Abstract
Aim:
Serotonin is crucial for proper foetal development, and the placenta has been described as a ‘donor’ of serotonin for the embryo/foetus. However, in later stages of gestation the foetus produces its own serotonin from maternally-derived tryptophan and placental supply is no longer needed. We propose a novel model of serotonin homeostasis in the term placenta with special focus on the protective role of organic cation transporter 3 (OCT3/SLC22A3).
Methods:
Dually perfused rat term placenta was employed to quantify serotonin/tryptophan transport and metabolism. Placental membrane vesicles isolated from human term placenta were used to characterize serotonin transporters on both sides of the syncytiotrophoblast.
Results:
We obtained the first evidence that serotonin is massively taken up from the foetal circulation by OCT3. This uptake is concentration-dependent and inhibitable by OCT3 blockers of endogenous (glucocorticoids) or exogenous (pharmaceuticals) origin. Population analyses in rat placenta revealed that foetal sex influences placental extraction of serotonin from foetal circulation. Negligible foetal serotonin levels were detected in maternal-to-foetal serotonin/tryptophan transport and metabolic studies.
Conclusion:
We demonstrate that OCT3, localized on the foetus-facing membrane of syncytiotrophoblast, is an essential component of foeto-placental homeostasis of serotonin. Together with serotonin degrading enzyme, monoamine oxidase-A, this offers a protective mechanism against local vasoconstriction effects of serotonin in the placenta. However, this system may be compromised by OCT3 inhibitory molecules, such as glucocorticoids or antidepressants. Our findings open new avenues to explore previously unsuspected/unexplained complications during pregnancy including prenatal glucocorticoid excess and pharmacotherapeutic risks of treating pregnant women with OCT3 inhibitors.
Keywords: foetal development, pharmacotherapy, placenta, pregnancy, serotonin, transport
1 |. INTRODUCTION
Serotonin (5-hydroxytryptamine, 5-HT) acts as a crucial monoamine neuromodulator in the central nervous system and a hormone in many other organs and tissues. It also exerts crucial trophic effects, hence adequate levels of 5-HT in the foetal circulation during pregnancy are essential for proper foetal development and programming.1 Placenta has been deemed an organ that, to a certain extent, controls embryo/foetal levels of 5-HT and, according to the latest studies, disruption of 5-HT pathways in the placenta may have profound and long-lasting consequences for the developing foetus.2 However, research on the placental handling of 5-HT from the 1960s3,4 onwards5–9 has yielded strikingly inconsistent and often contradictory results. Several older studies presented the placenta as a barrier against maternal monoamines, including 5-HT,4,10 while other studies have suggested that maternal blood 5-HT can freely cross the placental barrier.5 More recent work suggests that during a precise time window of pregnancy the placenta acts as a barrier to maternal 5-HT but also a transient source of 5-HT for the foetus, which is crucial for normal brain development.6,7
Early in pregnancy, before the serotonergic system is fully functional in the developing brain and gut, the foetus depends on external (maternal and/or placental) sources.5,7 Localization of 5-HT transporter (SERT/SLC6A4) in the apical membrane of the placental syncytiotrophoblast11 and associations between maternal genotypes that alter 5-HT production with perturbed foetal development5,12 have suggested that maternal 5-HT is indispensable for the foetus. However, recent evidence shows that placenta can synthesize 5-HT from maternal tryptophan (TRP) as early as E10.5 of mouse7 and 11 weeks of human gestation,6 via activity of placental tryptophan-hydroxylase 1 (TPH1) and subsequently provide placenta-derived 5-HT to the foetus.
Importantly, the placenta also expresses monoamine oxidase A (MAO-A), an enzyme that catalyses degradation of 5-HT to an inactive metabolite, 5-hydroxyindoleacetic acid (5-HIAA).3,7 The presence and activity of the two counteracting enzymes, 5-HT-producing TPH and 5-HT-degrading MAO-A, in one organ likely offers a mechanism for fine-tuning 5-HT homeostasis in the foeto-placental unit, which may change during the course of pregnancy. Accordingly, dramatic increases in MAO-A activity reportedly occur in murine 7 and rat placenta towards term,13,14 whereas TPH1 expression and 5-HT synthesis decreases.7
It should be stressed that in later stages of gestation the foetus can synthesize its own 5-HT from maternally-derived TRP as observed in mice 15 and rats,16,17 so placental supply is no longer needed. Since 5-HT causes potent dose-dependent vasoconstriction in the placenta,18 excessive 5-HT levels are toxic for both the foetus and placenta.19 In adults, plasma 5-HT is sequestrated by platelets via SERT-dependent uptake. However, such a mechanism has not been described in the foetal circulation; therefore, we cannot exclude that other mechanisms, including placental uptake, control foetal circulating levels of 5-HT.
