Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic has disrupted breast cancer control through short-term declines in screening and delays in diagnosis and treatments. We projected the impact of COVID-19 on future breast cancer mortality between 2020 and 2030.
Methods
Three established Cancer Intervention and Surveillance Modeling Network breast cancer models modeled reductions in mammography screening use, delays in symptomatic cancer diagnosis, and reduced use of chemotherapy for women with early-stage disease for the first 6 months of the pandemic with return to prepandemic patterns after that time. Sensitivity analyses were performed to determine the effect of key model parameters, including the duration of the pandemic impact.
Results
By 2030, the models project 950 (model range = 860-1297) cumulative excess breast cancer deaths related to reduced screening, 1314 (model range = 266-1325) associated with delayed diagnosis of symptomatic cases, and 151 (model range = 146-207) associated with reduced chemotherapy use in women with hormone positive, early-stage cancer. Jointly, 2487 (model range = 1713-2575) excess breast cancer deaths were estimated, representing a 0.52% (model range = 0.36%-0.56%) cumulative increase over breast cancer deaths expected by 2030 in the absence of the pandemic’s disruptions. Sensitivity analyses indicated that the breast cancer mortality impact would be approximately double if the modeled pandemic effects on screening, symptomatic diagnosis, and chemotherapy extended for 12 months.
Conclusions
Initial pandemic-related disruptions in breast cancer care will have a small long-term cumulative impact on breast cancer mortality. Continued efforts to ensure prompt return to screening and minimize delays in evaluation of symptomatic women can largely mitigate the effects of the initial pandemic-associated disruptions.
The novel coronavirus disease 2019 (COVID-19) pandemic has led to broad disruptions in health care in the United States, including major impacts on breast cancer control activities. Early in the pandemic, public health measures barred elective procedures, including screening mammography (1). Hospitals faced concerns with capacity and shortages of personal protective equipment, and women weighed the benefits of attending medical care facilities vs the risks of possible exposure.
In the initial months of the pandemic in 2020, there were severe declines in screening mammography (2-7) and reductions in diagnostic mammography of up to 80% (8,9). Breast cancer treatment protocols were also modified, with patient-reported delays in treatment (10) and reductions in chemotherapy administration (11).
Although weekly mammography volumes nearly recovered to prepandemic levels within 6 months (2,9,12-14), the impact of these disruptions on long-term breast cancer mortality remains unclear. We used 3 independently developed breast cancer simulation models from the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET) (15-18) to estimate the cumulative, long-term effect of COVID-19–related disruptions on breast cancer mortality. The results are intended to inform plans for mitigating these effects during and after the pandemic.
Methods
The study was determined to be other than human participant research by institutional review boards at each institution.
Model Descriptions
The study included 3 CISNET models (15): model D (Dana-Farber Cancer Institute) (19), model G-E (Georgetown University-Albert Einstein College of Medicine) (20), and model W-H (University of Wisconsin-Madison and Harvard Medical School) (21). The models are briefly summarized below; details are included in the Supplementary Methods (available online), previous publications, and online (15,16,18-22). CISNET breast cancer models have been previously used to inform breast cancer–screening guideline development by the US Preventive Services Task Force in 2009 and 2016 (17,18).
Briefly, the models estimate breast cancer incidence and mortality in the absence of screening and treatment and then overlay screening, diagnosis, and treatment effects to replicate US breast cancer trends over time (15). They account for differences in 4 molecular breast cancer subtypes based on estrogen receptors (ER) and HER2. All 3 models have the ability to follow multiple birth cohorts over time. Although the models share inputs, they utilize different structures, assumptions, and approaches (Supplementary Table 1, available online). For example, in model W-H, earlier diagnosis without a change in cancer staging can reduce mortality, whereas model G-E assumes a mortality benefit only if earlier diagnosis leads to a stage shift. The range of results across the models provides a measure of uncertainty due to unobservable factors such as breast cancer natural history and incidence in the absence of screening (16).
CISNET models use common inputs for key parameters informed by high-quality data sources (Table 1). The models successfully replicated US breast cancer incidence and mortality trends over time and were recently validated against the UK AGE trial results (16,18,23). In addition, the models have been validated individually. For example, model W-H was cross-validated against incidence and mortality data from Wisconsin Cancer Reporting System (21).
Table 1.
| Name | Description | Source |
|---|---|---|
| Population demographics and other-cause mortality | ||
| Population demographic characteristics | Cross-sectional female population in US organized by birth cohorts | US census data (34) |
| Other-case mortality | Death from other causes | CDC WONDER Database (41) |
| Natural history | ||
| Incidence in absence of screening | Breast cancer incidence in absence of screening and treatment | Age-period-cohort models (42, 43) |
| Survival in absence of screening and treatment | 25-y breast cancer survival before adjuvant treatment by joint ER/HER2 status, age group, AJCC/SEER stage, or tumor size | Meta-analyses (44) |
| Stage distribution | Stage distributions by mode of detection, age group (<50, 50-64, >65 y), screening round (first, subsequent), and screening interval | BCSC (40, 45) |
| ER/HER2 joint distribution | Probability of ER/HER2 conditional on age and stage/tumor size at diagnosis | BCSC (40, 45) |
| Screening and diagnosis | ||
| Mammography rates | Use of mammography by different ages over time | NHIS, BCSC (40, 45, 46) |
| Mammography performance | Sensitivity of initial and subsequent digital mammography by age group (<50, 50-64, >65 y) and screening interval | BCSC (40, 45) |
| Treatment | ||
| Treatment patterns | Treatments and rates of use by time period, ER/HER2, stage and age at time of breast cancer diagnosis | NCCN and meta-analyses (40, 47) |
| Treatment effects | Treatment efficacy by ER/HER2 for initial breast cancer diagnosis | Meta-analyses and clinical trial results (48–53) |
AJCC = American Joint Committee on Cancer; BCSC = Breast Cancer Surveillance Consortium; CDC = Centers for Disease Control and Prevention; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; NCCN = National Comprehensive Cancer Network; NHIS = National Health Information Survey; SEER = Surveillance, Epidemiology, and End Results; WONDER = Wide-ranging ONline Data for Epidemiologic Research.
