%) | 4 (14.8%) |
| Social anxiety disorder | 8 (21.1%) | 2 (7.4%) |
| Generalized anxiety disorder | 4 (10.5%) | — |
| Separation anxiety disorder | 1 (2.6%) | — |
| Major depressive disorder | — | 1 (3.7%) |
| Unspecified depressive disorder | 1 (2.6%) | 1 (3.7%) |
| Obsessive-compulsive disorder | 3 (7.9%) | — |
| Posttraumatic stress disorder | 1 (2.6%) | — |
| Attention-deficit/hyperactivity disorder | 1 (2.6%) | 3 (11.1%) |
| Oppositional defiant disorder | 1 (2.6%) | 1 (3.7%) |
| Enuresis | 1 (2.6%) | — |
| P-SID task performance (hit rate; %)—M (SE) | ||
| Reward cue | 50.2% (.01%) | 46.2% (.02%) |
| Punishment cue | 50.6% (.01%) | 48.8% (.02%) |
| Neutral cue | 44.9% (.01%) | 42.7% (.03%) |
Note. M = mean, SD = standard deviation, SE = standard error, P-SID = Peer Social Incentive Delay; Depressive symptoms were assessed using the MFQ-Child version.
Attained at the most recent diagnostic interview for the parent study prior the start of the COVID-19 follow-up study (time between most recent pre-COVID interview and COVID-19 study: M = 9.95 months, SD = 8.03, range = 0.10–43.33 months).
bAnnual income was reported by parents on a 0–10 scale with $10,000 increments (e.g., 0 = $0–10,000; 10 = $100,000+); No significant differences in task performance or any demographic or clinical variables were found between groups.
Measures
Temperament
Temperament was assessed using child and parent (parent report on child) versions of the short-form Early Adolescent Temperament Questionnaire-Revised (EATQ-R; Ellis & Rothbart, 2001) only during the baseline assessment of the parent study. The EATQ-R measures various reactive and regulative temperament traits in 9–15 years old. This measure includes 53 items loading onto 10 temperament scales and 12 additional items loading onto two behavioral (depression and aggression) scales. In this study, we used only the fear scale (six items; e.g., “I worry about getting into trouble”) and shyness scale (seven items; e.g., “It is a lot easier for me to talk to people I know than to strangers”). For each item, participants and their parent rate on a 5-point Likert-type scale whether a particular statement is true for the participant. Response options are “almost always untrue,” “usually untrue,” “sometimes true, sometimes untrue,” “usually true,” “almost always true.” Scores on each scale range from 1 to 5, with higher scores representing higher fear or shy temperament.
Participants with EATQ-R scores >0.75 SDs above the published age/sex-matched mean on the parent- or child-rated shyness scale (2.99 for parent-report, 3.16 for child-report) or fear scale (3.12 for parent-report, 3.48 for child-report) were classified as HTR. The remaining 1/3 of adolescents had a score below 0.75 SDs above the mean on the fear or shyness scales of both child and parent reports; for the purpose of group analysis, these participants were considered LTR. In this sample, internal consistency for the shyness scale was moderate for adolescent self-report (Cronbach’s α = .76) and high for parent report (α = .86). Internal consistency for the EATQ-R fear scale was low to moderate for adolescent self-report (α = .58) and parent report (α = .72).
Depressive Symptoms
Participants completed the 33-item Mood and Feelings Questionnaire (MFQ)-Child Version (Angold & Costello, 1987) to assess depressive symptoms (e.g., mood, appetite, sleep, psychomotor functioning, inappropriate guilt and feelings of worthlessness, suicidal ideation) over the past 2-week period. The measure was completed at baseline as part of the parent study and again as part of the COVID follow-up assessment. Each item on the MFQ is rated on a 3-point scale (0 = not true, 1 = sometimes true, and 2 = true) and summed to create a total score. Scores range from 0 to 66, with higher scores indicating greater depressive symptoms. Internal consistency was high at baseline (Cronbach = .89) and COVID follow-up (Cronbach = .90).
Peer Social Incentive Delay Task
The Peer Social Incentive Delay (P-SID) task (Kaurin et al., in press) is a social adaptation of the original monetary incentive delay task (Knutson et al., 2000), and was designed to measure brain activity related to social rewards and punishments. A novel “peer observation” version of the task was created to examine neural activation to social feedback from a virtual peer. At a laboratory visit prior to the scan, participants viewed fictional photos and autobiographical profiles (including hobbies and personality traits) of age-matched girls whom they were told were participating in the study at other institutions. Participants were asked to select and rank which girls they would most like to interact with during the fMRI scan.
To enhance the ecological validity of the task, participants were told that one of the girls they ranked highly (first or second) was participating in the study at another site and would be watching the participant complete the fMRI task and providing feedback after each trial by sending a smiling, frowning, or neutral (morphed) picture of themselves based on the participant’s performance. To increase believability, participants were also asked to view (via a mock video feed) and evaluate this peer’s performance on the P-SID task prior to completing the fMRI task themselves. The peer’s performance on the P-SID task was computer-generated.
