Abstract
This review highlights the convergence of three global health challenges at a crossroad where the pandemic of coronavirus disease 2019 (COVID-19) meets the tobacco epidemic and vaping. It begins with an overview of the current knowledge on the biology, pathophysiology and epidemiology of COVID-19. It then presents the state of smoking and vaping during the pandemic by summarizing the published data on prevalence, use patterns, product availability/accessibility, sales records and motivation to quit before and after the start of the pandemic. It highlights the state of evidence on the association of tobacco product use with COVID-19 infection and transmission rates, symptom severity and clinical outcomes. Also discussed are proposed biological mechanisms and behavioral factors that may modulate COVID-19 risk in tobacco product users. Furthermore, competing hypotheses on the protective effect of nicotine against COVID-19 as well as the claimed ‘smokers’ paradox’ are discussed. Considerations and challenges of COVID-19 vaccination in tobacco product users are underscored. Collectively, the present data show an ‘incomplete’ but rapidly shaping picture on the association of tobacco product use and COVID-19 infection, disease course and clinical outcomes. Evidence is also growing on the mechanisms by which tobacco product use may contribute to COVID-19 pathophysiology. Although we await definitive conclusions on the relative risk of COVID-19 infection in tobacco product users, compelling data confirm that many comorbidities associated with/caused by smoking predispose to COVID-19 infection, severe disease and poor prognosis. Additionally, it is becoming increasing clear that should smokers get the disease, they are more likely to have serious health consequences.
This is a comprehensive review on the interplay of COVID-19 and tobacco product use. The review provides detailed and up-to-date information on the biology, pathophysiology and epidemiology of COVID-19 in relation to smoking and vaping.
Graphical Abstract
Introduction
With an unabating global tobacco epidemic, killing nearly 8 million people annually (1), the US Surgeon General declared, in December 2018, youth vaping an epidemic in the USA (2). Earlier in September 2018, the US Food and Drug Administration (FDA) had called JUUL, the preeminent electronic cigarette (e-cig) on the market (3), a particular cause for concern (4). On 11 March 2020, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a global pandemic (5). Midway through the year 2021, the devastating global pandemic of COVID-19 continues to rage on (6). Since the outbreak of the disease in Wuhan, China, in December 2019, COVID-19 has spread to 192 countries and ravaged nations, causing infections and deaths at an unprecedented pace (6). As of 18 June 2021, COVID-19 has infected over 177.7 million and killed more than 3.8 million people, worldwide (6). The USA holds the unenviable record of having roughly one-fifth of the global cases and one-sixth of all deaths related to this disease (6). The pandemic has wreaked havoc on almost all aspects of daily life, including lifestyle habits, such as substance use, e.g. smoking and vaping.
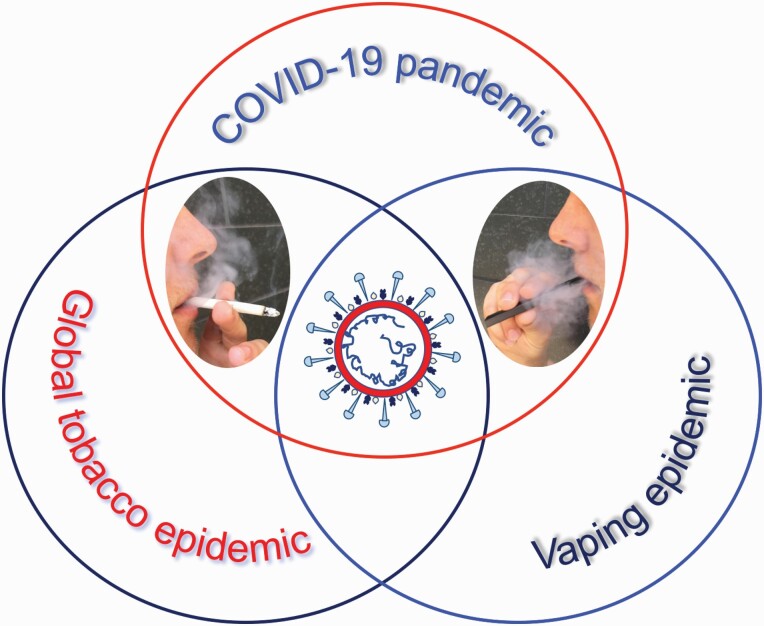
This review highlights the convergence of three global health challenges at a crossroad where the pandemic of COVID-19 meets the global tobacco epidemic and vaping. First, it provides an overview of the current knowledge on the biology, pathophysiology and epidemiology of COVID-19. It then summarizes the state of smoking and vaping during the pandemic by featuring the published data on prevalence, use patterns, product availability and accessibility, sales records and motivation to quit before and after the start of the pandemic. Moreover, it discusses the existing evidence on the association of tobacco product use with COVID-19 infection and transmission rates, symptom severity and clinical outcomes. The unique and overlapping clinical, laboratory and radiologic features of COVID-19 and ‘e-cig, or vaping, product use-associated lung injury’ (EVALI) (7) are also described. Furthermore, competing hypotheses on the potential utility of nicotine for prevention and treatment of COVID-19 as well as the claimed ‘smokers’ paradox’ are described. Also highlighted are considerations and challenges of COVID-19 vaccination in tobacco product users. As the global pandemic of COVID-19 continues to evolve, unparalleled public health challenges and unique research opportunities have emerged that will be discussed in detail.
Search strategy and selection criteria
PubMed search was conducted to identify references using the following terms: ‘COVID-19’, ‘SARS-CoV-2’, ‘coronavirus’, ‘smoking’, ‘vaping’, ‘tobacco’, ‘cigarette’, ‘electronic cigarette’ and ‘e-cigs’. The search terms were used both individually and in combination with each other. All English-written references, published on or before 18 June 2021, were considered. Where appropriate, publicly available databases and scientific reports from regulatory agencies and/or academia as well as news publications were considered; in all cases, cited sources were identified with a link to the published materials. To limit the number of citations, updated reviews were used rather than individual research articles, unless otherwise indicated.
COVID-19: disease course and outcomes
Symptoms of COVID-19 are variable but often include fever or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle or body aches, headache, loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting and diarrhea (8). COVID-19 most commonly spreads through close contact, from person to person, particularly when people are physically near each other in enclosed spaces (9). According to the Centers for Disease Control and Prevention (CDC) guidelines, the principal mode by which COVID-19 infection occurs is through exposure to respiratory fluids, containing infectious virus (10). There are three primary ways of exposure, including (i) ‘Inhalation’ of air carrying very small fine droplets and aerosol particles that contain infectious virus. Risk of transmission is greatest within 3–6 ft of an infectious source, where the concentration of these very fine droplets and particles is highest; (ii) ‘Deposition’ of virus carried in exhaled droplets and particles onto exposed mucous membranes, such as the mouth, nose or eye (e.g. being coughed on by ‘splashes and sprays’). Risk of transmission is likewise greatest close to an infectious source, where the concentration of these exhaled droplets and particles is highest; and (iii) ‘Touching’ mucous membranes with hands soiled by exhaled respiratory fluids containing virus or from touching inanimate surfaces contaminated with virus (10). Re-infection with COVID-19 has been reported but is relatively rare (10).
Symptoms of COVID-19 infection may appear a few to several days after exposure to the virus (i.e. incubation period) (8). The median incubation period for COVID-19 is 4–5 days (8). Most symptomatic people experience symptoms within 2–7 days after exposure, and almost all symptomatic people will develop one or more symptoms before day 12 (8). Although most infected people have mild symptoms, some develop acute respiratory distress syndrome (ARDS) (11,12). COVID-19 can spread as early as 2 days before infected persons show symptoms, as well as from individuals who never experience any noticeable symptoms (i.e. asymptomatic carriers) (8). The asymptomatic carriers tend not to get tested, and thus more likely to spread the disease (8). Infected individuals remain contagious for up to 10 days in moderate cases and 2 weeks in severe cases (8).
As COVID-19 progresses in its course, complications may follow and death may occur (13). Common complications of the disease include respiratory complications, such as pneumonia and ARDS, cardiovascular complications, such as heart failure, arrhythmia, heart inflammation and blood clots, multi-organ failure, kidney or liver injury and septic shock (12,13). Children infected with COVID-19 may develop pediatric multisystem inflammatory syndrome, which has symptoms similar to Kawasaki disease, that can be fatal (14). Treatment options for COVID-19 have been summarized in elegant articles, including references (15,16).
