Abstract
Context
Acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been related to subacute thyroiditis (SAT).
Objective
We compared SAT cases during the SARS-CoV-2 pandemic to those observed in the previous years.
Methods
A cross-sectional, retrospective study was conducted at the Endocrinology Unit of University Hospital of Pisa, Italy. We included all patients observed from January 2016 to December 2020 because of an untreated SAT, who had developed the disease within 15 days prior to the visit. SAT cases from 2016 to 2019 (N = 152) are referred to as pre-SARS-CoV-2, while 2020 SAT patients are classified as pos-SARS-CoV-2 (N = 18) or neg-SARS-CoV-2 (N = 28), according to positive or negative SARS-CoV-2 testing performed up to 45 days from SAT onset.
Results
While during 2016-2019, most SAT cases were observed in the third quarter, in 2020, 2 peaks were seen, superimposable to the SARS-CoV-2 outbreaks in the second and the fourth quarters. In the second and fourth quarters of 2020, we observed higher levels of free thyroxine (FT4), C-reactive protein (CRP), and thyroglobulin (Tg) compared with the same quarters of the years 2016-2019. Pos-SARS-CoV-2 patients had higher FT4 (28.4 vs 24.1 nmol/L), CRP (8.5 vs 3.6 mg/L), and Tg (155 vs 60 µg/L) (P < 0.05 for all) and more frequently had hypothyroidism (13/15 vs 30/152 at 3 months) (P < 0.001) than pre-SARS-CoV-2 patients. Neg-SARS-CoV-2 patients showed a clinical picture intermediate between the other 2 groups.
Conclusion
The SARS-CoV-2 pandemic has caused a shift in the annual timing and severity of SAT cases.
Keywords: SAT, thyroid dysfunction, viruses, thyroid, thyroiditis
The viral or postviral origin of subacute thyroiditis (SAT) is suggested by direct evidence (ie, identification of viruses in thyroid tissues) and mainly by epidemiological studies (ie, the association between SAT and positive antibodies to specific viruses) [1]. Most cases of SAT are reported in summer and fall, concomitantly with the spread of enteroviruses, coxsackieviruses, and echoviruses [1-3]. Given the self-limited course of the disease and the good response to anti-inflammatory treatment, the search for etiological viruses is not performed in clinical practice. The American Thyroid Association guidelines on the management of thyrotoxicosis and hyperthyroidism recommend that SAT treatment should be guided by its degree of severity, with steroids being advised in patients with moderate to severe SAT and nonsteroidal anti-inflammatory drugs (NSAIDs) in those with mild forms [4]. In clinical practice the severity of SAT is established on symptoms and the levels of inflammatory markers as well as of free thyroxine (FT4) and thyroglobulin (Tg). Furthermore, no study has ever evaluated whether the causative virus influence the severity of clinical presentation of SAT [1, 2]. In 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged as a respiratory virus with a pandemic spread and millions people worldwide experienced coronavirus disease 2019 (COVID-19) [5, 6]. After our first report in May 2020 [7], several outpatients affected by SAT associated with SARS-CoV-2 infection have been described worldwide [8-10]. In addition, some studies showed a destructive, painless thyroiditis in patients hospitalized for severe COVID-19 [11, 12].
The aim of the present study was to compare the features of SAT cases observed during the SARS-CoV-2 pandemic to those of SAT patients reported in previous years.
Methods
Study Design and Population
This was a retrospective, cross-sectional, observational study. From January 2016 to December 2020, 312 subjects were referred to the outpatient clinic of the Endocrinology Unit of University Hospital of Pisa, Italy, because of SAT. The diagnosis of SAT was based on clinical features (neck pain and systemic symptoms), laboratory tests (increased levels of FT4, associated with decreased levels of thyroid-stimulating hormone [TSH] and inflammatory markers, ie, C-reactive protein [CRP], and erythrocyte sedimentation rate [ESR]) and imaging features (diffuse hypoechoic areas and absent vascularization at neck ultrasound along with a reduced uptake at thyroid scintigraphy). We reviewed the charts of all patients with an untreated SAT who had developed the disease within 15 days prior to the visit and who had been evaluated at the Endocrinology Unit. Of the 312 SAT patients evaluated, some were excluded due to lacking a full evaluation (n = 37), others because they were referred to us more than 15 days after the onset of SAT (n = 45), and still others because they were already on anti-inflammatory drugs (n = 32), leaving 198 patients included in the study. The results of laboratory tests and the findings of neck ultrasound were available in the whole cohort while those of thyroid scintigraphy were available in 54 patients only. During the follow-up period, thyroid function was tested every 15 to 90 days, according to the patient’s clinical status. The finding of TSH levels > 10 mIU/L with normal or low FT4 levels 3 months after the onset of SAT established the diagnosis of hypothyroidism. Data publication was approved by the local institutional review committee (Comitato Etico di Area Vasta Nord Ovest—CEAVNO). Patients gave their informed consent to participate in the study.