The P-SID task (Supplementary Figure 1) consists of one run of 72 trials (27 social reward, 27 social punishment, and 18 control). Each trial proceeded in the following order: cue (500 ms), fixation cross (1,500–3,500 ms), target slide (500 ms), blank screen (1,000 ms), peer feedback (1,650 ms), and blank screen (2,500–5,000 ms). Participants were instructed to press a button with their index finger as quickly as possible when a target (white square) appeared on the screen. The target slide was always presented for 500 ms but target presentation on that slide was variable (160–500 ms) to ensure that hit rates in different conditions were similar across participants. At the start of each trial, a cue (circle, square, or triangle) signaled the possible outcomes when the participant pressed the button fast enough (i.e., response fell within the target presentation time) or was too slow. In the social reward condition, a circle signaled possible positive feedback (peer’s happy face) for a fast response or neutral feedback (peer’s morphed face) for a slow response. In the social punishment condition, a square signaled negative feedback (peer’s angry face) for a slow response or neutral feedback (peer’s morphed face) for a fast response. In the control condition, a triangle cued a neutral outcome (peer’s morphed face) regardless of performance. Average hit rates can be found in Table I. More information on task performance and stimuli can be found in the online supplement. Total duration of the P-SID task was 12 min 2 s (480 volumes). Participants practiced the task prior to the scan in a mock scanner.
We examined neural activity using two contrasts of interest: (a) social reward feedback (smiling face presented following the circle cue) versus neutral feedback (morphed face presented in the control condition) and (b) social punishment feedback (angry face presented following the square cue) versus neutral feedback (morphed face presented in the control condition).
For this study, we only include P-SID fMRI data from the baseline assessment of the parent study. Participants completed a preliminary debriefing protocol, in which they were asked what they thought about the virtual peers, following the administration of the fMRI scan at baseline. No participants verbally indicated suspicion. However, participants completed this task again (two years later) during the parent study, at which point they were fully debriefed.
Data Analysis
fMRI Acquisition and Analysis
Data were acquired using a Siemens 3T PRISMA with a 64-channel phase array coil. Anatomical images covering the entire brain were acquired first using a 3D magnetization-prepared rapid gradient-echo T1-weighted sequence (repetition time [TR] = 2,300 ms, echo time [TE] = 3.93 ms, flip angle 9°, inversion time [TI] = 900 ms, voxel size = 1 mm3). Functional images were acquired using multi-band gradient echo-planar imaging (EPI) sequences (60 slices, three-factor multiband) sensitive to blood-oxygen-level-dependent (BOLD) contrast [T2*] (TR = 1,500 ms, TE = 30 ms, flip angle 55°, voxel size = 2.3 × 2.3 × 2.3 mm). Field maps were acquired using gradient EPI sequences for correction of field distortions in the functional images with the following parameters: TR = 590ms, TE1 = 4.92 ms, TE2 = 7.38 ms, voxel size = 2.3 × 2.3 × 2.3 mm, flip angle 60°. Statistical Parametric Mapping software (SPM12; Wellcome Trust Centre for Neuroimaging, UK) was used to preprocess functional images. Preprocessing steps are detailed in the online supplement. Scans with >0.5 mm of incremental motion, >3 mm from the baseline image, and/or 3 SDs intensity shifts were considered outliers; outlier scans were replaced with a linear interpolation between the two nearest nonoutlier scans. Participants with >25% of volumes with excess movement (i.e., outliers) were excluded.
For the first-level analyses, individual effects were estimated using the general linear model approach implemented in SPM12. At the first level, we modeled anticipation trials (i.e., cues), social reward feedback (i.e., smiling face following reward cue), social punishment feedback (i.e., angry face following punishment cue), social reward miss feedback (morphed face following reward cue), social punishment hit feedback (morphed face following punishment cue), and neutral feedback (morphed face following neutral cue), with motion parameters included as nuisance regressors. Group analyses focused on several regions-of-interest (ROIs). The caudate, putamen, insula, amygdala, and nucleus accumbens (NAcc) masks were created using the AAL atlas in the WFU PickAtlas toolbox. The vmPFC mask was a 10 mm sphere centered on MNI coordinates [2 46 −8], identified from a meta-analysis on reward processing and subjective value (Bartra et al., 2013) and created using the PickAtlas toolbox. The sgACC ROI mask was created using Neurosynth (http://neurosynth.org; Yarkoni et al., 2011) and FSL v6.0.3. First, separate anatomical masks of bilateral subgenual, medial, and/or inferior portions of Brodmann areas 34, 24, and 25 (defined by the Talairach Daemon Labels in FSLeyes v0.31.2) were downloaded and summed using the fslmaths -add function. This mask was then multiplied by the Neurosynth activation map for the term “subgenual” to ensure specificity. The Neurosynth map was thresholded at false discovery rate (FDR)-corrected p < .001 by default. Images of all masks can be found in Supplementary Figure 2. Mean parameter estimates across each ROI were extracted from these regions for the contrasts of interest (social reward feedback>neutral feedback; social punishment feedback > neutral feedback) using MarsBar for SPM (Brett et al., 2002) and entered into SPSS v26.