Key Inputs and Assumptions
We used the same model inputs describing screening utilization and performance, clinical diagnosis, and treatment dissemination and effectiveness as prior analyses (16,18) to simulate breast cancer mortality from 2020 to 2030. In projecting outcomes for future years, we assumed that current mammography performance and use as well as treatment effectiveness and use remained constant for the 10-year period.
Our scenarios representing pandemic impacts on screening, diagnostic evaluation of breast cancer symptoms, and treatment were based on current literature and expert opinion. In our base case, we assumed a 6-month duration of pandemic-related disruptions (March to September 2020) in screening, diagnosis, and adjuvant chemotherapy, given reports that mammography use recovered to nearly 100% by the end of summer 2020 (2,9,13,14).
Disruptions in screening were informed by data from Epic Health Research Network, which pooled data from 60 health-care organizations representing 10 million women from 306 hospitals in 28 states (2). Based on their findings, we assumed that 50% of the women scheduled to undergo screening mammography missed their mammograms. Data from 2 Breast Cancer Surveillance Consortium registries (Vermont and San Francisco Bay Area) show that breast imaging volume for “evaluation of a breast problem” (ie, women presenting with symptoms for diagnostic imaging) decreased by 21% and 45%, respectively, during March-June 2020 compared with prepandemic levels in 2019 (Supplementary Figure 1, available online); therefore, we assumed that 25% of women delayed evaluation of breast cancer symptoms, resulting in delayed diagnosis and treatment. Finally, because there exist very limited data regarding COVID-19’s impact on breast cancer therapy, we based our treatment-related inputs on expert opinion. We base our assumption on treatment disruptions on the results of clinical trials including TAILORx and RxPonder that showed greater chemotherapy benefit for younger than older women, and of population-based studies showing that prepandemic treatment use coincided with these trial results (24-26). We assumed that oncologists would be more likely to recommend against cytotoxic chemotherapy for older women given their higher mortality rates from COVID-19 infection and concerns about treatment-related immunosuppression (27-29). We did not assume reductions in other systemic treatments (eg, endocrine therapy) because they are not immunosuppressive and, for certain medications, are taken at home and thus would be unlikely to be withheld for infection concerns. We modeled no chemotherapy reduction for patients with ER-negative and/or HER2-positive disease or for patients with stage IIB or higher cancer of any subtype because we assumed that oncologists recognize the more favorable risk–benefit ratio of chemotherapy for these higher-risk patients and recommend it despite the pandemic (30-32).
Pandemic Impact Scenarios
We simulated 6 scenarios (Table 2). Scenario 1 (no COVID-19 impact) assumed that the patterns in screening, diagnosis, and treatment between 2020 and 2030 would remain the same as in 2019. Scenario 2 represents the reduced screening scenario. Because it is not yet known how long women who missed screening during the pandemic will delay their screening, we simulated 3 different subscenarios for varying the time to return to screening (scenarios 2a-2c). In scenarios 2a-2c, women who missed their screening exams could be detected via clinical presentation and could start treatment during the pandemic period. Under scenario 2a (delayed screening), women who missed their screening exam resume screening 6 months after the missed mammogram. Under scenario 2b (skipped screening), women who missed their screening exam do not return until their next scheduled mammogram. Under scenario 2c (hybrid delayed/skipped screening), one-half of women who missed their screening mammogram resume screening 6 months after the missed mammogram, and one-half do not return until their next scheduled mammogram.
Table 2.
