We read with much interest the recent mini-review by Sun on the theoretically possible roles of cell surface sialic acids (Sias) in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (Sun 2021). Given the magnitude of this coronavirus disease 2019 (COVID-19) pandemic (Lu et al. 2020; Zhu et al. 2020) this question is not just of importance to the glycobiology community, but also to other scientists across many disciplines related to this crisis.
Numerous viruses are known to have hemagglutinin proteins that engage sialylated glycans as primary or secondary targets for infection, and many of them also display a “receptor-destroying enzyme”—either a neuraminidase (sialidase) that removes Sias, or an esterase that removes Sia O-acetyl groups required for virus binding (de Groot 2006; Lang et al. 2020). The latter types of viruses include some coronaviruses that cause mild infections and selectively recognize different kinds of Sias via hemagglutinin-esterase (HE) proteins (de Groot 2006; Huang et al. 2015; Tortorici et al. 2019; Lang et al. 2020).
Unlike many viruses that generally conserve their sialoglycan-recognizing properties or undergo subtle changes in binding preference, the coronaviruses seem to be involved in rapid evolution of binding specificity via convergent and divergent evolution, especially, with regard to their preferred ligands. Some coronaviruses have eliminated the esterase activity or even the entire HE protein and switched to sialic acid (Sia)-binding via a spike protein (Huang et al. 2015; Hulswit et al. 2019; Qing et al. 2020). A few appear to have evolved further to preferentially bind to very specific host proteins such as ACE2 for the SARS viruses (Li et al. 2005). Recently, some coronaviruses including SARS-CoV-2 have also been shown to bind heparan sulfate (Clausen et al. 2020; Kim et al. 2020) an interaction that appears necessary for infecting ACE2-positive cells.
In the initial sequencing of the SARS-Co-2 viral genome, it was noted that amino acid residues involved in Sia-based interactions were missing or modified (Wu et al. 2020). Another study showed that mutations at the putative Sia-binding sites did not completely abolish binding but led to reduced binding (Peng et al. 2012). Consistent with this finding, initial studies did not show evidence for sialoglycan recognition. However, a few recent papers have suggested that SARS-CoV-2 spike protein may also weakly recognize Sias, with a preference for glycolipids (Awasthi et al. 2020; Baker et al. 2020; Engin et al. 2020). Despite these and multiple other papers and reviews (Evans and Liu 2021; Sun 2021) discussing the potential role of Sias in binding by the SARS-CoV-2 spike protein, relatively little is known about which types of sialosides it bindsto.
To address this question, we probed a sialoglycan microarray presenting 139 glycans representing common terminal structures on vertebrate glycans, with a recombinant, soluble form of the SARS-CoV-2 spike protein (entire external domain) stabilized by six proline residues (HexaPro spike protein) (Hsieh et al. 2020) and secondary antibody StrepMAB Classic, anti-Twin-Strep-tag MoAb (IBA Life Sciences). The first experiment using the protein produced in the UT Austin lab of Jason McLellan showed low levels of binding to some of the sialoglycans on the microarray (data not shown). However, expression of the identical construct in our laboratory at UC San Diego gave a protein that did not bind to the microarray. We noticed that a major difference between the two preparations was that the UT Austin protein was produced in the presence of kifunensine (Elbein et al. 1990), an alkaloid that blocks N-linked glycan processing and thus prevents addition of Sias to the spike protein glycans, as a result of being a potent and specific mannosidase I inhibitor. In contrast the UC San Diego protein was generated in HEK293 cells (no kifunensine) that would add Sias to the spike protein glycans (Shajahan et al. 2021). A repeat experiment with the fresh batch of the UT Austin protein once again showed some binding. The most likely explanation is that the Sias on the heavily glycosylated spike protein are inhibiting the binding to the microarray due to cis inhibition. This type of “masking” has been known for a long time, e.g., with CD22 (Siglec-2) on B cells (Razi and Varki 1998) and also other immune cell types expressing other Siglecs (Razi and Varki 1999).
There are well-known species level differences in cell surface Sias that can affect pathogen binding (Dhar et al. 2019). While no clear pattern emerged regarding the underlying structure of the sialoglycans, there was a tendency toward preferential binding to Neu5Ac-containing glycans compared to Neu5Gc-containing glycans (Figure 1A). This binding preference was particularly noticed in glycans with α2-3-linked Neu5Ac (Figure 1A). There also was some tendency for binding to Neu5Ac-sialoglycans containing O-acetyl groups (particularly 4-O-acetylated ones) as has been hypothesized (Kim 2020). Additionally, there was preference for binding to sialoglycans synthetically replacing the O-acetyl group at C-9 position with an N-acetyl group. There appeared to be some binding to asialoglycans as well, particularly to Gal6Sβ4(Fucα3)GlcNAcβR1, an oligosaccharide that may be present in the airway mucus (Figure 1B shows the full microarray with the top three binding glycans labeled).