Test of the Full Model
Moderation analyses were conducted using the PROCESS macro for SPSS, version 3.1 (Hayes, 2018), which generates a regression model and simple slope effects. Temperament group (dummy coded) was a dichotomous moderator. Parameter estimates for each ROI were entered as the predictor in separate models. All analyses controlled for age, baseline depressive symptoms, and time (days) between the baseline assessment of depressive symptoms and COVID-19 data collection. Significance was evaluated using the change in R2 (R2) when the interaction term was added to the model. Separate models were run with parameter estimates for each ROI. Benjamini–Hochberg procedures (Benjamini & Hochberg, 1995) were used to correct for multiple tests with a FDR of 0.05. The jtools package (Long, 2020) in R v4.0.2 (R Core Team, 2020) was used to visualize significant interactions using partial residuals. With 65 participants, we were appropriately powered (0.80) to detect a medium-large effect of 0.36.
Results
Preliminary Findings
Increase in Depressive Symptoms During COVID-19 Pandemic
A repeated measures analysis of covariance (ANCOVA) was conducted using SPSS (v26) to test whether depressive symptoms during COVID-19 follow-up (late April to early May 2020) differed significantly from depressive symptoms at baseline measurement (2016–2018), controlling for days between measurement (centered). In the full sample of youth with baseline MFQ data and COVID-19 MFQ data (N = 92), depressive symptoms increased significantly from baseline to COVID-19 follow-up, controlling for unequal days between measurements (F[1, 90] = 13.30, p < .001, ηp2 = 0.13). Depressive symptoms did not increase from baseline to 18-month follow-up in this sample (F[1, 90] = 0.25, p = .62), which could suggest that the increase seen during COVID-19 is not attributable only to changes over time or with age. No interaction by temperament group was found; depressive symptoms increased during the pandemic across the entire sample. These findings replicated in the subsample of participants included in the following analyses (N = 65).
Differences in Neural Activity Based on Temperament Group
HTR and LTR participants (N = 65) differed significantly in neural activation to social reward feedback versus. neutral feedback in the insula (t [63] = 2.56, p = .013), amygdala (t [63] = 2.79, p = .007), caudate (t [63] = 2.58, p = .012), and putamen (t [63] = 2.86, p = .006). HTR participants showed reduced activity in these regions relative to LTR participants. HTR and LTR participants did not differ in neural activation to social punishment feedback versus neutral feedback (ps > .10).
Interactions Between Temperament and Neural Activation to Social Reward
Intercorrelations between variables included in these models can be found in Supplementary Table 1. Significant interactions (following FDR correction) between temperament and neural activation to social reward versus neutral feedback were found for three regions: (a) the bilateral caudate (interaction term R2 = .15, F [1, 58] = 13.02, uncorrected p < .001; Figure 1A), (b) the bilateral putamen (interaction term R2 = .13, F [1, 58] = 11.86, uncorrected p = .001; Figure 1B), and (c) the bilateral insula (interaction term R2 = .09, F [1, 58] = 7.43, uncorrected p = .009; Figure 1C). A main effect of baseline depressive symptoms was seen in all models (p < .005).
Figure 1.
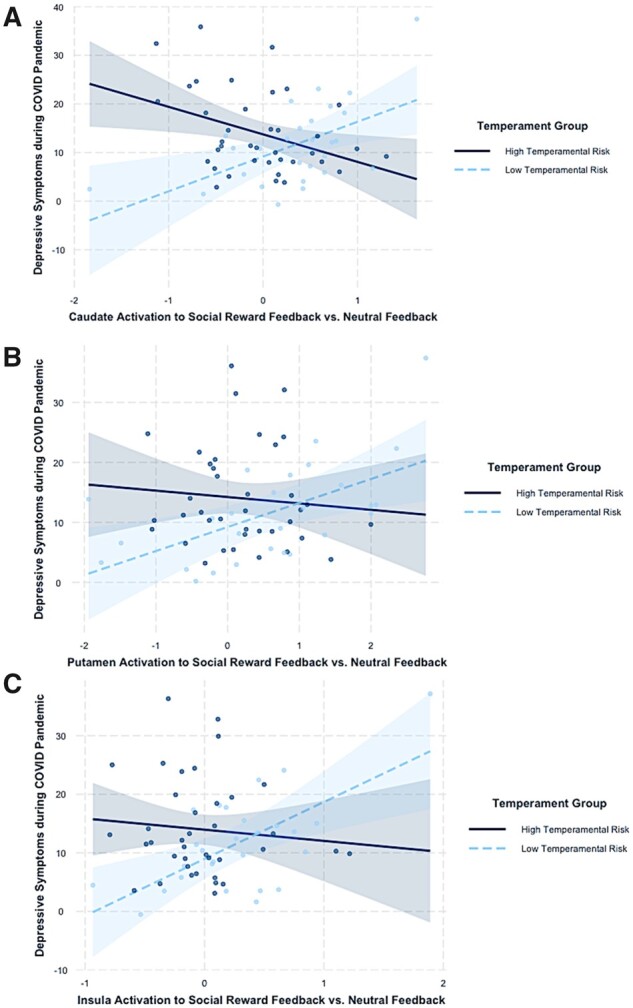
Interactions between temperamental risk type and neural activation to positive social feedback versus neutral feedback in the (A) caudate, (B) putamen, and (C) insula on depressive symptoms during the pandemic. Partial residuals are plotted with 95% CI bands.
