Abstract
Fibrosis is a major pathway to organ injury and failure, accounting for more than one-third of deaths worldwide. Intestinal fibrosis causes irreversible and serious clinical complications, such as strictures and obstruction, secondary to a complex pathogenesis. Under the stimulation of profibrotic soluble factors, excessive activation of mesenchymal cells causes extracellular matrix deposition via canonical transforming growth factor-β/Smads signaling or other pathways (eg, epithelial-to-mesenchymal transition and endothelial-to-mesenchymal transition) in intestinal fibrogenesis. In recent studies, the importance of noncoding RNAs (ncRNAs) stands out in fibrotic diseases in that ncRNAs exhibit a remarkable variety of biological functions in modulating the aforementioned fibrogenic responses. In this review, we summarize the role of ncRNAs, including the emerging long ncRNAs and circular RNAs, in intestinal fibrogenesis. Notably, the translational potential of ncRNAs as diagnostic biomarkers and therapeutic targets in the management of intestinal fibrosis is discussed based on clinical trials from fibrotic diseases in other organs. The main points of this review include the following:
• Characteristics of ncRNAs and mechanisms of intestinal fibrogenesis
• Wide participation of ncRNAs (especially the emerging long ncRNAs and circular RNAs) in intestinal fibrosis, including transforming growth factor-β signaling, epithelial-to-mesenchymal transition/endothelial-to-mesenchymal transition, and extracellular matrix remodeling
• Translational potential of ncRNAs in the diagnosis and treatment of intestinal fibrosis based on clinical trials from fibrotic diseases in other organs
Keywords: intestinal fibrosis, Crohn’s disease, noncoding RNA, extracellular matrix
BACKGROUND
Fibrosis stands out as a global medical challenge accounting for more than one-third of deaths worldwide.1 Specifically, intestinal fibrosis is a common and refractory pathology that leads to bowel strictures, perforation, fistula formation, and organ failure in many alimentary diseases, such as inflammatory bowel disease (IBD) and radiation enteritis. For example, approximately 50% of patients with Crohn's disease (CD) develop clinically relevant strictures and fistulas, and experience a nearly lifetime risk of surgery and heavy cost burden.2 More than two-thirds of patients with CD have endoscopic recurrence at 1 year, and nearly half still need treatment at 4 years.3 Nevertheless, there are few effective and reliable methods of identifying the early stages of fibrosis or reversing existing intestinal strictures. Current studies mainly focus on the sophisticated network of intestinal fibrosis including inflammatory cascades, extracellular matrix (ECM), profibrotic mediators, and gut microbiota. Thus far, noncoding RNAs (ncRNAs) have been proved to participate in the fibrotic diseases of multiple organs (eg, liver diseases, myocardial fibrosis, and renal fibrosis). The ncRNAs involved in fibrotic diseases mainly consist of microRNAs (miRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs). NcRNAs modulate the function of mesenchymal cells, inflammatory cascades, ECM, and microbiota via mechanisms of endogenous RNA competition, RNA transcription regulation, protein sponges, and translation regulation.4-6 In this review, we introduce the complicated roles of various ncRNAs, including the emerging lncRNAs and circRNAs, in intestinal fibrosis and explore their clinical value as biomarkers and therapeutic targets.
PATHOGENESIS OF INTESTINAL FIBROSIS
Similar to the fibrogenesis of other organs, intestinal fibrosis is triggered by autocrine and paracrine factors, pathogen- or damage-induced inflammation, and subsequent dysregulation of bowel mucosal healing.7 As a crucial factor in intestinal fibrogenesis, mesenchymal cells (eg, fibroblasts, myofibroblasts, and smooth muscle cells) are activated by multiple profibrotic soluble factors.8 Transforming growth factor-β (TGF-β), a major cytokine in intestinal fibrosis, is mainly secreted by macrophages in response to interleukin-4 (IL-4) and IL-13.9 Research has shown that TGF-β can transdifferentiate α-smooth muscle actin (α-SMA)-negative fibroblasts into α-SMA-positive myofibroblasts10 and activate the proliferation, migration, and contraction of myofibroblasts by a series of signaling pathways, including Smad2/3/4, ERK/JNK/p38/AKT, and rho/ROCK/actin/MRTF/SRF.11-14
In addition, other profibrotic cytokines, such as connective tissue growth factor (CTGF), platelet-derived growth factor, fibroblast growth factor, insulin-like growth factor (IGF), endothelin, IL-36, and tumor necrosis factor-like cytokine 1A (TL1A) also promote myofibroblast proliferation and ECM production.15-20 Because the balance between ECM production and degradation is disrupted, collagen-rich ECM is produced and excessively accumulates via fibrogenic responses,9 along with a significant upregulation of the collagens fibronectin and tenascin C,21 thereby ultimately leading to the pathologic thickening of all layers of the intestinal wall from the mucosa to the muscularis propria.
Meanwhile, microbiota dysbiosis is also associated with intestinal fibrosis. Gut infection by pathogens (eg, adherent-invasive Escherichia coli and Salmonella typhi), contributes to the pathogenesis of intestinal fibrosis.22 In bacterial infection, flagellin binds to Toll-like receptor 5 (TLR5) of the intestinal epithelium, induces the expression of IL-33 and its receptor, and therefore promotes IL-13 and TGF-β.23 The flagellin-induced MyD88 activation elicits increased collagen I and fibronectin production in intestinal myofibroblasts, and MyD88 deletion in α-SMA-positive cells alleviates fibrosis in a mouse model of chronic colitis.24
Epithelial-to-mesenchymal transition (EMT) is another potential fibrogenic mechanism in intestinal fibrosis in that it promotes epithelial-derived fibroblasts and ECM deposition in the fibrogenesis of many organs. Although the occurrence of EMT in intestinal fibrosis has been proved, its functional mechanism is still warranted.25
ncRNAS
The MiRNAs, lncRNAs, and circRNAs are the major entities of ncRNAs. Most of them selectively bind to other nucleic acids by base pairing and regulate gene transcription, RNA processing, and translation in various pathophysiological processes such as fibrosis.26 Defined as small fragments of RNA that comprise 20~25 nucleotides, miRNAs bind to the complementary sequences of targeted mRNAs and degrade them via cleavage, destabilization, or inhibition of mRNA translation, which finally represses the expression of target genes.27 NcRNAs with >200 nucleotides are classified as lncRNAs, which not only control gene transcription but also modulate regulate mRNA processing, stability, and translation via posttranscriptional regulation by acting as sponges for miRNAs or sources of other small RNAs.28 As another subclass of ncRNAs, circRNAs are generally produced by backsplicing and are highly stable, resulting from the formation of a covalently closed loop. CircRNAs have a similar function to lncRNAs, such as sponging miRNAs, sequestering RNA-binding proteins, and regulating mRNA transcription.29
Until now, many studies have focused on the relationship between ncRNAs and CD by means of high-throughput sequencing and microarray.30,31 Some ncRNAs have recently been developed as biomarkers of CD (eg, miR-146b-5p).30 Research has reported that ncRNAs participate in the inflammatory response by modulating the relevant cytokines or chemokines, activation, and differentiation of immune cells (eg, Th1 and Th17 cells).32,33 On the other hand, ncRNAs regulate tight junctions (eg, the claudin family) of the intestinal epithelium, mucus barrier, and immune homeostasis, therefore widely manipulating intestinal epithelial barrier function.34 In addition, the significance of ncRNAs in gut microbiota and fibrogenesis is gradually unveiled in the etiology of CD. In this review, we mainly elucidate the importance of miRNAs, along with lncRNAs and circRNAs, in the process of intestinal fibrosis in CD (Fig. 1).
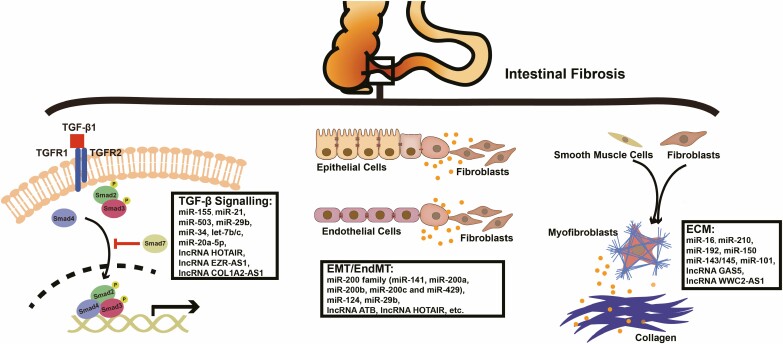
FIGURE 1.
Schematic diagram of ncRNAs involved in intestinal fibrosis. In intestinal fibrogenesis, excessive activation of mesenchymal cells causes ECM deposition via canonical TGF-β/Smads signaling or other pathways (eg, EMT/EndMT), and ncRNAs contribute to the aforementioned mechanisms.
DYSREGULATION OF NCRNAS IN INTESTINAL FIBROSIS
In intestinal fibrotic diseases, ncRNAs are often dysregulated. Lewis et al35 compared the serum level of 372 miRNAs of patients with stricturing CD (defined as Montreal criteria, n = 6) with those of patients with nonstricturing CD (n = 11) and healthy control patients (n = 5) and detected 94 differentially expressed miRNAs, such as miR-19-3p (miR-19a-3p and miR-19b-3p), miR-29a-3p, and miR-29c-3p. In accordance with the results of the aforementioned miRNA serum array, decreased levels of miR-19-3p and the miR-29 family have been further verified in the serum or tissues of intestinal strictures in patients with CD.35,36 Similarly, Zhou, Liang, et al37 analyzed the differential expression of lncRNAs in tissue samples from patients with radiation-induced intestinal fibrosis and reported that 76 lncRNAs (54 upregulated and 22 downregulated) exhibited 10-fold or more differences in comparison with nonradiation-induced intestinal fibrosis controls, such as lncRNA WWC2-AS1, lncRNA RP1-65 J11·1, lncRNA XLOC-004117, and lncRNA RP11-63P12·7. The changes of miRNA and lncRNA expression profiles suggest their underlying roles in modulating fibrogenic responses in different types of intestinal fibrotic diseases.
ncRNAs in TGF-β Signaling Modulation
TGF-β signaling modulates a wide spectrum of biological processes, such as tumor metastasis, tissue fibrosis, immune response, and cell proliferation and differentiation.38 Because TGF-β signaling not only alleviates inflammation but also drives organ fibrosis,39 miRNAs modulating TGF-β signaling are found dysregulated in inflammatory diseases (eg, miR-4448) and fibrotic diseases (eg, miR-21).38,40 For example, miR-155 increases in the inflamed duodenal mucosa and inhibits TGF-β signaling by targeting and downregulating Smad2 in inflammation.41,42 However, it decreases in the primary duodenal fibroblasts of pediatric patients with CD under TGF-β stimulation.41 The dual function of miR-155 partially reveals the sophisticated modulating network of ncRNAs in inflammation and fibrosis by modulating TGF-β signaling.