COVID-19 and tobacco product use: a protective role for smoking/vaping?
Some reports during the early stages of the pandemic have suggested that current smokers are less likely than never smokers to become infected with COVID-19 (17,18). There have also been claims, mostly circulating on social media, that vaping can protect against COVID-19 (19). Of relevance, promotion of e-cig products through social media and advocacy for vaping in virtual communities have greatly contributed to the popularity of e-cigs and the ongoing epidemic of youth vaping (20). The claimed protective or therapeutic effects of smoking/vaping in relation to COVID-19 have drawn media coverage; several mainstream media outlets and social media platforms have reported on those claims (19). In response, on 4 May 2020, the WHO released a warning on tobacco use during the pandemic (21). One week later, WHO issued a statement, which read, in part: ‘WHO urges researchers, scientists and the media to be cautious about amplifying unproven claims that tobacco or nicotine could reduce the risk of COVID-19. There is currently insufficient information to confirm any link between tobacco or nicotine in the prevention or treatment of COVID-19’ (22). Regardless of whether or not smokers are at higher risk of COVID-19, it is irrefutable that smoking is a primary cause or a major risk factor for many comorbidities that make people susceptible to COVID-19 infection, severe clinical outcomes and death (10). Therefore, more than 1 year after its release, the recommendation of WHO still remains valid.
Some have hypothesized that nicotine use is protective against COVID-19 infection (23,24), possibly explaining the lower percentage of smokers diagnosed with this disease, as reported in some studies (25,26). The adverse health consequences of smoking, however, are likely to exacerbate the course of COVID-19, once the infection has occurred (10). The above may explain, in part, why former smokers appear to be worse off than current smokers when infected with SARS-CoV-2, as reported in some studies (27). A hypothesis has been put forward stating that former smokers, who have incurred irreversible health damages due to prior smoking, lack the protective effect of nicotine as they no longer smoke (23). Detailed discussions on the claimed protective effect of nicotine against COVID-19 and the hypothesized ‘smokers’ paradox in COVID-19’ are provided in Association of COVID-19 infection, disease course and outcomes with tobacco product use and COVID-19 pathogenesis and transmission: importance of the biological and behavioral aspects of tobacco product use, respectively.
In a ‘Feature’ article, Horel et al. (28) have drawn attention to the issue of conflict of interest impacting the published research on COVID-19 and tobacco products use. The authors have argued that the tobacco/vaping industry, its affiliated researchers and harm reduction advocates have capitalized on the pandemic to promote the use of nicotine and alternative tobacco products. By way of example, the authors have highlighted the financial links between tobacco/vaping industry and the contributing authors of a provocative pre-print (17) and an ‘Editorial’ published in a scientific journal (29); these publications together with a related pre-print (18) have given rise to various claims on the protective effect of nicotine against COVID-19 and the low prevalence of smokers among people diagnosed with this disease, reported in some studies (see Association of COVID-19 infection, disease course and outcomes with tobacco product use and COVID-19 pathogenesis and transmission: importance of the biological and behavioral aspects of tobacco product use). Of significance is a high-profile paper, which reported no association of current smoking with adverse outcomes in patients admitted to hospital with COVID-19 and a significantly lower risk of smokers to contract the disease (30). In March 2021, this paper was retracted due to the undisclosed conflicts of interest of two of its contributing authors, with financial links to the tobacco industry (30). As we continue to investigate the relationship between smoking/vaping and COVID-19, it is incumbent upon the publishing community to require full disclosure of competing interests (financial or otherwise) from all authors before publishing their research and disseminating their results. The same requirement ‘must’ apply to all individuals involved in the peer review of research materials, specifically manuscripts submitted to scientific journals.
Association of COVID-19 infection, disease course and outcomes with tobacco product use
Initial studies investigating the associations between smoking and COVID-19 outcomes have yielded contradictory results, often due to various limitations that precluded firm conclusions (31,32). A systematic review, based on literature search on 17 March 2020, concluded that cigarette smoking is most likely associated with progression and adverse outcomes of COVID-19, while acknowledging the limited data available and not accounting for potential confounding factors (31). In contrast, preliminary results from a meta-analysis of data from Chinese patients, based on five studies published until 9 March 2020, suggested that active smoking is not a significant predictor of COVID-19 severity (32).
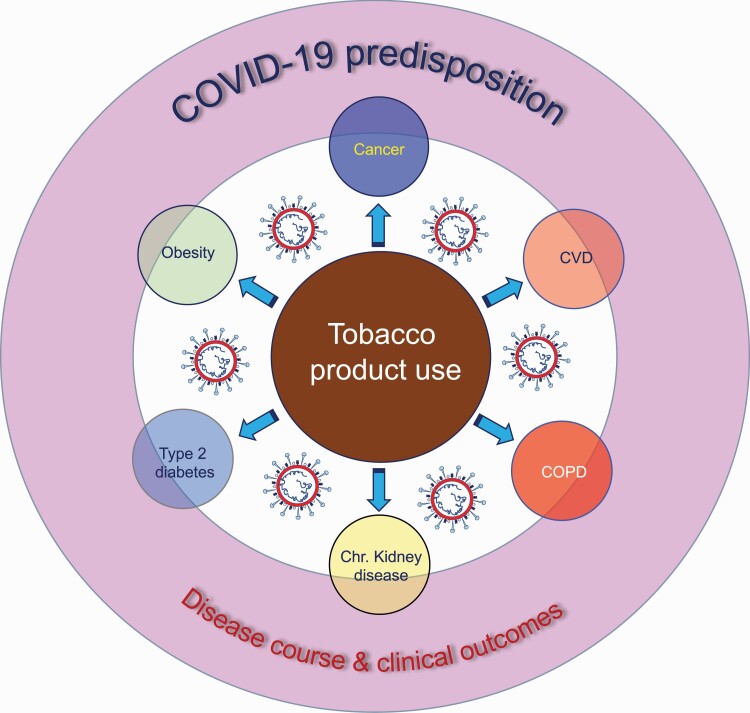
A French study proposed that nicotine may protect against COVID-19 infection (33), raising competing hypotheses that (i) nicotine exerts anti-inflammatory effects against COVID-19 by activating the nicotinic acetylcholine receptor (nAChR) and (ii) nicotine downregulates angiotensin-converting enzyme 2 (ACE2) expression through modulation of the renin–angiotensin–aldosterone system (see COVID-19 pathogenesis and transmission: importance of the biological and behavioral aspects of tobacco product use) (23,34). The authors proposed examination of the potential utility of nicotine for prevention and control of COVID-19 (33). The report garnered interest from the French Health Minister, prompting investigations into the utility of nicotine patches for COVID-19 prevention and/or treatment; clinical trials and observational studies in health care workers and patients are still underway (33). It is important to note that there is a clear distinction between ‘potential’ therapeutic or preventive utility of nicotine, when administered transdermally through patches [i.e. nicotine replacement therapy (NRT)], as opposed to the known dangers of nicotine, when inhaled via smoking or vaping (35,36). It should be emphasized that smoking is a primary cause/risk factor for many comorbidities that make people susceptible to COVID-19 infection and poor outcomes (10). These comorbidities include chronic obstructive pulmonary disease (COPD), cancer, cardiovascular disease (CVD) (e.g. cardiomyopathy, coronary artery disease and heart failure), chronic kidney disease, type 2 diabetes mellitus and obesity (see Figure 1) (10).
Figure 1.
The interplay of tobacco product use and COVID-19. Arrows indicate that comorbidities shown within circles are associated with tobacco product use (smoking). These comorbidities include COPD, cancer, CVD, chronic kidney disease, type 2 diabetes mellitus and obesity. The overlapping of ‘comorbidities’ with ‘COVID-19 risk and disease course and clinical outcomes’ represents ‘association’, not ‘causality’.
Subsequent systematic reviews and meta-analyses of data from considerably large populations have shown varying associations between smoking status and hospitalization rates, severity of symptoms and mortality from COVID-19 (37,38). The strength of associations has varied in different studies, dependent on the characteristics of the study population (e.g. age, gender, race and socioeconomic status) and their underlying health conditions and comorbidities (37,38). For example, existing comorbidities (as specified above), advanced age, male gender, being of minority races/ethnicities, working in service jobs and having low income are shown to disproportionately affect the severity of illness, hospitalization and death resulting from COVID-19 (37,39).