Full model results with simple slope analyses can be found in Table II. HTR participants showed a significant negative association between caudate activation to social reward and depressive symptoms, whereas LTR participants showed a positive association between caudate activation to social reward and depressive symptoms. A similar pattern of opposing associations by temperament group was seen for the putamen and insula. However, only the simple slopes for the LTR group were significant. At low levels (−1 SD) of neural activation in these regions, HTR girls reported significantly higher levels of depressive symptoms relative to LTR girls (p’s < .005). Although an interaction between NAcc activation and temperament group was found (R2 = .05, p = .037), this did not survive FDR correction. No significant interactions or main effects were found for the amygdala (R2 = .03, p = .13) or vmPFC (R2 = .00, p = .92).
Table II.
Summary of Significant PROCESS Regression Models Predicting COVID-19 Depressive Symptoms From Neural Activation to Social Reward Feedback Relative to Neutral Feedback
| Bilateral caudate | F | R 2 | ΔR2 | B | t | Uncorrected p | BH p |
|---|---|---|---|---|---|---|---|
| Model summary | 5.20** | .35 | .0002 | ||||
| Intercept | −23.54 | −1.47 | .146 | ||||
| Age | 2.22 | 1.71 | .093 | ||||
| TG | 3.15 | 1.51 | .137 | ||||
| MFQ baseline | 0.43 | 3.16 | .003 | ||||
| Time | 0.00 | −0.34 | .733 | ||||
| Caudate | −0.34 | −0.20 | .838 | ||||
| Caudate × TG | 13.02** | .15 | −12.81 | −3.61 | .0006 | .003 | |
| Simple slopes | B | SE | t (58) | p | 95% CI [lower, upper] | ||
| LTR | 7.15 | 2.49 | 2.87 | .006 | [2.17, 12.13] | ||
| HTR | −5.66 | 2.35 | −2.41 | .019 | [−10.36, −0.95] | ||
| Bilateral putamen | F | R 2 | ΔR2 | B | t | Uncorrected p | BH p |
| Model summary | 5.33** | .36 | .0002 | ||||
| Intercept | −18.33 | −1.14 | .260 | ||||
| Age | 1.99 | 1.52 | .134 | ||||
| TG | 3.52 | 1.69 | .097 | ||||
| MFQ baseline | 0.51 | 3.72 | .001 | ||||
| Time | 0.00 | −0.83 | .409 | ||||
| Putamen | 0.62 | 0.36 | .718 | ||||
| Putamen × TG | 11.86* | 0.13 | −11.48 | −3.44 | .0011 | .003 | |
| Simple slopes | B | SE | t (58) | p | 95% CI [lower, upper] | ||
| LTR | 7.34 | 2.22 | 3.31 | .002 | [2.89, 11.78] | ||
| HTR | −4.15 | 2.48 | −1.67 | .100 | [−9.12, 0.82] | ||
| Bilateral insula | F | R 2 | ΔR2 | B | t | Uncorrected p | BH p |
| Model summary | 4.88** | .34 | .0004 | ||||
| Intercept | −16.45 | −1.02 | .314 | ||||
| Age | 1.94 | 1.47 | .147 | ||||
| TG | 3.91 | 1.86 | .068 | ||||
| MFQ baseline | 0.52 | 3.76 | .0004 | ||||
| Time | −0.01 | −0.99 | .327 | ||||
| Insula | 2.92 | 1.34 | .314 | ||||
| Insula × TG | 7.43* | .09 | −11.64 | −2.72 | .0085 | .017 | |
| Simple slopes | B | SE | t (58) | p | 95% CI [lower, upper] | ||
| LTR | 9.73 | 2.91 | −3.34 | .002 | [3.90, 15.55] | ||
| HTR | −1.91 | 3.11 | −0.61 | .542 | [−8.15, 4.32] | ||
Note. BH = Benjamini–Hochberg; TG = temperament group; HTR = high temperamental risk; LTR = low temperamental risk; MFQ = Mood and Feelings Questionnaire (measure of depressive symptoms).
p < .01,
p < .001.
