Abstract
Context
High-residual C-peptide in longer-duration type 1 diabetes (T1D) is associated with fewer hypoglycemic events and reduced glycemic variability. Little is known about the impact of C-peptide close to diagnosis.
Objective
Using continuous glucose monitoring (CGM) data from a study of newly diagnosed adults with T1D, we aimed to explore if variation in C-peptide close to diagnosis influenced glycemic variability and risk of hypoglycemia.
Methods
We studied newly diagnosed adults with T1D who wore a Dexcom G4 CGM for 7 days as part of the Exercise in Type 1 Diabetes (EXTOD) study. We examined the relationship between peak stimulated C-peptide and glycemic metrics of variability and hypoglycemia for 36 CGM traces from 23 participants.
Results
For every 100 pmol/L-increase in peak C-peptide, the percentage of time spent in the range 3.9 to 10 mmol/L increased by 2.4% (95% CI, 0.5-4.3), P = .01) with a reduction in time spent at level 1 hyperglycemia (> 10 mmol/L) and level 2 hyperglycemia (> 13.9 mmol/L) by 2.6% (95% CI, –4.9 to –0.4, P = .02) and 1.3% (95% CI, –2.7 to –0.006, P = .04), respectively. Glucose levels were on average lower by 0.19 mmol/L (95% CI, –0.4 to 0.02, P = .06) and SD reduced by 0.14 (95% CI, –0.3 to –0.02, P = .02). Hypoglycemia was not common in this group and no association was observed between time spent in hypoglycemia (P = .97) or hypoglycemic risk (P = .72). There was no association between peak C-peptide and insulin dose–adjusted glycated hemoglobin A1c (P = .45).
Conclusion
C-peptide is associated with time spent in the normal glucose range and with less hyperglycemia, but not risk of hypoglycemia in newly diagnosed people with T1D.
Keywords: type 1 diabetes, C-peptide, hypoglycemia, CGM
Residual endogenous insulin production, as measured by serum C-peptide, is invariably present at the time of diagnosis with type 1 diabetes (T1D) [1]. These C-peptide levels are variable and fall exponentially in the first 7 years [2-5], with those diagnosed in adulthood more likely to retain significant levels of C-peptide years post diagnosis [1, 6, 7]. Evidence originally from the Diabetes Control and Complications Trial [8-10] and more recent studies [11-15] indicates that persistent detectable C-peptide is associated with reduced frequency and severity of self-reported hypoglycemia and fewer long-term microvascular complications. This has led to the adoption of mixed-meal stimulated C-peptide as a primary outcome measure of intervention trials that prevent or delay β-cell destruction [16].
Recently, increased use of flash glucose monitoring and continuous glucose monitoring (CGM) have highlighted the impact of persistent C-peptide on glycemic variability and hypoglycemia [12, 14, 15, 17-19]. Most data have been derived from studies of adults with long duration T1D or post islet transplantation where C-peptide persistence is associated with lower glycated hemoglobin A1c (HbA1c) and/or insulin dose, fewer low-glucose events, decreased variation, and more time spent in range (3.9-10 mmol/L) [11, 12, 14, 15, 19]. Less is known about the impact of C-peptide close to diagnosis. This is important because this has the potential to inform the most effective approach to supporting the newly diagnosed patient, and to identify early benefits of C-peptide preservation. A single study in newly diagnosed children demonstrated that the level of preserved peak C-peptide correlates with more time in the range of 3.9 to 7.8 mmol/L and less variability [18]. This study found no association between peak C-peptide and hypoglycemia (detected by CGM) in contrast to a study of adults with long-duration T1D, in which such an association was demonstrated [12]. No studies have looked at the impact of C-peptide on glucose control as measured by CGM in adults newly diagnosed with T1D.
In the present study we aimed to use CGM data from adults with recent-onset T1D to assess and describe the impact of variation in endogenous insulin secretion close to diagnosis on glycemic variability and hypoglycemia.
Materials and Methods
We performed a secondary analysis of peak (90-minute) mixed-meal tolerance test (MMTT) C-peptide and glycemic metrics of variability and hypoglycemia from a Dexcom G4 CGM measured as part of the Exercise in Type 1 Diabetes (EXTOD) Study (ISRCTN91388505) [20]. EXTOD was a pilot study undertaken to explore whether exercise can preserve β-cell function in adults newly diagnosed with T1D. It aimed to assess uptake, intervention adherence, dropout rates, and the rate of loss of β-cell function in a usual care group and exercise intervention group over 12 months [20, 21]. The EXTOD study was approved by the Birmingham East, North and Solihull Research Ethics Committee (No. 0/H1206/4), UK. All participants provided written informed consent in accordance with the Declaration of Helsinki.
Study Cohort
Participants were recruited between November 2011 and January 2014, from 19 UK National Health Service (NHS) hospitals. Eligible participants had a clinical diagnosis of T1D, were older than 16 years at diagnosis, and were self‐administering their insulin as part of a multiple-dose (basal/bolus) injection regimen. Participants included in the EXTOD pilot study were adults aged 18 to 60 years, diagnosed with T1D for less than 3 months, had C‐peptide greater than 200 pmol/L at 90 minutes following meal stimulation, had controlled blood pressure, were not pregnant or planning pregnancy, and were able to increase exercise levels and not on therapy that affect heart rate (β-blocker, calcium channel antagonist).