To the best of our knowledge, no attempt has been made to investigate placental handling of 5-HT (secretion/extraction) on the basal, foetus-facing membrane of the placenta. 5-HT is a substrate of several organic cation transporters (OCTs/SLC22As) 20 and plasma membrane monoamine transporter (PMAT/SLC29A4).21 While placental expression of OCT1 (SLC22A1), OCT2 (SLC22A2) and PMAT is only negligible, OCT3 (SLC22A3) is abundant in the foetus-facing basal membrane of syncytiotrophoblast.22 In recent studies, we quantified expression and activity of OCT3 in rat placenta and observed massive extraction of neurotoxin MPP+, an OCT3 substrate, from the foetal circulation.23 Subsequently, we detected increasing OCT3 expression and activity in the rat placenta and foetal brain towards the end of gestation, indicating that this transporter’s importance increases throughout gestation.24 In this study we hypothesized that the placenta removes 5-HT from foetal circulation via OCT3-mediated transport.
Based on results obtained from a set of various experimental approaches in rat and human placenta, we provide the first evidence that placental OCT3 extracts 5-HT from the foetal circulation into trophoblast cells, where it is degraded by MAO-A. This transport is concentration-dependent and inhibitable by both endogenous (glucocorticoids) and exogenous (pharmaceuticals) agents. Furthermore, in the rat term placenta we observed pronounced effects of foetal sex on extraction of 5-HT from the foetal circulation. Our data suggest that OCT3 plays an important role in 5-HT homeostasis in the foeto-placental unit. Therefore, any genetic, endocrine or pharmacological disruption of OCT3 function may perturb placental handling of 5-HT and hence foetal development/programming.
2 |. RESULTS
2.1 |. Placental uptake of 5-HT from foetal circulation in rat: Effect of concentration
The foetal side of the placenta was perfused with 5-HT at a range of concentrations (1 nM, 100 nM, 1 μM or 100 μM) with [3H]5-HT as a tracer. Organ clearance concept was applied to quantify placental uptake of 5-HT from the foetal circulation and is expressed as Cluptake, calculated according to Equation 2 and normalized to placental weight (Figure 1). A strong effect of inflow concentration on 5-HT placental clearance was detected. At low, physiological 5-HT concentration (1 nM), the average placental clearance for female and male placentas was 0.46 ml min−1 g−1 and 0.69 ml min−1 g−1 respectively, while at the highest supraphysiological concentration tested (100 μM), total clearance levels fell to 0.21 ml min−1 g−1, regardless of foetal sex (Figure 1). These results clearly demonstrate involvement of a capacity-limited transport mechanism of 5-HT uptake from foetal circulation; furthermore, effect of foetal sex on 5-HT placental uptake was observed at physiological concentrations.
FIGURE 1.
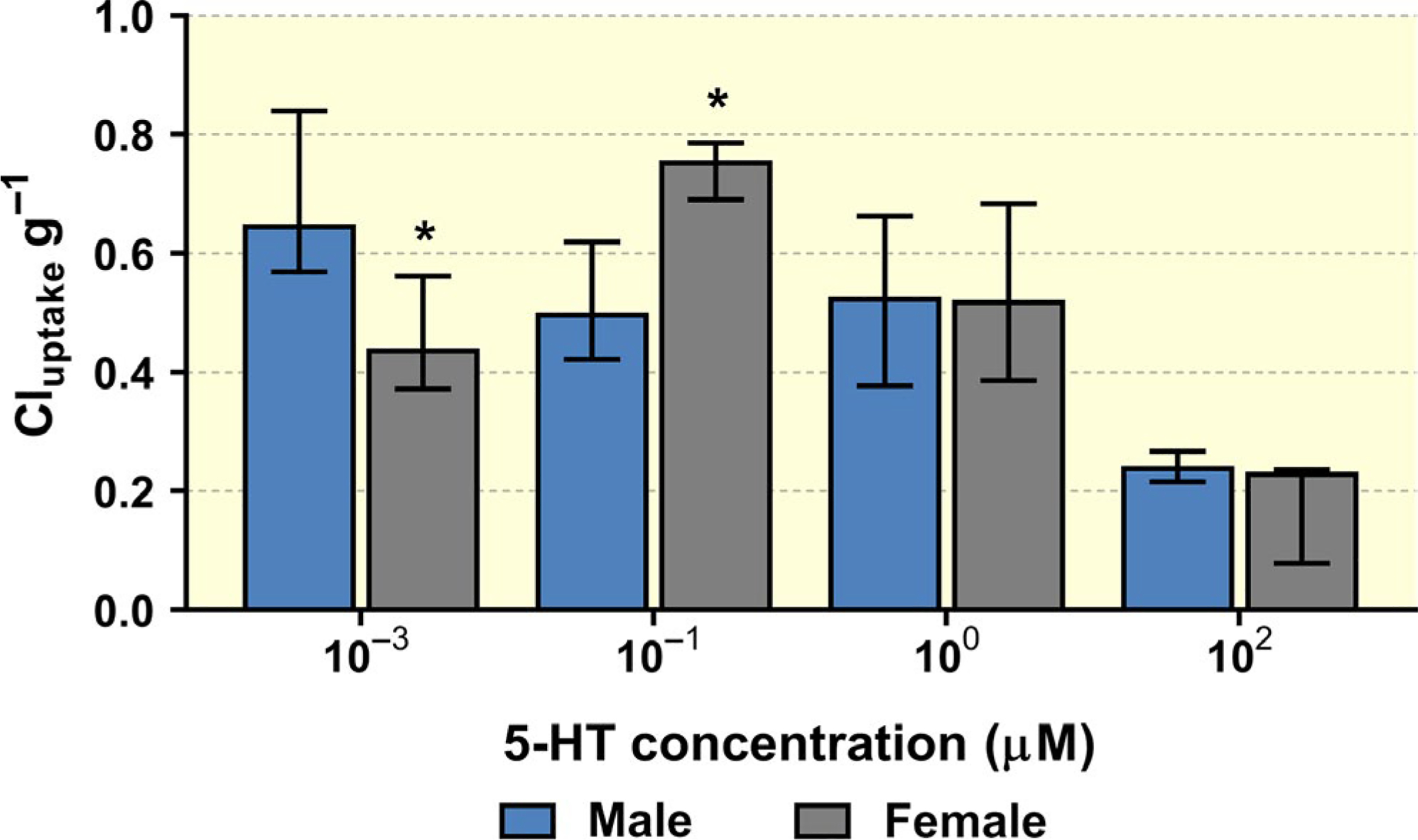