In canonical TGF-β signaling (Smad-dependent pathways), TGF-β triggers the phosphorylation of Smad2 and Smad3 by binding to TGF-β receptor (TGFBR) 1. Smad4 binds phosphorylated Smad2/3 and enables the nuclear translocation of the Smad2/3 complex, therefore activating the transcription of fibrosis-relevant genes. Smad7 competes with the Smad2/3 complex for TGFBR1 and exerts negative regulation on TGF-β signaling.38 As important transcription factors of TGF-β signaling, Smads are often targeted by ncRNAs. When treated with TGF-β, miR-21 expression is elevated in fibroblasts and epithelial cells depending on phosphorylated-Smad2/Smad3.43-45 MiR-21 also directly targets Smad7 and increases collagen expression in TGF-β activation,46,47 whereas the miR-21/Smad7 pathway can be further regulated by lncRNA COL1A2-AS1.48
Other ncRNAs have also been proven to manipulate every step of TGF-β signaling. MiR-503 modulates Smad2 differently because it mediates the ubiquitination of Smad2. It is known to upregulate Smad2 by directly targeting Smad ubiquitin regulatory factor 2, an E3 ubiquitin ligase that promotes the ubiquitination and degradation of phosphorylated Smad2.49 The miR-503-induced activation of TGF-β/Smad2 signaling further promotes downstream CTGF and collagen production.49,50 The antifibrotic role of miR-29b is attributed to its inhibition of the phosphorylation of Smad3 and the expression of collagen I and collagen III via the Sp1/TGF-β1/Smad/CTGF pathway.36,51 Different from miR-29b, lncRNA HOX transcript antisense RNA (HOTAIR) targets and downregulates the antifibrotic factor peroxisome proliferator-activated receptor γ (PPARγ) in fibrosis because it antagonizes Smad3 and interferes with TGF-β signaling.52,53 Regarding Smad4, miR-34 upregulation provides positive feedback under TGF-β stimulation and reciprocally activates TGF-β signaling by upregulating Smad4.54 Nevertheless, the function of miR-34 in fibrosis may be tissue-specific because the miR-34 downregulation triggered by lncRNA HOTAIR de-represses Notch signaling and elicits the enhanced expression of collagen I and α-SMA in dermal fibroblasts.55 In addition, the TGF-β receptor is another important target site for ncRNAs to modulate TGF-β signaling. Downregulated miR-20a-5p leads to the de-repression of TGFBR2 and activates TGF-β signaling in fibrogenesis56; Similarly, let-7b/c targets TGFBR1 and downregulates TGF-β signaling.57
Potential Involvement of ncRNAs in EMT and Endothelial-to-Mesenchymal Transition
Research has shown that EMT is a common pathological process of cellular transdifferentiation in fibrosis and cancer. Through EMT, epithelial cells acquire mesenchymal features, such as fibroblast-like morphology, downregulated epithelial markers (eg, E-cadherin, tight junction, and cytoskeleton proteins), upregulated mesenchymal markers (eg, α-SMA, vimentin, and collagens), and upregulated EMT transcription factors (eg, Twist, Snail, Slug, and zinc finger E-box binding homebox 1/2).25 The Wnt/β-catenin pathway is one of the most important signaling pathways that positively modulates the transcription of EMT-promoting genes.58 Because accumulating evidence has revealed the contribution of EMT to ECM deposition, EMT is believed to play a role in intestinal fibrogenesis.59 As a special form of EMT, endothelial-to-mesenchymal transition (EndMT) refers to the transdifferentiation of endothelium into mesenchymal cells, which exhibits a loss of endothelial markers and an upregulation of transcription factors and mesenchymal markers similar to EMT.60
Mainly modulating EMT/EndMT transcription factors, the miR-200 family takes on an antifibrotic role in intestinal fibrosis. Members of this family (miR-141, miR-200a, miR-200b, miR-200c, and miR-429) are all downregulated in the stricture-overlying mucosa of patients with CD.61,62 They inhibit TGF-β-induced EMT/EndMT by targeting zinc finger E-box binding homeobox 1 and 2,63–67 whereas lncRNA activated by TGF-β (lncRNA ATB) abrogates the antifibrotic function of the miR-200 family.68,69 MiR-200b-3p regulates microfibrial-associated glycoprotein 2 and the downstream expression of Slug, Snail, matrix metalloproteinase (MMP)-2, and MMP-9.70 The regulatory role of miR-200b has been further verified in in vivo experiments: miR-200b-containing microvesicles alleviate 2,4,6-Trinitrobenzenesulphonic acid–induced intestinal fibrosis in rats by inhibiting EMT.71 Because it plays an inhibitory role in EndMT, miR-200a may directly decrease the expression of growth factor receptor-bound 2.72
Different from the miR-200 family, lncRNA HOTAIR acts extensively in EMT and fibrosis mainly by regulating the expression of epithelial/mesenchymal markers and the Wnt/β-catenin pathway. Under the stimulation of TGF-β, HOTAIR targets antifibrotic miR-124 and upregulates Notch1 signaling, resulting in increased α-SMA, MMP-2, and MMP-9 in vitro.73,74 In addition, HOTAIR maintains the expression of IGF2 binding protein 2, therefore promoting IGF signaling–induced EMT.75 Furthermore, HOTAIR has an indirect function in the epigenetic regulation of fibrosis by inhibiting miR-29b because miR-29b is identified as targeting DNA methyltransferases in the methylation of EMT-relevant genes.76,77 MiR-29b-3p targets progranulin, a Wnt/β-catenin-signaling downstream adaptor, and significantly increases E-cadherin expression but downregulates vimentin and Snail.78 Other ncRNAs also form the sophisticated modulating network of EMT/EndMT and are listed in Table 1.
TABLE 1.
Potential Targets and Mechanisms of ncRNAs in EMT/EndMT
| Changes in EMT/EndMT | ncRNA | Target | Organ or Cell | Mechanism | Reference |
|---|---|---|---|---|---|
| EMT | |||||
| Down | miR-101 | ZEB1 | Liver | Suppresses ZEB1-induced EMT | 79 |
| Down | miR-147 | ZEB1 | Colon and lung | Reverses ZEB1-induced EMT and represses AKT phosphorylation | 80 |
| Down | miR-186-5p | ZEB1 | Colon | Reduces ZEB1 expression | 81 |
| Down | miR-205-5p | ZEB1 | Colon | Directly targets ZEB1 and raises E-cadherin and CDH1 levels | 82 |
| Down | miR-132 | ZEB2 | Colon | Suppresses ZEB2 expression | 83 |
| Down | miR-3653 | ZEB2 | Colon | Down-regulates ZEB2 level | 84 |
| Down | miR-34 | SNAIL | Colon, breast, and lung | Targets SNAIL and key SNAIL regulators (eg, CTNNB1, LEF1, and Axin2) | 85 |
| Down | miR-203 | TGF-β2, SNAIL | Colon | Reduces TGF-β2 and SNAIL expression | 86 |
| Down | miR-451 | SNAIL | Colon | Suppresses SNAIL expression | 87 |
| Down | miR-17~92 | CTNNB1 | Colon | Inhibits Wnt/β-catenin signaling and EMT | 88 |
| Down | miR-371-5p | SOX2 | Colon | Directly targets SOX2 and represses Wnt/β-catenin signaling | 89 |
| Down | miR-378 | SDAD1 | Colon | Directly targets SDAD1 and represses Wnt/β-catenin signaling | 90 |
| Down | miR-503 | p85 | Lung | Attenuates EMT by inhibiting PI3K/Akt/mTOR/Snail pathway | 91 |
| Up | lncRNA TUG1 | TWIST1 | Colon | Participates in the positive loop of TGF-β/TUG1/TWIST1 and activates EMT pathway | 92,93 |
| Up | lncRNA MALAT1 | TWIST, SNAIL, SLUG | Colon | Sponges miR-126-5p and promotes EMT genes (eg, SNAIL, SLUG, and TWIST) | 94,95 |
| Up | lncRNA SNHG15 | SLUG | Colon | Directly interacts with SLUG and impedes ubiquitination-induced SLUG degradation | 96 |
| Up | miR-675-5p | DDB2 | Colon | Targets DDB2, an EMT repressor of VEGF, ZEB1, and SNAIL | 97 |
| Up | lncRNA ZFAS1 | ZEB1 | Colon | Upregulates ZEB1 and induces EMT | 98 |
| Up | lncRNA CYTOR | CTNNB1 | Colon | Enables β-catenin nuclear translocation by impeding phosphorylation and is reciprocally upregulated by β-catenin activation, finally activates c | 99 |
| Up | lncRNA TCF7 | Unknown | Colon | Activates Wnt/β-catenin signaling | 100 |
| Up | lncRNA CASC21 | Unknown | Colon | Activates Wnt/β-catenin signaling | 101 |
| Up | hsa_circRNA_102610 | SMAD4 | Colon | Sponges miR-130a-3p and promotes TGF-β1-induced EMT | 102 |
| EndMT | |||||
| Down | miR-148b | TGFBR2, SMAD2 | Skin | Inhibits EndMT via diretly targeting TGFBR2 and Smad2 | 103 |
| Down | miR-18a-5p | NOTCH2 | Heart | Downregulates α-SMA, fibronectin, and vimentin via binding to Notch2 | 104 |
| Down | lncRNA H19 | Unknown | Retina | Suppresses TGF-β1-mediated EndMT through MAPK-ERK1/2 pathway | 105 |
| Down | let-7 | Unknown | Endothelial cells | Anti-EndMT effects | 106 |
| Up | lncRNA MALAT1 | TGFBR2, SMAD3 | Endothelial progenitor cells and human umbilical vein endothelial cells | Regulates TGFBR2 and Smad3 via sponging miR-145 and therefore modulates TGF-β1-induced EndMT of EPCs | 107,108 |
| Promotes nuclear translocation of CTNNB1 and activates Wnt/β-catenin signaling | |||||
| Up | lncRNA TUG1 | ATG5 | Liver | Sponges miR-142-3p and promotes autophagy and EndMT by upregulating ATG5 | 109 |
| Up | miR-199a-5p | Unknown | Human umbilical vein endothelial cells | Promotes radiation-induced EndMT and subsequent transdifferentiation of fibroblasts into myofibroblasts | 110 |
| Up | miR-21 | Unknown | Endothelial cells | Activates endothelial Akt pathway and increases SNAIL and MMP-2/-9 | 111,112 |
ATG5, autophagy-related 5; CDH1, cadherin 1; CTNNB1, catenin beta 1; DDB2, damage-specific DNA binding protein 2; EPC, endothlial progenitor cell; LEF1, lymphoid enhancer binding factor 1; SDAD1, SDA1 domain containing 1, Sox2, SRY-box transcription factor 2; TWIST1, twist family bHLH transcription factor 1; VEGF, vascular endothelial growth factor; ZEB1, zinc finger E-box binding homeobox 1; ZEB2, zinc finger E-box binding homeobox 2.