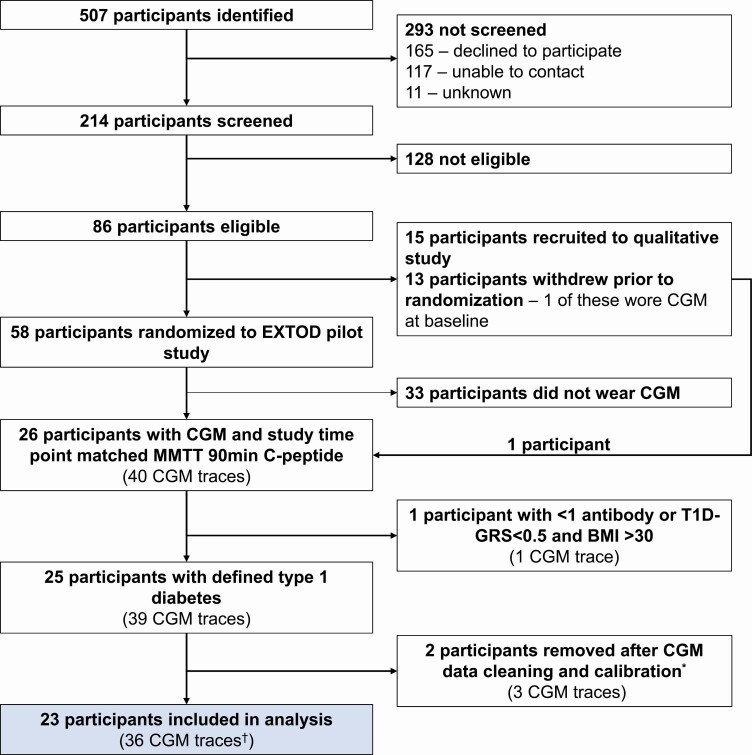
A total of 507 adults with new-onset T1D were identified; of these 214 were assessed for eligibility for the EXTOD pilot study. No participants were excluded from taking part because of low C-peptide. Eighty-six were eligible for face-to-face screening; of these, 15 participants were recruited into a distinct but linked study exploring barriers to exercise in newly diagnosed T1D, and 58 participants were randomly assigned to control (usual care) or intervention (exercise consultation every month + usual care) in a 1:1 ratio for 12 months (Fig. 1).
Figure 1.
Flow of participants included in this secondary analysis and breakdown of number of corresponding continuous glucose monitoring (CGM) traces analyzed by study time point. *Calibration is dependent on the sensor used in this study (Dexcom G4). Calibration excluded 1) whole traces if 2 blood glucose calibrations were not completed at the start of sensor wearing, 2) a day of wear if the mean absolute relative difference of the sensor glucose and blood glucose calibration that day is greater than 20% or if fewer than 2 blood glucose calibrations were completed that day. †Breakdown of CGM traces by study time point: baseline n = 21 CGM traces, 6 months n = 9 CGM traces, 12 months n = 6 CGM traces.
In this study we performed a secondary analysis of peak MMTT C-peptide and glycemic metrics of variability and hypoglycemia from 26 eligible participants of the EXTOD study who had consented to wearing a CGM at any study time point. We included 23 participants in this secondary analysis with defined T1D by 1 or more autoantibodies or a T1D genetic risk score (T1D-GRS) greater than the 50th percentile for T1D, and a body mass index (BMI) of less than 30, a validated CGM trace, and a 90-minute MMTT C-peptide matched to the study time point of CGM wear. A total of 36 CGM traces (4101 hours) were analyzed from 23 participants (see Fig. 1).
Procedures
β-Cell function was assessed at baseline (pre randomization), and at 6 and 12 months post randomization using a 240-mL Fortisip MMTT with blood taken for C‐peptide at –10, 0, 15, 30, 60, 90, and 120 minutes. Blood was immediately centrifuged and the plasma stored at –80 °C until analysis. Patients at each study visit had an option to wear a Dexcom G4 CGM with the aim of assessing the feasibility of using this as an outcome. The CGM was worn blinded with participants using usual care to monitor their blood glucose during the study. C‐peptide was measured using a direct electrochemiluminescence immunoassay at the Academic Department of Blood Sciences at the Royal Devon and Exeter NHS Foundation Trust as previously described [22]. The limit of the C‐peptide assay is 3.3 pmol/L. Antibodies were measured at the Research Laboratories of the School of Clinical Sciences, University of Bristol (Southmead Hospital, Bristol, UK). Insulin doses were used in calculation of the insulin dose–adjusted HbA1c (IDAA1c) as previously described [23]. We generated a T1D-GRS using a KASP genotyping assay (LGC Genomics) of 10 single-nucleotide variations as previously described [24].