A living rapid review of observational and experimental studies from around the world has been created to estimate the association of smoking status with rates of infection, hospitalization, disease severity and mortality from COVID-19 (27). The living review is updated regularly as new data from published studies become available. Version 7 of this review, based on search of MEDLINE and medRxiv for published articles and pre-prints up to 25 August 2020, identified a total of 347 new records, with 233 studies included in a narrative synthesis and 32 studies included in meta-analyses. The review found uncertainties in the majority of 233 studies, arisen from the recording of smoking status in the study subjects. More specifically, the vast majority of the analyzed studies either under-reported or did not report smoking status of their study population. As a result, recorded current smoking rates in most published studies from different countries were ‘lower’ than the corresponding national adults smoking prevalence estimates. So, under-reporting or not reporting smoking status proved to be a common problem in the overwhelming majority of the analyzed studies. In a subset of better-quality studies (n = 17), current smokers had a slightly reduced risk of testing positive for SARS-CoV-2 but appeared more likely to present for testing and/or receive a test as compared with never smokers. Data for current smokers on the risk of hospitalization, disease severity and mortality were inconclusive but favored a small but important increase in risk for severe disease. The data in current smokers, however, showed no trend of association between smoking status and hospitalization or mortality from the disease. Importantly, former smokers showed increased risk of hospitalization, disease severity and mortality from COVID-19 as compared with never smokers (27).
The findings of this living review underscore the need for better-designed studies with rigorous methodologies and adequate statistical power, which can produce reliable data and ensure reproducible results. Of high priority is collection of validated data on tobacco product use and frequency, e.g. by biochemical assays rather than self-reporting only. Equally important is the use of diagnostic tests with high sensitivity and specificity for COVID-19 diagnosis. As research groups around the world learn to navigate work in the midst of a global pandemic, we should aim to objectively investigate the relationship between tobacco product use and COVID-19 infection, transmission and clinical outcomes.
COVID-19 pathogenesis and transmission: importance of the biological and behavioral aspects of tobacco product use
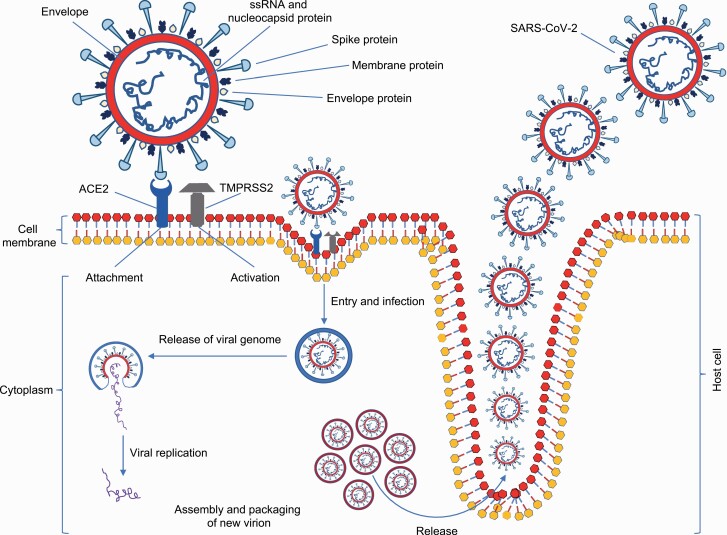
SARS-CoV-2 can target both the upper respiratory tract [nose, nasal passages, pharynx and larynx (throat)] and the lower respiratory tract [trachea and bronchi, bronchioles and alveoli (making up the lungs)] (12). The lungs are the most affected target organ for SARS-CoV-2 because the virus accesses host cells via ACE2, which is most abundant in type II alveolar cells of the lungs (40). The virus uses a distinct surface glycoprotein, named ‘spike’ protein (peplomer), to attach to ACE2 and enter the host cell; the ACE2 is interchangeably called ‘ACE2 receptor’ (Figure 2) (40). Using the spike protein on its surface, SARS-CoV-2 binds to ACE2 receptors—like a key being inserted into a lock—prior to entry and infection of the host cells (40). In addition to targeting the respiratory tract, SARS-CoV-2 can also affect gastrointestinal tract as ACE2 is abundantly present in the glandular cells of gastric, duodenal and rectal epithelium as well as endothelial cells and enterocytes of the small intestine (40). ACE2 is also highly expressed in the heart and involved in heart function (40).
Figure 2.
Schematic diagram of SARS-CoV-2 lifecycle and host cell infection. The spike protein of SARS-CoV-2 binds to ACE2 receptors in host cells (i.e. attachment). The proteolytic function of transmembrane protease, serine 2 (TMPRSS2) cleaves the spike protein (i.e. activation), thus allowing entry and viral replication in the host cell, and subsequent release of the infectious virus into the intercellular milieu. This is a simplified diagram of the highly complex life cycle of SARS-CoV-2, which causes COVID-19; interested readers are referred to elegant references (12,40) for detailed information.
It is biologically plausible that smokers and vapers may be at heightened risk of COVID-19 because chronic use of tobacco cigarettes and e-cigs can weaken respiratory system and compromise immune response (35,36). Inhalation of the toxicants and carcinogens present in both cigarette smoke and e-cig vapor (36) elicits inflammatory response in vivo (41) that may exacerbate the inflammation caused by SARS-CoV-2 infection. This may, in turn, trigger/promote ‘cytokine storms’ and hyperinflammatory immune response that are hallmarks of severe cases of COVID-19 (11). Also, as mentioned above, the spike protein of SARS-CoV-2 binds specifically to ACE2 receptors in type II alveolar cells in the respiratory tract (40). Of significance, ACE2 is over-expressed in smokers as compared with nonsmokers (42,43). Furthermore, chronic smoking is known to compromise mechanical barriers and other defense mechanisms in the respiratory system; these include filtration in the upper airway, mucociliary clearance of inhaled pathogens (e.g. disease-causing bacteria and viruses, such as SARS-CoV-2) and phagocytosis by macrophages (35). In addition, behaviors, such as sharing e-cig devices, which is common among vapers (20) (although likely reduced due to ‘stay-at-home’ mandates and restrictions on social gatherings), may help spread the disease, especially by facilitating transmission from asymptomatic cases.
Based on the proposed biological mechanisms underlying the claimed protective effect of nicotine against COVID (24,33,34), some have put forward the hypothesis of ‘smoker’s paradox in COVID-19’ (44–46). This hypothesis refers to a paradox, in which smokers are protected from infection and severe complications of COVID-19 (33,44–46). However, others (27,47,48) have argued that several plausible explanations and possible biases may be responsible for the under-representation of smokers among patients with COVID-19, and the lower severity of the disease among smokers, as reported in some studies (25,26). The reported low prevalence of active smokers among patients with COVID-19, often significantly lower than the prevalence of current smokers in the general population, can be ascribed to (i) inadequate collection of smoking history data from patients who were either intubated or in respiratory failure; (ii) coalescing former smokers and never smokers into ‘nonsmokers’ or former smokers and current smokers into ‘smokers’; (iii) inclusion of patients with missing smoking data; and (iv) lack of biochemical validation of the (self)-reported smoking status (27,47,48). This is further compounded by the fact that evaluating the effects of smoking has not been the primary goal of most conducted studies, considering the life-threatening nature of COVID-19, which made other research objectives less of a priority, especially during the early stages of the pandemic (27,47). Moreover, in the race to publish, particularly during the initial months of the pandemic (to some extent, still ongoing), limited scrutiny during editorial assessment and peer review has likely led to publication of many subpar research studies with less-than reproducible results (47).
Proposed biological mechanisms for susceptibility of smokers and vapers to COVID-19
The expression levels of ACE2 have been shown to be elevated in type II pneumocytes, alveolar macrophages and small airway epithelium of smokers and subjects with COPD in comparison with healthy control cells (49,50). The upregulation of ACE2 is thought to involve nicotinic receptors, mediated through α-7 subtype nicotinic acetylcholine receptor (α-7 nAChR) (51). Wang et al. (52) have demonstrated that subchronic exposure of mice to e-cig aerosol (containing nicotine) induced lung inflammation and dysregulated repair/extracellular matrix remodeling, mediated via α-7 nAChR in a sex-dependent manner. McAlinden et al. (53) have shown that in vitro treatment of immortalized human bronchial epithelial cells and primary human small airway epithelial cells with nicotine-containing e-cig aerosol-condensate and cigarette-smoke extract resulted in increased ACE2 protein expression as compared with untreated control cells.