ncRNA-Associated ECM Remodeling
Both EMT/EndMT and dysregulated TGF-β signaling finally lead to excessive ECM remodeling, which is crucial in fibrogenesis. The 2 entities of ECM, interstitial matrix and basement membrane, are different from each other in molecular composition and biological function.113 Consisting of proteins, glycosaminoglycans, proteoglycans, and enzymes, the heterogeneous ECM structure provides a dynamic microenvironment for collagen-producing cells (eg, fibroblasts, myofibroblasts, and smooth muscle cells).114 Fibronectin bridges ECM components (eg, collagens and cell surface integrins) to modulate ECM structural changes and signaling pathways.114 The collagen family, especially (myo-)fibroblast-produced collagen I/III, represents a major part of interstitial ECMs.115 Collagen-producing cells further organize the alignment of collagens under the stimulation of profibrotic cytokines or growth factors (eg, TGF-β and IL-13).116 In addition, ECM proteases, such as MMPs, tissue inhibitors of metalloproteinase, neutrophil elastases, and meprins, are the major mediators of ECM degradation.116 Because of the imbalance between ECM degradation and deposition, excessive ECM remodeling leads to intestinal fibrosis.
MiR-16 plays different parts in ECM remodeling because of different etiologies. In a mouse model resembling postsurgical intestinal inflammation and fibrosis, miR-16-1 increases at the site of anastomosis and exacerbates ileocolonic anastomotic fibrosis by de-repressing myofibroblast differentiation.117,118 In contrast, miR-16 is downregulated by lncRNA WWC2-AS1 in radiation-induced intestinal fibrosis. As a result, reduced miR-16 gives rise to the production of fibroblast growth factor 2, α-SMA, and collagen I, and therefore promotes fibroblast proliferation and fibrosis.37 Unlike miR-16, miR-210 is proven to be a profibrotic miRNA in radiation-induced intestinal fibrosis because it promotes collagen Iα1 expression in fibrotic smooth muscle cells.119,120 In addition, ECM deposition and degradation in IBD fibrogenesis are orchestrated by ncRNAs. Bioinformatic analysis indicates that miR-192 may participate in ECM remodeling in CD.121 Experiments have further shown that miR-192 is upregulated by TGF-β signaling and promotes the accumulation of matrix collagens.122 Apart from manipulating ECM components, the cotranscribed miR-143/145 functions as an upstream process and promotes the transdifferentiation of smooth muscle cells into myofibroblasts in that the knockout of miR-143/145 leads to morphological abnormality and dysfunction of myofibroblasts in a mouse model of chemically induced colitis.123
A few miRNAs also play a part in regulating the production of ECM proteins via similar targets. MiR-150 suppresses the expression of α-SMA, TGF-β1, and collagen fibers in ECM.124 MiR-101 suppresses the production of ECM components (eg, α-SMA, collagen I) in fibrosis by inhibiting PI3K/AKT/mTOR signaling.125 In addition, the negative correlation between lncRNA growth arrest–specific transcript 5 (lncRNA GAS5) and MMP-2/MMP-9 reveals the potential modulating mechanism of ncRNAs in ECM degradation.126
PERSPECTIVES
Challenges
Over recent years, research on ncRNA-modulated intestinal fibrosis has made substantial progress, but there are still challenges. First, although most studies reveal correlations instead of causal relationships between dysregulated ncRNAs and intestinal fibrotic diseases, whether these correlations differ across segments of gut and reflect the stages of fibrosis remains a question because there is no gold standard for diagnosis in radiology, pathology, or endoscopy. Second, few studies elaborate the underlying molecular mechanisms thoroughly, such as ncRNA localization via RNAscope or BaseScope and functional verification based on in vivo and in vitro experiments. Third, many ncRNAs have significant function in modulating EMT and profibrotic factors. For example, hsa_circRNA_102610 promotes TGF-β1-induced EMT by sponging miR-130a-3p.102 However, EMT in intestinal fibrogenesis and the contribution of EndMT should be further studied in in vivo experiments and clinical studies.9 Fourth, although some research reveals that circRNAs act in intestinal fibrosis, more convincing evidence is still warranted. Learning from studies on miRNAs and lncRNAs in intestinal fibrogenesis, researchers and clinicians could collect and analyze gut biopsy, serum, and fecal samples from patients with stricturing CD and those with nonstricturing CD. Based on screening from samples of large cohorts, potential circRNAs could be first profiled and subsequently validated in vitro and in vivo.
Finally, as a useful tool to unveil intestinal fibrogenic responses, spontaneous, induced, and gene-targeted animal models are developed and widely utilized,127 whereas the ncRNA-targeted model is rarely applied in studies.
Diagnosis
There is an urgent need for efficient and accurate biomarkers to diagnose and prognosticate intestinal fibrosis, especially for those that can be detected by noninvasive methods, such as blood and fecal tests. NcRNAs have been newly developed as diagnostic biomarkers for various diseases, including fibrosis diseases, in clinical trials and other studies (Tables 2 and 3). For example, miR-29c in urinary exosomes indicates early renal fibrosis in lupus nephritis (AUC = 0·946).128 Given the promising role of ncRNAs, certain ncRNAs (eg, miR-200 family, miR-29 family, and miR-19 family) could have clinical significance in patients. However, their diagnostic values should be further validated in larger cohort studies or multicenter clinical trials. In addition, even though intestinal fibrogenesis may be in part independent of inflammatory signal cascades, fibrogenic responses are indeed triggered by certain types of chronic intestinal inflammation (eg, IBD).16 NcRNAs in intestinal inflammation may present a unique opportunity for predicting the early stage of colitis-induced fibrosis.
TABLE 2.
ncRNAs as Diagnostic Biomarkers for Diseases in Registered Clinical Trials
| ncRNA | Disease | Reference/Clinical Trial |
|---|---|---|
| miR-10b | Gliomas | NCT01849952 |
| miR-100 | Breast cancer | NCT02950207 |
| miR-107 | Alzheimer disease | NCT01819545 |
| miR-122* | Chronic hepatitis C | NCT00980161/NCT03687229 |
| miR-122 | Drug-induced liver injury by chemotherapy | NCT03039062 |
| miR-126* | Postmyocardial infarction remodeling | NCT01875484 |
| miR-126 | Allergic contact dermatitis | NCT04365140 |
| miR-138 | Oral lichen planus | NCT02834520 |
| miR-146a | Chronic periodontitis and coronary heart disease | NCT03721159 |
| SNP rs2910164 in pre-miR-146a gene | Cancer | NCT04038996 |
| miR-142-3p | Synaptopathy in multiple sclerosis | NCT03999788 |
| miR-150/miR-155 | Multiple sclerosis | NCT04300543 |
| miR-155 | Preeclampsia | NCT04277390 |
| miR-155 | Nonmuscle invasive bladder cancer | NCT03591367 |
| miR-155 | Oral lichen planus | NCT03871114 |
| miR-192/miR-25* | Diabetic kidney disease | NCT04176276 |
| miR-200b/miR-21* | Diabetic wounds | NCT02581098 |
| miR-204 | Capillarization in limb muscles of patients with chronic obstructive pulmonary disease | NCT02903043 |
| miR-210 | Preeclampsia | NCT03193554 |
| miR-210* | Wound healing | NCT02024243 |
| miR-221/miR-222 | Hepatocellular carcinoma | NCT02928627 |
| miR-25 | Pancreatic cancer | NCT03432624 |
| miR-29 family* | Shoulder stiffness | NCT02534558 |
| miR-29 family | Head-and-neck squamous cell carcinoma | NCT01927354 |
| miR-29b | Oral squamous cell carcinoma | NCT02009852 |
| miR-30a | Childhood nephrotic syndrome | NCT03235128 |
| miR-30 family | Schizophrenia | NCT02650102/NCT03007303 |
| miR-31-3p/miR-31-5p | Colon cancer | NCT03362684 |
| miR-452 | Preeclampsia | NCT03258125 |
| miR-494 | Cerebral ischemia | NCT03577093 |
| lncRNA CCAT1 | Colorectal cancer | NCT04269746 |
| lncRNA HOTAIR | Thyroid cancer | NCT03469544 |
| lncRNA NBR2 | Sepsis | NCT04427371 |
| circRNA Uck2 | Acute myocardial infarction | NCT03170830 |
*Fibrotic diseases.
TABLE 3.