Classification of SAT According to SARS-CoV-2 Status
Patients of the period January 2016 to December 2019 are referred to as pre-SARS-CoV-2. Patients from 2020 are classified as pos-SARS-CoV-2 or neg-SARS-CoV-2, according to the finding of a positive or negative test for SARS-CoV-2 infection within 45 days prior to the onset of SAT. SARS-CoV-2 diagnosis was based on nucleic acid amplification tests obtained on nasopharyngeal swab or measurement of class M and class G antibodies to SARS-CoV-2 by highly specific assays.
Data on deaths related to SARS-CoV-2 infection were obtained from the public records of the “Istituto Superiore di Sanità, Rome, Italy” (https://www.iss.it/coronavirus, accessed on February 2, 2021).
Laboratory Examinations
Thyroid hormones and TSH were tested using immunoenzymatic assays (Ortho-clinical Diagnostic Inc., Rochester, NY). Reference ranges were 6 to 16 nmol/L for FT4, 2.3 to 4.2 pmol/L for free triiodothyronine (FT3), and 0.4 to 4.5 mIU/L for TSH. Thyroglobulin (Tg) was measured by an immunometric assay (Access Thyroglobulin assay; Beckman Coulter, Inc., Fullerton CA) (functional sensitivity 0.1 ng/mL) only in subjects showing thyroglobulin antibodies (TgAbs) below the interfering cutoff (see below). TgAbs were measured by a noncompetitive immunometric assay, Access Thyroglobulin Antibody (Tosoh Corporation, Tokyo, Japan); the analytic, functional, and positive cutoffs were 6, 8, and 30 IU/mL, respectively. In this assay, TgAbs interfere with Tg measurement when their levels are ≥ 9.3 IU/mL [13]. Thyroid peroxidase antibodies (TPOAbs) were checked by AIA-Pack 2000 TPOAb (Tosoh Corporation, Tokyo, Japan) (positive cutoff > 10 IU/mL). TSH-receptor antibodies (TRAbs) were tested by enzyme-linked immunosorbent assay (ElisaRSR TRAb third generation, Cardiff, UK) (positive cutoff > 1.5 IU/mL). ESR and CRP were measured using standard methods (reference range < 15 mm/h and < 1.5 mg/L, respectively). IgM to SARS-CoV-2 were measured by anti-SARS-CoV-2 kit (CLIA, Roche) (positive cutoff > 0.1 AU/mL); IgG to SARS-CoV-2 were assessed by anti-SARS-CoV-2 kit (CLIA, Roche) (positive cutoff > 1.4 AU/mL) or by Liaison SARS-CoV-2 S1/S2 IgG (Diasorin-Saluggia, Varese, Italy) (positive cutoff > 15 AU/mL).
Thyroid Imaging
Neck ultrasound was performed by Technos (Esaote Biomedica, Genova, Italy), with a 7.5-MHz linear transducer. Thyroid volume was calculated using the ellipsoid volume formula.
Statistical Analysis
Statistical data analysis was performed using SPSS 21 (IBM Corp., Armonk, NY). Data are presented as mean ± SD or median with interquartile range (IQR), as indicated. The Shapiro-Wilk test was used to assess normality of data distribution of continuous variables. Statistical tests used to compare groups included Student’s t-test for normally distributed variables and Mann-Whitney U tests for variables with skewed distribution. The Kruskal-Wallis test or one-way analysis of variance (ANOVA) with post-hoc correction was also applied, depending on the distribution of variables. The Chi-squared test or the Fisher exact test were used to compare counts and frequencies between groups for categorical variables, as appropriate.