Continuous Glucose Monitoring Processing
All CGM processing and analysis were performed in R statistical software version 3.6.1 (Foundation for Statistical Computing). Forty CGM traces from 26 participants went through processing. The CGM was expected to be worn for a minimum of 7 days, with the majority of the participants meeting this goal, with data cut off at 8 days if participants exceeded 7 days. As part of the processing, we calibrated CGM traces against an updated self-monitoring of blood glucose value every 12 hours as required by the Dexcom G4 sensor. Calibration excluded 1) whole traces if 2 blood glucose calibrations were not completed at the start of sensor wear, which are required to start the Dexcom G4 sensor (n = 1); 2) a whole day of wear from traces if the mean absolute relative difference (MARD) of blood glucose calibration reading and sensor glucose reading was greater than 20%, or if fewer than 2 blood glucoses calibrations were completed that particular day. The majority of traces after calibration had more than 95% of data remaining. Traces from participants were excluded if 12 hours or less of data remained after calibration (n = 2). We generated CGM-derived metrics of glycemic variability and hypoglycemia in accordance with, and using definitions of hypoglycemic/hyperglycemic episodes from, the International Consensus on Use of Continuous Glucose Monitoring [25]. We validated the CGM processing and analysis against results from manual processing completed by 2 individuals.
Statistical Analysis
Statistical analysis was performed in R statistical software version 3.6.1 (Foundation for Statistical Computing) using the nlme and afex packages. We assessed the association between MMTT C-peptide level and the consensus glycemic metrics of variability and hypoglycemia using repeated-measures, mixed-effects models, with glycemic metric as the outcome, C-peptide level (as picomole per liter [pmol/L]) as the predictor, with patient identification as the random effect. We report the modeling coefficients for 100-pmol/L change in C-peptide for clinical interpretation. Residual plots were examined for normality to ensure model assumptions were met. Significance was tested at the level of .05. Many clinical variables were not normally distributed, so data are presented as median and interquartile range (IQR).
Results
Participants and Characteristics
Twenty-one participants’ first point of CGM wear was at baseline, and 2 participants’ first point of CGM wear was at 6 months post trial randomization. Twenty-one participants wore a CGM at baseline, 10 at 6 months, and 6 at 12 months post trial randomization, totaling 36 CGM traces with a study time point–matched MMTT 90-minute C-peptide (Fig. 1). One participant was not randomly assigned in the primary EXTOD study. The characteristics of these participants at first wear of CGM are shown in Table 1. Participants had a median duration of disease of 2.4 months (IQR, 1.2-2.4 months), with the majority having a duration of symptoms of less than 1 month (median [IQR] 8 days [range, 4-12 days] with 14% presenting in diabetic ketoacidosis. Participants had a median peak MMTT C-peptide of 865 pmol/L (IQR, 684-1120 pmol/L) and an HbA1c of 67.5 mmol/mol (IQR, 48.2-76.2 mmol/mol) at their first wearing of CGM. Participants were all of White European descent with a BMI of (median [IQR] 23.5 [22.2-26.4]) and median insulin dose of 0.25 U/kg (IQR, 0.15-0.45 U/kg). Baseline characteristics of our sample were similar to the remaining participants recruited to the EXTOD study [20].
Table 1.
Characteristics of participants included in analysis from the Exercise in Type 1 Diabetes pilot study at first wearing of continuous glucose monitoring (N = 23 participants)
| N = 23a | |
|---|---|
| Age, y | 27.2 (23.4-36.5) |
| Duration of diabetes, month | 2.40 (1.20-2.40) |
| Sex | |
| Female | 10 (44%) |
| Male | 13 (57%) |
| Ethnic origin | |
| White British | 22 (96%) |
| Other White background | 1 (4.4%) |
| BMI | 23.5 (22.2-26.4) |
| HbA 1c , mmol/mol | 67.5 (48.2-76.2) |
| Insulin dose, U/kg | 0.25 (0.15-0.45) |
| Peak (90 min) MMTT C-peptide, pmol/L | 865 (684-1120) |
| T1D-GRS | 0.64 (0.55-0.76) |
| Presentation of diabetes | |
| Duration of symptoms pre diagnosis, d | 8.00 (4.00-12.0) |
| DKA | 3 (14%) |
| Hyperglycemia without acidosis | 19 (86%) |
| GAD-positive titer | 19 (83%) |
| IA 2A–positive titer | 13 (57%) |
| ZnT8-positive titer | 12 (52%) |
| Randomization arm | |
| Usual care | 9 (39%) |
| Intervention | 13 (57%) |
| Not randomly assigned | 1 (4%) |
Abbreviations: BMI, body mass index; DKA, diabetic ketoacidosis; GAD, glutamic acid decarboxylase; HbA1c, glycated hemoglobin A1c; MMTT, mixed-meal tolerance test; T1D-GRS, type 1 diabetes genetic risk score.
a Data presented as median (25th-75th), number (%).
Glycemic Characteristics
The glycemic characteristics of these participants during each time of CGM wear are shown in Table 2. Most participants had tight glucose control, with median percentage of time spent in the range of 3.9 to 10 mmol/L of 68% (IQR, 55%-76% mmol/L). Average glucose was 8.3 mmol/L (median [IQR], 7.1-9.3 mmol/L) with low levels of hypoglycemia (percentage of time spent in hypoglycemia; median [IQR], 0.0% [0.0%-0.6%]), little to no time in level 1 low events (percentage of time spent 3-< 3.9 mmol/L; median [IQR], 0.6% [0.2%-1.7%]), and even fewer level 2 low events (percentage of time spent < 3 mmol/l; median [IQR], 0.0% [0.0%-1.0%]). Overall, glycemia was considered stable (< 36%) [26] in these participants (coefficient of variation; median [IQR], 32% [26%-36%]).