Summary of the simulated scenarios
| Scenario | Name | Description | Screening | Diagnosis | Treatment use | Base value (range for sensitivity analysis) |
|---|---|---|---|---|---|---|
| Scenario 1 | No COVID-19 impact | Pandemic does not lead to any changes in breast cancer control | Normal | Normal | Normal | — |
| Scenario 2 | Reduced screening | 50% of women scheduled to undergo exams miss their screening mammography | Reduced | Normal | Normal | 50% (25%-75%) |
| Scenario 2a | Delayed screening | Catch-up screening exam in 6 mo and push all future screening exams by 6 mo | Reduced | Normal | Normal | — |
| Scenario 2b | Skipped screening | Never catches up missed mammography exam | Reduced | Normal | Normal | — |
| Scenario 2c | Hybrid delayed and skipped screening | 50% of women who missed their exams follow scenario 2a and 50% follows scenario 2b | Reduced | Normal | Normal | — |
| Scenario 3 | Delayed diagnosis of symptomatic cases | 25% of women who would normally be detected via symptoms delay diagnosis for 6 mo | Normal | Delayed | Normal | 25% (15%-40%) |
| Scenario 4 | Reduced chemotherapy treatment | Women with ER+/HER2− and stages I and II (node negative) receive reduced chemotherapy at 25% for <70 y and 50% for >70 y but no reduction in use of endocrine therapy | Normal | Normal | Reduced | 25% for ages <70 y; 50% for ages >70 y (12.5%-50% for ages <70 y and 25%-75% for ages >70 y) |
| Scenario 5 | Reduced screening and delayed diagnosis | — | Reduced | Delayed | Normal | — |
| Scenario 5a | Delayed screening and delayed diagnosis | Scenario 2a and scenario 3 combined | Reduced | Delayed | Normal | — |
| Scenario 5b | Skipped screening and delayed diagnosis | Scenario 2b and scenario 3 combined | Reduced | Delayed | Normal | — |
| Scenario 5c | Hybrid delayed/skipped screening and delayed diagnosis | Scenario 2c and scenario 3 combined | Reduced | Delayed | Normal | — |
| Scenario 6 | Reduced screening and delayed diagnosis and reduced chemotherapy treatment | — | Reduced | Delayed | Reduced | — |
| Scenario 6a | Delayed screening and delayed diagnosis and reduced chemotherapy treatment | Scenario 5a and scenario 4 combined | Reduced | Delayed | Reduced | — |
| Scenario 6b | Skipped screening and delayed diagnosis and reduced chemotherapy treatment | Scenario 5b and scenario 4 combined | Reduced | Delayed | Reduced | — |
| Scenario 6c | Hybrid delayed and skipped screening and delayed diagnosis and reduced chemotherapy treatment | Scenario 5c and scenario 4 combined | Reduced | Delayed | Reduced | — |
Scenario 3 represents the delayed diagnosis of symptomatic cases in which women who delayed evaluation of symptoms experienced a 6-month delay in breast cancer diagnosis relative to their expected diagnosis in the absence of the pandemic (Supplementary Table 2, available online). Scenario 4 represents reduced chemotherapy treatment. Under scenario 4, among women diagnosed with ER+ and HER2− tumors in stages I and IIA who would have received chemotherapy if not for the pandemic, 25% of those younger than age 70 years and 50% of those older than age 70 years did not receive clinically indicated adjuvant chemotherapy. Scenario 5 represents reduced screening and delayed diagnosis and therefore jointly models scenarios 2 and 3. Finally, scenario 6 represents reduced screening, delayed diagnosis, and reduced chemotherapy treatment and hence jointly models scenarios 2, 3, and 4.
Analysis
Each model estimated the effect of COVID-19 disruptions on breast cancer deaths among all women aged 30 to 84 years between 2020 and 2030 in the United States. We modeled each disruption independently (eg, screening only, diagnosis only, treatment only) and combinations of disruptions (Table 2). For all analyses, results were age adjusted to the US standard population (33,34). We calculated the cumulative number of excess breast cancer deaths from the pandemic as the difference between deaths in analysis vs usual care with no COVID impact. We also calculated the percent increase in cumulative number of additional breast cancer deaths in each scenario vs usual care. Results are reported as the median and range across the 3 models.
Sensitivity Analysis
We conducted a sensitivity analysis on the proportion of women who delayed (scenario 2a) vs skipped (scenario 2b) their mammography exams during the pandemic (ranging from 0% to 100%), who missed screening exams during the pandemic period, who experienced reduced chemotherapy, who had delays in diagnosis, and on the impact of COVID-19 on other-cause mortality. We also conducted a sensitivity analysis on the duration of the pandemic, given the pandemic situation is still evolving, in which we assumed a 12-month duration of pandemic-related disruptions instead of 6 months. In doing so, we extended the baseline assumptions on the effects of disruptions on screening, diagnosis, and treatment. We used only 1 model (model W-H) in the sensitivity analyses, which provided the median estimates by 2030 for the base case.
Results
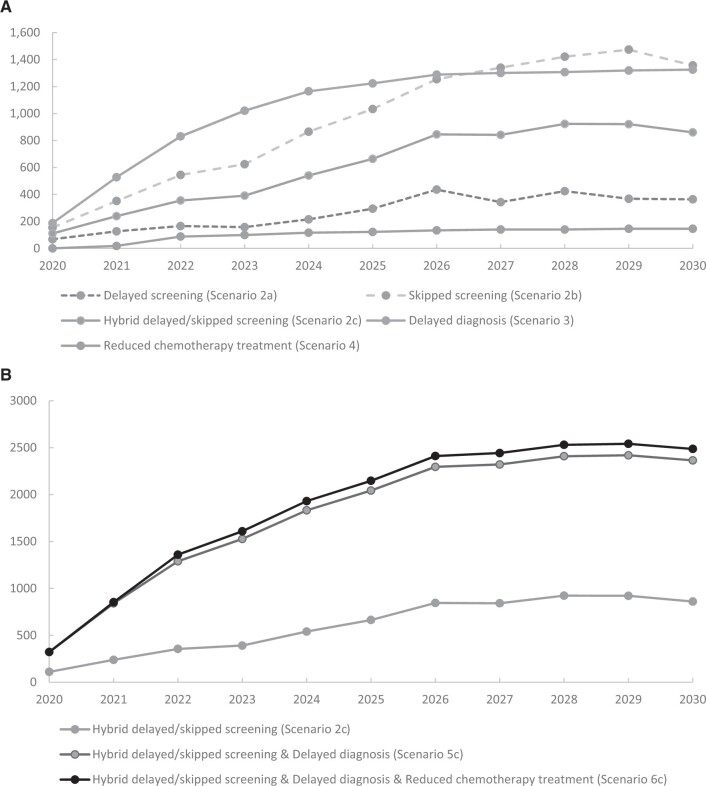
The models reproduced observed age-adjusted breast cancer mortality in the United States over time (Supplementary Figure 2, available online). The models predicted that the cumulative number of excess breast cancer deaths due to the COVID-19 pandemic’s impact on screening, diagnosis of symptomatic cases, and chemotherapy treatment could reach 2487 (model range = 1713-2575) by 2030 (Table 3; Supplementary Tables 3-7, and Supplementary Figures 3-4, available online). This corresponds to a 0.52% (model range = 0.36%-0.56%) increase in breast cancer deaths between 2020 and 2030 compared with usual care with no COVID-19 impact. By 2030, the models project 950 (model range = 860-1297) cumulative excess breast cancer deaths related to reduced screening; 1314 (model range = 266-1325) associated with delayed diagnosis of symptomatic cases, and 151 (model range = 146-207) associated with reduced chemotherapy use in women with hormone-positive, early-stage cancer. The effect of excess mortality associated with changes in screening, diagnosis, and treatment accelerated during 2020-2025 and leveled off thereafter (Table 3; Figure 1).