Results
Features of Study Population
Features of the 198 patients included in the present study are summarized in Table 1. Most patients were female; mean age was 44.6 years. Presence of neck pain was stated by all subjects and was bilateral in 48 patients, while fever was reported by 145 subjects. Fifty-six patients reported respiratory symptoms in the month preceding the onset of SAT. All subjects showed thyrotoxicosis, a high FT4/FT3 ratio, and high levels of ESR, CRP, and Tg. TgAbs and TPOAbs were positive, at low levels, in few patients whereas TRAbs were undetectable in the entire cohort. At neck ultrasound, most patients had an increased thyroid volume. All patients (n = 54) undergoing thyroid scintigraphy showed an absent or reduced uptake. Of 182 patients who were followed for at least 3 months, 57 developed hypothyroidism. Of the 46 patients evaluated in 2020, 18 were classified as pos-SARS-CoV-2 and 28 as neg-SARS-CoV-2. None of the 2020 patients had been previously hospitalized because of COVID-19.
Table 1.
Clinical, laboratory and imaging features of the entire cohort of SAT patients (n = 198) evaluated in the years 2016-2020
| Study population (N = 198) | Results |
|---|---|
| Sex | |
| - Female (%) | 167 (84) |
| - Male (%) | 31 (16) |
| Mean age (SD) | 44.6 (±12) |
| Patients with respiratory symptoms prior to SAT onset (%) | 56 (28) |
| Median time from respiratory symptoms to SAT (IQR) | 30 (28-45) |
| Patients with bilateral neck pain (%) | 48 (24) |
| Patients with fever (%) | 145 (73) |
| Laboratory and imaging findings | |
| FT4 (IQR) | 24.3 (4.6) |
| FT3 (IQR) | 5.8 (1.7) |
| FT4/FT3 (IQR) | 4.3 (1.2) |
| TSH (IQR) | 0.1 (0.2) |
| ESR (IQR) | 43 (45) |
| CRP (IQR) | 3.8 (2.2) |
| Tga (IQR) | 69 (70) |
| Patients with positive TgAbs (%) | 62 (31) |
| - Median titer (IQR) | 65 (52) |
| Patients with positive TPOAbs (%) | 27 (14) |
| - Median titer (IQR) | 35 (65) |
| Thyroid volume (IQR) | 22.3 (6) |
| Scintigraphy | |
| - Absent/reduced uptake | 54 |
| - Normal uptake | 0 |
| - Not available | 144 |
| Treatment and follow-up | |
| Type of treatment (%) | |
| - Steroids | 175 (89) |
| - NSAIDs | 15 (7) |
| - None | 8 (4) |
| Median duration of steroid treatment (days) (IQR) | 90 (40) |
| Hypothyroidism at 3 monthsb (%) | 57 (29) |
| SARS-CoV-2 status (year 2020) | |
| - pos-SARS-CoV-2 (%) | 18 (39) |
| - neg-SARS-CoV-2 (%) | 28 (61) |
Normal ranges: FT4 6-16 nmol/L; FT3 2.3-4.2 pmol/L; TSH 0.4-4.5 mIU/L; ESR < 15 mm/h; CRP < 1.5 mg/L; Tg < 35 µg/L; TgAbs < 30 IU/mL (positive cutoff); TPOAbs < 10 IU/mL (positive cutoff).
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FT3, free triiodothyronine; FT4, free thyroxine; IQR, interquartile range; NSAIDs, nonsteroidal anti-inflammatory drugs; SARS-CoV-2: severe acute respiratory syndrome virus 2; SAT, subacute thyroiditis; Tg, thyroglobulin; TgAbs, thyroglobulin antibodies; TPOAbs, thyroid peroxidase antibodies.
aMeasured in patients with TgAbs < 9.3 IU/mL (interfering cutoff).
bEvaluated in 182 subjects with a minimum follow-up of 3 months.