Eapen et al. (49) have reported concurrent rises in ACE2 protein expression and endosomal protein markers, specific for endocytic vacuoles, such as early/late endosomes and lysosomes that allow SARS-CoV-2 entry and deposition of viral RNA into host cells (12), in the small airways of smokers and COPD patients as compared with nonsmoking healthy controls. The authors proposed prominent expression of the ACE2 and elevation of the endosomal markers in type II pneumocytes and alveolar macrophages as an underlying mechanism that could make smokers and COPD patients susceptible to COVID-19 infection (49).
Lee et al. (43) mined three independent RNA expression datasets from smokers and vapers and concluded that tobacco smoking and use of nicotine and flavor-containing e-cigs are associated with upregulation of pro-inflammatory cytokines and inflammasome-related genes, while smoking is also associated with ACE2 upregulation. The authors suggested that smoking and possibly vaping may exacerbate COVID-19-related inflammation and/or increase susceptibility to COVID-19 infection through an ACE2-dependent mechanism (43).
Pathophysiological changes in COVID-19 patients: the impact of tobacco product use
The abundant expression of ACE2 in type II alveolar cells facilitates rapid viral expansion and local alveolar wall destruction, resulting in fast and progressive severe diffuse alveolar damage and hyperinflammation, otherwise known as cytokine storm (11). Furthermore, SARS-CoV-2 infection can cause oxidative stress in the lung as a result of activation of resident cells or inflammatory cells recruited to the site of tissue injury (54). Single-cell RNA sequencing of the human lungs has shown that specific components of the antioxidant defense system in the alveolar type II cells, such as superoxide dismutase 3 and activating transcription factor 4, are downregulated in healthy elderly donors as compared with young donors (55). This may explain, in part, the observed severity of COVID-19 and its poor prognosis in older patients (37,38). Of significance, smoking and, to a lesser extent, vaping are known to induce oxidative stress and inflammation and modulate antioxidant defense mechanisms in vivo (41,56,57).
Examination of autopsy lung tissues from patients who died from COVID-19, those who died from ARDS secondary to influenza A (H1N1) infection and age-matched, uninfected control lungs showed greater numbers of ACE2-positive endothelial cells in the lungs from patients with COVID-19 and from patients with influenza than those from uninfected controls. Endothelial cells in the specimens from patients with COVID-19 showed disruption of intercellular junctions, cell swelling and a loss of contact with the basal membrane, all indicative of vascular structural modification that promotes endothelial to mesenchymal transition (EndMT) (58). EndMT is a cellular plasticity state whereby endothelial cells respond to various endogenous stimuli or exogenous pathogens (59). EndMT occurs when endothelial cells undergo a series of molecular alterations and morphologic changes that transform them into a more aggressive mesenchymal state (e.g. myofibroblasts or smooth muscle cells), causing irreversible vascular damage or fibrosis (60). EndMT is widely considered a key contributor to several other pathological conditions, including CVDs, such as atherosclerosis, pulmonary arterial hypertension, valvular disease, fibroelastosis, arterial fibrosis and cardiac fibrosis, as well as malignancy (59). Accumulating evidence shows that SARS-CoV-2 may lead to endothelial cell dysfunction, possibly progressing to EndMT or pulmonary fibrosis (61).
Another cell type that may contribute to fibrosis post-infection in COVID-19 patients is type II pneumocytes [alveolar epithelial type II (AT II)]. These cells have been shown to be hyperplastic and hyperproliferative in idiopathic pulmonary fibrosis (62). Like EndMT, epithelial to mesenchymal transition (EMT) occurs when epithelial cells transform into a mesenchymal phenotype, accompanied by basement membrane degradation, loss of epithelial features and gain of mesenchymal proteins (63). EMT is known to be a key contributor to organ fibrosis and epithelial cell malignancy (63). Both epithelial and endothelial cells in the respiratory tract become compromised and susceptible to fibrotic conditions in a variety of smoking-associated diseases (63). It has been suggested that progressive EMT and endothelial cell dysfunction in the vasculature of smokers due to preferential targeting of alveolar type II cells and endothelial cells injury by SARS-CoV-2 may make them more vulnerable to post-COVID-19 pulmonary fibrosis and poor prognosis (61). Furthermore, endothelial dysfunction is a main determinant of comorbidities associated with severe clinical outcomes and worse prognosis of COVID-19; these include obesity, diabetes and systemic hypertension (10). SARS-CoV-2-related endothelial dysfunction has been suggested to contribute to venous thromboembolic disease, systemic vasculitis, endothelial cell apoptosis and inflammation in various organs in COVID-19 patients (64).
In a subset of COVID-19 patients, long-term lung impairment, including interstitial lung diseases, may follow after recovery from the disease (65). Interstitial lung diseases comprise a broad range of diffuse parenchymal lung disorders that are characterized by widespread and heterogeneous parenchymal lung abnormalities, which can lead to irreversible fibrosis (66). Pulmonary fibrosis is caused by progressive and irreversible destruction of lung architecture due to scar formation, which may lead to disruption of gas exchange, organ malfunction and ultimately death from respiratory failure (66). To date, therapeutic options for pulmonary fibrosis are limited, treatment response is relatively low, and as such, the disease has high mortality rates (66). The prevalence of pulmonary fibrosis as a post-infection complication of COVID-19 will be determined in time, but the existing data indicate that many COVID-19 survivors develop fibrotic lesions and pulmonary structural abnormalities and functional impairment (65). It is important to investigate the impact of COVID-19 on the progression of pre-existing interstitial lung diseases in patient populations (e.g. in COPD patients who are mostly current or former users of tobacco products (63)), as well as in non-clinically diagnosed individuals who are chronic smokers/vapers. These investigations will help elucidate the contribution of smoking/vaping to the development of pulmonary fibrosis in the aftermath of COVID-19 infection.
COVID-19 and EVALI: shared clinical, laboratory and imaging features
In August 2019, the CDC, FDA, state and local health departments and other clinical and public health partners reported a nationwide outbreak of vaping-related severe lung illnesses and deaths, also known as EVALI (7). National and state data showed a sharp increase in the number of EVALI cases, which reached a peak in September 2019 but gradually and persistently declined, afterward. Nationwide, a total of 2807 hospitalized cases of EVALI and 68 deaths were confirmed by 18 February 2020. Analysis of data from patient reports and product sample testing revealed that e-cigs or vaping products with tetrahydrocannabinol (THC) emulsified with vitamin E acetate (VEA), especially those obtained from informal sources like friends and family, or in-person or online dealers, were linked to most cases of EVALI. Of note, VEA is a viscous substance commonly used for diluting THC oil in vape cartridges or as a thickening agent in e-liquid (7). THC is the principal psychoactive component of cannabis, which produces the infamous ‘high’ in marijuana/weed users (3). Testing of product samples and analysis of lung fluid from EVALI patients, but not from healthy controls, showed detectable levels of VEA. However, evidence was not sufficient to rule out the contribution of other suspected chemicals, including constituents of THC- or non-THC-containing vaping products, to some of the reported EVALI cases (7). The continued drop in the number of EVALI cases has been attributed to (i) increased public awareness of the risk associated with THC-containing e-cigs or vaping products; (ii) removal of VEA from some vaping products; and (iii) law enforcement actions taken against the sale and distribution of black market e-cigs and vaping products (3).
To test whether aerosolized VEA causes lung injury in mice and damages human alveolar epithelial cells, Matsumoto et al. (67) exposed adult mice and primary human AT II cells to an aerosol of VEA generated by an atomizer designed for vaping oils. Whereas the in vivo exposure of mice to VEA consisted of 1 h twice daily for 6 or 15 days, in vitro exposure of the surfactant-producing human AT II cells was performed for 20–120 min daily for 3 days in air–liquid interface conditions. Mice exposed to VEA developed diffuse lung injury, with dose-dependent increases in lung water and BAL protein in association with mixed neutrophilic and monocytic alveolar and interstitial inflammation in a bronchiolocentric pattern, including the presence of numerous lipid-laden and large multinucleated macrophages. Consistently, AT II cells readily absorbed VEA aerosol and showed direct signs of injury, manifest as dose-dependent cytotoxicity and release of monocyte and neutrophil chemokines in the context of major inflammatory changes in gene expression. The VEA-induced effects in both mouse and cell culture models recapitulate several key pathological features of EVALI in patient populations. As such, the authors suggested an important causative role for VAE in the etiology of EVALI (67).