Table 3.
Median cumulative excess breast cancer mortalitya by 2022, 2025, and 2030 due to the COVID-19 pandemic effect for selected scenarios across 3 models (range across 3 models)
| Scenario | 2022 |
2025 |
2030 |
|||
|---|---|---|---|---|---|---|
| Scenario 1: no COVID-19 impact, median cumulative no. of deaths (range across models) | 122 675 (110 406-125 042) | 250 633 (228 585-257 537) | 473 903 (444 352-493 595) | |||
| Excess deaths (range across models), No. | Increase (range across models), % | Excess deaths (range across models), No. | Increase (range across models), % | Excess deaths (range across models), No. | Increase (range across models), % | |
| Scenario 2a: delayed screening | 166 | 0.15 | 294 | 0.13 | 364 | 0.08 |
| (131-209) | (0.11-0.17) | (265-413) | (0.10-0.16) | (269-404) | (0.05-0.09) | |
| Scenario 2b: skipped screening | 545 | 0.49 | 1158 | 0.45 | 1631 | 0.33 |
| (156-810) | (0.13-0.65) | (1033-1382) | (0.45-0.55) | (1357-2191) | (0.31-0.46) | |
| Scenario 2c: hybrid delayed and skipped screening | 355 | 0.32 | 711 | 0.29 | 950 | 0.19 |
| (144-509) | (0.12-0.41) | (664-898) | (0.28-0.36) | (860-1297) | (0.19-0.27) | |
| Scenario 3: delayed diagnosis | 411 | 0.33 | 728 | 0.28 | 1314 | 0.27 |
| (134-830) | (0.11-0.75) | (233-1223) | (0.09-0.54) | (266-1325) | (0.06-0.30) | |
| Scenario 4: reduced chemotherapy treatment | 39 | 0.03 | 100 | 0.04 | 151 | 0.03 |
| (27–88) | (0.02-0.08) | (84–122) | (0.03-0.05) | (146–207) | (0.03-0.04) | |
| Scenario 5a: disruptions in screening and diagnosis: best case scenario | 623 | 0.50 | 997 | 0.39 | 1589 | 0.32 |
| (267-1100) | (0.22-1.00) | (656-1674) | (0.26-0.73) | (675-1868) | (0.14-0.42) | |
| Scenario 5b: disruptions in screening and diagnosis: worst case scenario | 1236 | 0.99 | 1904 | 0.74 | 2861 | 0.60 |
| (302-1479) | (0.25-1.34) | (1632-2412) | (0.65-1.06) | (2476-2966) | (0.52-0.64) | |
| Scenario 5c: disruptions in screening and diagnosis | 930 | 0.74 | 1450 | 0.56 | 2277 | 0.46 |
| (285-1289) | (0.23-1.17) | (1144-2043) | (0.46-0.89) | (1576-2365) | (0.33-0.53) | |
| Scenario 6a: disruptions in screening and diagnosis and treatment: best case scenario | 701 | 0.56 | 1167 | 0.45 | 1896 | 0.38 |
| (291-1170) | (0.24-1.06) | (744-1778) | (0.30-0.78) | (826-1990) | (0.17-0.45) | |
| Scenario 6b: disruptions in screening and diagnosis and treatment: worst case scenario | 1311 | 1.05 | 2067 | 0.80 | 2983 | 0.66 |
| (315-1549) | (0.26-1.40) | (1700-2516) | (0.68-1.10) | (2599-3255) | (0.55-0.67) | |
| Scenario 6c: disruptions in screening and diagnosis and treatment | 1006 | 0.80 | 1617 | 0.63 | 2487 | 0.52 |
| (303-1360) | (0.25-1.23) | (1222-2147) | (0.49-0.94) | (1713-2575) | (0.36-0.56) | |
The excess mortality is expressed in terms of both the number of breast cancer deaths and percent increase compared with cumulative number of breast cancer deaths without pandemic effect. The excess number of deaths in a row for a particular scenario is calculated by subtracting the cumulative number of deaths without COVID-19 pandemic (scenario 1) as given in the first row from that obtained under that scenario. Similarly, the percent increase is calculated by dividing this difference by the cumulative number of deaths without COVID-19 pandemic.
Figure 1.
Cumulative excess breast cancer mortality according to exemplar model (University of Wisconsin-Madison and Harvard Medical School model) over time. A) The number of cumulative excessive deaths when each disruption is modeled separately. B) The number of excessive deaths when disruptions are combined.