Seasonal Distribution of SAT and SARS-CoV-2 Incidence
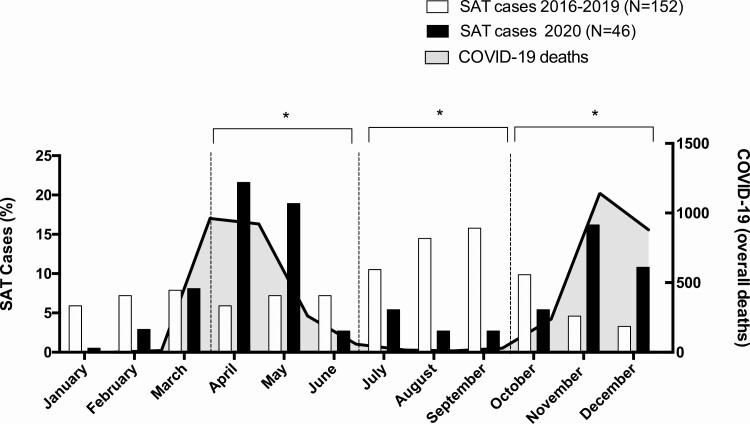
We observed a comparable number of SAT patients per year throughout the period 2016-2020 (40 in 2016, 34 in 2017, 43 in 2018, 35 in 2019 and 46 in 2020). However, seasonal distribution of SAT cases was different in the second (Q2), third (Q3), and fourth (Q4) quarters. During Q3 of the years 2016-2019, cases were more common compared with 2020 (68/152 vs 6/46) (P = 0.01); however, in both Q2 and Q4 they were less frequent during the years 2016-2019 than in 2020 (31/152 vs 16/46 in Q2 [P = 0.01] and 21/152 vs 17/46 in Q4 [P = 0.03]) (Fig. 1).
Figure 1.
Percentage distribution of cases of SAT in years 2016-2019 (white columns) and in 2020 (black columns). The absolute number of cases of deaths for SARS-COV-2 in 2020 in Tuscany is reported as solid gray curve. The dashed vertical line identifies each quarter. Data on deaths related to SARS-CoV-2 infection were obtained from the public records of the “Istituto Superiore di Sanità, Rome, Italy” (https://www.iss.it/coronavirus, access on February 2, 2021) *P <.05 between the 2 groups in the quarter.
The 2 waves of SAT of 2020 were superimposable to the 2 peaks of SARS-CoV-2 deaths observed in Tuscany (Fig. 1). It is worth noting that the number of SARS-CoV-2 deaths from January to March is probably underestimated because of the poor awareness of the disease during the initial phase of the pandemic.
Severity of SAT According to the Quarters of the Year
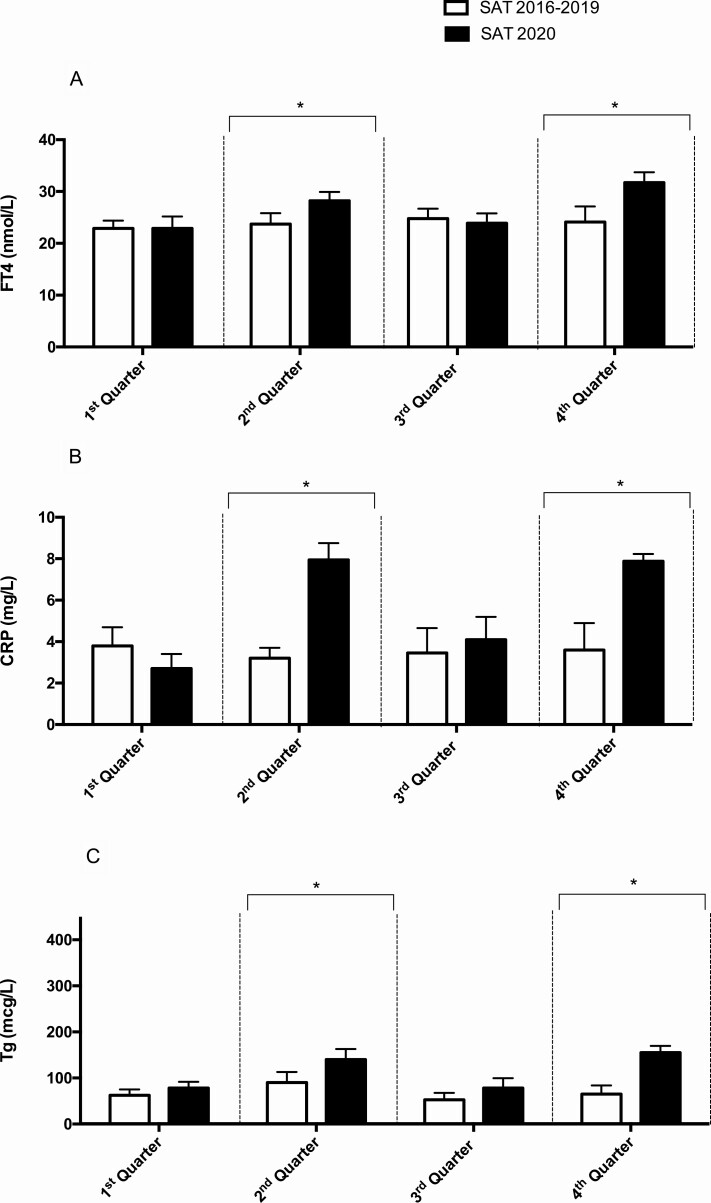
The levels of FT4, CRP, and Tg were similar across the quarters in the period 2016-2019 (Fig. 2, Panels A-C).
