Abstract
Background
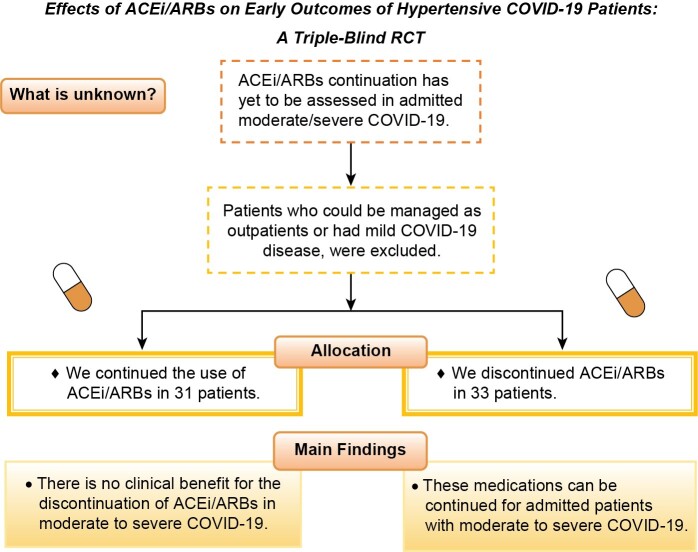
The role of angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) has been addressed in some studies related to the current coronavirus disease-2019 (COVID-19) pandemic with possible higher severity and mortality in patients with hypertension. A triple-blind randomized controlled trial was designed to evaluate the effects of these medications on the COVID-19 progression.
Methods
Patients were enrolled in this trial between April and September 2020. They were randomized in two groups. The former dosage of ACEis/ARBs was continued in one group while in another group, the ACEis/ARBs were replaced by amlodipine ± carvedilol according to the dose equivalents. The primary outcomes were length of stay in hospitals and intensive care units. Other outcomes include mechanical ventilation, non-invasive ventilation, readmission, and COVID-19 symptoms after discharge.
Results
We randomized 64 patients with COVID-19 into two groups. Most patients were aged 66-80 and 46-65 years-old, 33 (51.6%) and 27 (42.2%), respectively. The study groups were nearly similar in baseline vital signs and characteristics. In addition, there was no significant difference in terms of recorded systolic and diastolic blood pressure measurements between groups. Furthermore, we did not find a significant difference between the days of intensive care unit or ward admission, the discharge rate, or readmission rates between the two groups.
Conclusions
This randomized triple-blind multi-centric clinical trial did not show any deleterious effects of ACEi/ARB medications in hypertensive COVID-19 patients.
Keywords: Angiotensin Receptor Antagonists, Angiotensin-Converting Enzyme Inhibitors, Blood Pressure, Coronavirus Disease-2019, Hypertension, Adverse Outcome
Graphical Abstract
Graphical Abstract.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.