Concentration-dependent 5-HT uptake clearance by the rat placenta from foetal circulation normalized to placental weight. Decreased clearance with increasing 5-HT concentration indicates the involvement of a saturable, transporter-mediated mechanism. Moreover at 1 and 10 nM 5-HT concentrations, we observe statistically significant differences in placental clearance between male and female placentas. Data presented are median + IQR (n > 3) for each sex/concentration. Statistical analysis was evaluated using non-parametric Mann-Whitney test: * (P ≤ .05)
With Cluptake being modelled as a function of passive (Clpassive) and transporter-mediated uptake (Equation 3), data obtained from these perfusion experiments were pooled for population-based statistical analysis. Expected values of parameters for the entire population of subjects were estimated and used to draw a mean curve (Figure S1). The resulting variability in the dataset can be partitioned into interindividual or between-subject variability (the differences between individual subjects and the population means), and the unexplained residual or random variability. In the analysis, the interindividual and residual variability were described by additive and exponential error models respectively. This base model provided an acceptable fit of the data.
2.2 |. Placental uptake of 5-HT from foetal circulation in rat: effects of foetal sex and placental weight
To improve understanding of sources of variability, we investigated the influence of several covariates on parameter estimates; we detected strong influence of placental weight on passive transport (Clpassive) (Figure 2A) as well as effect of foetal sex on transporter-mediated uptake of 5-HT (Km and Vmax) (Figure 2B and C). For this, covariate models were constructed with foetal sex and placental weight as predictors of the interindividual variability in estimated parameters, and various choices of function types to model the dependence on covariates. Step-wise forward comparisons - based on the likelihood ratio test with corresponding objective function, Akaike information criterion (AIC) and Bayesian information criterion (BIC) (see Table S7) - of the models identified a covariate model with linear dependence and no intercept as best in terms of the goodness-of-fit criteria (Figure S2). The standard errors of the estimated slopes, which quantify the dependence on sex and placental weight, are below 100%, but not small enough to guarantee that they are statistically significantly different from zero. This may have been partly because the sample size was too small, but it is also possible that some variability was due to effects of other covariates that were not included in the analysis. On the other hand, direct comparison of the estimates for Vmax and Km evaluated using the non-parametric Mann-Whitney test revealed that these values were significantly higher for placentas of female offspring than for placentas of male offspring (Figure 2B and C).
FIGURE 2.