Validated ncRNAs as Biomarkers in Clinical Studies on Fibrotic Diseases
| ncRNA | Disease | Sample | Result | Reference |
|---|---|---|---|---|
| miR-29 family | Hepatic fibrosis | Serum | ↓ | 129-132 |
| miR-122 | Hepatic fibrosis | Liver tissue and serum | ↓ | 130,133-136 |
| miR-34a-5p | Hepatic fibrosis | Serum | ↑ | 137-139 |
| miR-378 family | Hepatic fibrosis | Liver tissue | ↑ | 140,141 |
| let-7 | Hepatic fibrosis | Serum | ↓ | 142,143 |
| miR-223 | Hepatic fibrosis | Serum | ↑ | 144,145 |
| miR-21 | Hepatic fibrosis | Liver tissue and serum | ↑ | 132,139,146,147 |
| lncRNA H19 | Hepatic fibrosis | Liver tissue and serum | ↑ | 148-150 |
| lncRNA MALAT1 | Hepatic fibrosis | Liver tissue and serum | ↑ | 151-153 |
| lncRNA HOTAIR | Hepatic fibrosis | Liver tissue | ↑ | 76,77 |
| lincRNA p21 | Hepatic fibrosis | Liver tissue and serum | ↓ | 154-156 |
| lncRNA APTR | Hepatic fibrosis | Serum | ↑ | 157,158 |
| lncRNA ATB | Hepatic fibrosis | Liver tissue and serum | ↑ | 68,159 |
| miR-21 | Renal fibrosis | Renal tissue, urine, and serum | ↑ | 160-166 |
| miR-214 | Renal fibrosis | Renal tissue | ↑ | 165,167 |
| miR-29 family | Renal fibrosis | Urine | ↓ | 128,164,166,168 |
| miR-29 family | Cardiac fibrosis | Cardiac tissue and serum | ↓ | 169-175 |
| miR-21 | Cardiac fibrosis | Cardiac tissue and serum | ↑ | 173,176-182 |
| miR-208 | Cardiac fibrosis | Cardiac tissue and serum | ↑ | 173,183,184 |
| miR-133 | Cardiac fibrosis | Cardiac tissue and serum | ↑ | 185-187 |
| miR-155 | Cardiac fibrosis | Cardiac tissue and serum | ↑ | 174,187 |
| miR-146 | Cardiac fibrosis | Cardiac tissue and serum | ↑ | 176,188,189 |
| miR-21 | Pulmonary fibrosis | Lung tissue and serum | ↑ | 190-195 |
| miR-200 family | Pulmonary fibrosis | Lung tissue and serum | ↓ | 191,196 |
| miR-155 | Pulmonary fibrosis | Lung tissue and serum | ↑ | 197,198 |
| miR-101 | Pulmonary fibrosis | Lung tissue | ↑ | 199,200 |
| miR-31 | Pulmonary fibrosis | Serum and bronchoalveolar lavage fluid | ↓ | 191,201 |
| miR-21 | Skin fibrosis | Skin tissue | ↑ | 202,203 |
| miR-29 | Skin fibrosis | Skin tissue | ↓ | 202,204,205 |
| miR-145 | Skin fibrosis | Skin tissue | ↑ | 205,206 |
| lncRNA HOXA11-AS | Skin fibrosis | Skin tissue | ↑ | 207,208 |
| lncRNA CACNA1G-AS1 | Skin fibrosis | Skin tissue | ↑ | 207,209 |
| miR-29 | Intestinal fibrosis | Gut tissue | ↓ | 36,210 |
| miR-200 | Intestinal fibrosis | Gut tissue and serum | ↓ | 61-63 |
| miR-19 | Intestinal fibrosis | Serum | ↓ | 35 |
We only include studies on specific ncRNAs in fibrosis diseases screened and validated by multicenter studies or no less than 2 studies.
Therapeutic Potential
Owing to the extensive participation of ncRNAs in intestinal fibrogenesis, more attention should be paid to their potential role as therapeutic targets to prevent early-stage fibrosis or reverse existing fibrosis.211 Since Miravirsen (SPC3649, a miR-122 inhibitor) was first used for hepatitis C in clinical trials, ncRNA-based therapies have become feasible and attractive212,213 (Table 4). As for fibrotic diseases, Remlarsen (MRG-201), an anti-fibrotic miR-29 mimic,204 has been applied to keloids in phase 2 clinical trials (eg, ClinicalTrials.gov identifier: NCT03601052). Because miR-29 is also an inhibitor in intestinal fibrosis because it inhibits TGF-β signaling, whether Remlarsen can be used to treat intestinal fibrosis is worth a discussion. However, the design of ncRNA-based drugs needs further consideration for optimized curative effects. First, a successful delivery system (eg, nanoparticles and liposome-bearing microvesicles) of artificial miRNAs is prerequisite because of their vulnerability to degradation, especially in the alimentary tract. Second, effectiveness and efficiency should be considered in the choice of drug administration method for patients with intestinal obstruction. Because of varied tissue enrichment among different organs, choosing a gut-specific ncRNA for alimentary fibrotic diseases is important. In addition, an intestine-targeting delivery system for ncRNA-based drugs remains to be developed. Third, because of the intricate network of ncRNA, 1 ncRNA usually plays multiple roles in different organs and needs to be thought over as a whole system. For example, in spite of its antifibrotic role, miR-200 has been shown to promote the malignant transformation of tumors by inducing EMT in hepatocellular carcinoma214 and to enhance the proliferative and invasive capacities of ovarian cancer cells.215 Therefore, whether the application of ncRNA-based drugs may cause adverse effects remains a problem.
TABLE 4.
ncRNAs as Therapeutic Targets for Diseases in Registered Clinical Trials
| Drug | Disease | Target | Phase | Reference/Clinical Trial |
|---|---|---|---|---|
| RG-125 (AZD4076) | Type 2 diabetes with nonalcoholic fatty liver disease | miR-103/miR-107 | Phase 1/2a | NCT02826525 |
| RG-125 (AZD4076) | Non-alcoholic steatohepatitis | miR-103/miR-107 | Phase 1 | NCT02612662 |
| Miravirsen (SPC3649)* | Hepatitis C | miR-122 | Phase 2 | NCT01200420/ NCT01727934/NCT01872936/NCT01646489/NCT02452814/NCT02508090/NCT00979927/ NCT00688012 |
| Cobomarsen (MRG-106) | Mycosis fungoides | miR-155 | Phase 2 | NCT03713320/NCT03837457 |
| TargomiRs | Malignant pleural mesothelioma, Non-small cell lung cancer | miR-16 | Phase 1 | NCT02369198 |
| Lademirsen (SAR339375, RG-012) | Alport syndrome | miR-21 | Phase 2 | NCT02855268/NCT03373786 |
| Remlarsen (MRG-201)* | Keloids | miR-29 | Phase 2 | NCT03601052/NCT02603224 |
| MRX34 | Primary liver cancer, Lymphoma, melanoma, non–small cell lung cancer, small cell lung cancer | miR-34a | Phase 1 | NCT01829971 |
| Multiple myeloma, renal cell carcinoma | ||||
| MRX34 | Melanoma | miR-34a | Phase 1/2; withdrawn | NCT02862145 |
| MRG-110* | Heart failure | miR-92 | Phase 1 | NCT03603431 |
*Fibrosis diseases.
CONCLUSIONS
As the biology of ncRNA-modulated intestinal fibrosis is gradually unveiled, ncRNAs may present as promising biomarkers and therapeutic targets in the future.
Glossary
Abbreviations
- α-SMA
α-smooth muscle actin
- CD
Crohn’s disease
- circRNA
circular RNA
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- EMT
epithelial-to-mesenchymal transition
- EndMT
endothelial-to-mesenchymal transition
- EPC
endothlial progenitor cell
- HOTAIR
HOX transcript antisense RNA
- IBD
inflammatory bowel disease
- IGF
insulin-like growth factor
- IL
interleukin
- lncRNA
long noncoding RNA
- miRNA
microRNA
- MMP
matrix metalloproteinase
- ncRNA
noncoding RNA
- TGF-β
transforming growth factor-β
- TGFBR
transforming growth factor-β receptor
- VEGF
vascular endothelial growth factor
Author contributions: R. Mao had the idea for the article. Y. Zhou and S.N. Lin performed the literature search. L.Y. Zhou drew the figures and tables. L.Y. Zhou and S.N. Lin drafted the article, and M.H. Chen, F. Rieder, S.H. Zhang, and R. Mao critically revised the manuscript. All authors approved the final version of the manuscript.
Supported by: This study was supported by National Natural Science Foundation of China (81970483).
Conflict of interest: All authors declared no conflict of interest.
REFERENCES
- 1. Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. AJP Cell Physiology. 2013;304:C216–C225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li J, Mao R, Kurada S, et al. . Pathogenesis of fibrostenosing Crohn’s disease. Translational Research. 2019;209:39–54. [DOI] [PubMed] [Google Scholar]
- 4. Xu M, Xie F, Tang X, et al. . Insights into the role of circular RNA in macrophage activation and fibrosis disease. Pharmacol Res. 2020;156:104777. [DOI] [PubMed] [Google Scholar]
- 5. Iacob DG, Rosca A, Ruta SM. Circulating microRNAs as non-invasive biomarkers for hepatitis B virus liver fibrosis. World J Gastroenterol. 2020;26:1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He Z, Yang D, Fan X, et al. . The roles and mechanisms of lncRNAs in liver fibrosis. Int J Mol Sci. 2020;21:1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan WPW, Mourad F, Leong RW. Crohn’s disease associated strictures. J Gastroenterol Hepatol. 2018;33:998–1008. [DOI] [PubMed] [Google Scholar]
- 8. Latella G, Rogler G, Bamias G, et al. . Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis. 2014;8:1147–1165. [DOI] [PubMed] [Google Scholar]
- 9. Lenti MV, Di Sabatino A. Intestinal fibrosis. Mol Aspects Med. 2019;65:100–109. [DOI] [PubMed] [Google Scholar]
- 10. Rieder F, Fiocchi C. Intestinal fibrosis in inflammatory bowel disease—current knowledge and future perspectives. J Crohn’s and Colitis 2008;2:279–290. [DOI] [PubMed] [Google Scholar]
- 11. Sanders YY, Cui Z, Le Saux CJ, et al. . SMAD-independent down-regulation of caveolin-1 by TGF-β: effects on proliferation and survival of myofibroblasts. Plos One. 2015;10:e0116995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. [DOI] [PubMed] [Google Scholar]
- 13. Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. [DOI] [PubMed] [Google Scholar]
- 14. Johnson LA, Rodansky ES, Haak AJ, et al. . Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-β-induced fibrogenesis in human colonic myofibroblasts. Inflamm Bowel Dis. 2014;20:154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burke JP, Mulsow JJ, O’Keane C, et al. . Fibrogenesis in Crohn’s disease. Am J Gastroenterol. 2007;102:439–448. [DOI] [PubMed] [Google Scholar]
- 16. Fiocchi C, Rieder F. Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastro Hepat. 2009;6:228–235. [DOI] [PubMed] [Google Scholar]
- 17. Lawrance IC, Rogler G, Bamias G, et al. . Cellular and molecular mediators of intestinal fibrosis. J Crohn’s Colitis. 2017;11:1491–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheibe K, Kersten C, Schmied A, et al. . Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156:1082–1097.e11. [DOI] [PubMed] [Google Scholar]
- 19. Scheibe K, Backert I, Wirtz S, et al. . IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 2017;66:823–838. [DOI] [PubMed] [Google Scholar]