Kleinman et al. (68) reported an EVALI-like condition in rats acutely exposed to tobacco-flavored e-cig liquid without THC, VEA or nicotine (i.e. propylene glycol | vegetable glycerin, 50% each). The exposure protocol consisted of a single, 2-h, nose-only exposure to e-liquid, vaporized by a nichrome heating element, operated at ‘high’ power setting. Lung lesions in the exposed rats consisted of thickening of the alveolar wall with foci of inflammation, red blood cell congestion, obliteration of alveolar spaces and pneumonitis in some cases; bronchi showed accumulation of fibrin, inflammatory cells and mucus plugs. The authors concluded that thermal decomposition of e-liquid at high temperature, even in the absence of THC, VEA or nicotine, may lead to an EVALI-like syndrome in vivo (68).
Since the early stages of the pandemic, concerns have been raised about the overlapping clinical, laboratory and radiologic features of COVID-19 and EVALI, which can make accurate diagnosis a challenge, especially among e-cig users (69). Also, the convergence of the two diseases and their possible synergistic effects have become a matter of concern (69). The shared clinical characteristics of EVALI and COVID-19, like many other respiratory illnesses, include fever (>100.4°F), cough, shortness of breath and gastrointestinal symptoms, such as nausea, vomiting and diarrhea (70). Both EVALI and COVID-19 can present as interstitial pneumonia leading to ARDS (70). Chest computed tomography imaging findings may considerably overlap, with ground glass opacities present in both EVALI and COVID-19, and organizing pneumonia as the imaging feature that is most common between the two diseases (71). Laboratory findings in EVALI include nonspecific leukocytosis, elevated inflammatory markers, such as erythrocyte sedimentation rate and procalcitonin, and increased levels of liver enzymes (72). Elevation of liver enzymes and inflammatory markers can also be seen in COVID-19; however, patients with COVID-19 typically exhibit lymphopenia as opposed to lymphocytosis in EVALI (72).
Because of the remarkably similar presentation of acute lung injury secondary to COVID-19 and EVALI, and considering the shared laboratory and imaging findings of the two diseases, clinicians are advised to pay particular attention during patients’ history taking (69). Specifically, physicians and nurses are encouraged to inquire about patients’ vaping history, particularly use of e-cig products or marijuana vaping, as vaping is often a social activity during which wearing of masks and maintaining social distancing become less frequent, if not, unlikely (70). Moreover, as the pandemic-related restrictions on social gatherings are being lifted, and schools, colleges and universities are re-opening, e-cigs users, specifically those who use VAE/THC-containing e-cig products, should be monitored for pneumonia-like symptoms, not only for COVID-19 diagnosis but also for EVALI evaluation (72).
The impact of COVID-19 pandemic on tobacco products sales
For the past several decades, there has been an accelerating fall in USA cigarette unit sales, consistent with the drop in smoking prevalence; adult smoking rates have dropped from 42% in 1965 to 14% in 2019 (73). The decline in smoking rates among adults has been attributed to public health campaigns, raising awareness of the health consequences of smoking, implementation of comprehensive smoke-free laws, higher cigarette prices and increased tobacco taxes, advertising bans, availability and accessibility of smoking-cessation programs and switching of smokers to alternative tobacco products, such as e-cigs, IQOS and other heat-not-burn tobacco products, among other factors (35,36,74).
Reports released in January–March 2021 indicate that the decades-long decline in USA cigarette sales has halted during the pandemic of COVID-19 (75,76). Data from US Treasury Department show that cigarette sales increased 1% in 2020 after dropping 4–5% each year since 2015 (76). Altria, the largest USA tobacco company, reported 4.9% increase in sales to $6.3 billion in the quarter ended 31 December 2020, compared with $6.0 billion in 2019. Altria’s revenue from cigarette and cigar sales was $5.6 billion in 2020 (75). The rise in cigarette sales during the pandemic might be due to a variety of factors, including pandemic-related stress, anxiety, depression and isolation, among others. A North American Quitline Consortium reported a sharp decline in calls (27%), with ~190 000 fewer Americans who called toll-free smoking-cessation help lines in 2020 compared with the year before (77). The greatest drop of 39% in calls was during the period of April through June 2020 when states enacted widespread lockdowns and strict restrictions to curb the spread of COVID-19 (77). The 1-800-QUIT-NOW line is a national portal that directs callers to state smoking-cessation help lines.
There may have been other barriers for smokers interested in quitting during the pandemic. Non-emergency counseling appointments were canceled during the shutdowns, and loss of jobs for many smokers resulted in termination of employer-provided health insurance plans that often cover (wholly or in part) counseling fees and costs of smoking-cessation medications (76). Furthermore, state and local health departments, which were overwhelmed during the early stages of the pandemic, might have redirected their resources, at least, temporarily and partially, from tobacco control intervention programs to other initiatives and campaigns, exclusively centered on COVID-19 (75,76).
Of significance, the rise in USA tobacco cigarette sales during the pandemic has coincided with a drop in vaping product sales (75,76). Juul Labs, the leader of vaping industry and manufacturer of the most popular e-cigs in the market (20), reported $1.1 billion in sales in the first 9 months of 2020 as compared with $1.9 billion during the same period in 2019 (75). The estimated revenue for Juul Labs in the last quarter of 2020 was $340 million (75). Although the underlying reasons for the observed changes in JUUL’s sales remain to be investigated, concerns about vaping safety, bans on flavored vaping products (78), together with stay-at-home mandates and pandemic-related restrictions, possibly affecting accessibility, purchase and use environment for e-cigs, may deserve special attention.
Altogether, the highly complex relationship between vaping and smoking, which existed pre-COVID-19 era, has further evolved during the pandemic, with multiple factors driving the prevalence of smoking and vaping, in similar or different directions, in diverse populations. The projection for tobacco cigarette and e-cig sales in the USA and around the world for the coming years will depend on a wide range of determinants of which COVID-19 vaccine acceptance and rollout as well as behavioral changes of smokers/vapers post-vaccination are critically important (see COVID-19 vaccination: considerations for tobacco product users).
Tobacco product use frequency and patterns, accessibility and motivation to quit during the pandemic
The pandemic of COVID-19 has had an unprecedented impact on virtually all aspects of daily life, including lifestyle habits, such as substance use, e.g. smoking, vaping and alcohol consumption. A growing body of research shows that prevalence, frequency and patterns of substance use, specifically smoking and vaping, have changed, to different degrees, in various populations following the pandemic of COVID-19 (see Table 1). Concerns about smoking and vaping predisposing to COVID-19 have influenced, to varying extents, tobacco cigarette and e-cig use frequency and patterns as well as motivation to quit. Also, stay-at-home mandates and pandemic-related restrictions, especially on social gatherings, where tobacco product use and sharing of e-cig devices are most common, have varyingly impacted the accessibility, point of purchase and use environment for e-cigs and cigarettes. A critically important target group has been the adolescents. Having to stay at home, mostly with parents during the lockdowns, teens have likely lost some, if not most, of their privacy and freedom for using or sharing tobacco products. Understandably, housebound teens have not been able to practice ‘stealth’ vaping or smoking with peers in classrooms or other school premises as before (20). Also, implementation of the pandemic rules and orders has led to retail store closures, disrupted supply chains and restrictions on movement outside one’s home to purchase tobacco products. This, in turn, has affected, to varying degrees, the availability and accessibility of tobacco products, as well as users’ preference for source and location of purchase (e.g. online suppliers versus vape shops or gas stations). Table 1 summarizes the results of selected studies on vaping and smoking prevalence, use frequency and patterns, product accessibility and motivation to quit before and after the start of COVID-19 pandemic. Detailed description of the studies and discussion of their findings are provided in Supplementary Materials.
Table 1.