Figure 1.
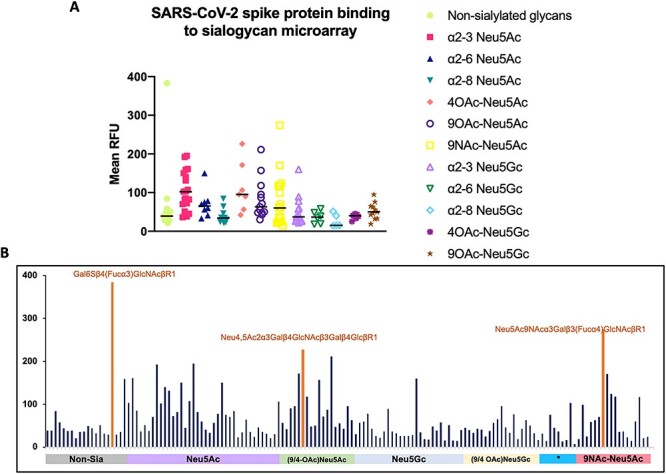
SARS-CoV-2 spike protein (25 μg/mL) binding on the sialoglycan microarray. (A) Binding to Neu5Ac- versus Neu5Gc-containing glycans. Mean RFU values were grouped into non-sialosides and sialosides based on the terminal sialic acid, linkage and acetylation status to identify outlier glycans as well as median binding tendency. Outlier binding was seen in Neu5Ac-containing glycans, N-acetyl Neu5Ac-glycans and non-sialylated glycans. (B) Binding pattern on complete sialoglycan microarray. Individual bars on the X-axis represent individual glycans. Some of the strongest binding glycans are labeled [Gal6Sβ4(Fucα3)GlcNAcβR1, Neu5Ac9NAcα3Galβ3(Fucα4)GlcNAcβR1 and Neu4,5Ac2α3Galβ4GlcNAcβ3Galβ4GlcβR1]. *Asterisked group represent ganglioside type sialoglycans.
Looking at limited literature regarding SARS-CoV-2 infection of various animals suggests a tendency for more severe/lethal disease in those that do not express Neu5Gc-containing glycans and instead have an excess of Neu5Ac due to genomic mutations (Altman and Gagneux 2019) inactivating CMAH e.g., humans, ferrets (Stout et al. 2021), minks (Oude Munnink et al. 2021) and white-tailed deer (Palmer et al. 2021). Notably, the bat species that are thought to be the origin of this virus are also deficient in Cmah (Cagliani et al. 2020).
It is currently unclear whether this weak binding to sialoglycans is important in actual SARS-CoV-2 infections. But considering that the first contact of incoming viruses is likely to be with heavily sialylated mucins, even such interactions in the form of viral “surfing” (Seyran et al. 2020) could be very important and determine the outcome. The same may be true for other viruses that are being spread by small aerosol particles (Greenhalgh et al. 2021). There is also a possibility that other variations of Sias found in the humans (e.g., 9-O-lactyl sialic acids) (Corfield et al. 1993) serve as potential targets. Yet another factor to be considered is the presence of sialylated glycans on the ACE2 glycoprotein receptor (Pruimboom 2020; Allen et al. 2021; Mehdipour and Hummer 2021).
We recognize that our glycan array does not mimic the in vivo situation with regard to underlying glycan structures nor the glycoproteins/glycolipids as the aglycone entities. Indeed, a novel cell-based glycan array recently confirmed (Narimatsu et al. 2019) an earlier prediction of higher order binding of microbial adhesins to “clustered saccharide patches” of sialylated O-glycans, organized by their presentation on proteins (Cohen and Varki 2014). The array also does not take into account the complex organization of the glycocalyx and secretory glycoproteins, especially mucins. Additionally, sialylation of spike protein (dependent on cell type producing the virus) might affect binding as discussed earlier. Further studies are needed to confirm and expand these results, and comparison of binding to heparan sulfate is needed. The possibility must be considered that the individual variations in sialoglycan complexities might contribute to the extreme range of differences in severity and lethality in individual humans (Silva-Filho et al. 2020; Jiang et al. 2021). Furthermore, pathogen evolution may also be altering Sia binding tendency. While an early paper suggested that the SARS-CoV-2 spike protein lacked the amino acid residues needed for Sia recognition (Wu et al. 2020), it remains to be seen if some of the more recent infectious variants have regained these residues. There may also be other effects of Sias such as on immune recognition given that sialoglycans serve as self-associated molecular patters (SAMPs) (Varki 2011) and/or in COVID-19-associated inflammation (Siddiqui et al. 2021).