Figure 2.
Comparison of the median levels of FT4 (Panel A), CRP (Panel B), and Tg (Panel C) at the onset of SAT in the years 2016-2019 (N = 152) and in the year 2020 (N = 46). The dashed vertical line identifies each quarter. *P <.05 between the 2 groups in the quarter.
Higher levels of FT4 were observed in SAT 2020 patients compared to SAT 2016-2019 patients in Q2 (28.3 vs 23.7 nmol/L) (P < 0.001) and in Q4 (31.6 vs 24.1 nmol/L) (P = 0.007) but not in Q3 (23.9 vs 24.8 nmol/L) (P = 0.1) (Fig. 2, Panel A). The levels of CRP were higher in SAT 2020 patients in comparison with SAT 2016-2019 patients in the Q2 (7.9 vs 3.2 mg/L) (P < 0.001) and in the Q4 (7.9 vs 3.6 mg/L) (P = 0.006) and similar in the Q3 (4.1 vs 3.4 mg/L) (P = 0.63) (Fig. 2, Panel B). Higher levels of Tg were retrieved in SAT 2020 patients as compared with SAT 2016-2019 patients in the Q2 (141 vs 90 µg/L) (P = 0.002) and in the Q4 (153 vs 65 µg/L) (P = 0.01) quarters and similar in the Q3 (78 vs 52 µg/L) (P = 0.12) (Fig. 2, Panel C). The values of ESR in Q2 were also higher in SAT 2020 compared with Q2 SAT 2016-2019 (94 mm/h vs 48 mm/h, P < 0.001).
Clinical and Laboratory Features of pre-SARS-CoV-2, neg-SARS-CoV-2, and pos-SARS-CoV-2 Patients
Comparisons between pre-SARS-CoV-2, neg-SARS-CoV-2, and pos-SARS-CoV-2 cohorts are reported in Table 2. Sex distribution was similar in the 3 groups, whereas mean age was lower in pos-SARS-CoV-2 than in pre-SARS-CoV-2 patients. Previous respiratory symptoms as well as bilateral (as opposite to unilateral) neck pain were more common in pos-SARS-CoV-2 patients compared with pre-SARS-CoV-2 and neg-SARS-CoV-2. Fever was more common in pos-SARS-CoV-2 compared with pre-SARS-CoV-2. FT4, FT3, ESR, and Tg were higher in neg-SARS-CoV-2 than in pre-SARS-CoV-2 subjects. Pos-SARS-CoV-2 showed higher levels of FT4, FT3, ESR, CRP, and Tg and lower levels of TSH compared with pre-SARS-CoV-2 subjects. Finally, compared with neg-SARS-CoV-2, pos-SARS-CoV-2 had higher CRP levels. Hypothyroidism at 3 months was more frequently diagnosed in pos-SARS-CoV-2 than in pre-SARS-CoV-2 and in neg-SARS-CoV-2 patients. At the end of the follow-up period (median 28 months), 92% of 2016-2019 SAT patients who developed hypothyroidism at 3 months were still hypothyroid. In 2020, 20 (9 pos-SARS-CoV-2 and 11 neg-SARS-CoV-2) of the 27 patients who were hypothyroid at 3 months were checked at 6 months and were all still hypothyroid.
Table 2.