Summary results of selected studies on the associations of vaping and smoking prevalence, use frequency and patterns, product accessibility and motivation to quit before and after the start of the pandemic
| Ref. | Study design | Population | Location | Timeframe | Aim(s) | Key findings |
|---|---|---|---|---|---|---|
| Gaiha et al. (2020) JAMA Netw. Open, 3, e2027572 | National cross-sectional online survey | Adolescent and young adult e-cig ever-users, aged 13–24 years (n = 2167) | USA | From 6 May 2020 to 14 May 2020 | To evaluate changes in youth e-cig use, point of purchase and ability to purchase e-cigs without age verification during the pandemic as compared with the pre-pandemic era | - Over half of the respondents reported change in their e-cig use since the beginning of COVID-19 pandemic - Of those who reported on the type of change, nearly one-third quit vaping, another one-third cut down on vaping and the reminder either increased e-cig- or cannabis use or switched to other nicotine or cannabis products - Approximately one-fifth of the respondents reported changes in point of purchase of e-cigs, from retail stores to online sources, another one-fifth reported switching to alternative retail stores and the remainder overwhelmingly reported no change in their point of purchase of e-cigs - Over a quarter of the underage respondents (13–20 years) reported access to e-cigs without age verification - Those who reported higher nicotine dependence and more frequent use of e-cigs were less likely to quit vaping or reduce e-cig use - Compliance with the stay-at-home mandates was significantly associated with quitting vaping or reduced e-cig use - Users of pod-based devices (e.g. JUUL) were less likely to quit vaping or reduce e-cig use |
| Klemperer et al. (2020) Nicotine Tob. Res., 22, 1662–1663 | Cross-sectional web-based survey | Adult dual tobacco cigarette and e-cig users, aged 21 or older (n = 366) | USA | 10 April 2020 | To assess changes in cigarette and e-cig use and motivation to quit due to concerns about COVID-19 | - Participants reported similar and positively correlated concerns that smoking and vaping increased their risk of harm from COVID-19 - Participants reported similar and positively correlated changes in motivation to quit smoking and vaping due to COVID-19 concerns - Participants reported similar and positively correlated changes in use of cigarettes and e-cigs - Nearly a quarter of participants reported decreased access to both cigarettes and e-cigs following the pandemic, half reported no change in access to either product and another quarter reported increased access to both products |
| Sharma et al. (2020) SAGE Open Med., 8, 2050312120965321 | Cross-sectional online survey | Young adults, aged 18–25 years (n = 1018) | USA | April 2020 | To assess changes in substance use and pattern (vaping, smoking, alcohol and/or marijuana use) during the stay-at-home period of COVID-19 (i.e. March–April 2020) | - Over half of all respondents reported a form(s) of substance use, of whom one-third confirmed a change in use pattern during the stay-at-home period - Of those reporting changes in use pattern, 44 and 47% confirmed decreased vaping and smoking, respectively and 69% acknowledged increased alcohol drinking. More than one-third of the respondents reported increased marijuana use, whereas another one-third reported decreased use - The extent of changes in substance use patterns was significantly and directly related to the self-reported levels of anxiety, depression and loneliness. |
Detailed description of the listed studies and discussion of their findings are provided in Supplementary Materials.
COVID-19 diagnosis, prevalence and prognosis among tobacco product users
As discussed in Association of COVID-19 infection, disease course and outcomes with tobacco product use, numerous studies have investigated the incidence, symptom severity and clinical outcomes of COVID-19 among tobacco product users. Table 2 summarizes the results of selected studies on COVID-19 testing, diagnosis and disease outcomes among tobacco product users. Detailed description of the studies and discussion of their findings are provided in Supplementary Materials. Although we await definitive conclusions on the relative risk of COVID-19 infection in tobacco product users, the evidence is roundly growing on the severity of the disease in smokers as compared with nonsmokers (37,38). Evaluation of the overall published studies indicates that there is a need for high-quality investigations with improved study designs, rigorous methodologies and high statistical power, which can generate reproducible and conclusive results. Ideally, these investigations should use accurate diagnostic tests, clinical examination, histopathology and imaging analysis to confirm cases of the disease, courses of its progress and outcomes, while also validating smoking/vaping status by biochemical assays. Completion of these studies should help definitively demonstrate the influence of tobacco cigarette and e-cig use in COVID-19 infection, transmission and clinical outcomes, as well as uncover the mechanistic interactions between these lifestyle factors and the course, severity and prognosis of this illness.
Table 2.
Summary results of selected studies on COVID-19 testing, diagnosis and disease outcomes among tobacco product users
| Ref. | Study design | Population | Location | Timeframe | Aim(s) | Key findings |
|---|---|---|---|---|---|---|
| Li et al. (2020) Prev. Med. Rep., 20, 101254 | Integrated national surveys and online COVID-19 repository data | Residents of 34 US states | USA | From 21 January 2020 to 25 April 2020 | To assess associations between vaping prevalence and number of COVID-19 cases and deaths at the state level | - The proportion of vapers was positively and significantly associated with daily number of COVID-19 cases and deaths in each state, after adjustment for proportion of smokers and other relevant confounding factors - With every 1% increase in weighted proportion of vapers in each state, the number of COVID-19 cases increased by 0.3139 and the number of COVID-19 deaths increased by 0.3730 in log scale in each US state |
| Gaiha et al. (2020) J. Adolesc. Health, 67, 519–523 | National cross-sectional online survey | Adolescents and young adults, aged 13–24 years (n = 4351) | USA | From 6 May 2020 to 14 May 2020 | To assess associations between youth vaping and smoking and self-reported COVID-19 testing and diagnosis | - Exclusive vapers were 2.6 times more likely to be tested for COVID-19, and 5 times more likely to be diagnosed with COVID-19, after adjustment for relevant confounding factors - Dual users (vapers and smokers) were nine times more likely to be tested for COVID-19, and seven times more likely to be diagnosed with COVID-19, after adjustment for relevant confounding factors |
| Tattan-Birch et al. (2021) Addiction, 116, 1186–1195 | National cross-sectional household survey (phone interviews with one household member) | Residents of UK, aged 18 years or older (n = 3179) | UK | From April 2020 to May 2020 | To assess associations between smoking status, e-cig use and NRT use, and self-reported COVID-19 diagnosis To find the relationship between COVID-19 diagnosis and quit attempts for smoking or vaping |
- Current smokers and long-term ex-smokers (>1 year), but not vapers or NRT users, were more likely to report COVID-19 diagnosis as compared with never smokers, after controlling for relevant confounding factors - Approximately, 1 in 12 smokers and 1 in 11 vapers reported attempt to quit smoking and vaping, respectively, in the past 3 months due to concerns about COVID-19 |
Detailed description of the listed studies and discussion of their findings are provided in Supplementary Materials.
COVID-19 vaccination: considerations for tobacco product users
A pre-print study (79) has reported that smokers and vapers are more likely than nonsmokers to believe that smoking/vaping has ‘no impact’ or ‘decreases risk’ for severe COVID-19 symptoms. It is plausible that if tobacco product users become aware of a ‘claimed’ protective effect of smoking/vaping against COVID-19, and believe it to be true, they may feel a sense of enhanced immunity, which could make them less likely to take up a vaccine when offered. This might elevate their risk for contracting COVID-19, especially those who have a pre-existing comorbidity/comorbidities. At the same time, it could make it more difficult to achieve the vaccination coverage required for population immunity. Complicating matters further, smokers (by definition) are less likely to accept public health advice, and more prone to poor health choices, such as hesitancy for vaccination (e.g. against influenza), when compared with nonsmokers (80). As discussed in COVID-19 and tobacco product use: a protective role for smoking/vaping?, transparency and disclosure of competing interests for researchers claiming protection by smoking against COVID-19 (28) should not only facilitate non-biased evaluation of such claims but also boost public trust and willingness for considering COVID-19 vaccination.
In a population survey of UK adults (n = 29 148), current smokers reported the greatest levels (and never smokers the lowest levels) of mistrust in the benefit of vaccines, worries about unforeseen future effects, concerns about commercial profiteering and preference for natural immunity (81). When asked whether they would take up the offer of a COVID-19 vaccine when one becomes available, current smokers were the most likely to report being uncertain or unwilling, with just 51% reporting an intention to vaccinate, compared with 66% of former smokers and never smokers. These differences were independent of age, gender, ethnicity, income, key worker status or chronic physical health conditions. Of note, the data for this survey were collected during the period of 7 September to 5 October 2020, which precedes the reports of positive results for COVID-19 vaccine trials and the announcement of first vaccine being approved for use in the UK (81). With a disproportionately large number of smokers having comorbidities predisposing to COVID-19 (10), and also belonging to disadvantaged socioeconomic groups that have been hit hardest by the pandemic (82), vaccination hesitancy could further exacerbate the existing health disparities among tobacco product users.