Interactions Between Temperament and Neural Activation to Social Punishment
No significant main effects or interactions between temperament group and neural activation to social punishment emerged for the amygdala (R2 = .02, uncorrected p = .26), insula (R2 = .02, uncorrected p = .18), or sgACC (R2 = .05, uncorrected p = .054).
Exploratory Analyses: Extension to Pre-COVID Depressive Symptoms
To test whether findings may be specific to depressive symptoms during COVID-19, follow-up analyses were run with MFQ scores collected most recently prior to COVID (between May 2019 and January 2020) entered as the dependent variable. Controlling for age, baseline depressive symptoms, and number of days between the baseline MFQ and most recent pre-COVID MFQ, no interactions between neural activity and temperament group were significant.
Discussion
Surges in depressive symptoms among adolescents during the COVID-19 pandemic are a public health concern requiring additional research, particularly longitudinal research, and attention and action from the health care system and policy makers (Singh et al., 2020). In this study, we capitalized on longitudinal data collection to examine factors that contribute to increases in depressive symptoms during COVID-19 in adolescent girls. We observed significant increases in depressive symptoms during the pandemic (relative to 2–4 years ago) in mid-adolescent girls differing in early adolescent shy/fearful temperament. Further, we found that interactions between shy/fearful temperament and neural reactivity to positive social feedback in early adolescence conferred risk for depressive symptoms during the pandemic.
As hypothesized, girls with histories of high shy/fearful temperaments and blunted activation to social reward in the dorsal striatum (i.e., caudate and putamen) and insula reported higher levels of depressive symptoms during the pandemic. The caudate and putamen are reliably implicated in responding to social and nonsocial rewards in adolescence (Silverman et al., 2015) and may play a key role in reward learning (Chase et al., 2015). Reduced activity in the caudate and putamen to positive stimuli could support social anhedonia in those at risk for depression (Keedwell et al., 2005), which may be especially harmful during the COVID-19 pandemic due to greater social isolation. Shy/fearful girls with blunted neural activation to social rewards may be less motivated to seek out and participate in potentially rewarding virtual interactions with peers (e.g., videochats) during the pandemic, which may be detrimental in combination with restrictions on in-person interactions. Notably, reduced activation in insula, amygdala, caudate, and putamen to social reward in HTR compared with LTR was also found. Blunted sensitivity to social reward in shy/fearful girls could be formed through a history of social avoidance in childhood that contributes to depression risk in adolescence, potentially through increased loneliness or social disconnectedness (Silk et al., 2012).
Blunted striatal and insula activation to social rewards has been found in young adults with subthreshold depression (He et al., 2019), youth at risk for MDD (Monk et al., 2008), and adults with remitted depression (e.g., Dichter et al., 2012). Reduced neural reward reactivity also prospectively predicts depressive symptoms in at-risk adolescents (Bress et al., 2013). Blunted reward responsivity could thus be a key biomarker of depression for those at-risk (Keren et al., 2018). However, more large-scale, longitudinal research is needed to test whether blunted reward responsivity is truly trait-like, developing early in life and remaining stable over adolescence, or a symptom of depression present before self-reported symptoms (Bress et al., 2013). Of note, research suggests that neural activity derived from reward fMRI tasks has acceptable stability over a 1- to 2-year period (Baranger et al., 2021), supporting the short-term predictive ability of present fMRI data.
In contrast to HTR girls, LTR girls showed positive associations between striatal and insula activation to social reward and depressive symptoms during the COVID-19 pandemic. Adolescent girls low in shy or fearfulness with heightened neural activation to social rewards may be most likely to attempt to engage with peers and to enjoy this engagement. This may be formed in part through a history of positive experiences interacting with peers. Though elevated neurobiological responses to social reward may typically be associated with socioemotional health and well-being, the paucity of opportunities to engage and connect with others during the pandemic may actually confer risk for depression in those most motivated to seek out positive social experiences. Moreover, high reactivity to social rewards could be detrimental during the pandemic because these girls find their quality and/or quantity of social interactions diminished. Importantly, these findings did not replicate when depressive symptoms measured in the year to month prior to the pandemic were entered as the dependent variable. This could support the hypothesis that the unique nature of the COVID-19 pandemic contributes to present findings.