Table 2.
Metrics of glycemic variability and hypoglycemia for participants for continuous glucose monitoring (CGM) wearing at any study time point (N = 36 CGM traces, 23 participants)
| N = 36a | |
|---|---|
| Percentage expected wear | 97.5 (83.9-105) |
| Percentage of good data remaining post calibration | 97.4 (92.7-100) |
| No. of days of good data | 6.35 (5.26-7.18) |
| Average glucose, mmol/L | 8.27 (7.12-9.30) |
| SD, mmol/L | 2.60 (1.97-3.42) |
| CV, % | 32.0 (26.0-36.0) |
| MAGE | 4.99 (4.02-6.63) |
| Estimated HbA1c, mmol/mol | 51.4 (43.2-57.7) |
| Time spent > 10 mmol/L, level 1 elevated, % | 22.9 (9.43-37.3) |
| Time spent level 1 hyperglycemia, > 10 mmol/L ≥ 15 min, % | 25.2 (9.56-38.5) |
| Time spent > 13.9 mmol/L, level 2 elevated, % | 5.98 (1.59-13.2) |
| Time spent level 2 hyperglycemia, > 13.9 mmol/L ≥ 15 min, % | 2.62 (0.28-8.70) |
| HBGI | 7.43 (3.28-10.4) |
| Time spent 3.9-10 mmol/L, % | 68.3 (55.1-76.2) |
| Time spent 3-< 3.9 mmol/L, level 1 low, % | 0.63 (0.23-1.70) |
| Time spent < 3 mmol/L, level 2 low, % | 0.00 (0.00-1.00) |
| Time spent in hypoglycemia, % | 0.00 (0.00-0.58) |
| LBGI | 1.96 (1.28-2.64) |
Abbreviations: CV, coefficient of variation; HbA1c, glycated hemoglobin A1c; HBGI, high blood glucose index; LBGI, low blood glucose index; MAGE, mean amplitude glycemic excursion.
a Data presented as median (25th-75th).
C-Peptide at Diagnosis Is Associated With Less Glucose Variability, More Time in Range, and Less Hyperglycemia
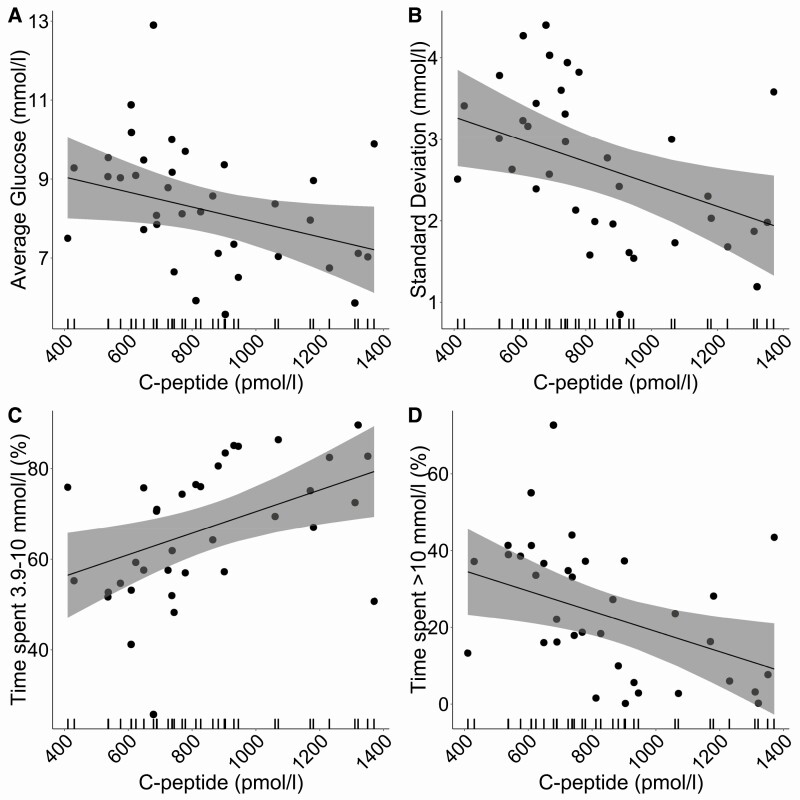
For every 100-pmol/L increase in peak C-peptide, glucose levels were on average lower by 0.2 mmol/L (95% CI, –0.4 to 0.02, P = .06) (Fig. 2A, Table 3). In addition, there was a reduced SD in glucose of 0.1 (95% CI, –0.3 to –0.02, P = .02) (Fig. 2B, see Table 3). Percentage of time spent in the range of 3.9 to 10 mmol/L increased by 2.4% (95% CI, 0.5-4.3, P = .01) (Fig. 2C, see Table 3) with a reduction in the amount of time (percentage) spent at level 1 (> 10 mmol/L) elevated glucose levels by 2.6% (95% CI, –4.9 to –0.4, P = .02) (Fig. 2D, see Table 3) and level 2 (> 13.9 mmol/l) elevated glucose levels by 1.3% (95% CI, –2.7 to –0.006], P = .04) (see Table 3). Coefficients for all metrics of glycemic variability are outlined in Table 3, and all followed the same direction in a reduction of variability for every 100-pmol/L change in C-peptide.
