Abstract
Aims
The COVID-19 pandemic has resulted in excess mortality due to both COVID-19 directly and other conditions, including cardiovascular (CV) disease. We aimed to explore the excess in-hospital mortality, unrelated to COVID-19 infection, across a range of CV diseases.
Methods and results
A systematic search was performed for studies investigating in-hospital mortality among patients admitted with CV disease without SARS-CoV-2 infection compared with a period outside the COVID-19 pandemic. Fifteen studies on 27 421 patients with CV disease were included in the analysis. The average in-hospital mortality rate was 10.4% (n = 974) in the COVID-19 group and 5.7% (n = 1026) in the comparator group. Compared with periods outside the COVID-19 pandemic, the pooled risk ratio (RR) demonstrated increased in-hospital mortality by 62% during COVID-19 [95% confidence interval (CI) 1.20–2.20, P = 0.002]. Studies with a decline in admission rate >50% during the COVID-19 pandemic observed the greatest increase in mortality compared with those with <50% reduction [RR 2.74 (95% CI 2.43–3.10) vs. 1.21 (95% CI 1.07–1.37), P < 0.001]. The observed increased mortality was consistent across different CV conditions (P = 0.74 for interaction).
Conclusions
In-hospital mortality among patients admitted with CV diseases was increased relative to periods outside the pandemic, independent of co-infection with COVID-19. This effect was larger in studies with the biggest decline in admission rates, suggesting a sicker cohort of patients in this period. However, studies were generally poorly conducted, and there is a need for further well-designed studies to establish the full extent of mortality not directly related to COVID-19 infection.
Keywords: COVID-19, In-hospital mortality, Cardiovascular disease, Meta-analysis
Introduction
The COVID-19 pandemic has directly caused significant excess mortality on a global scale, accounting for ∼2.5 million deaths.1 Moreover, the pandemic has resulted in excess mortality beyond those infected by the SARS-CoV2 virus. There is emerging evidence that cardiovascular (CV) mortality has increased during the pandemic, independent of COVID infection.2–4 This has been attributed to several factors, including patients avoiding healthcare environments to avoid nosocomial infection with SARS-CoV2, redeployment of specialist healthcare staff to support COVID-19 services, and reduced availability of routine investigations and procedures.5 However, most studies evaluating this question have been small and underpowered to detect significant changes in mortality, and the collateral impact of the pandemic on patients with CV disease remains unclear.
The aim of this meta-analysis is to quantify the reported effects of the COVID-19 pandemic on in-hospital mortality in patients admitted with CV disease but without SARS-CoV2 infection. Furthermore, we aimed to examine the determinants of outcomes compared with periods outside the pandemic.
Methods
Search strategy and eligibility criteria
The project was performed in accordance with Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.6 A systematic search of Medline (2016 to January Week 2 2021) and Embase (1974–2021 Week 1) was performed on 16 January 2021. The literature search was developed in an iterative manner by D.I.B. and A.D. using previously published guidelines.7–9 The search strategy included keywords and MeSH terms relating to CV disease, COVID-19, and in-hospital mortality (Supplementary material online). The search was limited to reports available in English due to time constraints and lack of access to translators. Review or commentary articles, abstracts, unpublished material, and reports without available full text were excluded. Duplicates were removed using Endnote (Thomas Reuters, USA) and all remaining results subjected to eligibility screening.
Study eligibility criteria were selected using PICOS criteria.10 Observational studies of humans with CV disease were included if they reported in-hospital mortality during the COVID-19 pandemic, compared with a period outside the pandemic, regardless of any service changes during the pandemic. Exclusion criteria were: firstly, studies that did not report absolute numbers for admissions and mortality; secondly, studies that included or did not specifically exclude patients with concomitant COVID-19 infection; thirdly, studies of stroke, cardiac surgery, vascular disease, or congenital heart disease; finally, studies including outpatients.
For this analysis, there was no involvement of patients and public. The research complies with the Declaration of Helsinki and ethical approval was not needed.
Data collection, synthesis, and study quality
Retrieved records were screened for eligibility using the title and abstract. Next, eligibility assessment was performed, independently and un-blinded, by A.C. and D.I.B. Disagreements were resolved by examining the full text of the article and a consensus between reviewers in all cases were reached. Admission rates and basic demographic variables (age and sex) were extracted, independently and un-blinded, by A.C. and D.I.B. We attempted to acquire key missing information by contacting the report authors.11 A full list of assumptions is available in the Supplementary material online.
Risk of bias
The risk of bias was assessed for each report using the ROBINS-I tool.12 This validated tool evaluates the risk of bias in estimates of the comparative effectiveness of interventions from reports that do not use randomization. The relevant confounding domains were defined a priori and included patient demographics, use of a matched comparator period, selection of specific CV diagnoses, CV disease severity, and comorbidities. The treatment received by patients was considered as a co-intervention that could be different between groups. All bias domains were assessed for each report using the signalling questions provided by the tool. The judgements made within each bias domain were used to determine an overall risk of bias score. Missing information provided by report authors, but not included in the published report, was considered as part of the risk of bias assessment, which may not be reflected in the published study. Reports with critical risk of bias were excluded. The risk of bias was assessed independently from other assessors and data extraction in an un-blinded manner by S.A.W. and P.A.S. Disagreements were resolved by consensus or discussion with the senior author.