Among the modeled scenarios, reductions in screening use and delays in diagnosis of symptomatic cases contributed the largest numbers of excess deaths. For example, disruptions for these 2 components (scenario 5c) resulted in 2277 (model range = 1576-2365) additional deaths, representing over 90% of the cumulative excess deaths associated with the modeled disruptions in screening, diagnosis, and chemotherapy treatment combined (scenario 6c) during this period (Table 3). The models suggest that the contribution of the modeled delay in diagnosis of symptomatic cases and reduced screening to the additional breast cancer deaths is similar. Disruptions in screening alone (scenario 2c) would lead to 950 (model range = 860-1297) additional deaths, representing 42% of the total excess deaths due to disruptions in screening and diagnosis (Table 3). However, cumulative breast cancer deaths by 2030 were fourfold higher if women skipped their mammogram rather than delayed screening by 6 months (1631 vs 364).
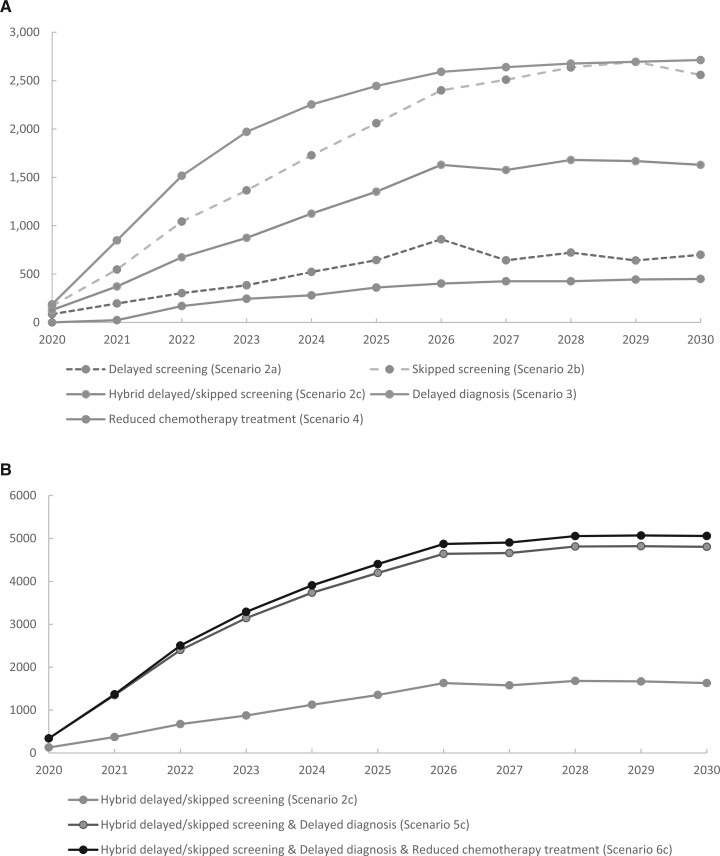
Varying assumptions about the proportion of women experiencing delays did not change the overall patterns of impact of the pandemic on 2030 breast cancer mortality (Table 4; Supplementary Tables 8-14, available online). Under all modeled scenarios, the increase in breast cancer deaths due to pandemic-related disruptions is not predicted to exceed 1% by 2030. In addition, if cancer diagnosis is delayed by 6 months for only 15% of women during the pandemic, the number of excess deaths exceeds the number observed if 50% of asymptomatic women delay screening for 6 months (758 vs 364) (Supplementary Table 11, available online; Table 3). If the modeled pandemic effects on screening, diagnosis, and treatment lasted for 12 months instead of 6 months, the number of additional deaths approximately doubles (Figure 2; Table 4; Supplementary Figures 5-6, available online). Sensitivity analysis on pandemic-related other-cause mortality input did not lead to any major changes in the results (Supplementary Table 15, available online).
Table 4.
Excess cumulative mortality projections for the sensitivity analyses varying scenario assumptions about magnitude of disruptions for the exemplar model (University of Wisconsin-Madison and Harvard Medical School model)
| Sensitivity analysis (SA) scenario | 2022 | 2025 | 2030 | |||
|---|---|---|---|---|---|---|
| No COVID-19 impact (scenario 1), cumulative no. of deaths | 110 406 | 228 585 | 473 903 | |||
| Excess deaths, No.a | Increase, % | Excess deaths, No.a | Increase, % | Excess deaths, No.a | Increase, % | |
| Base case results | 1360 | 1.23 | 2147 | 0.94 | 2487 | 0.56 |
| SA1: pandemic effects last 12 mo | 2504 | 2.27 | 4402 | 1.93 | 5058 | 1.14 |
| SA2: screening reduction is 25% | 1157 | 1.05 | 1818 | 0.80 | 2067 | 0.47 |
| SA3: screening reduction is 75% | 1545 | 1.40 | 2412 | 1.06 | 2860 | 0.64 |
| SA4: 15% of symptomatic cases are delayed | 970 | 0.88 | 1551 | 0.68 | 1832 | 0.41 |
| SA5: 40% of symptomatic cases are delayed | 1646 | 1.49 | 2556 | 1.12 | 2969 | 0.67 |
| SA6: smaller reduced chemotherapy treatment (12.5% for ages <70 y and 25% for ages >70 y) | 1324 | 1.20 | 2100 | 0.92 | 2433 | 0.55 |
| SA7: larger reduced chemotherapy treatment (50% for ages <70 y and 75% for ages >70 y) | 1394 | 1.26 | 2217 | 0.97 | 2580 | 0.58 |
The cumulative excess number of deaths are reported for each scenario representing disruptions in screening, diagnosis, and treatment (scenario 6c). The excess number of deaths for a sensitivity analysis in a row is calculated by subtracting the cumulative number of deaths without the COVID-19 pandemic (scenario 1) as given in the first row from that obtained under that sensitivity analysis scenario. Similarly, the percent increase is calculated by dividing this difference by the cumulative number of deaths without the COVID-19 pandemic. For each of the sensitivity analyses, the cumulative number of deaths without the COVID-19 pandemic (scenario 1) is the same.