Importantly, smokers/vapers are inclined to cluster in social networks whose members’ attitudes toward health choices could be influenced by other members (83). It is prudent to adequately address issues related to vaccine hesitancy in the general population, although tobacco product users may require ‘targeted’ interventions and educational campaigns. These efforts should raise awareness of the greater benefits of vaccination against COVID-19 than any claimed protective effect by smoking against this illness. The accumulating data on the safety and excellent efficacy of COVID-19 vaccines against severe disease, hospitalization and death (84) should persuade smokers, particularly those with pre-existing health conditions who are more vulnerable to those outcomes (10), to take up a vaccine when it becomes available.
Concluding remarks
At this point in the pandemic, the evidence on the association of tobacco product use and COVID-19 infection, disease course and clinical outcomes is ‘incomplete’ but rapidly growing. So is the knowledge on the mechanistic involvement of smoking and vaping in the pathophysiology of COVID-19. Nearly 15 months into the pandemic, we have learned that comorbidities caused by or associated with tobacco product use are risk factors for COVID-19 infection, severe symptoms and poor disease outcomes (10). At the same time, we have begun to understand the associations between smoking and vaping and COVID-19 disease course and clinical outcomes. But we still have much to learn about the mechanistic involvement of tobacco product use in the pathophysiology of this disease. Although we await fully characterizing the smokers’ risk for COVID-19, it is becoming increasing clear that should smokers get the disease, they are more likely to have serious consequences (37,38). For now, the ‘take home message’ of this review is to mitigate risk of developing health conditions known to predispose to COVID-19 and to minimize chances of having severe symptoms and poor prognosis if/when infected with COVID-19, smokers and possibly vapers should seriously consider quitting or, at least, reduce their tobacco product use. Lastly, we should be aware of the conflicts of interest and the role of the tobacco industry in claims surrounding COVID-19 and smoking (28), which create the illusion that there is more controversy on some points than there actually is in the non-conflicted literature.
Supplementary Material
Acknowledgements
I would like to thank Dr Stella Tommasi, PhD, for critically reading this manuscript and providing invaluable comments. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report or in the decision to submit for publication.
Glossary
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- ARDS
acute respiratory distress syndrome
- AT II
alveolar epithelial type II
- CDC
Centers for Disease Control and Prevention
- COPD
chronic obstructive pulmonary disease
- COVID-19
coronavirus disease 2019
- CVD
cardiovascular disease
- e-cig
electronic cigarette
- EMT
epithelial to mesenchymal transition
- EndMT
endothelial to mesenchymal transition
- EVALI
e-cig, or vaping, product use-associated lung injury
- FDA
Food and Drug Administration
- nAChR
nicotinic acetylcholine receptor
- NRT
nicotine replacement therapy
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- THC
tetrahydrocannabinol
- VEA
vitamin E acetate
- WHO
World Health Organization
Funding
National Institute of Dental and Craniofacial Research of the National Institutes of Health (1R01DE026043); University of California Tobacco-Related Disease Research Program (28IR-0058 and T31IR-1839 to A.B.).
Conflict of Interest Statement: None declared.
Author’s contributions
A.B.: conceived the study, performed literature search and wrote the manuscript.
References
- 1.World Health Organization (WHO) (2019) Who Report on the Global Tobacco Epidemic 2019: Offer Help to Quit Tobacco Use. https://www.who.int/tobacco/global_report/en/ (18 June 2021, date last accessed).
- 2.US Department of Health and Human Services (2018) Surgeon General’s Advisory on E-Cigarette Use Among Youth. https://e-cigarettes.surgeongeneral.gov/documents/surgeon-generals-advisory-on-e-cigarette-use-among-youth-2018.pdf (18 June 2021, date last accessed).
- 3.Besaratinia, A., et al. (2020) Vaping epidemic: challenges and opportunities. Cancer Causes Control, 31, 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration (FDA) (2018) FDA Takes New Steps to Address Epidemic of Youth E-Cigarette Use, Including a Historic Action Against More Than 1,300 Retailers and 5 Major Manufacturers for Their Roles Perpetuating Youth Access. https://www.fda.gov/news-events/press-announcements/fda-takes-new-steps-address-epidemic-youth-e-cigarette-use-including-historic-action-against-more (11 September 2018, date last accessed).
- 5.Cucinotta, D., et al. (2020) WHO declares COVID-19 a pandemic. Acta Biomed., 91, 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.JHU CSSE COVID-19 Data. (2020) COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://github.com/CSSEGISandData/COVID-19 (18 June 2021, date last accessed).
- 7.Centers for Disease Control and Prevention (CDC). (2019). Outbreak of Lung Injury Associated With E-Cigarette Use, or Vaping. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (18 June 2021, date last accessed).
- 8.Wiersinga, W.J., et al. (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA, 324, 782–793. [DOI] [PubMed] [Google Scholar]
- 9.Fennelly, K.P. (2020) Particle sizes of infectious aerosols: implications for infection control. Lancet. Respir. Med., 8, 914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). (2020) COVID-19: People With Certain Medical Conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (18 June 2021, date last accessed).
- 11.Mehta, P., et al. ; HLH Across Speciality Collaboration, UK (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet, 395, 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta, A., et al. (2020) Extrapulmonary manifestations of COVID-19. Nat. Med., 26, 1017–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, T., et al. (2020) Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet, 395, e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowley, A.H. (2020) Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol., 20, 453–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders, J.M., et al. (2020) Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA, 323, 1824–1836. [DOI] [PubMed] [Google Scholar]
- 16.Murthy, S., et al. (2020) Care for critically ill patients with COVID-19. JAMA, 323, 1499–1500. [DOI] [PubMed] [Google Scholar]
- 17.Chageux, J.P., et al. (2020) A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. Qeios. FXGQSB. Preprint. [DOI] [PubMed] [Google Scholar]
- 18.Miyara, M., et al. (2020) Low rate of daily active tobacco smoking in patients with symptomatic COVID-19. Qeiso. WPP19W. Preprint. [Google Scholar]
- 19.Majmundar, A., et al. (2020) Public health concerns and unsubstantiated claims at the intersection of vaping and COVID-19. Nicotine Tob. Res., 22, 1667–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besaratinia, A., et al. (2021) The consequential impact of JUUL on youth vaping and the landscape of tobacco products: the state of play in the COVID-19 era. Prev. Med. Rep., 22, 101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) (2020) Tobacco Users May Be at an Increased Risk of #COVID19, Both in Contracting the Disease and Complications. https://twitter.com/WHO_Europe/status/1257255102634745857 (4 May 2020, 3:25 a.m., date posted) (18 June 2021, date last accessed).
- 22.World Health Organization (WHO) (2020) WHO Statement: Tobacco Use and COVID-19. https://www.who.int/news/item/11-05-2020-who-statement-tobacco-use-and-covid-19 (11 May 2020, date last accessed).