Findings did not extend to neural activation to social punishment, failing to replicate previous research showing positive associations between sgACC activation to social threat and increases in depressive symptoms in adolescents (e.g., Masten et al., 2011). This could again be related to the unique nature of the pandemic, in which the frequency of socially threatening interactions is likely reduced for adolescents due to social distancing. Thus, individual differences in neural reactivity to social punishment may not play a strong role in the development of depressive symptoms during the pandemic. Another potential explanation for present findings could be related to the nature of performance-based social punishment on the P-SID task. This approach differs from most prior studies using social interactive tasks (e.g., Masten et al., 2011, Silk et al., 2014) to probe neural activity to more explicit social rejection and exclusion. Of note, temperament groups did not differ in neural activation to social punishment.
Findings should be viewed in light of several limitations, including a relatively small sample of adolescent girls with unequal distribution of girls in the LTR and HTR groups, though this was done by design given the sampling strategy. Additionally, the median income of the sample was high, and over two-thirds of the sample were white. We know that the pandemic is disproportionately harming Black, Latinx, and Native American communities (Tai et al., 2021), related to deeply rooted structural racism in America, and we do not assume or suggest that the risk and protective factors examined in this study will generalize across race, ethnicity, or culture. In addition, it is unknown how findings might extend to adolescent males. It should be noted that the majority of girls who did not respond to recruitment initiatives for this study were in the high shy/fearful (HTR) group, which could have biased the present sample towards lower depressive severity and impacted generalizability. This study was also underpowered to examine how diagnostic status might influence present findings. Given higher rates of social anxiety disorder in the HTR group (N = 8) relative to the LTR group (N = 2), as expected, one hypothesis could be that HTR girls with blunted reactivity to social rewards are at risk for depressive symptoms during the pandemic due to high levels of social anxiety. Future research could test whether reduced neural activation to reward is associated with real-world social behavior (e.g., avoidance) in girls high in social anxiety, and whether this confers risk for depression. Finally, while we speculate that blunted neural responses to social reward could support social disengagement, this is only one possible interpretation of findings. One additional interpretation could be that blunted neural responses to reward indicate a deficit in reward learning, which may play a role in the development of depression in youth (e.g., Morris et al., 2015).
Nonetheless, examining factors that contribute to increases in depressive symptoms during COVID-19 is critical for understanding how and why social distancing and reduced in-person interactions with peers confer risk for depression and elucidating targets for intervention. Improving social engagement and connectedness in adolescents, even virtually, may be critical for improving mental health during and following the COVID-19 pandemic. Pediatricians, clinicians, and school teachers and counselors will play a critical role in assessing the degree to which youth are engaging in social interaction during the pandemic, and may provide encouragement to parents to help youth engage in safe modes of social interaction. More broadly, this work adds to the literature examining neural threat and reward reactivity in shy, inhibited youth and shows how dramatic changes to the adolescent social context may promote depressive symptoms, particularly in those at higher risk.
Supplementary Data
Supplementary data can be found at: https://academic.oup.com/jpepsy.
Funding
This work was supported by National Institute of Mental Health grant R01 MH103241 (MPI’s: J.S.S. and C.D.L.) and a National Science Foundation Graduate Research Fellowship awarded to S.L.S. under Grant No. 1747452.
Conflicts of interest: None declared.
Supplementary Material
Footnotes
The parent study was a 3-year longitudinal study; semistructured clinical interviews (Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version; Kaufman et al., 1997) were conducted at baseline, 2-, and 3-year follow-up. Time between the most recent pre-COVID interview and COVID-19 study ranged from 0.10 to 43.33 months (M = 9.95 months, SD = 8.03 months).
References
- Angold A., Costello E. J. (1987). Mood and feelings questionnaire (MFQ). Developmental Epidemiology Program, Duke University. [Google Scholar]
- Baranger D. A., Lindenmuth M., Nance M., Guyer A. E., Keenan K., Hipwell A. E., Daniel S. H.& Forbes E. E. (2021). The longitudinal stability of fMRI activation during reward processing in adolescents and young adults. NeuroImage, 232, 117872. [DOI] [PMC free article] [PubMed]
- Bartra O., McGuire J. T., Kable J. W. (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bolling D. Z., Pitskel N. B., Deen B., Crowley M. J., Mayes L. C., Pelphrey K. A. (2011). Development of neural systems for processing social exclusion from childhood to adolescence. Developmental Science, 14(6), 1431–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress J. N., Foti D., Kotov R., Klein D. N., Hajcak G. (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology, 50(1), 74–81. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J. L., Valabregue R., Poline J. B. (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage, 16(2), S497. [Google Scholar]
- Brechwald W. A., Prinstein M. J. (2011). Beyond homophily: A decade of advances in understanding peer influence processes. Journal of Research on Adolescence, 21(1), 166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma L. M., Morris B. H., Rottenberg J. (2008). A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review, 28(4), 676–691. [DOI] [PubMed] [Google Scholar]
- Chase H. W., Kumar P., Eickhoff S. B., Dombrovski A. Y. (2015). Reinforcement learning models and their neural correlates: An activation likelihood estimation meta-analysis. Cognitive, Affective, & Behavioral Neuroscience, 15(2), 435–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caouette J. D., Guyer A. E. (2014). Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Developmental Cognitive Neuroscience, 8, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski J. M., Frank E., Young E., Shear M. K. (2000). Adolescent onset of the gender difference in lifetime rates of major depression: A theoretical model. Archives of General Psychiatry, 57(1), 21–27. [DOI] [PubMed] [Google Scholar]
- Dichter G. S., Kozink R. V., McClernon F. J., Smoski M. J. (2012). Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. Journal of Affective Disorders, 136(3), 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis W. E., Dumas T. M., Forbes L. M. (2020). Physically isolated but socially connected: Psychological adjustment and stress among adolescents during the initial COVID-19 crisis. Canadian Journal of Behavioural Science/Revue Canadienne Des Sciences du Comportement, 52(3), 177–187. [Google Scholar]
- Ellis L. K., Rothbart M. K. (2001, April). Revision of the early adolescent temperament questionnaire. Poster presented at the 2001 Biennial Meeting of the Society for Research in Child Development, Minnesota.