Another unexpected link to sialic acids is the alarmin molecule HMGB1, a major pathogenic and prognostic factor in severe sepsis (Andersson and Tracey, 2011), which is also elevated in COVID-19 sepsis (Chen et al., 2020; Andersson et al., 2020). Low serum zinc is another well-known risk factor for increased severity of sepsis (Hoeger et al., 2017), and hypozincemia was noted in patients with poor clinical outcomes from COVID (Yasui et al., 2020). While prospective trials of zinc supplementation are underway (Perera et al., 2020), existing retrospective meta-analyses have given inconsistent results (Fromonot et al., 2021; Dubourg et al., 2021; Wessels et al., 2021; Szarpak et al., 2021). We recently discovered that HMGB1 can be functionally sequestered away from its activating receptors by plasma sialoglycoproteins in a zinc-dependent manner, a protective effect that is lost when blood pH falls due to lactic acidosis (Siddiqui et al., 2021). Current trials independently studying zinc supplementation, HMGB1 inhibition, or pH normalization may be more successful if these approaches are combined and perhaps supplemented by infusions of heavily sialylated forms of glycoproteins like CD52 (Shathili et al., 2019).
Finally, it is notable that this virus appears to be best transmitted by very small long-lived aerosol particles generated by the human upper airways (Greenhalgh et al. 2021). It is possible that the weak but highly multivalent Sia-binding properties of the virus contribute to the formation of these particles.
Acknowledgements
We thank Ching-Lin Hsieh and Jason McLellan (UT Austin) for providing samples of HexaPro spike protein produced in the presence of kifunensine, and for expression constructs producing the same protein. Space does not allow thorough citation of the voluminous literature on this subject. For some additional references on sialic acid binding by coronaviruses we direct the readers to a mini-review by Sun (Sun 2021).
Contributor Information
Chirag Dhar, Departments of Medicine and Cellular and Molecular Medicine, UC San Diego, 9500 Gilman Drive, La Jolla, CA, USA; Glycobiology Research and Training Center (GRTC), UC San Diego, La Jolla, CA, USA.
Aniruddha Sasmal, Departments of Medicine and Cellular and Molecular Medicine, UC San Diego, 9500 Gilman Drive, La Jolla, CA, USA; Glycobiology Research and Training Center (GRTC), UC San Diego, La Jolla, CA, USA.
Sandra Diaz, Departments of Medicine and Cellular and Molecular Medicine, UC San Diego, 9500 Gilman Drive, La Jolla, CA, USA; Glycobiology Research and Training Center (GRTC), UC San Diego, La Jolla, CA, USA.
Andrea Verhagen, Departments of Medicine and Cellular and Molecular Medicine, UC San Diego, 9500 Gilman Drive, La Jolla, CA, USA; Glycobiology Research and Training Center (GRTC), UC San Diego, La Jolla, CA, USA.
Hai Yu, Department of Chemistry, 1 Shields Ave, Davis, CA, USA.
Wanqing Li, Department of Chemistry, 1 Shields Ave, Davis, CA, USA.
Xi Chen, Department of Chemistry, 1 Shields Ave, Davis, CA, USA.
Ajit Varki, Departments of Medicine and Cellular and Molecular Medicine, UC San Diego, 9500 Gilman Drive, La Jolla, CA, USA; Glycobiology Research and Training Center (GRTC), UC San Diego, La Jolla, CA, USA.
Funding
National Institute of Health R01GM32373 (A.V.) and R01AI130684 (X.C. and A.V.).
Conflict of interest statement
None declared.