Comparison of clinical, laboratory, and imaging features between Pre-SARS-CoV-2, Neg-SARS-CoV-2, and Pos-SARS-CoV-2 cohorts
| Pre-SARS-CoV-2 | Neg-SARS-CoV-2 | Pos-SARS-CoV-2 | Overall | Pre-SARS-CoV-2 vs Neg-SARS-CoV-2 | Pre-SARS-CoV-2 vs Pos-SARS-CoV-2 | Neg-SARS-CoV-2 vs Pos-SARS-CoV-2 | |
|---|---|---|---|---|---|---|---|
| N = 152 | N = 28 | N = 18 | P value | P value | P value | P value | |
| Sex | 0.11 | 0.38 | 0.12 | 0.43 | |||
| - Female (%) | 130 (86) | 19 (68) | 18 (100) | ||||
| - Male (%) | 22 (14) | 9 (32) | 0 (0) | ||||
| Mean age (SD) | 46 (±9) | 43 (±14) | 34 (±14) | 0.006 | 0.32 | <0.001 | 0.08 |
| Respiratory symptoms preceding SAT onset (%) | 30 (20) | 12 (43) | 14 (78) | <0.001 | 0.008 | 0.01 | 0.01 |
| Median time from respiratory symptoms to SAT (IQR) | 30 (15) | 30 (18) | 29 (12) | 0.08 | 0.86 | 0.06 | 0.33 |
| Bilateral neck pain (%) | 21 (14) | 11 (39) | 16 (89) | <0.001 | 0.0025 | <0.001 | 0.016 |
| Fever (%) | 105 (69) | 23 (82) | 17 (94) | 0.005 | 0.12 | 0.002 | 0.34 |
| FT4 (IQR) | 24.1 (3.8) | 26.9 (8.9) | 28.4 (5.6) | <0.001 | 0.001 | <0.001 | 0.29 |
| FT3 (IQR) | 5.8 (1.88) | 7.0 (2) | 8.2 (1.6) | <0.001 | <0.001 | <0.001 | 0.13 |
| TSH (IQR) | 0.1 (0.23) | 0.02 (0.29) | 0.01 (0.19) | <0.001 | 0.11 | 0.004 | 0.25 |
| ESR (IQR) | 43.5 (29) | 71.5 (49.8) | 91 (25) | <0.001 | <0.001 | <0.001 | 0.06 |
| CRP (IQR) | 3.6 (1.2) | 5.3 (5.3) | 8.5 (1.1) | <0.001 | 0.13 | <0.001 | 0.005 |
| Tga (IQR) | 60 (40) | 94.5 (27) | 155 (150) | <0.001 | <0.001 | <0.001 | 0.45 |
| Thyroid volume (IQR) | 22 (6) | 23.3 (5.4) | 22 (5) | 0.37 | 0.45 | 0.58 | 0.39 |
| Treatment (%) | 0.54 | 0.61 | 0.39 | 0.53 | |||
| - Steroids | 135 (89) | 24 (86) | 16 (92) | ||||
| - NSAIDs | 12 (8) | 2 (7) | 1 (0) | ||||
| - None | 5 (3) | 2 (7) | 1 (8) | ||||
| Median duration (days) of steroid treatment (IQR) | 90 (40) | 90 (40) | 85 (13) | 0.28 | 0.43 | 0.29 | 0.54 |
| Hypothyroidism at 3 months (%) | 30 (20) | 14b (66) | 13c (87) | <0.001 | <0.001 | <0.001 | 0.01 |
Normal ranges: FT4 6-16 nmol/L; FT3 2.3-4.2 pmol/L; TSH 0.4-4.5 mIU/L; ESR < 15 mm/h; CRP < 1.5 mg/L; Tg < 35 µg/L.
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FT3, free triiodothyronine; FT4, free thyroxine; IQR, interquartile range; NA, not applicable; NAATs, nucleic acid amplification tests; NS, not significant; NSAIDs, nonsteroidal anti-inflammatory drugs; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SAT, subacute thyroiditis; Tg, thyroglobulin; TSH, thyrotropin (thyroid-stimulating hormone).
aMeasured in patients with TgAbs < 9.3 IU/mL (interfering cutoff).
bEstablished in the 21 subjects with a follow-up of 3 months.
cEstablished in the 15 subjects with a follow-up of 3 months.