Figure 2.
Cumulative excess breast cancer mortality according to exemplar model (University of Wisconsin-Madison and Harvard Medical School model) over time when the pandemic-related disruptions last for 12 months. A) The number of cumulative excessive deaths when each disruption is modeled separately. B) The number of excessive deaths when disruptions are combined.
Discussion
This collaborative modeling study provides useful information regarding the likely effects of initial pandemic-related disruptions on breast cancer mortality over time. Three independent models found that the cumulative impact of initial pandemic disruptions would be less than a 1% increase in cumulative breast cancer mortality over the next decade. This result is likely to be related to the rapid response of care providers to reinstituting screening and the assumption that women diagnosed with advanced-stage and/or poor prognosis cancers did not experience any delays in chemotherapy initiation. If the pandemic effects on care disruptions persist for 12 months, excess mortality would approximately double.
To our knowledge, no previous modeling study has conducted a comprehensive analysis of the impact of disruptions due to the COVID-19 pandemic on breast cancer mortality in the United States. A prior commentary used a preliminary analysis by 1 of the models used in this study (model W-H) and reported a higher mortality impact than that seen in this study (5391 vs 2487 cumulative excess deaths from 2020 to 2030) (35). However, that early analysis assumed higher reduction in screening (75% vs 50%) and greater delays in diagnosis (delay in diagnosis of all case s vs only symptomatic cases). A modeling study from Canada found that when all scheduled mammograms are skipped for 6 months and are made up gradually, the number of deaths increases by 0.48% by 2029, which is slightly higher than our estimate because we do not assume that all screening stopped during the pandemic (36). Another study focusing on the United Kingdom estimated that the number of breast cancer deaths could increase by 7.9%-9.6% in 5 years due to the pandemic; however, that study assumed that cancers could not be diagnosed after a screening exam but only through urgent referrals during the pandemic (37).
Our results suggest that the rapid adaptations of health-care facilities to devise strategies to resume breast cancer screening, diagnosis, and treatment services within a 6-month period greatly mitigated the potential impact on breast cancer mortality (2,9,13). The overall impact of disruptions in screening and delays in diagnosis of symptomatic evaluation were estimated to be similar, because most women due for routine screening will not have breast cancer. Even small disruptions to diagnostic evaluation of symptomatic women translate to substantial numbers of excess breast cancer deaths as demonstrated in the sensitivity analysis. As such, our results reinforce the importance of prompt evaluation of women with breast cancer symptoms during periods of reduced health-care access and capacity. This is consistent with a recent Breast Cancer Surveillance Consortium study, which found that during periods of reduced capacity, triaging individuals most likely to have cancer, including women with symptoms, could result in detecting the most cancers while performing the fewest examinations compared with a nonrisk-based approach (38).
Our findings also suggest that excess breast cancer mortality due to reduced access to screening during the pandemic could be mitigated by facilities giving priority to women who missed a screen during the pandemic. Although imaging volumes had returned to normal or above normal by September 2020, there remains a substantial cumulative deficit in screening and diagnostic evaluations compared with prepandemic years (9,13,39). This deficit may be due to multiple factors, including limited capacity of breast-imaging facilities to accommodate the number of women whose evaluation has been delayed, ongoing concerns from women about the safety of health-care facilities due the continued pandemic, or reduced access to health care due to COVID-19–related loss of employment-based health insurance. Many of these factors are likely to continue to affect receipt of diagnostic and screening services until control of the COVID-19 pandemic is achieved. Thus, the true duration of disruption to breast cancer control activities and the impact on long-term breast cancer mortality may ultimately prove to be more substantial than our current models suggest.
Despite the strength of consistent results from 3 established CISNET models, there are several limitations that should be considered in interpreting our results. First, in the absence of detailed information on breast cancer treatment patterns during the pandemic, we based our treatment assumptions on expert opinion. We assumed that during the pandemic, oncologists rationally limited chemotherapy use among patients least likely to benefit (early stage, ER-positive or HER2-negative disease) and at greatest risk for COVID-19–related complications (women older than 70 years) (27-29). We also assumed that oncologists ensured chemotherapy receipt among poor prognosis subtypes (ER-negative and/or HER2-positive or more advanced stages), assuming the benefit of adjuvant chemotherapy outweighed the risk of death due to COVID-19. Furthermore, because oral endocrine therapies (eg, tamoxifen or aromatase inhibitors) neither compromise the immune system nor require in-person visits for administration, we assumed the pandemic did not disrupt their use. It is possible that there were delays or nonuse of treatment, especially with losses of health-care insurance. In this case, the impact of pandemic-related treatment changes on excess breast cancer mortality may be greater than we expect. More data on cancer treatment patterns and longer follow-up will be essential to refine the mortality projections.