Figure 2.
Distribution of 4 key glycemic metrics: A, average glucose; B, SD; C, percentage of time in range 3.9 to 10 mmol/L; and D, percentage of time spent at greater than 10 mmol/L with peak mixed-meal tolerance test (MMTT) C-peptide. The line represents repeated-measures, mixed-effects regression modeling between glycemic metric and peak MMTT C-peptide with 95% CI shown as a shaded bar.
Table 3.
Associations from repeated-measures, mixed-effects regression modeling of glycemic variability and hypoglycemia metrics with peak mixed-meal tolerance test C-peptide (N = 36 continuous glucose monitoring traces, 23 participants)
| Coefficient for 100 pmol/L change in C-peptide, N = 36a | P b | |
|---|---|---|
| Average glucose, mmol/L | –0.19 (–0.39 to 0.015) | .06 |
| SD, mmol/L | –0.14 (–0.25 to –0.023) | .02 |
| CV, % | –1.00 (–2.16 to 0.15) | .08 |
| MAGE | –0.34 (–0.63 to –0.050) | .02 |
| Estimated HbA1c, mmol/mol | –0.14 (–0.25 to –0.023) | .06 |
| Time spent > 10 mmol/L, level 1 elevated, % | –2.64 (–4.87 to –0.41) | .02 |
| Time spent level 1 hyperglycemia, > 10 mmol/L excursion ≥ 15 min, % | –3.53 (–6.64 to –0.42) | .02 |
| Time spent > 13.9 mmol/L, level 2 elevated, % | –1.33 (–2.66 to –0.0057) | .04 |
| Time spent level 2 hyperglycemia, > 13.9 mmol/L excursion ≥ 15 min, % | 0.92 (–2.02 to 0.19) | .09 |
| HBGI | –0.71 (–1.27 to –0.14) | .01 |
| Time spent 3.9-10 mmol/L, % | 2.39 (0.51 to 4.26) | .01 |
| Time spent 3-< 3.9 mmol/L, level 1 low, % | –0.015 (–0.22 to 0.19) | .88 |
| Time spent < 3 mmol/L, level 2 low, % | –0.028 (–0.17 to 0.11) | .66 |
| Time spent in hypoglycemia, % | 0.0052 (–0.27 to 0.26) | .97 |
| LBGI | –0.062 (–0.41 to 0.29) | .72 |
Abbreviations: CV, coefficient of variation; HbA1c, glycated hemoglobin A1c; HBGI, high blood glucose index; LBGI, low blood glucose index; MAGE, mean amplitude glycemic excursion.
a Data presented as coefficient (95% CI).
b Kenward-Roger approximation for degrees of freedom.
C-Peptide at Diagnosis Is not Associated With Hypoglycemia
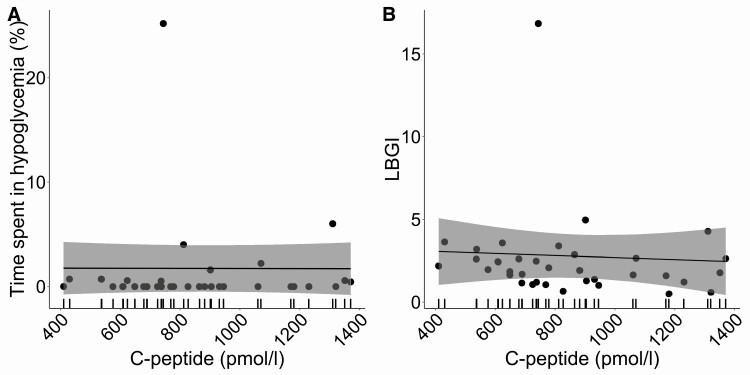
In this cohort hypoglycemic events were rare during all times of CGM wear (Fig. 3, see Table 2). There was no association with peak C-peptide and percentage time spent in hypoglycemic ranges (P = .97) or hypoglycemic risk, as measured by low blood glucose index (P = .72) (Fig. 3, see Table 3).
Figure 3.
Distribution of hypoglycemic metrics: percentage of time spent in A, hypoglycemia, and B, low blood glucose index (LBGI) with peak mixed-meal tolerance test (MMTT) C-peptide. The line represents repeated-measures, mixed-effects regression modeling between glycemic metric and peak MMTT C-peptide with 95% CI shown as a shaded bar.
There was also no association between peak C-peptide and insulin dose (P = .71), HbA1c (P = .36), or IDAA1c, P = .45) (Table 4).
Table 4.
Associations for clinical measures with peak mixed-meal tolerance test C-peptide (N = 36 observations, 23 participants)
| Coefficient for 100 pmol/L change in C-peptide, N = 36a | P b | |
|---|---|---|
| IDAA1cc | –0.091 (–0.34 to 0.16) | .45 |
| Insulin dose | –0.005 (–0.033 to 0.023) | .71 |
| HbA1cc, mmol/mol | –1.02 (–3.37 to 1.32) | .36 |
Abbreviations: HbA1c, glycated hemoglobin A1c; IDAA1c, and insulin dose–adjusted glycated hemoglobin A1c.
a Data presented as coefficient (95% CI).
b Kenward-Roger approximation for degrees of freedom.
c Missing for one participant (one observation).