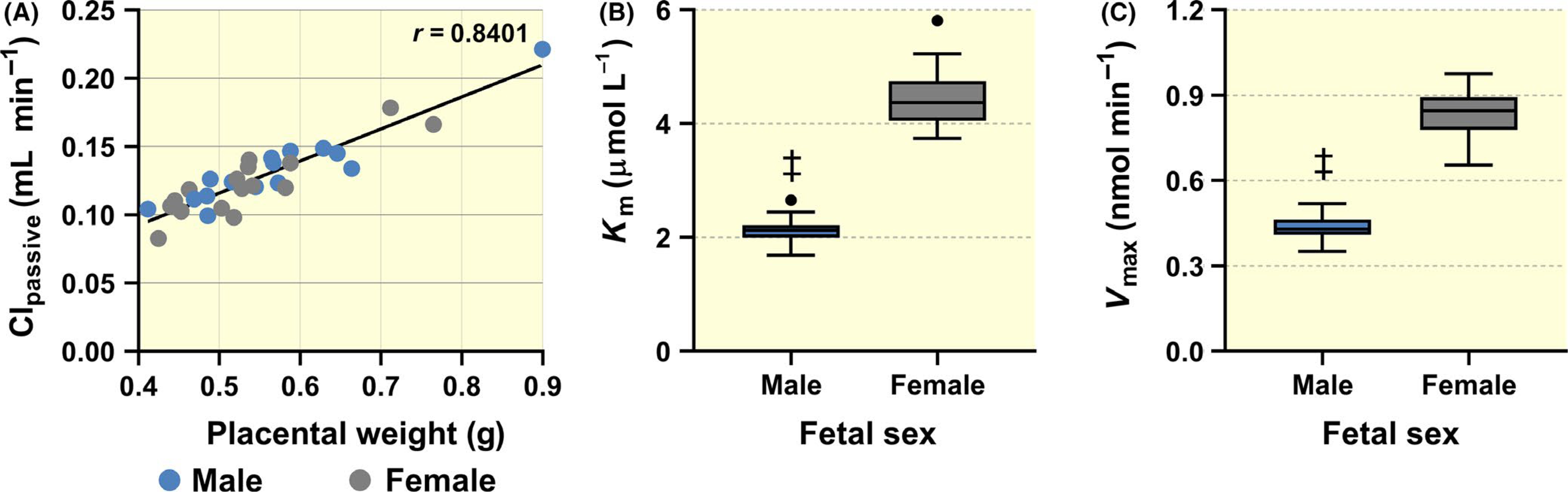
Identification of covariates affecting total Cluptake as a function of Clpassive and transporter-mediated uptake (see Equation 3). While a linear correlation between placental weight and Clpassive was found (A), foetal sex influences the kinetic parameters Km (B) and Vmax (C). Specifically, male placentas show statistically significant lower Km and Vmax compared to female placentas. Data are presented as Tukey boxplots (1.5-times IQR). Statistical analysis was performed using non-parametric Mann-Whitney test: ‡ (P ≤ .001)
2.3 |. Placental uptake of 5-HT from foetal circulation in rat: effects of inhibitors
To characterize the mechanism of 5-HT uptake from foetal circulation, paroxetine (an inhibitor of both SERT and OCT3), cimetidine (an OCT3 inhibitor) and corticosterone (an OCT3 inhibitor) were co-administered with [3H]5-HT at physiological, non-saturating concentration (1 nM). All inhibitors tested significantly decreased placental extraction ratio (ER) (Figure 3), with paroxetine showing the highest inhibitory potential (ER = 5%).
FIGURE 3.