Outcomes and statistical methods
The a priori primary outcome was in-hospital mortality. Absolute admission rates and in-hospital mortality were collected for each period and used to calculate a risk ratio (RR). We assumed heterogeneity between studies and pooled in-hospital mortality rates using random-effects meta-analysis, using the method of DerSimonian and Laird.13 Pooled in-hospital mortality rates were compared using the RR and corresponding 95% confidence interval (CI).
Heterogeneity was quantified using χ2, τ2, and I2 tests. We considered heterogeneity to be significant if I2 > 75%.14,15 To investigate sources of heterogeneity, the outcome was assessed in pre-defined subgroups and quality indicators, including change in admission rate between the COVID-19 and comparator periods, where a decline in admissions greater than 50% was used as the cut-off, presence or absence of matched comparator periods, CV condition, geographical location (by continent and by country), and risk of bias.
Publication bias was assessed graphically by generating a funnel plot of the logarithm of effect size against the standard error for each trial. Variables are expressed as median and interquartile range (IQR), or count and percentage, as appropriate. A two-tailed P-value of 0.01 was considered statistically significant. All analyses were performed with Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) or IBM SPSS Statistics Version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Study characteristics
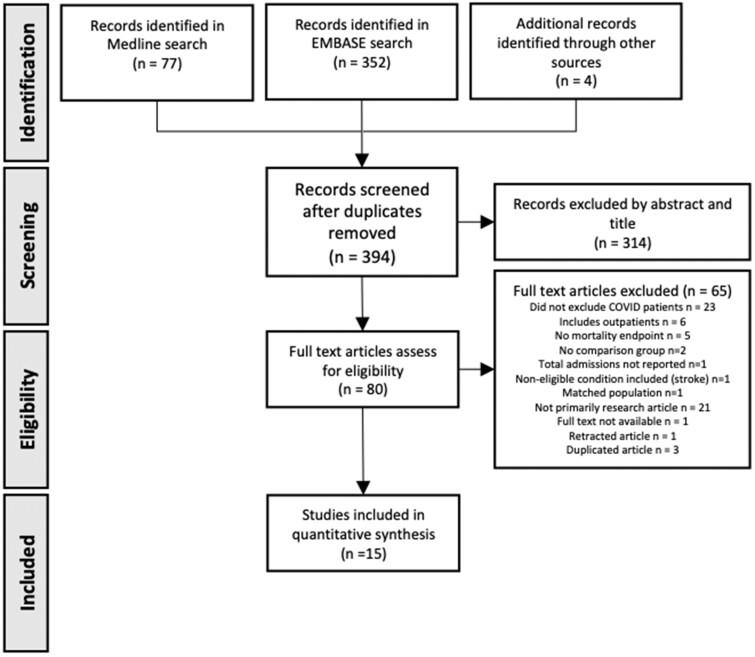
The systematic search identified 433 references and retained 394 after the removal of duplicates (Figure 1). Following screening of title and abstract, 80 reports underwent full-text review for eligibility. Of these, 23 were excluded because they either included or did not specifically exclude patients with concurrent COVID-19, 6 included outpatients, 5 did not report a mortality endpoint, 2 had no comparison group, 1 did not report total admissions, and 1 included a non-eligible condition (stroke). In addition, 21 reports were excluded as they were not primary research articles, 1 reported a matched population, 1 did not have full text available, 1 was retracted,16 and 3 were duplicate references.
Figure 1.
Flow chart of the study selection process. A systematic review yielded 433 reports. After the removal of duplicates and the application of inclusion and exclusion criteria, 15 studies were included in the meta-analysis.
The remaining 15 studies were included in our review (Figure 1). The main study characteristics are described in Table 1. Of these, the majority were from Europe with one from Australia17 and two from Asia.18,19 One reported overall CV disease, which included heart failure (HF), acute myocardial infarction, and arrhythmias,20 eight described acute coronary syndromes (ACS), and six described HF. Most studies used the same period in a preceding year for comparison, to account for seasonal variability. However, three used a different period,18,19,21 and two used composite numbers for different periods over previous years.17,22
Table 1.
Characteristics of the included studies.