Discussion
We report that higher levels of C-peptide at diagnosis are associated with lower glycemic variability, more time in range, and less hyperglycemia, but not with hypoglycemia or HbA1c. Variations in the high levels of residual C-peptide present at the time of diagnosis with T1D are associated with key clinical outcomes and could potentially inform the most effective approach to supporting the newly diagnosed patient.
Our findings support and enhance the understanding of the benefits of preserved C-peptide in patients with newly diagnosed T1D. Our results are consistent with the one other study in newly diagnosed participants by Buckingham et al comparing CGM-measured glucose variability with MMTT C-peptide; however, Buckingham’s study was conducted in a largely pediatric cohort and did not compare C-peptide with CGM metrics as the primary analysis outcome [18]. The study by Buckingham et al also found lower glucose variability is associated with higher levels of C-peptide, with increased time spent in range (3.9-7.8 mmol/L) and decreased variation for higher levels of C-peptide, with no associations demonstrated with hypoglycemia. Both studies estimated C-peptide at peak following meal stimulation with similar values in the range of 100 to 1500 pmol/L. We did not find an association of C-peptide with HbA1c and insulin dose in our study, in contrast with the study by Buckingham and colleagues, as well as studies in new-onset T1D [27] in which immunomodulation has resulted in some preservation in C-peptide being associated with lower HbA1c and insulin doses. It is possible that the lower numbers in our study prevented us from observing an impact on HbA1c and insulin dose; another possibility is that alterations of HbA1c and insulin dose may be influenced by study protocol or clinical care.
Studies exploring the benefits of preserved C-peptide in long-duration T1D demonstrate a similar impact on time in range and glucose variability, but also commonly show protection from hypoglycemia [11, 12, 28] in addition to lower HbA1c [13, 15] and lower insulin doses [11]. However, we and others [18] do not find an association between variations in C-peptide level present at diagnosis and hypoglycemia. It is possible that the C-peptide levels at diagnosis, in addition to other factors related to a short duration of T1D, may offer more protection from hypoglycemia than in long-duration T1D, when endogenous insulin secretion is much lower or absent.
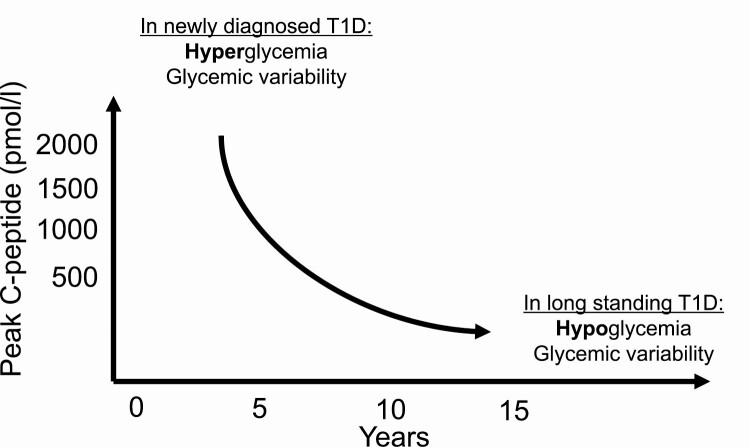
We therefore propose that the impact on glucose control associated with preserved C-peptide appears to vary across duration of disease in people with T1D (Fig. 4). Early after diagnosis when stimulated C-peptide values can reach higher than 1500 pmol/L, relatively higher levels of C-peptide reduce hyperglycemia and glucose variability, but not hypoglycemia. Later in the natural history, when stimulated C-peptide values are around 500 pmol/L or lower, higher values in this range reduce hypoglycemia and potentially also HbA1c and insulin dose. The benefits of less glycemic variability and greater time in range are present across the spectrum. Furthermore, preserved C-peptide in longer-duration T1D is associated, presumably through consistent tight glucose control, with fewer microvascular complications [5, 13]. Our study, combined with others [18], highlights that hypoglycemia is rare around the time of diagnosis.
Figure 4.
Summary of the impact variation in C-peptide level has on glucose control in people with type 1 diabetes (T1D), across diabetes duration. In newly diagnosed T1D, C-peptide variation impacts do not affect hypoglycemia, as demonstrated in longer-duration T1D. C-peptide variation affects glycemic variability near to diagnosis of T1D and at long-duration disease.