Another limitation is that many aspects of screening behavior and diagnostic evaluation during the pandemic remain poorly understood, including whether patterns in resumption of care are differential by age or perceived cancer risk. For example, disruptions in screening continue as woman are being encouraged to reschedule screening mammography after receiving the COVID-19 vaccination to prevent false-positive callbacks for vaccination-related lymphadenopathy. Additionally, although mammography volumes rebounded over the summer to reach prepandemic levels (2,9,13), it is not clear what portion of these exams are for missed mammograms or regularly scheduled exams. We tested a range of assumptions about these patterns, and results were similar in magnitude across different scenarios. Moreover, our models did not account for potential disparities in the resumption of breast cancer care services. Our recent work has shown that the recovery of breast cancer–screening and diagnostic services has not been equal for all women, with a slower rebound in use among Hispanic and Asian women as of July 2020 (13). Thus, although the overall impact of the pandemic on cancer outcomes may be small, it may disproportionately affect women in underserved populations and exacerbate health inequities. Therefore, it will be important to focus on resumption of access to screening for racial and ethnic minority women.
Finally, we assumed that future incidence, accuracy of screening and diagnosis, effectiveness of treatment, and other-cause mortality would carry forward at levels before the pandemic. Because these assumptions applied to the scenarios representing both COVID-19 disruptions and usual care, the relative difference in outcomes is unlikely to be affected.
In conclusion, in this collaborative simulation modeling study, we projected a small number of additional breast cancer deaths among US women from 2020 to 2030 due to the COVID-19 pandemic–related disruptions in breast cancer screening, diagnosis, and treatment. Efforts to ensure prompt access to screening, diagnostic evaluation, and treatment should mitigate the impact of the COVID-19 pandemic on breast cancer mortality.
Funding
This work was supported by the National Institutes of Health under National Cancer Institute Grants U01CA152958, U01CA253911, and R01CA248068. Supported in part by National Cancer Institute at the National Institutes of Health grant R35CA197289 to J.M. Collection of Breast Cancer Surveillance Consortium (BCSC; http://www.bcsc-research.org/) data used in this study was supported by National Cancer Institute Grants P01CA154292 and U54CA163303; grant R01 HS018366-01A1 from the Agency for Healthcare Research and Quality; and award PCS-1504–30370 from the Patient-Centered Outcomes Research Institute (PCORI).
Notes
Role of the funder: The funding agencies had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Disclosures: OA is a paid consultant for Bristol-Myers Squibb, outside of the submitted work. AK serves as an unpaid consultant for Ambry Genetics, Color Genomics, GeneDx/BioReference, Genentech, and InVitae, outside of the submitted work. KL has research funding from Association of University Radiologists/GE Healthcare, outside of the submitted work. No other conflicts to report.
Author contributions: Conception and design: OA, KL, AK, JM, BS, NS. Analysis and interpretation of the data: OA, KL, AK, JM, ME, HH, SL, CS, AT, DM, ATD, SN, KK, BS, NS. Drafting of the article: OA, KL, AK, JM, BS, NS. Critical revision for important intellectual content: OA, KL, AK, JM, ME, HH, SL, CS, AT, DM, ATD, SN, KK, BS, NS. Final approval of the article: OA, KL, AK, JM, ME, HH, SL, CS, AT, DM, ATD, SN, KK, BS, NS. Programming: ME, CS, HH. Analysis of data: OA.
Disclaimers: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Agency for Health Research and Quality, or PCORI or its Board of Governors or Methodology Committee.
Acknowledgements: The authors are grateful for the early feedback received from Rocky Feuer, PhD from the National Cancer Institute, which helped to improve this manuscript.
Data Availability
Additional details about the CISNET simulations models are available at https://cisnet.cancer.gov/breast/profiles.html and in references (15-23). All data underlying this study as well as model outputs are available from the corresponding author.
Supplementary Material
References
- 1.American College of Radiology. States with elective medical procedures guidance in effect. https://www.acr.org/-/media/ACR/Files/COVID19/May-18_States-With-Elective-Medical-Procedures-Guidance-in-Effect.pdf. Accessed November 20, 2020.
- 2.EPIC Health Research Network. Delayed cancer screenings--a second look. https://ehrn.org/articles/delayed-cancer-screenings-a-second-look. Accessed December 29, 2020.