|
|
Comparator period |
COVID-19 period |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author | Journal | Country | Condition | Period (days) | Mortality (n) | Total admissions (n) | Mean age | Male (%) | Daily admissions (n) | Period (days) | Mortality (n) | Total admissions (n) | Mean age | Male (%) | Daily admissions (n) | Difference in admissions (%) | Comparator perioda |
| Cannata | Eur J Heart Failure | UK | HF | 158 | 67 | 794 | 77 | 54 | 5 | 159 | 62 | 578 | 78 | 50 | 4 | −28 | 1 |
| Chew | Circ J | Singapore | STEMI | 128 | 12 | 208 | 57 | 64 | 2 | 53 | 4 | 95 | 59 | 57 | 2 | 10 | 2 |
| Choudhary | Emerg Med J | India | ACS | 30 | 40 | 1488 | 61 | 71 | 50 | 30 | 21 | 289 | 62 | 89 | 10 | −81 | 2 |
| Colivicchi | J Cardiac Fail | Italy | HF | 59 | 382 | 6060 | 73 | 57 | 103 | 60 | 466 | 2711 | 78 | 79 | 45 | −56 | 1 |
| Cosentino | EHJ CV Pharma | Italy | STEMI | 73 | 2 | 43 | 65 | 47 | 1 | 74 | 15 | 76 | 64 | 83 | 1 | 74 | 1 |
| Coughlan | IJC Heart Vasc | Republic of Ireland | STEMI | 21 | 0 | 14 | 59 | 100 | 1 | 21 | 2 | 9 | 58 | 55 | 0 | −36 | 1 |
| De Rosa | Eur Heart J | Italy | ACS | 7 | 17 | 618 | 67 | 72 | 88 | 7 | 25 | 286 | 68 | 76 | 41 | −54 | 1 |
| Doolub | ESC HF | UK | HF | 55 | 16 | 160 | 82 | 51 | 3 | 55 | 21 | 112 | 80 | 59 | 2 | −30 | 2 |
| Konig | Eur J Heart Failure | Germany | HF | 69 | 288 | 4799 | n/a | 48 | 70 | 69 | 242 | 3501 | n/a | 50 | 51 | −27 | 1 |
| Little | Open Heart | UK | STEMI | 60 | 38 | 440 | 63 | 78 | 7 | 60 | 28 | 302 | 63 | 80 | 5 | −31 | 1 |
| Popovic | Cath CV Interv | France | STEMI | 3652 | 67 | 1552 | 60 | 76 | 0 | 74 | 4 | 72 | 63 | 74 | 1 | 129 | 3 |
| Rodriguez-Leor | Rev Esp Cardio | Spain | STEMI | 29 | 67 | 1305 | 64 | 78 | 45 | 29 | 61 | 946 | 63 | 78 | 33 | −28 | 1 |
| Salzano | Eur J Heart Failure | Italy | HF | 54 | 3 | 104 | 68 | 82 | 2 | 54 | 2 | 103 | 68 | 82 | 2 | −1 | 1 |
| Toner | JACC HF | Australia | HF | 120 | 13 | 217 | 80 | 52 | 2 | 30 | 3 | 32 | 80 | 44 | 1 | −41 | 3 |
| Zorzi | J Cardiovasc Med | Italy | Cardiovascular | 91 | 14 | 207 | 69 | 68 | 2 | 91 | 18 | 210 | 70 | 69 | 2 | 1 | 1 |
Comparator (1 = same period different year, 2 = different period, 3 = different periods summed).
ACS, acute coronary syndrome; HF, heart failure; STEMI, ST-elevation myocardial infarction.
The majority of studies reported a decline in admission rate during the COVID-19 pandemic, ranging from a 1%23 to 81%19 reduction, with only four reporting an increase in admission rate.18,20,22,24 The mean age of patients was 68 years in both COVID-19 and comparator cohorts and the percentage of male patients was 68% and 67%, respectively. The median length of the COVID-19 observation period was 55 [IQR 30–54] days compared with 60 [IQR 30–120] days in the comparator period.
Study quality
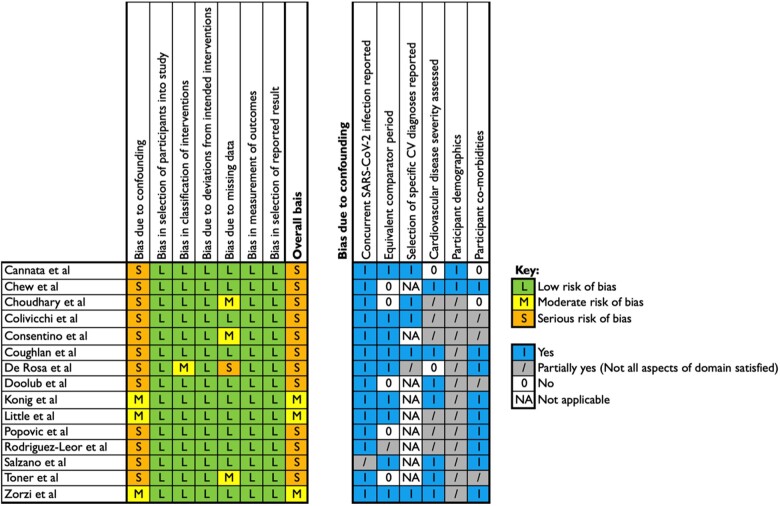
We classified 12 studies as being at serious risk of bias due to confounding and 3 studies to be at moderate risk (Figure 2). The principal reason for the elevated risk of bias was the use of non-equivalent comparator periods in 33% of studies. All studies were at low risk of bias according to the selection of participants into the study, deviation from intended interventions, measurement of outcomes, and selective reporting. One study had moderate risk of bias due to the classification of interventions, specifically due to the absence of justification for the selected time period.25 One had severe25 and three moderate risk of bias17,19,24 due to missing data or the potential for missing data. All other domains were at low risk of bias.
Figure 2.
ROBINS-I risk of bias score. The risk of bias was assessed using the ROBINS-I tool (A). Confounding domain scores are shown in (B).