- Forbes E. E., Christopher May J., Siegle G. J., Ladouceur C. D., Ryan N. D., Carter C. S., Birmaher B., Axelson D. A., Dahl R. E. (2006). Reward‐related decision‐making in pediatric major depressive disorder: An fMRI study. Journal of Child Psychology and Psychiatry 47(10), 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E. E., Hariri A. R., Martin S. L., Silk J. S., Moyles D. L., Fisher P. M., Brown S. M., Ryan N. D., Birmaher B., Axelson D. A., Dahl R. E. (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry, 166(1), 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E. E., Ryan N. D., Phillips M. L., Manuck S. B., Worthman C. M., Moyles D. L., Tarr J. A., Sciarrillo S. R., Dahl R. E. (2010). Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry, 49(2), 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone G. L., Parker G. B. (2006). Is behavioral inhibition a risk factor for depression? Journal of Affective Disorders, 95(1-3), 85–94. [DOI] [PubMed] [Google Scholar]
- Guyer A. E., Benson B., Choate V. R., Bar-Haim Y., Perez-Edgar K., Jarcho J. M., Pine D. S., Ernst M., Fox N. A., Nelson E. E. (2014). Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology, 26(1), 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A. E., Nelson E. E., Perez-Edgar K., Hardin M. G., Roberson-Nay R., Monk C. S., Bjork J. M., Henderson H. A., Pine D. S., Fox N. A., Ernst M. (2006). Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. The Journal of Neuroscience, 26(24), 6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A. E., Silk J. S., Nelson E. E. (2016). The neurobiology of the emotional adolescent: From the inside out. Neuroscience and Biobehavioral Reviews, 70, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarasilta L. M., Marttunen M. J., Kaprio J. A., Aro H. M. (2004). Correlates of depression in a representative nationwide sample of adolescents (15–19 years) and young adults (20–24 years). The European Journal of Public Health, 14(3), 280–285. [DOI] [PubMed] [Google Scholar]
- Hayes A. F. (2018). Partial, conditional, and moderated moderated mediation: Quantification, inference, and interpretation. Communication Monographs, 85(1), 4–40. [Google Scholar]
- He Z., Zhang D., Muhlert N., Elliott R. (2019). Neural substrates for anticipation and consumption of social and monetary incentives in depression. Social Cognitive and Affective Neuroscience, 14(8), 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein S. M., Benson B., Perez-Edgar K., Bar-Haim Y., Detloff A., Pine D. S., Fox N. A., Ernst M. (2011). Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia, 49(3), 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkelman L. (2020). Findings from 1,273 U.S. girls on school, technology, relationships and stress since COVID-19. Ruling Our eXperiences (ROX; ). [Google Scholar]
- Jarcho J. M., Davis M. M., Shechner T., Degnan K. A., Henderson H. A., Stoddard J., Fox N. A., Leibenluft E., Pine D. S., Nelson E. E. (2016). Early-childhood social reticence predicts brain function in preadolescent youths during distinct forms of peer evaluation. Psychological Science, 27(6), 821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kaurin A., Sequeira S. L., Ladouceur C. D., McKone K. M. P., Rosen D., Jones N., Wright A. G. C., Silk J. S. (in press). Modeling sensitivity to social threat in adolescent girls: A psychoneurometric approach. Journal of Abnormal Psychology. [DOI] [PMC free article] [PubMed]
- Keedwell P. A., Andrew C., Williams S. C., Brammer M. J., Phillips M. L. (2005). The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry, 58(11), 843–853. [DOI] [PubMed] [Google Scholar]
- Keenan-Miller D., Hammen C. L., Brennan P. A. (2007). Health outcomes related to early adolescent depression. The Journal of Adolescent Health, 41(3), 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KerenH., , O'callaghanG., , Vidal-RibasP., , BuzzellG. A., , BrotmanM. A., , LeibenluftE., , PanP. M., , MeffertL., , KaiserA., , WolkeS., , PineD. S., & , Stringaris A. (2018). Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. American Journal of Psychiatry, 175(11), 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KingeryJ. N., , ErdleyC. A., , MarshallK. C., , WhitakerK. G., & , Reuter T. R. (2010). Peer experiences of anxious and socially withdrawn youth: An integrative review of the developmental and clinical literature. Clinical Child and Family Psychology Review, 13(1), 91–128. [DOI] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–27. [DOI] [PubMed] [Google Scholar]
- Loades M. E., Chatburn E., Higson-Sweeney N., Reynolds S., Shafran R., Brigden A., Linney C., McManus M. N., Borwick C., Crawley E. (2020). Rapid systematic review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. Journal of the American Academy of Child and Adolescent Psychiatry, 59(11), 1218–1239.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. A. (2020). _jtools: Analysis and Presentation of Social Scientific Data_. R package version 2.1.0. https://cran.r-project.org/package=jtools. Retrieved 12 September 2020.