The strengths of this study include the careful cleaning and interpretation of the CGM data. To ensure a high standard of accuracy in our CGM data for analysis, we developed in-house CGM processing that compared the MARD of each self-monitored blood glucose calibration and the 15-minute later CGM sensor glucose reading, since intestinal glucose trails blood glucose by 5 to 20 minutes [29-31]. We used the assumption that an entire day of glucose readings had a systematic error if the self-monitored blood glucose calibration reading and CGM sensor glucose reading had a MARD of greater than 20%, and therefore removed it from the CGM trace before analysis and generation of CGM-derived glycemic metrics. We developed in-house CGM analysis to generate these metrics in accordance with the International Consensus on Use of Continuous Glucose Monitoring [25]. Our in-house processing and analysis were validated against manual processing and analysis conducted by 2 people. Furthermore, the CGM data obtained from the participants were blinded at the time of wear, ensuring measured sensor glucose was not highly influenced by patient reactivity. Also, MMTT-measured C-peptide, obtained according to protocol, offered reduced variation of C-peptide levels, which is more likely with randomly measured C-peptide. Since misclassification of diabetes is common at diagnosis, occurring in 7% to 15% of cases [32], we used a specific criteria for defining T1D that included clinical diagnosis and either positive autoantibodies or T1D-GRS in addition to BMI in our definition.
A notable limitation of this study is that this is a retrospective analysis of data collected as part of a randomized controlled trial, using CGM data and peak MMTT C-peptide from participants involved in the EXTOD study, a randomized exercise trial. Participants who enroll in exercise trials may not be wholly representative of the T1D population because of their levels of activity and the effect exercise may have on blood glucose. This may have affected the average glucose metrics that we demonstrate in this cohort. Our sample size was limited to the consent rate to CGM monitoring during the study, and a high dropout rate of CGM monitoring over the 12 months of study. This may have affected the power to detect associations with C-peptide and CGM metrics that describe glycemic variability. Nevertheless, it is reassuring that we observed the same directional associations in all CGM metrics that describe glycemic variability and hyperglycemia with peak MMTT C-peptide, with no associations observed with the CGM metrics that describe hypoglycemia and hypoglycemia risk. As we previously highlighted, the higher C-peptide levels present close to diagnosis may exceed a threshold needed to protect from hypoglycemia (minimal islet transplant function has been shown to protect from hypoglycemia [17]), which would explain the low rate of hypoglycemia commonly found post diagnosis and the lack of association found with postdiagnosis C-peptide by us and others.
Notwithstanding these limitations, our findings are important because they suggest that the benefits of C-peptide retention have a measurable impact from the point of diagnosis for a person with T1D. Our findings also highlight that metabolic or physiological differences between individuals may have more of an impact on glycemic variability than previously thought, with C-peptide playing a part in defining the manifestation of their T1D.
As we propose in Fig. 4, our results add to findings from previous studies of longer-duration diabetes, offering a more complete picture of the impact that variation in C-peptide levels has on glucose control in people with T1D. We suggest that managing newly diagnosed patients, informed by a current estimate of their C-peptide reserve, will influence how they are managed. Those with a lower C-peptide are likely to experience less time in glucose range, greater glucose variability, and more hyperglycemia and would be earmarked for earlier and more intensive support. Diabetes is currently the only endocrine condition for which the hormone in question is not measured as part of routine care. We suggest there is now increasing evidence to start doing so.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Parts of this work were presented at the American Diabetes Association online conference, June 25-29, 2021.
The study from which the data used in the paper comes received ethical opinion approval from Birmingham, East, North and Solihull Research Ethics committees in February 2010 (reference No. 10/H1206/4). The study was sponsored by the University of Birmingham.
Glossary
Abbreviations
- BMI
body mass index
- CGM
continuous glucose monitoring
- EXTOD
Exercise in Type 1 Diabetes
- HbA1c
glycated hemoglobin A1c
- IDAA1c
insulin dose–adjusted glycated hemoglobin A1c
- IQR
interquartile range
- MARD
mean absolute relative difference
- MMTT
mixed-meal tolerance test
- NHS
UK National Health Service
- T1D
type 1 diabetes
- T1D-GRS
type 1 diabetes genetic risk score.
Financial Support
This work was supported by a Diabetes UK Harry Keen Fellowship (No. 16/0005529 to R.A.O.). The study from which the data for the paper came was funded by the National Institute of Health Research (grant No. PB-PG-0609-19093). The content and views expressed are those of the authors.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Palmer JP. C-peptide in the natural history of type 1 diabetes. Diabetes Metab Res Rev. 2009;25(4):325-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shields BM, McDonald TJ, Oram R, et al. ; TIGI Consortium . C-peptide decline in type 1 diabetes has two phases: an initial exponential fall and a subsequent stable phase. Diabetes Care. 2018;41(7):1486-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greenbaum CJ, Beam CA, Boulware D, et al. ; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61(8):2066-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dabelea D, Mayer-Davis EJ, Andrews JS, et al. . Clinical evolution of beta cell function in youth with diabetes: the SEARCH for diabetes in youth study. Diabetologia. 2012;55(12):3359-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGee P, Steffes M, Nowicki M, et al. ; DCCT/EDIC Research Group . Insulin secretion measured by stimulated C-peptide in long-established Type 1 diabetes in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) cohort: a pilot study. Diabet Med. 2014;31(10):1264-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis AK, DuBose SN, Haller MJ, et al. ; T1D Exchange Clinic Network . Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38(3):476-481. [DOI] [PubMed] [Google Scholar]