Quantitative synthesis
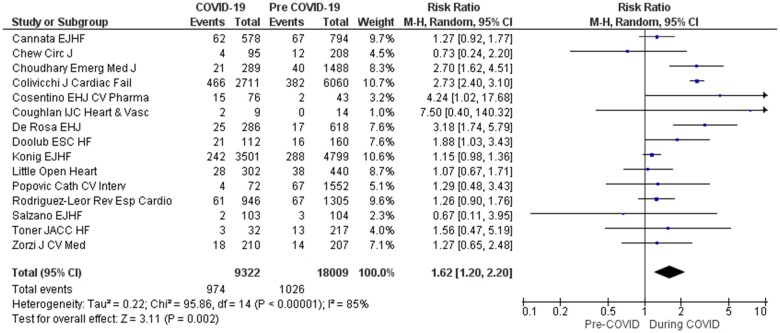
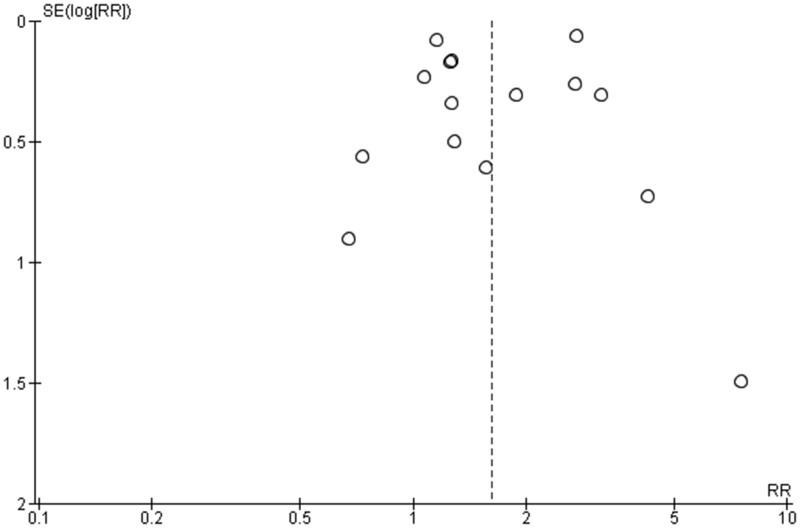
A total of 9322 and 18 009 admissions were included in the COVID-19 and comparator groups, respectively. Of these, 974 (10.4%) died in hospital in the COVID-19 group and 1026 (5.7%) in the comparator group. Overall, there was a significant 62% increase in in-hospital mortality in the COVID-19 period compared with the control period (RR 1.62, 95% CI 1.20–2.20, P = 0.002; Figure 3). Significant heterogeneity was observed in the pooled analysis (τ2 0.22 and I2 85%, P < 0.001). The funnel plot was symmetrical, demonstrating no evidence of significant publication bias (Figure 5).
Figure 3.
Summary plot of meta-analysis of in-hospital mortality. Forest plot of the effect of the COVID-19 pandemic on in-hospital mortality, pooled using random-effects meta-analysis. Overall, 15 studies were included. The diamonds represent the pooled difference using a random-effects model. I2 is the percentage of total variation across studies due to heterogeneity. CI, confidence interval.
Figure 5.
Funnel plot to look for publication bias.RR, risk ratio; SE, study effect.
Subgroup analysis
We performed pre-specified subgroup analysis according to the change in admission rate between the COVID-19 and comparator periods. This showed that studies with a drop in admission rate >50% (n = 3) had a significantly greater increase in mortality compared with those with <50% reduction (n = 12) [RR 2.74 (95% CI 2.43–3.10) vs. 1.21 (95% CI 1.07–1.37, P < 0.001]. This result also demonstrated that the heterogeneity observed in the overall pooled analysis was sensitive to changes in admission rate. Excluding the three studies with a >50% drop in admission rate abolished the heterogeneity (τ2 <0.01 and I2 0%) but had no effect on the overall result [RR 1.21 (95% CI 1.05–1.39), P = 0.01, Supplementary material online, Figure S1].
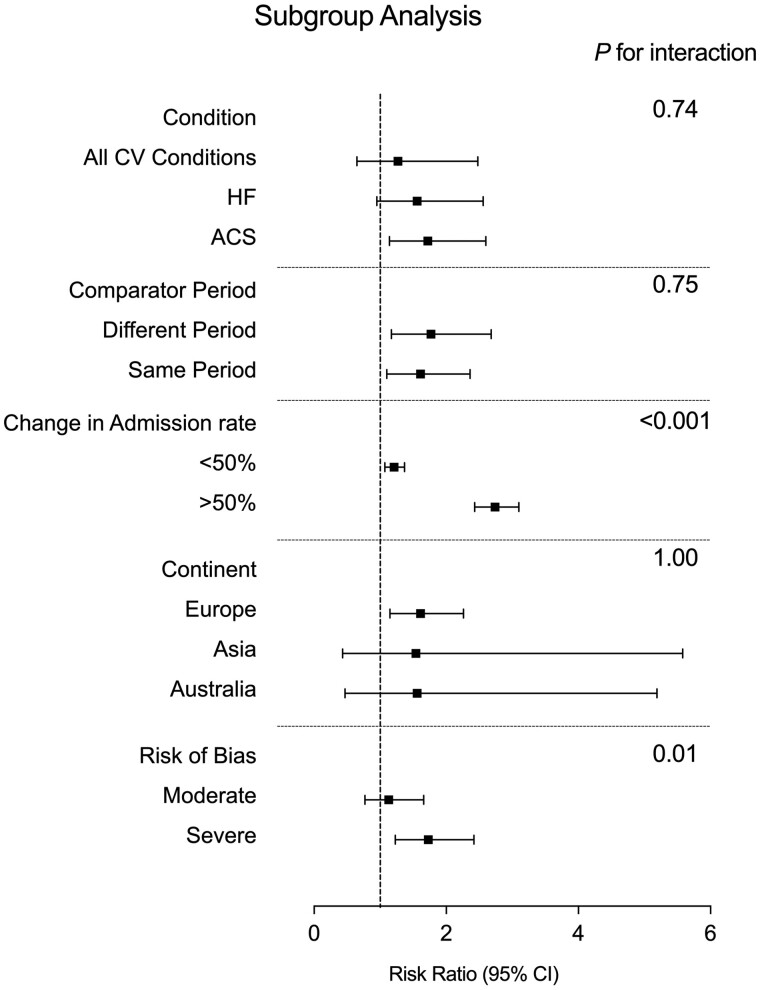
Other subgroup analyses did not explain the heterogeneity found in the overall analysis and, other than the risk of bias analysis, demonstrated consistent results within subgroups (Figure 4). We observed no difference in RR between studies using the same or different comparator periods, to account for seasonal variation [1.61 (95% CI 1.10–2.36) and 1.77 (95% CI 1.17–2.68), respectively, P = 0.75], or between different CV diseases at presentation [ACS 1.72 (95% CI 1.14–2.60), HF 1.56 (95 CI 0.95–2.56) and studies investigating combined CV conditions 1.27 (95% CI 0.65–2.48), P = 0.74]. Furthermore, the finding of increased in-hospital mortality was consistent across all geographical locations [Europe RR 1.61 (95% CI 1.15–2.26), Asia 1.54 (95 CI 0.43–5.58) and Australia 1.56 (95% CI 0.47–5.19), P = 1.00]. All countries, other than Singapore, where there was only one small study (n = 303), had higher in-hospital mortality during than pre-COVID and there was no clear relationship between the incidence of COVID infection (cases/million) and the increase in in-hospital mortality in each country. However, the number of studies per country was too small to draw any firm conclusions. However, studies with severe risk of bias described a greater risk of in-hospital mortality compared with those with moderate risk of bias [RR 1.84 (95% CI 1.33–2.54) vs. 1.15 (95 CI 0.99–1.34), respectively, P = 0.01].
Figure 4.
Summary forest plot of subgroup analyses. Summary forest plot of subgroup analyses, including condition, comparator period, change in admission rate, geographical location by continent and risk of bias, pooled using random-effects meta-analysis. CI, confidence interval.
Discussion
The main finding of our study is that during the COVID-19 pandemic, in-hospital mortality for patients admitted with CV conditions, but not infected with SARS-CoV2, increased significantly. Based on data from 15 studies and 27 331 admissions, we found that mortality was 62% higher during COVID-19 compared with pre-pandemic levels.