- 20. Shih DQ, Zheng L, Zhang X, et al. . Inhibition of a novel fibrogenic factor Tl1a reverses established colonic fibrosis. Mucosal Immunol. 2014;7:1492–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borley NR, Mortensen NJ, Kettlewell MG, et al. . Connective tissue changes in ileal Crohn’s disease: relationship to disease phenotype and ulcer-associated cell lineage. Dis Colon Rectum. 2001;44:388–396. [DOI] [PubMed] [Google Scholar]
- 22. Ellermann M, Gharaibeh RZ, Fulbright L, et al. . Yersiniabactin-producing adherent/invasive Escherichia coli promotes inflammation-associated fibrosis in gnotobiotic Il10(-/-) mice. Infect Immun. 2019;87:e00587-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imai J, Kitamoto S, Sugihara K, et al. . Flagellin-mediated activation of IL-33-ST2 signaling by a pathobiont promotes intestinal fibrosis. Mucosal Immunol. 2019;12:632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao S, Dejanovic D, Yao P, et al. . Selective deletion of MyD88 signaling in α-SMA positive cells ameliorates experimental intestinal fibrosis via post-transcriptional regulation. Mucosal Immunol. 2020;13:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lovisa S, Genovese G, Danese S. Role of epithelial-to-mesenchymal transition in inflammatory bowel disease. J Crohns Colitis. 2019;13:659–668. [DOI] [PubMed] [Google Scholar]
- 26. Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. 2019;179:1033–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20:5–20. [DOI] [PubMed] [Google Scholar]
- 28. Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kristensen LS, Andersen MS, Stagsted LVW, et al. . The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–691. [DOI] [PubMed] [Google Scholar]
- 30. Chen P, Li Y, Li L, et al. . Circulating microRNA146b-5p is superior to C-reactive protein as a novel biomarker for monitoring inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:733–743. [DOI] [PubMed] [Google Scholar]
- 31. Yael H, Marina BS, Ayelet DS, et al. . Long ncRNA landscape in the ileum of treatment-naive early-onset Crohn disease. Inflamm Bowel Dis. 2018;24:346–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Braga-Neto MB, Gaballa JM, Bamidele AO, et al. . Deregulation of long intergenic non-coding RNAs in CD4+ T cells of lamina propria in Crohn’s disease through transcriptome profiling. J Crohns Colitis. 2020;14:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nie J, Zhao Q. Lnc-ITSN1-2, derived from RNA sequencing, correlates with increased disease risk, activity and promotes CD4+ T cell activation, proliferation and Th1/Th17 cell differentiation by serving as a ceRNA for IL-23R via sponging miR-125a in inflammatory bowel disease. Front Immunol. 2020;11:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li L, Huang S, Wang H, et al. . Cytokine IL9 triggers the pathogenesis of inflammatory bowel disease through the miR21-CLDN8 pathway. Inflamm Bowel Dis. 2018;24:2211–2223. [DOI] [PubMed] [Google Scholar]
- 35. Lewis A, Mehta S, Hanna LN, et al. . Low serum levels of MicroRNA-19 are associated with a stricturing Crohn’s disease phenotype. Inflamm Bowel Dis. 2015;21:1926–1934. [DOI] [PubMed] [Google Scholar]
- 36. Nijhuis A, Biancheri P, Lewis A, et al. . In Crohn’s disease fibrosis-reduced expression of the miR-29 family enhances collagen expression in intestinal fibroblasts. Clin Sci (Lond). 2014;127:341–350. [DOI] [PubMed] [Google Scholar]
- 37. Zhou JM, Liang R, Zhu SY, et al. . LncRNA WWC2-AS1 functions AS a novel competing endogenous RNA in the regulation of FGF2 expression by sponging miR-16 in radiation-induced intestinal fibrosis. BMC Cancer. 2019;19:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. [DOI] [PubMed] [Google Scholar]
- 39. Yun SM, Kim SH, Kim EH. The molecular mechanism of transforming growth factor-β signaling for intestinal fibrosis: a mini-review. Front Pharmacol. 2019;10:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohammadi A, Kelly OB, Filice M, et al. . Differential expression of microRNAs in peripheral blood mononuclear cells identifies autophagy and TGF-beta-related signatures aberrantly expressed in inflammatory bowel disease. J Crohns Colitis. 2018;12:568–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szűcs D, Béres NJ, Rokonay R, et al. . Increased duodenal expression of miR-146a and -155 in pediatric Crohn’s disease. World J Gastroenterol. 2016;22:6027–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Louafi F, Martinez-Nunez RT, Sanchez-Elsner T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-{beta}. J Biol Chem. 2010;285:41328–41336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yao Q, Cao S, Li C, et al. . Micro-RNA-21 regulates TGF-β-induced myofibroblast differentiation by targeting PDCD4 in tumor-stroma interaction. Int J Cancer. 2011;128:1783–1792. [DOI] [PubMed] [Google Scholar]
- 44. Zhong X, Chung AC, Chen HY, et al. . Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol. 2011;22:1668–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. García R, Nistal JF, Merino D, et al. . p-SMAD2/3 and DICER promote pre-miR-21 processing during pressure overload-associated myocardial remodeling. Biochim Biophys Acta. 2015;1852:1520–1530. [DOI] [PubMed] [Google Scholar]
- 46. Wang H, Nie L, Wu L, et al. . NR2F2 inhibits Smad7 expression and promotes TGF-β-dependent epithelial-mesenchymal transition of CRC via transactivation of miR-21. Biochem Biophys Res Commun. 2017;485:181–188. [DOI] [PubMed] [Google Scholar]
- 47. Wu X, Ding X, Ding Z, et al. . Total flavonoids from leaves of carya cathayensis ameliorate renal fibrosis via the miR-21/Smad7 signaling pathway. Cell Physiol Biochem. 2018;49:1551–1563. [DOI] [PubMed] [Google Scholar]
- 48. Nong Q, Li S, Wu Y, et al. . LncRNA COL1A2-AS1 inhibits the scar fibroblasts proliferation via regulating miR-21/Smad7 pathway. Biochem Biophys Res Commun. 2018;495:319–324. [DOI] [PubMed] [Google Scholar]
- 49. Cao S, Xiao L, Rao JN, et al. . Inhibition of Smurf2 translation by miR-322/503 modulates TGF-β/Smad2 signaling and intestinal epithelial homeostasis. Mol Biol Cell. 2014;25:1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou Y, Deng L, Zhao D, et al. . MicroRNA-503 promotes angiotensin II-induced cardiac fibrosis by targeting Apelin-13. J Cell Mol Med. 2016;20:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li J, Du S, Sheng X, et al. . MicroRNA-29b inhibits endometrial fibrosis by regulating the Sp1-TGF-β1/Smad-CTGF axis in a rat model. Reprod Sci. 2016;23:386–394. [DOI] [PubMed] [Google Scholar]
- 52. Zhi SC, Chen SZ, Li YY, et al. . Rosiglitazone inhibits activation of hepatic stellate cells via up-regulating micro-RNA-124-3p to alleviate hepatic fibrosis. Dig Dis Sci. 2019;64:1560–1570. [DOI] [PubMed] [Google Scholar]
- 53. Speca S, Rousseaux C, Dubuquoy C, et al. . Novel PPARγ modulator GED-0507-34 levo ameliorates inflammation-driven intestinal fibrosis. Inflamm Bowel Dis. 2016;22:279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang Y, Qi Y, Du JQ, et al. . MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin Ther Targets. 2014;18:1355–1365. [DOI] [PubMed] [Google Scholar]
- 55. Wasson CW, Abignano G, Hermes H, et al. . Long non-coding RNA HOTAIR drives EZH2-dependent myofibroblast activation in systemic sclerosis through miRNA 34a-dependent activation of NOTCH. Ann Rheum Dis. 2020;79:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fu X, Qie J, Fu Q, et al. . miR-20a-5p/TGFBR2 axis affects pro-inflammatory macrophages and aggravates liver fibrosis. Front Oncol. 2020;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Choi H, Park JS, Kim D, et al. . PGC-1α suppresses the activation of TGF-β/Smad signaling via targeting TGFβRI downregulation by let-7b/c upregulation. Int J Mol Sci. 2019;20:5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51:1638–1649. [DOI] [PubMed] [Google Scholar]
- 59. Zhao J, Liang F, Li J, et al. . Role of non-inflammatory factors in intestinal fibrosis. J Dig Dis. 2020;21:315–318. [DOI] [PubMed] [Google Scholar]
- 60. Hulshoff MS, Del Monte-Nieto G, Kovacic J, et al. . Non-coding RNA in endothelial-to-mesenchymal transition. Cardiovasc Res. 2019;115:1716–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mehta SJ, Lewis A, Nijhuis A, et al. . Epithelial down-regulation of the miR-200 family in fibrostenosing Crohn’s disease is associated with features of epithelial to mesenchymal transition. J Cell Mol Med. 2018;22:5617–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zidar N, Boštjančič E, Jerala M, et al. . Down-regulation of microRNAs of the miR-200 family and up-regulation of Snail and Slug in inflammatory bowel diseases—hallmark of epithelial-mesenchymal transition. J Cell Mol Med. 2016;20:1813–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen Y, Ge W, Xu L, et al. . miR-200b is involved in intestinal fibrosis of Crohn’s disease. Int J Mol Med. 2012;29:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lamprecht S, Kaller M, Schmidt EM, et al. . PBX3 is part of an EMT regulatory network and indicates poor outcome in colorectal cancer. Clin Cancer Res. 2018;24:1974–1986. [DOI] [PubMed] [Google Scholar]
- 65. Hur K, Toiyama Y, Takahashi M, et al. . MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62:1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Edvardsson K, Nguyen-Vu T, Kalasekar SM, et al. . Estrogen receptor β expression induces changes in the microRNA pool in human colon cancer cells. Carcinogenesis. 2013;34:1431–1441. [DOI] [PubMed] [Google Scholar]
- 67. Feng B, Cao Y, Chen S, et al. . miR-200b mediates endothelial-to-mesenchymal transition in diabetic cardiomyopathy. Diabetes. 2016;65:768–779. [DOI] [PubMed] [Google Scholar]
- 68. Fu N, Zhao SX, Kong LB, et al. . LncRNA-ATB/microRNA-200a/β-catenin regulatory axis involved in the progression of HCV-related hepatic fibrosis. Gene. 2017;618:1–7. [DOI] [PubMed] [Google Scholar]
- 69. Liu Y, Li Y, Xu Q, et al. . Long non-coding RNA-ATB promotes EMT during silica-induced pulmonary fibrosis by competitively binding miR-200c. Biochim Biophys Acta Mol Basis Dis. 2018;1864:420–431. [DOI] [PubMed] [Google Scholar]