References
- Allen JD, Watanabe Y, Chawla H, Newby ML, Crispin M. 2021. Subtle Influence of ACE2 Glycan Processing on SARS-CoV-2 Recognition. ’, J Mol Biol. 433(4):166762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman MO, Gagneux P. 2019. Absence of Neu5Gc and Presence of Anti-Neu5Gc Antibodies in Humans-An Evolutionary Perspective. Front Immunol. 10:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Tracey KJ. 2011. HMGB1 is a therapeutic target for sterile inflammation and infection.. Annu Rev Immunol. 29:139–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Ottestad W, Tracey KJ. 2020. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19. Mol Med. 26(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi M, Gulati S, Sarkar DP, Tiwari S, Kateriya S, Ranjan P, Verma SK. 2020. The Sialoside-Binding Pocket of SARS-CoV-2 Spike Glycoprotein Structurally Resembles MERS-CoV. Viruses. 12(9):909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AN, Richards SJ, Guy CS, Congdon TR, Hasan M, Zwetsloot AJ, Gallo A, Lewandowski JR, Stansfeld PJ, Straube A et al. 2020. The SARS-COV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device. ACS Cent Sci. 6(11):2046–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliani R, Forni D, Clerici M, Sironi M. 2020. Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2. J Virol. 94(12):e00411–e00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L et al. 2020. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell Mol Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, Narayanan A, Majowicz SA, Kwong EM, McVicar RN et al. 2020. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell. 183(4):1043–1057.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Varki A. 2014. Modulation of glycan recognition by clustered saccharide patches. Int Rev Cell Mol Biol. 308:75–125. [DOI] [PubMed] [Google Scholar]
- Corfield AP, Wagner SA, Safe A, Mountford RA, Clamp JR, Kamerling JP, Vliegenthart JFG, Schauer R. 1993. Sialic acids in human gastric aspirates: detection of 9-O-lactyl- and 9-O-acetyl-N-acetylneuraminic acids and a decrease in total sialic acid concentration with age. Clin Sci (Lond). 84(5):573–579. [DOI] [PubMed] [Google Scholar]
- de Groot RJ. 2006. Structure, function and evolution of the hemagglutinin-esterase proteins of corona- and toroviruses. Glycoconj J. 23(1–2):59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar C, Sasmal A, Varki A. 2019. From “Serum Sickness” to “Xenosialitis”: Past, Present, and Future Significance of the Non-human Sialic Acid Neu5Gc. Front Immunol. 10:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg G et al. 2021. Low blood zinc concentrations in patients with poor clinical outcome during SARS-CoV-2 infection: is there a need to supplement with zinc COVID-19 patients. J Microbiol Immunol Infect. S1684–1182(21)00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein AD, Tropea JE, Mitchell M, Kaushal GP. 1990. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem. 265(26):15599–15605. [PubMed] [Google Scholar]
- Engin AB, Engin ED, Engin A. 2020. Dual function of sialic acid in gastrointestinal SARS-CoV-2 infection. Environ Toxicol Pharmacol. 79:103436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP, Liu SL. 2021. Role of host factors in SARS-CoV-2 entry. J Biol Chem. 297(1):100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromonot J et al. 2021. Hypozincemia in the early stage of COVID-19 is associated with an increased risk of severe COVID-19. Clin Nutr. S0261–5614(21)00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. 2021. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 397(10285):1603–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Goldsmith JA, Schaub JM, DiVenere AM, Kuo HC, Javanmardi K, le KC, Wrapp D, Lee AG, Liu Y et al. 2020. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 369(6510):1501–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeger J et al. 2017. Persistent low serum zinc is associated with recurrent sepsis in critically ill patients – A pilot study. PLoS One. 12(5):e0176069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Dong W, Milewska A, Golda A, Qi Y, Zhu QK, Marasco WA, Baric RS, Sims AC, Pyrc K et al. 2015. Human Coronavirus HKU1 Spike Protein Uses O-Acetylated Sialic Acid as an Attachment Receptor Determinant and Employs Hemagglutinin-Esterase Protein as a Receptor-Destroying Enzyme. J Virol. 89(14):7202–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulswit RJG, Lang Y, Bakkers MJG, Li W, Li Z, Schouten A, Ophorst B, van Kuppeveld FJM, Boons GJ, Bosch BJ et al. 2019. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc Natl Acad Sci U S A. 116(7):2681–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Tan M, Xia M, Huang P, Kennedy MA. 2021. Intra-species sialic acid polymorphism in humans: a common niche for influenza and coronavirus pandemics. Emerg Microbes Infect. 10(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH. 2020. SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus-Host Interaction. Int J Mol Sci. 21(12):4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Jin W, Sood A, Montgomery DW, Grant OC, Fuster MM, Fu L, Dordick JS, Woods RJ, Zhang F et al. 2020. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. 