- 7. Barker A, Lauria A, Schloot N, et al. . Age-dependent decline of β-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes Obes Metab. 2014;16(3):262-267. [DOI] [PubMed] [Google Scholar]
- 8. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26(3):832-836. [DOI] [PubMed] [Google Scholar]
- 9. Lachin JM, Orchard TJ, Nathan DM; DCCT/EDIC Research Group . Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the Diabetes Control and Complications Trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann Intern Med. 1998;128(7):517-523. [DOI] [PubMed] [Google Scholar]
- 11. Marren SM, Hammersley S, McDonald TJ, et al. ; TIGI Consortium . Persistent C-peptide is associated with reduced hypoglycaemia but not HbA1c in adults with longstanding Type 1 diabetes: evidence for lack of intensive treatment in UK clinical practice? Diabet Med. 2019;36(9):1092-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gibb FW, McKnight JA, Clarke C, Strachan MWJ. Preserved C-peptide secretion is associated with fewer low-glucose events and lower glucose variability on flash glucose monitoring in adults with type 1 diabetes. Diabetologia. 2020;63(5): 906-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeyam A, Colhoun H, McGurnaghan S, et al. . SDRNT1BIO Investigators. Clinical impact of residual C-peptide secretion in type 1 diabetes on glycemia and microvascular complications. Diabetes Care. 2021;44(2):390-398. [DOI] [PubMed] [Google Scholar]
- 14. Brooks AM, Oram R, Home P, Steen N, Shaw JAM. Demonstration of an intrinsic relationship between endogenous C-peptide concentration and determinants of glycemic control in type 1 diabetes following islet transplantation. Diabetes Care. 2015;38(1):105-112. [DOI] [PubMed] [Google Scholar]
- 15. Rickels MR, Evans-Molina C, Bahnson HT, et al. ; T1D Exchange β-Cell Function Study Group . High residual C-peptide likely contributes to glycemic control in type 1 diabetes. J Clin Invest. 2020;130(4):1850-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palmer JP, Fleming GA, Greenbaum CJ, et al. . C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes. 2004;53(1):250-264. [DOI] [PubMed] [Google Scholar]
- 17. Vantyghem MC, Raverdy V, Balavoine AS, et al. . Continuous glucose monitoring after islet transplantation in type 1 diabetes: an excellent graft function (β-score greater than 7) Is required to abrogate hyperglycemia, whereas a minimal function is necessary to suppress severe hypoglycemia (β-score greater than 3). J Clin Endocrinol Metab. 2012;97(11):E2078-E2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buckingham B, Cheng P, Beck RW, et al. ; Diabetes Research in Children Network (DirecNet) and Type 1 Diabetes TrialNet Study Groups . CGM-measured glucose values have a strong correlation with C-peptide, HbA1c and IDAAC, but do poorly in predicting C-peptide levels in the two years following onset of diabetes. Diabetologia. 2015;58(6):1167-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forbes S, Olateju TO, Lam A, CGM shows islet transplantation prevents hypoglycemia, correcting time in range and reducing glycemic variability, despite subnormal beta-cell function. Diabetes. 2018;67(Suppl 1):143-OR. [Google Scholar]
- 20. Narendran P, Jackson N, Daley A, et al. . Exercise to preserve β-cell function in recent-onset Type 1 diabetes mellitus (EXTOD)—a randomized controlled pilot trial. Diabet Med. 2017;34(11):1521-1531. [DOI] [PubMed] [Google Scholar]
- 21. Lascar N, Kennedy A, Jackson N, et al. . Exercise to preserve beta cell function in recent-onset type 1 diabetes mellitus (EXTOD)—a study protocol for a pilot randomized controlled trial. Trials. 2013;14:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDonald TJ, Perry MH, Peake RW, et al. . EDTA improves stability of whole blood C-peptide and insulin to over 24 hours at room temperature. PLoS One. 2012;7(7):e42084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mortensen HB, Hougaard P, Swift P, et al. ; Hvidoere Study Group on Childhood Diabetes . New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32(8):1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oram RA, Patel K, Hill A, et al. . A type 1 diabetes genetic risk score can aid discrimination between type 1 and type 2 diabetes in young adults. Diabetes Care. 2016;39(3):337-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Danne T, Nimri R, Battelino T, et al. . International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monnier L, Colette C, Wojtusciszyn A, et al. . Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40(7):832-838. [DOI] [PubMed] [Google Scholar]
- 27. Herold KC, Hagopian W, Auger JA, et al. . Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1692-1698. [DOI] [PubMed] [Google Scholar]
- 28. Gubitosi-Klug RA, Braffett BH, Hitt S, et al. . DCCT/EDIC Research Group. Residual β cell function in long-term type 1 diabetes associates with reduced incidence of hypoglycemia. J Clin Invest. 2021;131(3):e143011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes. 2003;52(11):2790-2794. [DOI] [PubMed] [Google Scholar]
- 30. Stout PJ, Peled N, Erickson BJ, Hilgers ME, Racchini JR, Hoegh TB. Comparison of glucose levels in dermal interstitial fluid and finger capillary blood. Diabetes Technol Ther. 2001;3(1):81-90. [DOI] [PubMed] [Google Scholar]
- 31. Cengiz E, Tamborlane WV. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol Ther. 2009;11(Suppl 1):S11-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shields BM, Peters JL, Cooper C, et al. . Can clinical features be used to differentiate type 1 from type 2 diabetes? A systematic review of the literature. BMJ Open. 2015;5(11):e009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.