- 70. Feifei W, Hui G, Ruiqiang Z, et al. . MAGP2, a component of extracellular matrix, is upregulated in colorectal cancer and negatively modulated by miR-200b-3p. Technol Cancer Res Treat. 2019;18:1533033819870777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang J, Zhou CZ, Zhu R, et al. . miR-200b-containing microvesicles attenuate experimental colitis associated intestinal fibrosis by inhibiting epithelial-mesenchymal transition. J Gastroenterol Hepatol. 2017;32:1966–1974. [DOI] [PubMed] [Google Scholar]
- 72. Zhang H, Hu J, Liu L. MiR-200a modulates TGF-β1-induced endothelial-to-mesenchymal shift via suppression of GRB2 in HAECs. Biomed Pharmacother. 2017;95:215–222. [DOI] [PubMed] [Google Scholar]
- 73. Zhou H, Qiu ZZ, Yu ZH, et al. . Paeonol reverses promoting effect of the HOTAIR/miR-124/Notch1 axis on renal interstitial fibrosis in a rat model. J Cell Physiol. 2018;234:14351–14363. [DOI] [PubMed] [Google Scholar]
- 74. Zhou H, Gao L, Yu ZH, et al. . LncRNA HOTAIR promotes renal interstitial fibrosis by regulating Notch1 pathway via the modulation of miR-124. Nephrology. 2019;24:472–480. [DOI] [PubMed] [Google Scholar]
- 75. Wu XL, Lu RY, Wang LK, et al. . Long noncoding RNA HOTAIR silencing inhibits invasion and proliferation of human colon cancer LoVo cells via regulating IGF2BP2. J Cell Biochem. Published online ahead of print October 18, 2018. doi:10.1002/jcb.27079. [DOI] [PubMed] [Google Scholar]
- 76. Bian EB, Wang YY, Yang Y, et al. . Hotair facilitates hepatic stellate cells activation and fibrogenesis in the liver. Biochim Biophys Acta Mol Basis Dis. 2017;1863:674–686. [DOI] [PubMed] [Google Scholar]
- 77. Yu F, Chen B, Dong P, et al. . HOTAIR epigenetically modulates PTEN expression via MicroRNA-29b: a novel mechanism in regulation of liver fibrosis. Mol Ther. 2017;25:205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ding D, Li C, Zhao T, et al. . LncRNA H19/miR-29b-3p/PGRN axis promoted epithelial-mesenchymal transition of colorectal cancer cells by acting on wnt signaling. Mol Cells. 2018;41:423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao S, Zhang Y, Zheng X, et al. . Loss of MicroRNA-101 promotes epithelial to mesenchymal transition in hepatocytes. J Cell Physiol. 2015;230:2706–2717. [DOI] [PubMed] [Google Scholar]
- 80. Lee CG, McCarthy S, Gruidl M, et al. . MicroRNA-147 induces a mesenchymal-to-epithelial transition (MET) and reverses EGFR inhibitor resistance. Plos One. 2014;9:e84597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li J, Xia L, Zhou Z, et al. . MiR-186-5p upregulation inhibits proliferation, metastasis and epithelial-to-mesenchymal transition of colorectal cancer cell by targeting ZEB1. Arch Biochem Biophys. 2018;640:53–60. [DOI] [PubMed] [Google Scholar]
- 82. Gulei D, Magdo L, Jurj A, et al. . The silent healer: miR-205-5p up-regulation inhibits epithelial to mesenchymal transition in colon cancer cells by indirectly up-regulating E-cadherin expression. Cell Death Dis. 2018;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zheng YB, Luo HP, Shi Q, et al. . miR-132 inhibits colorectal cancer invasion and metastasis via directly targeting ZEB2. World J Gastroenterol. 2014;20:6515–6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhu W, Luo X, Fu H, et al. . MiR-3653 inhibits the metastasis and epithelial-mesenchymal transition of colon cancer by targeting Zeb2. Pathol Res Pract. 2019;215:152577. [DOI] [PubMed] [Google Scholar]
- 85. Kim NH, Kim HS, Li XY, et al. . A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol. 2011;195:417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sells E, Pandey R, Chen H, et al. . Specific microRNA-mRNA regulatory network of colon cancer invasion mediated by tissue kallikrein-related peptidase 6. Neoplasia. 2017;19:396–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mamoori A, Wahab R, Vider J, et al. . The tumour suppressor effects and regulation of cancer stem cells by macrophage migration inhibitory factor targeted miR-451 in colon cancer. Gene. 2019;697:165–174. [DOI] [PubMed] [Google Scholar]
- 88. Jiang H, Wang P, Wang Q, et al. . Quantitatively controlling expression of miR-17~92 determines colon tumor progression in a mouse tumor model. Am J Pathol. 2014;184:1355–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li Y, Lv Z, He G, et al. . The SOX17/miR-371-5p/SOX2 axis inhibits EMT, stem cell properties and metastasis in colorectal cancer. Oncotarget. 2015;6:9099–9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zeng M, Zhu L, Li L, et al. . miR-378 suppresses the proliferation, migration and invasion of colon cancer cells by inhibiting SDAD1. Cell Mol Biol Lett. 2017;22:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yan W, Wu Q, Yao W, et al. . MiR-503 modulates epithelial-mesenchymal transition in silica-induced pulmonary fibrosis by targeting PI3K p85 and is sponged by lncRNA MALAT1. Sci Rep. 2017;7:11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Shen X, Hu X, Mao J, et al. . The long noncoding RNA TUG1 is required for TGF-β/TWIST1/EMT-mediated metastasis in colorectal cancer cells. Cell Death Dis. 2020;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhu QQ, Ma C, Wang Q, et al. . The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumour Biol. 2016;37:185–197. [DOI] [PubMed] [Google Scholar]
- 94. Sun Z, Ou C, Liu J, et al. . YAP1-induced MALAT1 promotes epithelial-mesenchymal transition and angiogenesis by sponging miR-126-5p in colorectal cancer. Oncogene. 2019;38:2627–2644. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95. Kan JY, Wu DC, Yu FJ, et al. . Chemokine (C-C Motif) ligand 5 is involved in tumor-associated dendritic cell-mediated colon cancer progression through non-coding RNA MALAT-1. J Cell Physiol. 2015;230:1883–1894. [DOI] [PubMed] [Google Scholar]
- 96. Jiang H, Li T, Qu Y, et al. . Long non-coding RNA SNHG15 interacts with and stabilizes transcription factor Slug and promotes colon cancer progression. Cancer Lett. 2018;425:78–87. [DOI] [PubMed] [Google Scholar]
- 97. Costa V, Lo Dico A, Rizzo A, et al. . MiR-675-5p supports hypoxia induced epithelial to mesenchymal transition in colon cancer cells. Oncotarget. 2017;8:24292–24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fang C, Zan J, Yue B, et al. . Long non-coding ribonucleic acid zinc finger antisense 1 promotes the progression of colonic cancer by modulating ZEB1 expression. J Gastroenterol Hepatol. 2017;32:1204–1211. [DOI] [PubMed] [Google Scholar]
- 99. Yue B, Liu C, Sun H, et al. . A positive feed-forward loop between LncRNA-CYTOR and Wnt/β-catenin signaling promotes metastasis of colon cancer. Mol Ther. 2018;26:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li T, Zhu J, Wang X, et al. . Long non-coding RNA lncTCF7 activates the Wnt/β-catenin pathway to promote metastasis and invasion in colorectal cancer. Oncol Lett. 2017;14:7384–7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang Q, Bian Y, Zhu Y, et al. . Integrative analysis and validation of dysregulated long non-coding RNAs in colon cancer. J Cell Mol Med. 2020;24:2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yin J, Ye YL, Hu T, et al. . Hsa_circRNA_102610 upregulation in Crohn’s disease promotes transforming growth factor-β1-induced epithelial-mesenchymal transition via sponging of hsa-miR-130a-3p. World J Gastroenterol. 2020;26:3034–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Miscianinov V, Martello A, Rose L, et al. . MicroRNA-148b targets the TGF-β pathway to regulate angiogenesis and endothelial-to-mesenchymal transition during skin wound healing. Mol Ther. 2018;26:1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Geng H, Guan J. MiR-18a-5p inhibits endothelial–mesenchymal transition and cardiac fibrosis through the Notch2 pathway. Biochem Bioph Res Commun. 2017;491:329–336. [DOI] [PubMed] [Google Scholar]
- 105. Thomas AA, Biswas S, Feng B, et al. . lncRNA H19 prevents endothelial-mesenchymal transition in diabetic retinopathy. Diabetologia. 2019;62:517–530. [DOI] [PubMed] [Google Scholar]
- 106. Terzuoli E, Nannelli G, Giachetti A, et al. . Targeting endothelial-to-mesenchymal transition: the protective role of hydroxytyrosol sulfate metabolite. Eur J Nutr. 2020;59:517–527. [DOI] [PubMed] [Google Scholar]
- 107. Xiang Y, Zhang Y, Tang Y, et al. . MALAT1 modulates TGF-β1-induced endothelial-to-mesenchymal transition through downregulation of miR-145. Cell Physiol Biochem. 2017;42:357–372. [DOI] [PubMed] [Google Scholar]
- 108. Li H, Zhao Q, Chang L, et al. . LncRNA MALAT1 modulates ox-LDL induced EndMT through the Wnt/β-catenin signaling pathway. Lipids Health Dis. 2019;18:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang R, Huang XQ, Jiang YY, et al. . LncRNA TUG1 regulates autophagy-mediated endothelial-mesenchymal transition of liver sinusoidal endothelial cells by sponging miR-142-3p. Am J Transl Res. 2020;12:758–772. [PMC free article] [PubMed] [Google Scholar]
- 110. Yi M, Liu B, Tang Y, et al. . Irradiated human umbilical vein endothelial cells undergo endothelial-mesenchymal transition via the Snail/miR-199a-5p axis to promote the differentiation of fibroblasts into myofibroblasts. Biomed Res Int. 2018;2018:4135806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kumarswamy R, Volkmann I, Jazbutyte V, et al. . Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361–369. [DOI] [PubMed] [Google Scholar]
- 112. Guo Y, Li P, Bledsoe G, et al. . Kallistatin inhibits TGF-β-induced endothelial-mesenchymal transition by differential regulation of microRNA-21 and eNOS expression. Exp Cell Res. 2015;337:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Villesen IF, Daniels SJ, Leeming DJ, et al. . Review article: the signalling and functional role of the extracellular matrix in the development of liver fibrosis. Aliment Pharmacol Ther. 2020;52:85–97. [DOI] [PubMed] [Google Scholar]
- 114. Theocharis AD, Skandalis SS, Gialeli C, et al. . Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. [DOI] [PubMed] [Google Scholar]
- 115. Karsdal MA, Nielsen SH, Leeming DJ, et al. . The good and the bad collagens of fibrosis—their role in signaling and organ function. Adv Drug Deliver Rev. 2017;121:43–56. [DOI] [PubMed] [Google Scholar]