181:104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Y, Li W, Li Z, Koerhuis D, van den Burg ACS, Rozemuller E, Bosch BJ, van Kuppeveld FJM, Boons GJ, Huizinga EG et al. 2020. Coronavirus hemagglutinin-esterase and spike proteins coevolve for functional balance and optimal virion avidity. Proc Natl Acad Sci U S A. 117(41):25759–25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S, Wong SK, Huang IC, Xu K, Vasilieva N et al. 2005. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 24(8):1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N et al. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdipour AR, Hummer G. 2021. Dual nature of human ACE2 glycosylation in binding to SARS-CoV-2 spike. Proc Natl Acad Sci U S A. 118(19):e2100425118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narimatsu Y, Joshi HJ, Nason R, van Coillie J, Karlsson R, Sun L, Ye Z, Chen YH, Schjoldager KT, Steentoft C et al. 2019. An Atlas of Human Glycosylation Pathways Enables Display of the Human Glycome by Gene Engineered Cells. Mol Cell. 75(2):394–407.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink BB, Sikkema RS, Nieuwenhuijse DF, Molenaar RJ, Munger E, Molenkamp R, van der Spek A, Tolsma P, Rietveld A, Brouwer M et al. 2021. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to humans. Science. 371(6525):172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MV, Martins M, Falkenberg S, Buckley A, Caserta LC, Mitchell PK, Cassmann ED, Rollins A, Zylich NC, Renshaw RW et al. 2021. Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. J Virol. 95(11):e00083-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Xu L, Lin YL, Chen L, Pasquarella JR, Holmes KV, Li F. 2012. Crystal structure of bovine coronavirus spike protein lectin domain. J Biol Chem. 287(50):41931–41938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera M et al. 2020. Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: trial protocol. BMJ Open. 10(12):e040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruimboom L. 2020. SARS-CoV 2; Possible alternative virus receptors and pathophysiological determinants. Med Hypotheses. 146:110368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing E, Hantak M, Perlman S, Gallagher T. 2020. Distinct Roles for Sialoside and Protein Receptors in Coronavirus Infection. mBio. 11(1):e02764-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi N, Varki A. 1998. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci USA. 95:7469–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi N, Varki A. 1999. Cryptic sialic acid binding lectins on human blood leukocytes can be unmasked by sialidase treatment or cellular activation. Glycobiology. 9:1225–1234. [DOI] [PubMed] [Google Scholar]
- Seyran M, Takayama K, Uversky VN, Lundstrom K, Palù G, Sherchan SP, Attrish D, Rezaei N, Aljabali AAA, Ghosh S et al. 2020. The structural basis of accelerated host cell entry by SARS-CoV-2†. FEBS J. febs.15651. 10.1111/febs.15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajahan A, Archer-Hartmann S, Supekar NT, Gleinich AS, Heiss C, Azadi P. 2021. Comprehensive characterization of N- and O- glycosylation of SARS-CoV-2 human receptor angiotensin converting enzyme 2. Glycobiology. 31(4):410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shathili AM et al. 2019. Specific sialoforms required for the immune suppressive activity of human soluble CD52. Front Immunol. 10:1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui SS et al. 2021. Sialoglycan recognition is a common connection linking acidosis, zinc, and HMGB1 in sepsis. Proc Natl Acad Sci U S A. 118(10):e2018090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Filho JC, Melo CGF, Oliveira JL. 2020. The influence of ABO blood groups on COVID-19 susceptibility and severity: A molecular hypothesis based on carbohydrate-carbohydrate interactions. Med Hypotheses. 144:110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AE, Guo Q, Millet JK, de Matos R, Whittaker GR. 2021. Coronaviruses Associated with the Superfamily Musteloidea. mBio. 12(1):e02873–e02820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XL. 2021. The role of cell surface sialic acids for SARS-CoV-2 infection. Glycobiology. cwab032. 10.1093/glycob/cwab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarpak L et al. 2021. Should we supplement zinc in COVID-19 patients? Evidence from meta-analysis. Pol Arch Intern Med. [DOI] [PubMed] [Google Scholar]
- Tortorici MA, Walls AC, Lang Y, Wang C, Li Z, Koerhuis D, Boons GJ, Bosch BJ, Rey FA, de Groot RJ et al. 2019. Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol. 26(6):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. 2011. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology. 21(9):1121–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I et al. 2021. Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of Corona Virus Disease 19. Br J Nutr. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY et al. 2020. A new coronavirus associated with human respiratory disease in China. Nature. 579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y et al. 2020. Analysis of the predictive factors for a critical illness of COVID-19 during treatment – relationship between serum zinc level and critical illness of COVID-19. Int J Infect Dis. 100:230–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R et al. 2020. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 382(8):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]