To our knowledge, this is the first meta-analysis to investigate in-hospital mortality for CV conditions during the COVID-19 pandemic. Several previous studies have evaluated the impact of COVID-19 on in-hospital CV mortality, with mixed results.3,4,26 Moreover, many reports have been significantly confounded by the COVID-19 status of the included patients, as SARS-CoV-2 infection is itself associated with a high case fatality rate, especially in patients with CV comorbidities.27,28 Importantly, in our analysis, we were careful to exclude studies where concurrent COVID-19 was not specifically excluded.
We observed high levels of methodological heterogeneity between the included studies. However, despite this heterogeneity, almost all studies reported similar findings—an increase in CV mortality during the COVID pandemic. Using subgroup analysis, we evaluated the impact of the underlying CV condition, geographical location, and comparator period. Increased in-hospital mortality during the COVID-19 pandemic was consistent across all tested subgroups, including the underlying cardiac condition, with comparable mortality increases in both ACS and HF. Furthermore, the use of non-matched comparator periods did not alter the overall effect, suggesting increased in-hospital CV mortality during the pandemic regardless of confounding due to seasonal variation.
Most studies reported a decline in hospital admissions for CV disease, which is consistent with other reports in both CV disease and other conditions.29–32 We also performed subgroup analysis evaluating the effect of the change in admission rate on CV mortality. In studies reporting a decline in admissions of >50% during the COVID-19 pandemic (n = 3), the CV mortality rate was significantly higher than those with lower reductions in admission rate (n = 12). Excluding these three studies from the analysis abolished statistical heterogeneity in the remaining 12 studies without impacting the overall pooled result, which remained significant. Our finding of a significant relationship between change in admission rate and observed in-hospital CV mortality rate is important. More extreme declines in hospital admission rate may reflect patients with less severe disease staying at home, and hospital admissions including a smaller number of sicker patients with a resulting higher in-hospital mortality rate. This observation, at least in part, may underlie some of the observed increase in CV mortality seen in our analysis and warrants further investigation, including changes in mortality at community level.
The SARS-CoV2 pandemic has had an enormous impact on healthcare systems and, consequently, on the care of people with conditions other than COVID-19, including CV diseases.33–35 There has been widespread redeployment of specialist healthcare staff to support COVID-19 services, and a reduced availability of routine CV investigations and procedures. In addition, many patients are likely to have avoided healthcare environments to prevent nosocomial infection with SARS-CoV2, which may have led to patients delaying seeking medical care for CV conditions. All these factors are potentially important and may contribute to the increased CV mortality reported here.
Patients admitted during the COVID-19 pandemic were generally sicker and presented later compared with previous years.3,36,37 Specifically, for patients with ACS, door to balloon time was increased and patients more often presented with cardiogenic shock and more advanced conditions.18,19,24,38 Hospital admissions for ACS with signs of HF were more frequent during the pandemic and the complication rate for invasive procedures was increased.18–20 Delayed presentation might be partially justified by the reluctance of patient to seek medical attention as well as reduced availability of emergency services.35 This might also account for reports of increased of out-of-hospital cardiac arrest.39,40 Similarly, hospital management of patients admitted with HF changed during the first wave of the pandemic, including more frequent management in non-specialist wards.4
The present analysis included reports investigating in-hospital outcomes during the first wave of the pandemic. It is possible that, as the pandemic has progressed, in-hospital outcomes for patients admitted with CV conditions have improved, but there are currently little data available to address this question.