Discussion
Subacute thyroiditis, a thyroid disease of viral or postviral origin, is characterized by neck pain and systemic symptoms, namely fever, asthenia, malaise, high levels of inflammatory markers, and thyrotoxicosis [2, 14]. Many respiratory viruses, including coxsackievirus, echovirus, rhinovirus, and adenovirus have been associated with SAT by means of high titers of virus-specific antibodies or positive virus swabs, whereas studies based on virus culture from thyroid tissue have yielded conflicting results [1]. Furthermore, the association of SAT with viral outbreaks has been occasionally reported [3, 15]. The course of SAT is usually self-limited and responds excellently to anti-inflammatory treatment. Because specific antiviral treatment is not required, diagnostic tools aimed at identifying the etiological viruses are not routinely employed [1, 2]. In 2020, SARS-CoV-2, which originated in Wuhan, China, spread quickly worldwide, emerging as the cause of a respiratory disease (COVID-19) of various severity [5, 6]. Other tissues may be also involved in SARS-CoV-2 infection [16, 17]. After our first report in May 2020 [7], several cases of SAT associated with SARS-CoV-2 infection have been described by our and additional groups [8-10, 18-22]. In order to investigate the effect of SARS-CoV-2 pandemic on the clinical picture of SAT, we performed a cross-sectional study which included the cases of SAT observed in the year 2020 and those observed in the years 2016-2019, prior to the SARS-CoV-2 pandemic.
The overall number of SAT cases referred to our institution in 2020 was similar to that observed in each year of the period 2016-2019. Patients’ age and the female-to-male ratio of the 2020 cohort was also comparable to patients observed at our institution in the years 2016-2019 as well as to those described in previous studies [2, 23]. While similarly to previous reports, most cases of SAT in years 2016-2019 occurred in the third quarter [2, 3], in 2020 most cases were recorded in the second and fourth quarters, within a month from the 2 main SARS-COV-2 outbreaks in Tuscany. It is likely that, in 2020, both social distancing and the face masks used in order to prevent SARS-CoV-2 pandemic had reduced the spread of other viruses.
By evaluating the severity of SAT, we observed that the levels of FT4, CRP, Tg, and ESR in the period 2016-2019 were similar across the quarters and comparable to those reported in previous studies [2, 23, 24]. The observation that the levels of FT4, CRP, Tg, and ESR were significantly higher in SAT occurring in the Q2 and Q4 of 2020 suggests that the clinical picture of SAT induced by SARS-CoV-2 is more severe compared to that related to other viruses. In addition, compared to pre-SARS-CoV-2 patients, more pos-SARS-CoV-2 patients had experienced respiratory symptoms in the previous month and had bilateral (in comparison to unilateral) neck pain. Furthermore, compared with pre-SARS-CoV-2, the pos-SARS-CoV-2 patients presented with a more severe thyrotoxicosis and higher levels of inflammatory markers. Finally, more pos-SARS-CoV-2 patients than pre-SARS-CoV-2 patients experienced hypothyroidism. All these findings point to a higher severity of SAT cases induced by SARS-CoV-2 as compared to those previously observed, caused by other viruses. Particularly noteworthy is the observation that, as with pos-SARS-CoV-2, most neg-SARS-CoV-2 cases occurred in the second and the fourth quarters of 2020. The clinical and biochemical features and the rate of evolution to hypothyroidism of these patients were intermediate between pre-SARS-CoV-2 and pos-SARS-CoV-2 subjects. Although not conclusive, some of these findings suggest that these SAT cases were likely due to SARS-CoV-2 infections which had gone undiagnosed.
We did not observe a rise in total number of SAT in 2020 compared to the previous years, even though a large part of Tuscan population has been affected by SARS-CoV-2 infection. This indicates that only a small portion of subjects infected by SARS-CoV-2 experienced SAT and supports the notion that only predisposed people develop this disease [25]. Moreover, our findings suggest that the severity of SAT and the risk of developing hypothyroidism is correlated with the causative virus. In conclusion, our study demonstrates that SARS-CoV-2 pandemic, although not associated with a rise in the total number of SAT cases, has influenced the severity of the disease, leading to more severe forms of the disease.
Acknowledgments
Financial Support: This research was supported by “Fondi di Ateneo” (No. 539901 to F.L.), University of Pisa to Francesco Latrofa.
Author Contributions: A.B. and F.L planned the study. A.B., N.V., and D.S. extracted the data. A.B. and G.R. performed the statistical analysis. A.B., F.S., and F.L wrote the manuscript. All authors discussed the results of the study.