- 23.Farsalinos, K., et al. (2020) Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern. Emerg. Med., 15, 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagoumintzis, G., et al. (2021) Nicotinic cholinergic system and COVID-19: in silico identification of interactions between α7 nicotinic acetylcholine receptor and the cryptic epitopes of SARS-Co-V and SARS-CoV-2 spike glycoproteins. Food Chem. Toxicol., 149, 112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meini, S., et al. (2020) The Paradox of the low prevalence of current smokers among covid-19 patients hospitalized in non-intensive care wards: results from an Italian multicenter case–control study. Nicotine Tob. Res., ntaa188. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paleiron, N., et al. (2021) Impact of tobacco smoking on the risk of COVID-19. A large scale retrospective cohort study. Nicotine Tob. Res., ntab004. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons, D., et al. (2020) The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: a living rapid evidence review with Bayesian meta-analyses (version 7). Addiction, 116, 1319–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horel, S., et al. (2021) Covid 19: how harm reduction advocates and the tobacco industry capitalised on the pandemic to promote nicotine. BMJ, 373, n1303. [DOI] [PubMed] [Google Scholar]
- 29.Farsalinos, K., et al. (2020) Editorial: nicotine and SARS-CoV-2: COVID-19 may be a disease of the nicotinic cholinergic system. Toxicol. Rep., 7, 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Retraction notice for: “Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory-confirmed COVID-19 cases.” (2021) Theodoros V. Giannouchos, Roberto A. Sussman, José M. Mier, Konstantinos Poulas and Konstantinos Farsalinos. Eur Respir J 2020; in press. Eur. Respir. J., 57, 2002144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vardavas, C.I., et al. (2020) COVID-19 and smoking: a systematic review of the evidence. Tob. Induc. Dis., 18, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippi, G., et al. (2020) Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur. J. Intern. Med., 75, 107–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Changeux, J.P., et al. (2020) A nicotinic hypothesis for Covid-19 with preventive and therapeutic implications. C. R. Biol., 343, 33–39. [DOI] [PubMed] [Google Scholar]
- 34.Tindle, H.A., et al. (2020) Beyond smoking cessation: investigating medicinal nicotine to prevent and treat COVID-19. Nicotine Tob. Res., 22, 1669–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The US Surgeon General (2014) The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/ (18 June 2021, date last accessed). [Google Scholar]
- 36.National Academies of Sciences, Engineering, and Medicine (2018) Public Health Consequences of E-Cigarettes. Washington, DC: National Academies of Sciences, Engineering, and Medicine. 10.17226/24952 [DOI] [Google Scholar]
- 37.Williamson, E.J., et al. (2020) Factors associated with COVID-19-related death using OpenSAFELY. Nature, 584, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patanavanich, R., et al. (2020) Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob. Res., 22, 1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pijls, B.G., et al. (2021) Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open, 11, e044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Letko, M., et al. (2020) Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol., 5, 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caliri, A.W., et al. (2021) Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res., 787, 108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, J.C., et al. (2020) Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev. Cell, 53, 514–529.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, A.C., et al. (2020) Tobacco, but not nicotine and flavor-less electronic cigarettes, induces ACE2 and immune dysregulation. Int. J. Mol. Sci., 21, 5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farsalinos, K., et al. (2020) COVID-19 and the nicotinic cholinergic system. Eur. Respir. J., 56, 2001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farsalinos, K., et al. (2020) Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther. Adv. Chronic Dis., 11, 2040622320935765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lombardi, C., et al. (2021) Smoking and COVID-19, the paradox to discover: an Italian retrospective, observational study in hospitalized and non-hospitalized patients. Med. Hypotheses, 146, 110391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Usman, M.S., et al. (2020) Is there a smoker’s paradox in COVID-19? BMJ Evid. Based Med., bmjebm-2020-111492. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Haddad, C., et al. (2021) Smoking and COVID-19: a scoping review. Tob. Use Insights, 14, 1179173X21994612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eapen, M.S., et al. (2021) Dysregulation of endocytic machinery and ACE2 in small airways of smokers and COPD patients can augment their susceptibility to SARS-CoV-2 (COVID-19) infections. Am. J. Physiol. Lung Cell. Mol. Physiol., 320, L158–L163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai, G., et al. (2020) Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med., 201, 1557–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russo, P., et al. (2020) COVID-19 and smoking: is nicotine the hidden link? Eur. Respir. J., 55, 2001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, Q., et al. (2020) E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: role of nAChR α7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation. Respir. Res., 21, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McAlinden, K.D., et al. (2021) Electronic cigarette aerosol is cytotoxic and increases ACE2 expression on human airway epithelial cells: implications for SARS-CoV-2 (COVID-19). J. Clin. Med., 10, 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huertas, A., et al. (2020) Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J., 56, 2001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abouhashem, A.S., et al. (2020) Is low alveolar type II cell SOD3 in the lungs of elderly linked to the observed severity of COVID-19? Antioxid. Redox Signal., 33, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caliri, A.W., et al. (2020) Hypomethylation of LINE-1 repeat elements and global loss of DNA hydroxymethylation in vapers and smokers. Epigenetics, 15, 816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tommasi, S., et al. (2019) Deregulation of biologically significant genes and associated molecular pathways in the oral epithelium of electronic cigarette users. Int. J. Mol. Sci., 20, 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ackermann, M., et al. (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med., 383, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kovacic, J.C., et al. (2019) Endothelial to mesenchymal transition in cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol., 73, 190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaikwad, A.V., et al. (2020) Endothelial to mesenchymal transition (EndMT) and vascular remodeling in pulmonary hypertension and idiopathic pulmonary fibrosis. Expert Rev. Respir. Med., 14, 1027–1043. [DOI] [PubMed] [Google Scholar]
- 61.Eapen, M.S., et al. (2020) Endothelial to mesenchymal transition: a precursor to post-COVID-19 interstitial pulmonary fibrosis and vascular obliteration? Eur. Respir. J., 56, 2003167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selman, M., et al. (2006) Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc. Am. Thorac. Soc., 3, 364–372. [DOI] [PubMed] [Google Scholar]
- 63.Sohal, S.S. (2017) Epithelial and endothelial cell plasticity in chronic obstructive pulmonary disease (COPD). Respir. Investig., 55, 104–113. [DOI] [PubMed] [Google Scholar]
- 64.Poissy, J., et al. ; Lille ICU Haemostasis COVID-19 Group (2020) Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation, 142, 184–186. [DOI] [PubMed] [Google Scholar]
- 65.Morin, L., et al. (2021) Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA, 325, 1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wynn, T.A. (2011) Integrating mechanisms of pulmonary fibrosis. J. Exp. Med., 208, 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsumoto, S., et al. (2020) Dose-dependent pulmonary toxicity of aerosolized vitamin E acetate. Am. J. Respir. Cell Mol. Biol., 63, 748–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kleinman, M.T., et al. (2020) E-cigarette or vaping product use-associated lung injury produced in an animal model from electronic cigarette vapor exposure without tetrahydrocannabinol or vitamin E oil. J. Am. Heart Assoc., 9, e017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kazachkov, M., et al. (2020) Diagnosis of EVALI in the COVID-19 era. Lancet Respir. Med., 8, 1169–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hassoun, A., et al. (2021) Vaping-associated lung injury during COVID-19 multisystem inflammatory syndrome outbreak. J. Emerg. Med., 60, 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ansari-Gilani, K., et al. (2020) COVID-19 pneumonia versus EVALI, distinguishing the overlapping CT features in the COVID-19 era. Heart Lung, 49, 885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kichloo, A., et al. (2020) Habit mimics the illness: EVALI during the era of the COVID-19 pandemic. J. Investig. Med. High Impact Case Rep., 8, 2324709620972243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention (CDC) (2019) Current Cigarette Smoking Among Adults in the United States. Smoking & Tobacco Use. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm (18 June 2021, date last accessed).
- 74.Hartmann-Boyce, J., et al. (2020) Electronic cigarettes for smoking cessation. Cochrane Database Syst. Rev., 10, CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maloney, J. (2021) Smoking’s long decline is over. The Wall Street Journal. https://www.google.com/amp/s/www.wsj.com/amp/articles/during-covid-19-lockdowns-people-went-back-to-smoking-11611829803 [Google Scholar]
- 76.Alltucker, K. (2021) Cigarette sales increased during pandemic as fewer smokers sought help quitting. USA Today. https://www.google.com/amp/s/amp.usatoday.com/amp/4664323001 [Google Scholar]
- 77.North American Quitline Consortium (2021) In Bailey, L. et al. (eds) Report on the Impact of the COVID-19 Pandemic on Smoking Cessation. North American Quitline Consortium, Phoenix, AZ.https://cdn.ymaws.com/www.naquitline.org/resource/resmgr/reports-naqc/report_impact__of_covid-19_p.pdf [Google Scholar]
- 78.Besaratinia, A., et al. (2019) Vaping: a growing global health concern. EClinicalMedicine, 17, 100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herbec, A., et al. (2020) PsyArXiv. doi: 10.17605/OSF.IO/392SP, 1–17. Preprint. [DOI] [Google Scholar]
- 80.Lu, P.J., et al. (2013) Influenza A (H1N1) 2009 monovalent and seasonal influenza vaccination among adults 25 to 64 years of age with high-risk conditions—United States, 2010. Am. J. Infect. Control, 41, 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jackson, S.E., et al. (2021) Negative vaccine attitudes and intentions to vaccinate against Covid-19 in relation to smoking status: a population survey of UK adults. Nicotine Tob. Res., ntab039. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patel, J.A., et al. (2020) Poverty, inequality and COVID-19: the forgotten vulnerable. Public Health, 183, 110–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christakis, N.A., et al. (2008) The collective dynamics of smoking in a large social network. N. Engl. J. Med., 358, 2249–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baden, L.R., et al. ; COVE Study Group. (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med., 384, 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.