- 116. Mortensen JH, Lindholm M, Langholm LL, et al. . The intestinal tissue homeostasis—the role of extracellular matrix remodeling in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2019;13:977–993. [DOI] [PubMed] [Google Scholar]
- 117. Hou HW, Wang JM, Wang D, et al. . Triptolide exerts protective effects against fibrosis following ileocolonic anastomosis by mechanisms involving the miR-16-1/HSP70 pathway in IL-10-deficient mice. Int J Mol Med. 2017;40:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bellaye PS, Burgy O, Causse S, et al. . Heat shock proteins in fibrosis and wound healing: good or evil? Pharmacol Ther. 2014;143:119–132. [DOI] [PubMed] [Google Scholar]
- 119. Hamama S, Noman MZ, Gervaz P, et al. . MiR-210: a potential therapeutic target against radiation-induced enteropathy. Radiother Oncol. 2014;111:219–221. [DOI] [PubMed] [Google Scholar]
- 120. Delanian S, Chatel C, Porcher R, et al. . Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherol-clodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80:832–839. [DOI] [PubMed] [Google Scholar]
- 121. Zhao H, Chen J, Chen J, et al. . miR-192/215-5p act as tumor suppressors and link Crohn’s disease and colorectal cancer by targeting common metabolic pathways: an integrated informatics analysis and experimental study. J Cell Physiol. 2019;234:21060–21075. [DOI] [PubMed] [Google Scholar]
- 122. Chung AC, Huang XR, Meng X, et al. . miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol. 2010;21:1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chivukula RR, Shi G, Acharya A, et al. . An essential mesenchymal function for miR-143/145 in intestinal epithelial regeneration. Cell. 2014;157:1104–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Li Y, Ren W, Wang X, et al. . MicroRNA-150 relieves vascular remodeling and fibrosis in hypoxia-induced pulmonary hypertension. Biomed Pharmacother. 2019;109:1740–1749. [DOI] [PubMed] [Google Scholar]
- 125. Lei Y, Wang QL, Shen L, et al. . MicroRNA-101 suppresses liver fibrosis by downregulating PI3K/Akt/mTOR signaling pathway. Clin Res Hepatol Gas. 2019;43:575–584. [DOI] [PubMed] [Google Scholar]
- 126. Lucafò M, Pugnetti L, Bramuzzo M, et al. . Long non-coding RNA GAS5 and intestinal MMP2 and MMP9 expression: a translational study in pediatric patients with IBD. Int J Mol Sci. 2019;20:5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rieder F, Kessler S, Sans M, et al. . Animal models of intestinal fibrosis: new tools for the understanding of pathogenesis and therapy of human disease. Am J Physiol. Gastroint Liver Physiol. 2012;303:G786–G801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Solé C, Cortés-Hernández J, Felip ML, et al. . miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol Dial Transplant. 2015;30:1488–1496. [DOI] [PubMed] [Google Scholar]
- 129. Roderburg C, Urban GW, Bettermann K, et al. . Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. [DOI] [PubMed] [Google Scholar]
- 130. Xing TJ, Jiang DF, Huang JX, et al. . Expression and clinical significance of miR-122 and miR-29 in hepatitis B virus-related liver disease. Genet Mol Res. 2014;13:7912–7918. [DOI] [PubMed] [Google Scholar]
- 131. Huang C, Zheng JM, Cheng Q, et al. . Serum microRNA-29 levels correlate with disease progression in patients with chronic hepatitis B virus infection. J Dig Dis. 2014;15:614–621. [DOI] [PubMed] [Google Scholar]
- 132. Besheer T, Elalfy H, Abd El-Maksoud M, et al. . Diffusion-weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus. World J Gastroenterol. 2019;25:1366–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Trebicka J, Anadol E, Elfimova N, et al. . Hepatic and serum levels of miR-122 after chronic HCV-induced fibrosis. J Hepatol. 2013;58:234–239. [DOI] [PubMed] [Google Scholar]
- 134. Appourchaux K, Dokmak S, Resche-Rigon M, et al. . MicroRNA-based diagnostic tools for advanced fibrosis and cirrhosis in patients with chronic hepatitis B and C. Sci Rep. 2016;6:34935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Halász T, Horváth G, Pár G, et al. . miR-122 negatively correlates with liver fibrosis as detected by histology and FibroScan. World J Gastroenterol. 2015;21:7814–7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Waidmann O, Köberle V, Brunner F, et al. . Serum microRNA-122 predicts survival in patients with liver cirrhosis. Plos One. 2012;7:e45652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Liu XL, Pan Q, Zhang RN, et al. . Disease-specific miR-34a as diagnostic marker of non-alcoholic steatohepatitis in a Chinese population. World J Gastroenterol. 2016;22:9844–9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Feili X, Wu S, Ye W, et al. . MicroRNA-34a-5p inhibits liver fibrosis by regulating TGF-β1/Smad3 pathway in hepatic stellate cells. Cell Biol Int. 2018;42:1370–1376. [DOI] [PubMed] [Google Scholar]
- 139. Amaral AED, Rode MP, Cisilotto J, et al. . MicroRNA profiles in serum samples from patients with stable cirrhosis and miRNA-21 as a predictor of transplant-free survival. Pharmacol Res. 2018;134:179–192. [DOI] [PubMed] [Google Scholar]
- 140. Zhang T, Hu J, Wang X, et al. . MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-κB-TNFα pathway. J Hepatol. 2019;70:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hyun J, Wang S, Kim J, et al. . MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat Commun. 2016;7:10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Matsuura K, De Giorgi V, Schechterly C, et al. . Circulating let-7 levels in plasma and extracellular vesicles correlate with hepatic fibrosis progression in chronic hepatitis C. Hepatology. 2016;64:732–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Matsuura K, Aizawa N, Enomoto H, et al. . Circulating let-7 levels in serum correlate with the severity of hepatic fibrosis in chronic hepatitis C. Open Forum Infect Dis. 2018;5:ofy268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Schueller F, Roy S, Loosen SH, et al. . miR-223 represents a biomarker in acute and chronic liver injury. Clin Sci (Lond). 2017;131:1971–1987. [DOI] [PubMed] [Google Scholar]
- 145. Shaker OG, Senousy MA. Serum microRNAs as predictors for liver fibrosis staging in hepatitis C virus-associated chronic liver disease patients. J Viral Hepat. 2017;24:636–644. [DOI] [PubMed] [Google Scholar]
- 146. Zhang Z, Zha Y, Hu W, et al. . The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. J Biol Chem. 2013;288:37082–37093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ning Z, Luo X, Wang G, et al. . MicroRNA-21 mediates angiotensin II-induced liver fibrosis by activating NLRP3 inflammasome/IL-1β axisvia targeting Smad7 and Spry1. Antioxid Redox Sign. 2017;27:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhang Y, Liu C, Barbier O, et al. . Bcl2 is a critical regulator of bile acid homeostasis by dictating Shp and lncRNA H19 function. Sci Rep. 2016;6 :20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Li X, Liu R, Huang Z, et al. . Cholangiocyte-derived exosomal long noncoding RNA H19 promotes cholestatic liver injury in mouse and humans. Hepatology. 2018;68:599–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhu Y, Ni T, Lin J, et al. . Long non-coding RNA H19, a negative regulator of microRNA-148b-3p, participates in hypoxia stress in human hepatic sinusoidal endothelial cells via NOX4 and eNOS/NO signaling. Biochimie. 2019;163:128–136. [DOI] [PubMed] [Google Scholar]
- 151. Dai X, Chen C, Xue J, et al. . Exosomal MALAT1 derived from hepatic cells is involved in the activation of hepatic stellate cells via miRNA-26b in fibrosis induced by arsenite. Toxicol Lett. 2019;316:73–84. [DOI] [PubMed] [Google Scholar]
- 152. Leti F, Legendre C, Still CD, et al. . Altered expression of MALAT1 lncRNA in nonalcoholic steatohepatitis fibrosis regulates CXCL5 in hepatic stellate cells. Transl Res. 2017;190:25–39.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yu F, Lu Z, Cai J, et al. . MALAT1 functions as a competing endogenous RNA to mediate Rac1 expression by sequestering miR-101b in liver fibrosis. Cell Cycle. 2015;14:3885–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zheng J, Dong P, Mao Y, et al. . lincRNA-p21 inhibits hepatic stellate cell activation and liver fibrogenesis via p21. Febs J. 2015;282:4810–4821. [DOI] [PubMed] [Google Scholar]
- 155. Yu F, Lu Z, Chen B, et al. . Identification of a novel lincRNA-p21-miR-181b-PTEN signaling cascade in liver fibrosis. Mediators Inflamm. 2016;2016:9856538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yu F, Zhou G, Huang K, et al. . Serum lincRNA-p21 as a potential biomarker of liver fibrosis in chronic hepatitis B patients. J Viral Hepat. 2017;24:580–588. [DOI] [PubMed] [Google Scholar]
- 157. Yu F, Zheng J, Mao Y, et al. . Long non-coding RNA APTR promotes the activation of hepatic stellate cells and the progression of liver fibrosis. Biochem Biophys Res Commun. 2015;463:679–685. [DOI] [PubMed] [Google Scholar]
- 158. Yu S, Qi Y, Jiang J, et al. . APTR is a prognostic marker in cirrhotic patients with portal hypertension during TIPS procedure. Gene. 2018;645:30–33. [DOI] [PubMed] [Google Scholar]
- 159. Fu N, Niu X, Wang Y, et al. . Role of LncRNA-activated by transforming growth factor beta in the progression of hepatitis C virus-related liver fibrosis. Discov Med. 2016;22:29–42. [PubMed] [Google Scholar]
- 160. Liang S, CaiG, Duan Z, et al. . Urinary sediment miRNAs reflect tubulointerstitial damage and therapeutic response in IgA nephropathy. BMC Nephrol. 2017;18:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Nafar M, Samavat S, Shahraki E. Downregulation of profibrotic gene expression by angiotensin receptor blockers. Iran J Kidney Dis. 2018;12:369–375. [PubMed] [Google Scholar]
- 162. Chau BN, Xin C, Hartner J, et al. . MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4:121ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Glowacki F, Savary G, Gnemmi V, et al. . Increased circulating miR-21 levels are associated with kidney fibrosis. Plos One. 2013;8:e58014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lv CY, Ding WJ, Wang YL, et al. . A PEG-based method for the isolation of urinary exosomes and its application in renal fibrosis diagnostics using cargo miR-29c and miR-21 analysis. Int Urol Nephrol. 2018;50:973–982. [DOI] [PubMed] [Google Scholar]