- 3. London JW, Fazio-Eynullayeva E, Palchuk MB, et al. Effects of the COVID-19 pandemic on cancer-related patient encounters. J Clin Oncol Clin Cancer Inform. 2020;4:657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naidich JJ, Boltyenkov A, Wang JJ, et al. Impact of the Coronavirus Disease 2019 (COVID-19) pandemic on imaging case volumes. J Am Coll Radiol. 2020;17(7):865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parikh KD, Ramaiya NH, Kikano EG, et al. COVID-19 pandemic impact on decreased imaging utilization: a single institutional experience. Acad Radiol. 2020;27(9):1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin K, Singh P, Drohan B, et al. Breast imaging, breast surgery, and cancer genetics in the age of COVID-19. Cancer. 2020;126(20):4466–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whaley CM, Pera MF, Cantor J, et al. Changes in health services use among commercially insured US populations during the COVID-19 pandemic. JAMA Netw Open. 2020;3(11):e2024984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norbash AM, Moore AV Jr, Recht MP, et al. Early-stage radiology volume effects and considerations with the coronavirus disease 2019 (COVID-19) pandemic: adaptations, risks, and lessons learned. J Am Coll Radiol. 2020;17(9):1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song H, Bergman A, Chen AT, et al. Disruptions in preventive care: mammograms during the COVID-19 pandemic. Health Serv Res. 2021;56(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papautsky EL, Hamlish T.. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184(1):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. J Clin Oncol Clin Cancer Inform. 2020;4:1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaufman HW, Chen Z, Niles J, et al. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3(8):e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sprague BL, Lowry KP, Miglioretti DL, et al. Changes in mammography utilization by women’s characteristics during the first five months of the COVID-19 pandemic. J Natl Cancer Inst. 2021;113(9):1161--1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nyante SJ, Benefield TS, Kuzmiak CM, et al. Population‐level impact of coronavirus disease 2019 on breast cancer screening and diagnostic procedures. Cancer. 2021. doi:10.1002/cncr.33460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alagoz O, Berry DA, de Koning HJ, et al. Introduction to the Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models. Medical Decision Making. 2018;38(suppl 1):3S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. JAMA. 2018;319(2):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164(4):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee SJ, Li X, Huang H, et al. The Dana-Farber CISNET model for breast cancer screening strategies: an update. Medical Decision Making. 2018;38(suppl 1):44S–53S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schechter CB, Near AM, Jayasekera J, et al. Structure, function, and applications of the Georgetown-Einstein (GE) breast cancer simulation model. Med Decis Making. 2018;38(suppl 1):66S–77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alagoz O, Ergun MA, Cevik M, et al. The University of Wisconsin breast cancer epidemiology simulation model: an update. Med Decision Making. 2018;38(suppl 1):99S–111S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trentham-Dietz A, Kerlikowske K, Stout NK, et al. Tailoring breast cancer screening intervals by breast density and risk for women aged 50 years or older: collaborative modeling of screening outcomes. Ann Intern Med. 2016;165(10):700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Broek JJ, van Ravesteyn NT, Mandelblatt JS, et al. Comparing CISNET breast cancer incidence and mortality predictions to observed clinical trial results of mammography screening from ages 40 to 49. Med Decis Making. 2018;38(suppl 1):140S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. New Engl J Med. 2018;379(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurian AW, Bondarenko I, Jagsi R, et al. Recent trends in chemotherapy use and oncologists’ treatment recommendations for early-stage breast cancer. J Natl Cancer Inst. 2018;110(5):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalinsky K, Barlow WE, Meric-Bernstam F, et al. First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy and chemotherapy in patients with 1-3 positive nodes, hormone receptor-positive and HER2-negative breast cancer with recurrence score less than 25: SWOG S1007 (RxPonder). Abstract presented at San Antonio Breast Cancer Symposium; December 2020; San Antonio, TX.
- 27. Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369;m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee LY, Cazier J-B, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295(14):1658–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Early Breast Cancer Trialists' Collaborative Group. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lambertini M, Pondé NF, Solinas C, et al. Adjuvant trastuzumab: a 10-year overview of its benefit. Expert Rev Anticancer Ther. 2017;17(1):61–74. [DOI] [PubMed] [Google Scholar]
- 33.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program: standard populations (millions) for age-adjustment. https://seer.cancer.gov/stdpopulations/. Accessed February 2, 2021.
- 34.United States Census Bureau. 2017 National Population Projections Datasets. https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html. Accessed January 30, 2021.
- 35. Sharpless NE. COVID-19 and cancer. Science. 2020;368(6497):1290–1290. [DOI] [PubMed] [Google Scholar]
- 36. Yong JH, Mainprize JG, Yaffe MJ, et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2020;0969141320974711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miglioretti DL, Bissell MCS, Kerlikowske K, et al. A risk-based approach for triaging mammography examinations during periods of reduced capacity. JAMA Network Open. 2021;4(3):e211974–e211974. [DOI] [PMC free article] [PubMed]
- 39.American College of Radiology. “Return to Mammography Care” toolkit. https://www.acr.org/Clinical-Resources/Breast-Imaging-Resources/Mammography-Care-Toolkit. Accessed January 18, 2021.
- 40. Mandelblatt JS, Near AM, Miglioretti DL, et al. Common model inputs used in CISNET collaborative breast cancer modeling. Med Decis Making. 2018;38(suppl 1):9S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gangnon RE, Stout NK, Alagoz O, et al. Contribution of breast cancer to overall mortality for US women. Med Decis Making. 2018;38(suppl 1):24S–31S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gangnon RE, Sprague BL, Stout NK, et al. The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer Epidemiol Biomarkers Prev. 2015;24(6):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holford TR, Cronin KA, Mariotto AB, et al. Chapter 4: changing patterns in breast cancer incidence trends. JNCI Monographs. 2006;2006(36):19–25. [DOI] [PubMed] [Google Scholar]
- 44. Munoz DF, Plevritis SK.. Estimating breast cancer survival by molecular subtype in the absence of screening and adjuvant treatment. Med Decis Making. 2018;38(suppl 1):32s–43s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breast Cancer Surveillance Consortium. About the BCSC. https://www.bcsc-research.org/about. Accessed February 24, 2021.
- 46. Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the breast cancer surveillance consortium. Radiology. 2017;283(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caswell-Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectr. 2018;2(4):pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Early Breast Cancer Trialists' Collaborative Group, Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Early Breast Cancer Trialists' Collaborative Group, Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Early Breast Cancer Trialists' Collaborative Group, Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. [DOI] [PubMed] [Google Scholar]
- 52.Early Breast Cancer Trialists' Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. [DOI] [PubMed] [Google Scholar]
- 53.Early Breast Cancer Trialists' Collaborative Group. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional details about the CISNET simulations models are available at https://cisnet.cancer.gov/breast/profiles.html and in references (15-23). All data underlying this study as well as model outputs are available from the corresponding author.