Glossary
Abbreviations
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- FT3
free triiodothyronine
- FT4
free thyroxine
- IQR
interquartile range
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SAT
subacute thyroiditis
- Tg
thyroglobulin
- TgAbs
thyroglobulin antibodies
- TPOAbs
thyroid peroxidase antibodies
- TRAb
TSH-receptor antibodies
- TSH
thyrotropin (thyroid-stimulating hormone)
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Informed Consent: Written informed consent was obtained from the patients for publication of this study.
References
- 1. Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishihara E, Ohye H, Amino N, et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med. 2008;47(8):725-729. [DOI] [PubMed] [Google Scholar]
- 3. Martino E, Buratti L, Bartalena L, et al. High prevalence of subacute thyroiditis during summer season in Italy. J Endocrinol Invest. 1987;10(3):321-323. [DOI] [PubMed] [Google Scholar]
- 4. Ross DS, Burch HB, Cooper DS, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421. [DOI] [PubMed] [Google Scholar]
- 5. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. 2020;105(7):2367-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brancatella A, Ricci D, Cappellani D, et al. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J Clin Endocrinol Metab. 2020;105(10):e3742-e3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruggeri RM, Campennì A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones (Athens, Greece). 2021;20(1):219-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ippolito S, Dentali F, Tanda ML. SARS-CoV-2: a potential trigger for subacute thyroiditis? Insights from a case report. J Endocrinol Investig. 2020;43(8):1171-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020;183(4):381-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muller I, Cannavaro D, Dazzi D, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Latrofa F, Ricci D, Bottai S, et al. Effect of thyroglobulin autoantibodies on the metabolic clearance of serum thyroglobulin. Thyroid. 2018;28(3):288-294. [DOI] [PubMed] [Google Scholar]
- 14. Ricci D, Brancatella A, Marinò M, et al. The detection of serum IgMs to thyroglobulin in subacute thyroiditis suggests a protective role of IgMs in thyroid autoimmunity. J Clin Endocrinol Metab. 2020;105(6):e2261-e2270. [DOI] [PubMed] [Google Scholar]
- 15. Stancek D, Stanceková-Gressnerová M, Janotka M, Hnilica P, Oravec D. Isolation and some serological and epidemiological data on the viruses recovered from patients with subacute thyroiditis de Quervain. Med Microbiol Immunol. 1975;161(2):133-144. [DOI] [PubMed] [Google Scholar]
- 16. Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dhakal BP, Sweitzer NK, Indik JH, Acharya D, William P. SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ. 2020;29(7):973-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chong WH, Shkolnik B, Saha B, Beegle S. Subacute thyroiditis in the setting of coronavirus disease 2019. Am J Medical Sci. 2021;361(3):400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mattar SAM, Koh SJQ, Rama Chandran S, Cherng BPZ. Subacute thyroiditis associated with COVID-19. BMJ Case Rep. 2020;13(8):e237336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Campos-Barrera E, Alvarez-Cisneros T, Davalos-Fuentes M. Subacute thyroiditis associated with COVID-19. Case Rep Endocrinol. 2020;2020:8891539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sohrabpour S, Heidari F, Karimi E, Ansari R, Tajdini A, Heidari F. Subacute thyroiditis in COVID-19 patients. Eur Thyroid J. 2021;9(6):321-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christensen J, O’Callaghan K, Sinclair H, et al. Risk factors, treatment and outcomes of subacute thyroiditis secondary to COVID-19: a systematic review. Intern Med J. Published online ahead of print June 17, 2021. doi:10.1111/imj.15432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishihara E, Amino N, Kudo T, et al. Moderate frequency of anti-thyroglobulin antibodies in the early phase of subacute thyroiditis. Eur Thyroid J. 2019;8(5):268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Latrofa F, Ricci D, Montanelli L, et al. Thyroglobulin autoantibodies of patients with subacute thyroiditis are restricted to a major B cell epitope. J Endocrinol Invest. 2012;35(8):712-714. [DOI] [PubMed] [Google Scholar]
- 25. Ohsako N, Tamai H, Sudo T, et al. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing. J Clin Endocrinol Metab. 1995;80(12):3653-3656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Informed Consent: Written informed consent was obtained from the patients for publication of this study.