- MastenC. L., , EisenbergerN. I., , BorofskyL. A., , PfeiferJ. H., , McnealyK., , MazziottaJ. C., & , Dapretto M. (2009). Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience, 4(2), 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C. L., Eisenberger N. I., Borofsky L. A., McNealy K., Pfeifer J. H., Dapretto M. (2011). Subgenual anterior cingulate responses to peer rejection: A marker of adolescents’ risk for depression. Development and Psychopathology, 23(1), 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C. S., Klein R. G., Telzer E. H., Schroth E. A., Mannuzza S., Moulton J. L., Guardino M., Masten C. L., McClure-Tone E. B., Fromm S., Blair R. J., Pine D. S., Ernst M. (2008). Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry, 165(1), 90–98. [DOI] [PubMed] [Google Scholar]
- Morris B. H., Bylsma L. M., Yaroslavsky I., Kovacs M., Rottenberg J. (2015). Reward learning in pediatric depression and anxiety: Preliminary findings in a high‐risk sample. Depression and Anxiety, 32(5), 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B. D., Perlman G., Klein D. N., Kotov R., Hajcak G. (2016). Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. American Journal of Psychiatry, 173(12), 1223–1230. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. (2002). Gender differences in depression. In Gotlib I. H. ?0026; Hammen C. L. (Eds.), Handbook of depression. (pp. 492–509). The Guilford Press. [Google Scholar]
- Olino T. M., McMakin D. L., Morgan J. K., Silk J. S., Birmaher B., Axelson D. A., Williamson D. E., Dahl R. E., Ryan N. D., Forbes E. E. (2014). Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Developmental Cognitive Neuroscience, 8, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino T. M., Silk J. S., Osterritter C., Forbes E. E. (2015). Social reward in youth at risk for depression: A preliminary investigation of subjective and neural differences. Journal of Child and Adolescent Psychopharmacology, 25(9), 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Roberson-Nay R., Hardin M. G., Poeth K., Guyer A. E., Nelson E. E., McClure E. B., Henderson H. A., Fox N. A., Pine D. S., Ernst M. (2007). Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage, 35(4), 1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team R. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/. Retrieved 12 September 2020. [Google Scholar]
- Racine N., Cooke J. L., Eirich R., Korczak D. J., McArthur B., Madigan S. (2020). Child and Adolescent Mental Illness during COVID-19: A Rapid Review. Psychiatry Research, 292, 113307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K. D., Conley C. S. (2005). The socioemotional costs and benefits of social‐evaluative concerns: Do girls care too much? Journal of Personality, 73(1), 115–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. E., Wright C. I., Shin L. M., Kagan J., Rauch S. L. (2003). Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science (New York, N.Y.), 300(5627), 1952–1953. [DOI] [PubMed] [Google Scholar]
- Silk J. S., Davis S., McMakin D. L., Dahl R. E., Forbes E. E. (2012). Why do anxious children become depressed teenagers?: The role of social evaluative threat and reward processing. Psychological Medicine, 42(10), 2095–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J. S., Siegle G. J., Lee K. H., Nelson E. E., Stroud L. R., Dahl R. E. (2014). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience, 9(11), 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M. H., Jedd K., Luciana M. (2015). Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage, 122, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Roy D., Sinha K., Parveen S., Sharma P., Joshi G. (2020). Impact of COVID-19 and lockdown on mental health of children and adolescents: A narrative review with recommendations. Psychiatry Research, 293, 113429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai D. B. G., Shah A., Doubeni C. A., Sia I. G., Wieland M. L. (2021). The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clinical Infectious Diseases, 72(4), 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YarkoniT., , PoldrackR. A., , NicholsT. E., , Van EssenD. C., & , WagerT. D. (2011).Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