The studies included in our analysis were generally at serious risk of bias. This was predominantly due to confounding, related to the use of non-matched periods for comparison. Although subgroup analysis did not demonstrate a significant impact of the choice of comparator period on the results, this high risk of bias needs to be considered when interpreting our results.
Limitations
Our study was a meta-analysis of observational studies and, as such, has several important limitations. First, the studies included in our analysis demonstrated significant methodological heterogeneity, most importantly concerning patient populations and comparator periods. We have tried to mitigate this by using random-effects models and performing subgroup analyses, but this needs to be considered when interpreting our findings. Second, some subgroups contained a small number of studies and these should be interpreted with caution. Third, all studies included in our analysis were observational and most studies did not report patients’ characteristics in detail. As a result, there are likely to be unmeasured, potentially confounding variables that could impact our results and limit our ability to ascertain the reasons for the observed increase in mortality. Fourth, this analysis investigated only in-hospital mortality, while the effect of the pandemic on out-of-hospital mortality is still under investigation, resulting in possible collider bias. Further studies are needed to assess the impact of the pandemic on the whole spectrum of CV disease. Lastly, while we made every effort to exclude studies that did not adequately account for concurrent SARS-CoV2 infection, it is likely that some included patients will have had COVID-19. This may have impacted our results.
Conclusions
This is the first meta-analysis investigating in-hospital mortality for CV conditions during the COVID-19 pandemic, independent of co-infection with SARS-CoV2. We report a significantly higher risk of in-hospital mortality in this cohort compared with periods outside the pandemic. This effect was largest in studies with the biggest decline in admission rates, where only the sickest patients may have presented. However, studies were generally poorly conducted and there is a need for further well-designed studies to establish the full extent of mortality not directly related to COVID-19 infection. More detailed and comprehensive analysis investigating the role of COVID-19 co-infection, healthcare reconfiguration and disruption of services are warranted to describe the magnitude of collateral damage caused by the pandemic.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the authors of all the studies included in this meta-analysis, with special thanks to those who supplied information not included in their published manuscript.
Funding
D.I.B. has received funding from an NIHR Clinical Lectureship and Academy of Medical Sciences Starter Grant for Clinical Lecturers. A.M.S. is supported by the British Heart Foundation (CH/1999001/11735). The funders played no role in study design, collection, analysis, interpretation of data, writing of the report, or in the decision to submit the paper for publication. They accept no responsibility for the contents.
Conflict of interest: none declared.
Contributor Information
Antonio Cannata, School of Cardiovascular Medicine & Sciences, King's College London British Heart Foundation Centre of Excellence, 125 Coldharbour Lane, London SE5 9NU, UK; Department of Cardiology, King’s College Hospital NHS Foundation Trust, London, SE5 9RS, UK.
Samuel A Watson, School of Cardiovascular Medicine & Sciences, King's College London British Heart Foundation Centre of Excellence, 125 Coldharbour Lane, London SE5 9NU, UK; Department of Cardiology, King’s College Hospital NHS Foundation Trust, London, SE5 9RS, UK.
Allen Daniel, Department of Cardiology, King’s College Hospital NHS Foundation Trust, London, SE5 9RS, UK.
Mauro Giacca, School of Cardiovascular Medicine & Sciences, King's College London British Heart Foundation Centre of Excellence, 125 Coldharbour Lane, London SE5 9NU, UK.
Ajay M Shah, School of Cardiovascular Medicine & Sciences, King's College London British Heart Foundation Centre of Excellence, 125 Coldharbour Lane, London SE5 9NU, UK.
Theresa A McDonagh, Department of Cardiology, King’s College Hospital NHS Foundation Trust, London, SE5 9RS, UK.
Paul A Scott, Department of Cardiology, King’s College Hospital NHS Foundation Trust, London, SE5 9RS, UK.
Daniel I Bromage, School of Cardiovascular Medicine & Sciences, King's College London British Heart Foundation Centre of Excellence, 125 Coldharbour Lane, London SE5 9NU, UK; Department of Cardiology, King’s College Hospital NHS Foundation Trust, London, SE5 9RS, UK.
References
- 1.Coronavirus disease 2019 (COVID-19) Situation Report: World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (25 May 2021).