- 165. Hennino MF, Buob D, Van der Hauwaert C, et al. . miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Sci Rep. 2016;6:27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang G, Kwan BC, Lai FM, et al. . Urinary miR-21, miR-29, and miR-93: novel biomarkers of fibrosis. Am J Nephrol. 2012;36:412–418. [DOI] [PubMed] [Google Scholar]
- 167. Denby L, Ramdas V, Lu R, et al. . MicroRNA-214 antagonism protects against renal fibrosis. J Am Soc Nephrol. 2014;25:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lv LL, Cao YH, Ni HF, et al. . MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305:F1220–F1227. [DOI] [PubMed] [Google Scholar]
- 169. van Rooij E, Sutherland LB, Thatcher JE, et al. . Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sassi Y, Avramopoulos P, Ramanujam D, et al. . Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat Commun. 2017;8:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rubiś P, Totoń-Żurańska J, Wiśniowska-Śmiałek S, et al. . Relations between circulating microRNAs (miR-21, miR-26, miR-29, miR-30 and miR-133a), extracellular matrix fibrosis and serum markers of fibrosis in dilated cardiomyopathy. Int J Cardiol. 2017;231:201–206. [DOI] [PubMed] [Google Scholar]
- 172. Roncarati R, Viviani Anselmi C, Losi MA, et al. . Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2014;63:920–927. [DOI] [PubMed] [Google Scholar]
- 173. Zile MR, Mehurg SM, Arroyo JE, et al. . Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. Circ Cardiovasc Genet. 2011;4:614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Leistner DM, Boeckel JN, Reis SM, et al. . Transcoronary gradients of vascular miRNAs and coronary atherosclerotic plaque characteristics. Eur Heart J. 2016;37:1738–1749. [DOI] [PubMed] [Google Scholar]
- 175. Wang Y, Li M, Xu L, et al. . Expression of Bcl-2 and microRNAs in cardiac tissues of patients with dilated cardiomyopathy. Mol Med Rep. 2017;15:359–365. [DOI] [PubMed] [Google Scholar]
- 176. Liu X, Dong Y, Chen S, et al. . Circulating microRNA-146a and microRNA-21 predict left ventricular remodeling after ST-elevation myocardial infarction. Cardiology. 2015;132:233–241. [DOI] [PubMed] [Google Scholar]
- 177. Villar AV, García R, Merino D, et al. . Myocardial and circulating levels of microRNA-21 reflect left ventricular fibrosis in aortic stenosis patients. Int J Cardiol. 2013;167:2875–2881. [DOI] [PubMed] [Google Scholar]
- 178. Watanabe K, Narumi T, Watanabe T, et al. . The association between microRNA-21 and hypertension-induced cardiac remodeling. PLos One. 2020;15:e226053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Fang L, Ellims AH, Moore XL, et al. . Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J Transl Med. 2015;13:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Nonaka CKV, Macêdo CT, Cavalcante BRR, et al. . Circulating miRNAs as potential biomarkers associated with cardiac remodeling and fibrosis in Chagas disease cardiomyopathy. Int J Mol Sci. 2019;20:4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Thum T, Gross C, Fiedler J, et al. . MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. [DOI] [PubMed] [Google Scholar]
- 182. Adam O, Löhfelm B, Thum T, et al. . Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. 2012;107:278. [DOI] [PubMed] [Google Scholar]
- 183. Satoh M, Minami Y, Takahashi Y, et al. . Expression of microRNA-208 is associated with adverse clinical outcomes in human dilated cardiomyopathy. J Card Fail. 2010;16:404–410. [DOI] [PubMed] [Google Scholar]
- 184. Wang J, Pei Y, Zhong Y, et al. . Altered serum microRNAs as novel diagnostic biomarkers for atypical coronary artery disease. Plos One. 2014;9:e107012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rubiś P, Totoń-Żurańska J, Wiśniowska-Śmiałek S, et al. . The relationship between myocardial fibrosis and myocardial microRNAs in dilated cardiomyopathy: a link between mir-133a and cardiovascular events. J Cell Mol Med. 2018;22:2514–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Duisters RF, Tijsen AJ, Schroen B, et al. . miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–17 8. [DOI] [PubMed] [Google Scholar]
- 187. Besler C, Urban D, Watzka S, et al. . Endomyocardial miR-133a levels correlate with myocardial inflammation, improved left ventricular function, and clinical outcome in patients with inflammatory cardiomyopathy. Eur J Heart Fail. 2016;18:1442–1451. [DOI] [PubMed] [Google Scholar]
- 188. Kin K, Miyagawa S, Fukushima S, et al. . Tissue- and plasma-specific microRNA signatures for atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc. 2012;1:e000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wang J, Wang Y, Han J, et al. . Integrated analysis of microRNA and mRNA expression profiles in the left atrium of patients with nonvalvular paroxysmal atrial fibrillation: role of miR-146b-5p in atrial fibrosis. Heart Rhythm. 2015;12:1018–1026. [DOI] [PubMed] [Google Scholar]
- 190. Budding K, Rossato M, van de Graaf EA, et al. . Serum miRNAs as potential biomarkers for the bronchiolitis obliterans syndrome after lung transplantation. Transpl Immunol. 2017;42:1–4. [DOI] [PubMed] [Google Scholar]
- 191. Yang G, Yang L, Wang W, et al. . Discovery and validation of extracellular/circulating microRNAs during idiopathic pulmonary fibrosis disease progression. Gene. 2015;562:138–144. [DOI] [PubMed] [Google Scholar]
- 192. Kwon OS, Kim KT, Lee E, et al. . Induction of MiR-21 by stereotactic body radiotherapy contributes to the pulmonary fibrotic response. Plos One. 2016;11:e0154942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Makiguchi T, Yamada M, Yoshioka Y, et al. . Serum extracellular vesicular miR-21-5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respir Res. 2016;17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Li P, Zhao GQ, Chen TF, et al. . Serum miR-21 and miR-155 expression in idiopathic pulmonary fibrosis. J Asthma. 2013;50:960–964. [DOI] [PubMed] [Google Scholar]
- 195. Liu G, Friggeri A, Yang Y, et al. . miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Yang S, Banerjee S, de Freitas A, et al. . Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012;180:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Christmann RB, Wooten A, Sampaio-Barros P, et al. . miR-155 in the progression of lung fibrosis in systemic sclerosis. Arthritis Res Ther. 2016;18:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Li P, Li J, Chen T, et al. . Expression analysis of serum microRNAs in idiopathic pulmonary fibrosis. Int J Mol Med. 2014;33:1554–1562. [DOI] [PubMed] [Google Scholar]
- 199. Hassan F, Nuovo GJ, Crawford M, et al. . MiR-101 and miR-144 regulate the expression of the CFTR chloride channel in the lung. Plos One. 2012; 7:e50837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Viart V, Bergougnoux A, Bonini J, et al. . Transcription factors and miRNAs that regulate fetal to adult CFTR expression change are new targets for cystic fibrosis. Eur Respir J. 2015;45:116–128. [DOI] [PubMed] [Google Scholar]
- 201. Weldon S, McNally P, McAuley DF, et al. . miR-31 dysregulation in cystic fibrosis airways contributes to increased pulmonary cathepsin S production. Am J Respir Crit Care Med. 2014;190:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Zhu H, Li Y, Qu S, et al. . MicroRNA expression abnormalities in limited cutaneous scleroderma and diffuse cutaneous scleroderma. J Clin Immunol. 2012;32:514–522. [DOI] [PubMed] [Google Scholar]
- 203. Zhou R, Wang C, Wen C, et al. . miR-21 promotes collagen production in keloid via Smad7. Burns. 2017;43:555–561. [DOI] [PubMed] [Google Scholar]
- 204. Gallant-Behm CL, Piper J, Lynch JM, et al. . A MicroRNA-29 mimic (Remlarsen) represses extracellular matrix expression and fibroplasia in the skin. J Invest Dermatol. 2019;139:1073–1081. [DOI] [PubMed] [Google Scholar]
- 205. Bi S, Cao C, Chai LL, et al. . Regulatory mechanism of miR-29 over TGF-β1 and COL1 in scar cells. Eur Rev Med Pharmacol Sci. 2017;21:2512–2517. [PubMed] [Google Scholar]
- 206. Gras C, Ratuszny D, Hadamitzky C, et al. . miR-145 contributes to hypertrophic scarring of the skin by inducing myofibroblast activity. Mol Med. 2015;21:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Sun XJ, Wang Q, Guo B, et al. . Identification of skin-related lncRNAs as potential biomarkers that involved in Wnt pathways in keloids. Oncotarget. 2017;8:34236–34244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Jin J, Zhai HF, Jia ZH, et al. . Long non-coding RNA HOXA11-AS induces type I collagen synthesis to stimulate keloid formation via sponging miR-124-3p and activation of Smad5 signaling. Am J Physiol Cell Physiol. 2019;317:C1001–C1010. [DOI] [PubMed] [Google Scholar]
- 209. Liang X, Ma L, Long X, et al. . LncRNA expression profiles and validation in keloid and normal skin tissue. Int J Oncol. 2015;47:1829–1838. [DOI] [PubMed] [Google Scholar]
- 210. Nijhuis A, Curciarello R, Mehta S, et al. . MCL-1 is modulated in Crohn’s disease fibrosis by miR-29b via IL-6 and IL-8. Cell Tissue Res. 2017;368: 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Matsui M, Corey DR. Non-coding RNAs as drug targets. Nat Rev Drug Discov. 2017;16:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Janssen HL, Reesink HW, Lawitz EJ, et al. . Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. [DOI] [PubMed] [Google Scholar]
- 213. Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. . Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Xue X, Zhang Y, Zhi Q, et al. . MiR200-upregulated Vasohibin 2 promotes the malignant transformation of tumors by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. Cell Commun Signal. 2014;12:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Jiang JH, Lv QY, Yi YX, et al. . MicroRNA-200a promotes proliferation and invasion of ovarian cancer cells by targeting PTEN. Eur Rev Med Pharmacol Sci. 2018;22:6260–6267. [DOI] [PubMed] [Google Scholar]