- 2. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;5:831–840. [DOI] [PubMed] [Google Scholar]
- 3. Bromage DI, Cannatà A, Rind IA, Gregorio C, Piper S, Shah AM, McDonagh TA. The impact of COVID-19 on heart failure hospitalization and management: report from a Heart Failure Unit in London during the peak of the pandemic. Eur J Heart Fail 2020;22:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cannatà A, Bromage DI, Rind IA, Gregorio C, Bannister C, Albarjas M, Piper S, Shah AM, McDonagh TA. Temporal trends in decompensated heart failure and outcomes during COVID-19: a multisite report from heart failure referral centres in London. Eur J Heart Fail 2020;22:2219–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boukhris M, Hillani A, Moroni F, Annabi MS, Addad F, Ribeiro MH, Mansour S, Zhao X, Ybarra LF, Abbate A, Vilca LM, Azzalini L. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can J Cardiol 2020;36:1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flores-Mir C, Major MP, Major PW. Search and selection methodology of systematic reviews in orthodontics (2000-2004). Am J Orthod Dentofacial Orthop 2006;130:214–217. [DOI] [PubMed] [Google Scholar]
- 8. Major MP, Major PW, Flores-Mir C. An evaluation of search and selection methods used in dental systematic reviews published in English. J Am Dent Assoc 2006;137:1252–1257. [DOI] [PubMed] [Google Scholar]
- 9. Major MP, Major PW, Flores-Mir C. Benchmarking of reported search and selection methods of systematic reviews by dental speciality. Evid Based Dent 2007;8:66–70. [DOI] [PubMed] [Google Scholar]
- 10. O’Connor D, Green S, Higgins JPT. Chapter 5: Defining the review question and developing criteria for including studies. In: Cochrane Handbook for Systematic Reviews of Interventions Version 510 [Internet]. The Cochrane Collaboration; 2011. http://handbook.cochrane.org/ (11 August 2015).
- 11. Rodríguez-Leor O, Cid-Álvarez B, Pérez de Prado A, Rossello X, Ojeda S, Serrador A, López-Palop R, Martín-Moreiras J, Rumoroso JR, Cequier Á, Ibáñez B, Cruz-González I, Romaguera R, Moreno R, Villa M, Ruíz-Salmerón R, Molano F, Sánchez C, Muñoz-García E, Íñigo L, Herrador J, Gómez-Menchero A, Gómez-Menchero A, Caballero J, Ojeda S, Cárdenas M, Gheorghe L, Oneto J, Morales F, Valencia F, Ruíz JR, Diarte JA, Avanzas P, Rondán J, Peral V, Pernasetti LV, Hernández J, Bosa F, Lorenzo PLM, Jiménez F, Hernández J. M D L T, Jiménez-Mazuecos J, Lozano F, Moreu J, Novo E, Robles J, Moreiras JM, Fernández-Vázquez F, Amat-Santos IJ, Gómez-Hospital JA, García-Picart J, Blanco B. G D, Regueiro A, Carrillo-Suárez X, Tizón H, Mohandes M, Casanova J, Agudelo-Montañez V, Muñoz JF, Franco J, del Castillo R, Salinas P, Elizaga J, Sarnago F, Jiménez-Valero S, Rivero F, Oteo JF, Alegría-Barrero E, Sánchez-Recalde Á, Ruíz V, Pinar E, Pinar E, Planas A, Ledesma BL, Berenguer A, Fernández-Cisnal A, Aguar P, Pomar F, Jerez M, Torres F, García R, Frutos A, Nodar JMR, García K, Sáez R, Torres A, Tellería M, Sadaba M, Mínguez JRL, Merchán JCR, Portales J, Trillo R, Aldama G, Fernández S, Santás M, Pérez MPP. Impact of COVID-19 on ST-segment elevation myocardial infarction care. The Spanish experience. Rev Esp Cardiol (Engl Ed) 2020;73:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 14. Hooijmans CR, IntHout J, Ritskes-Hoitinga M, Rovers MM. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J 2014;55:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, Antonic A, Howells DW, Macleod MR. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 2014;221:92–102. [DOI] [PubMed] [Google Scholar]
- 16. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Retraction: cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020;382:2582. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Toner L, Koshy AN, Ko J, Driscoll A, Farouque O. Clinical characteristics and trends in heart failure hospitalizations: an Australian experience during the COVID-19 lockdown. JACC Heart Fail 2020;8:872–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chew NW, Sia C-H, Wee H-L, Benedict LJ-D, Rastogi S, Kojodjojo P, Chor WPD, Leong BS-H, Koh BC-P, Tam H, Quek L-S, Sia WC, Saw KW, Tung BW-L, Ng ZZ-Y, Ambhore A, Tay EL-W, Chan K-H, Lee C-H, Loh JP-Y, Low AF-H, Chan MY-Y, Yeo T-C, Tan H-C, Loh P-H. Impact of the COVID-19 pandemic on door-to-balloon time for primary percutaneous coronary intervention- results from the Singapore Western STEMI Network. Circ J 2021;85:139–149. [DOI] [PubMed] [Google Scholar]
- 19. Choudhary R, Gautam D, Mathur R, Choudhary D. Management of cardiovascular emergencies during the COVID-19 pandemic. Emerg Med J 2020;37:778–780. [DOI] [PubMed] [Google Scholar]
- 20. Zorzi A, Vio R, Rivezzi F, Falzone PV, Giordani AS, Condello C, Dellino CM, Deola P, Gallucci M, Giannattasio A, Licchelli L, Lupasco D, Montonati C, Ravagnin A, Sinigiani G, Torreggiani G, Vianello R, Migliore F, Famoso G, Babuin L, Cacciavillani L, Iliceto S. Characteristics and hospital course of patients admitted for acute cardiovascular diseases during the coronavirus disease-19 outbreak. J Cardiovasc Med (Hagerstown) 2021;22:29–35. [DOI] [PubMed] [Google Scholar]
- 21. Doolub G, Wong C, Hewitson L, Mohamed A, Todd F, Gogola L, Skyrme-Jones A, Shahid A, Sammut E, Dastidar A. Impact of COVID-19 on inpatient referral of acute heart failure: a single-centre experience from the south-west of the UK. ESC Heart Fail 2021; 8:1691–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Popovic B, Varlot J, Metzdorf PA, Jeulin H, Goehringer F, Camenzind E. Changes in characteristics and management among patients with ST-elevation myocardial infarction due to COVID-19 infection. Catheter Cardiovasc Interv 2021;97: E319–E326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salzano A, D'Assante R, Stagnaro FM, Valente V, Crisci G, Giardino F, Arcopinto M, Bossone E, Marra AM, Cittadini A. Heart failure management during the COVID-19 outbreak in Italy: a telemedicine experience from a heart failure university tertiary referral centre. Eur J Heart Fail 2020;22:1048–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cosentino N, Bartorelli AL, Marenzi G. Time to treatment still matters in ST-elevation myocardial infarction: a call to maintain treatment effectiveness during the COVID-19 pandemic. Eur Heart J Cardiovasc Pharmacother 2020;6:408–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Rosa S, Spaccarotella C, Basso C, Calabro MP, Curcio A, Filardi PP, Mancone M, Mercuro G, Muscoli S, Nodari S, Pedrinelli R, Sinagra G, Indolfi C; Società Italiana di Cardiologia and the CCU Academy Investigators Group. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;41:2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiss P, Carcel C, Hockham C, Peters SAE. The impact of the COVID-19 pandemic on the care and management of patients with acute cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes 2021;7:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, Curnis A, Faggiano P, Gorga E, Lombardi CM, Milesi G, Vizzardi E, Volpini M, Nodari S, Specchia C, Maroldi R, Bezzi M, Metra M. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J 2020;41:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020;323:1775–1776. [DOI] [PubMed] [Google Scholar]
- 29. Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, Hollings S, Roebuck C, Gale CP, Mamas MA, Deanfield JE, de Belder MA, Luescher TF, Denwood T, Landray MJ, Emberson JR, Collins R, Morris EJA, Casadei B, Baigent C. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020;396:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butt JH, Fosbøl EL, Østergaard L, Yafasova A, Andersson C, Schou M, Gerds TA, Phelps M, Kruuse C, Gislason GH, Torp-Pedersen C, Køber L. Effect of COVID-19 on first-time acute stroke and transient ischemic attack admission rates and prognosis in Denmark: a nationwide cohort study. Circulation 2020;142:1227–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clerici M, Durbano F, Spinogatti F, Vita A, de Girolamo G, Micciolo R. Psychiatric hospitalization rates in Italy before and during COVID-19: did they change? An analysis of register data. Ir J Psychol Med 2020;37:283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dopfer C, Wetzke M, Zychlinsky Scharff A, Mueller F, Dressler F, Baumann U, Sasse M, Hansen G, Jablonka A, Happle C. COVID-19 related reduction in pediatric emergency healthcare utilization—a concerning trend. BMC Pediatr 2020;20:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crisafulli A, Pagliaro P. Physical activity/inactivity and COVID-19. Eur J Prev Cardiol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agostoni P, Mapelli M, Conte E, Baggiano A, Assanelli E, Apostolo A, Alimento M, Berna G, Guglielmo M, Muratori M, Susini F, Palermo P, Pezzuto B, Salvioni E, Sudati A, Vignati C, Merlino L. Cardiac patient care during a pandemic: how to reorganise a heart failure unit at the time of COVID-19. Eur J Prev Cardiol 2020;27:1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cannata A, Bromage DI, McDonagh TA. The collateral cardiovascular damage of COVID-19: only history will reveal the depth of the iceberg. Eur Heart J 2021;42:1524–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pareek N, Yeoh J, Macaya F, Cannata S, Kanyal R, Bharucha A, Adamo M, Salinas P, Shah AM, Dworakowski R, MacCarthy P, Byrne J. Association of social containment on ST-segment elevation myocardial infarction presentations during the COVID-19 pandemic. Coron Artery Dis 2021;32:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Banerjee A, Chen S, Pasea L, Lai AG, Katsoulis M, Denaxas S, Nafilyan V, Williams B, Keong Wong W, Bakhai A, Khunti K, Pillay D, Noursadeghi M, Wu H, Pareek N, Bromage DI, McDonagh TA, Byrne J, Teo JTH, Shah AM, Humberstone B, Tang LV, Shah ASV, Rubboli A, Guo Y, Hu Y, Sudlow CLM, Lip GYH, Hemingway H. Excess deaths in people with cardiovascular diseases during the COVID-19 pandemic. Eur J Prev Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colivicchi F, Di Fusco SA, Magnanti M, Cipriani M, Imperoli G. The impact of the coronavirus disease-2019 pandemic and Italian lockdown measures on clinical presentation and management of acute heart failure. J Card Fail 2020;26:464–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baldi E, Sechi GM, Mare C, Canevari F, Brancaglione A, Primi R, Klersy C, Palo A, Contri E, Ronchi V, Beretta G, Reali F, Parogni P, Facchin F, Bua D, Rizzi U, Bussi D, Ruggeri S, Oltrona Visconti L, Savastano S; Lombardia CARe Researchers. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med 2020;383:496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marijon E, Karam N, Jost D, Perrot D, Frattini B, Derkenne C, Sharifzadehgan A, Waldmann V, Beganton F, Narayanan K, Lafont A, Bougouin W, Jouven X. Out-of-hospital cardiac arrest during the COVID-19 pandemic in Paris, France: a population-based, observational study. Lancet Public Health 2020;5:e437–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.