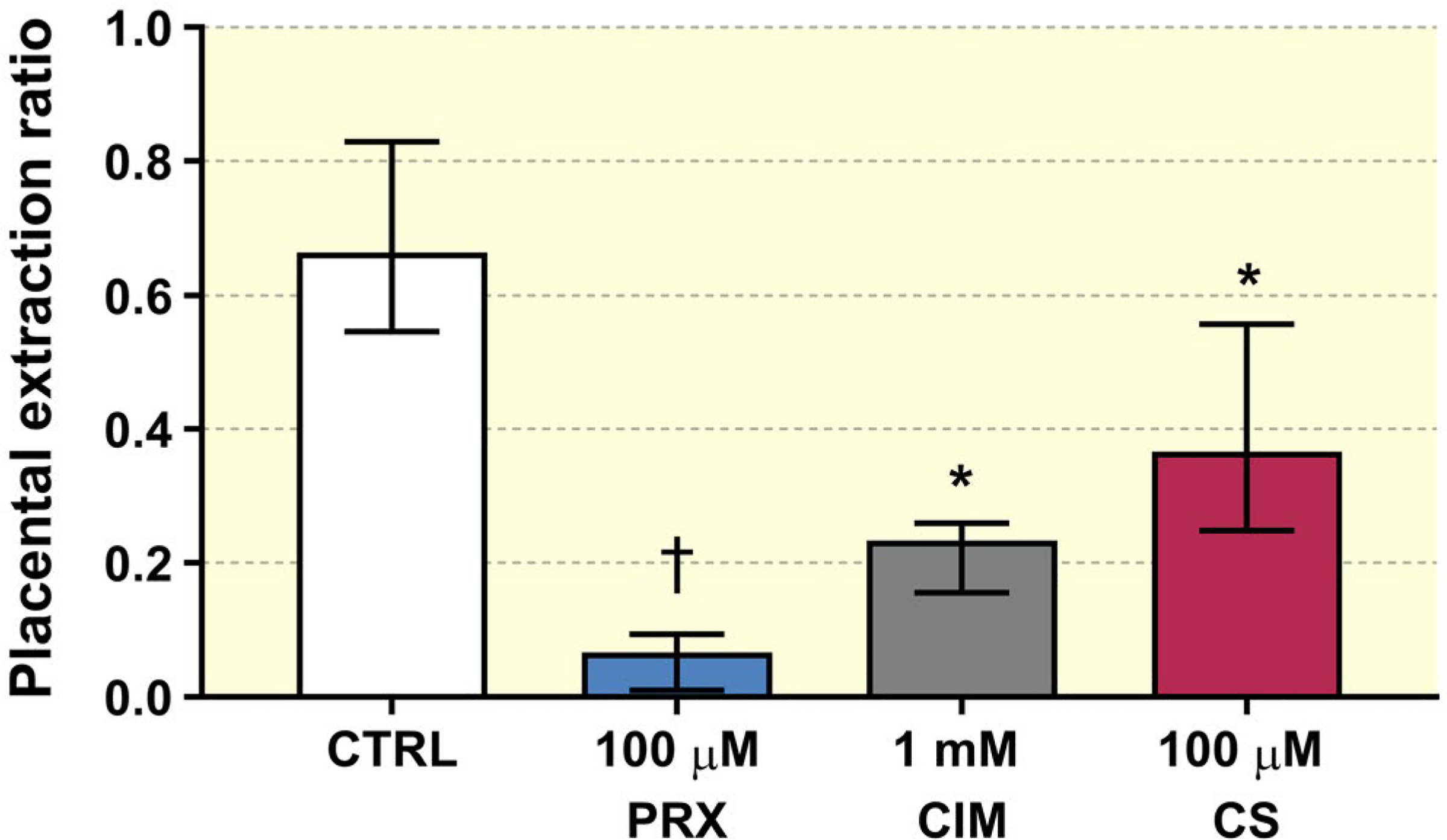
Rat term placenta extracted on average 66% of 5-HT (supplied at 1 nM concentration) from the foetal circulation during a single pass. This extraction was significantly inhibited by two tested pharmacological agents (paroxetine and cimetidine: PRX and CIM respectively) and the endogenous compound corticosterone (CS). Data presented are median + IQR (n > 3) for each test condition. Statistical analysis was evaluated using non-parametric Kruskal-Wallis test followed by Dunn’s multiple comparisons test: * (P ≤ .05), † (P ≤ .01)
2.4 |. Mother-to-foetus 5-HT transport and metabolism in rat term placenta
Mother-to-foetus 5-HT transport was tested in three concentrations, revealing low transport rate and high metabolism within the placenta (Figure 4). Specifically, perfusion of maternal circulation with 1 nM 5-HT for 20 minutes resulted in foetal steady-state 5-HT concentrations that were only 4% of maternal levels. Upon inhibition of MAO-A by phenelzine, foetal 5-HT concentrations increased to 33% of maternal levels, highlighting the crucial role of MAO-A in 5-HT metabolism. In contrast, maternal perfusion with 10 and 100 nM 5-HT resulted in foetal steady state levels up to 2% of maternal levels before inhibition of MAO-A, increasing up to 10% following its inhibition. The reduction in transplacental 5-HT transport with increasing substrate concentration could be due to increases in MAO-A activity and/or limitations in transport capacity of the basal membrane. In addition, at 100 nM 5-HT we often experienced difficulties with perfusions such as increases in maternal pressure and oedema formation, indicating a vasoconstrictive effect of 5-HT on the maternal side of the placenta.
FIGURE 4.
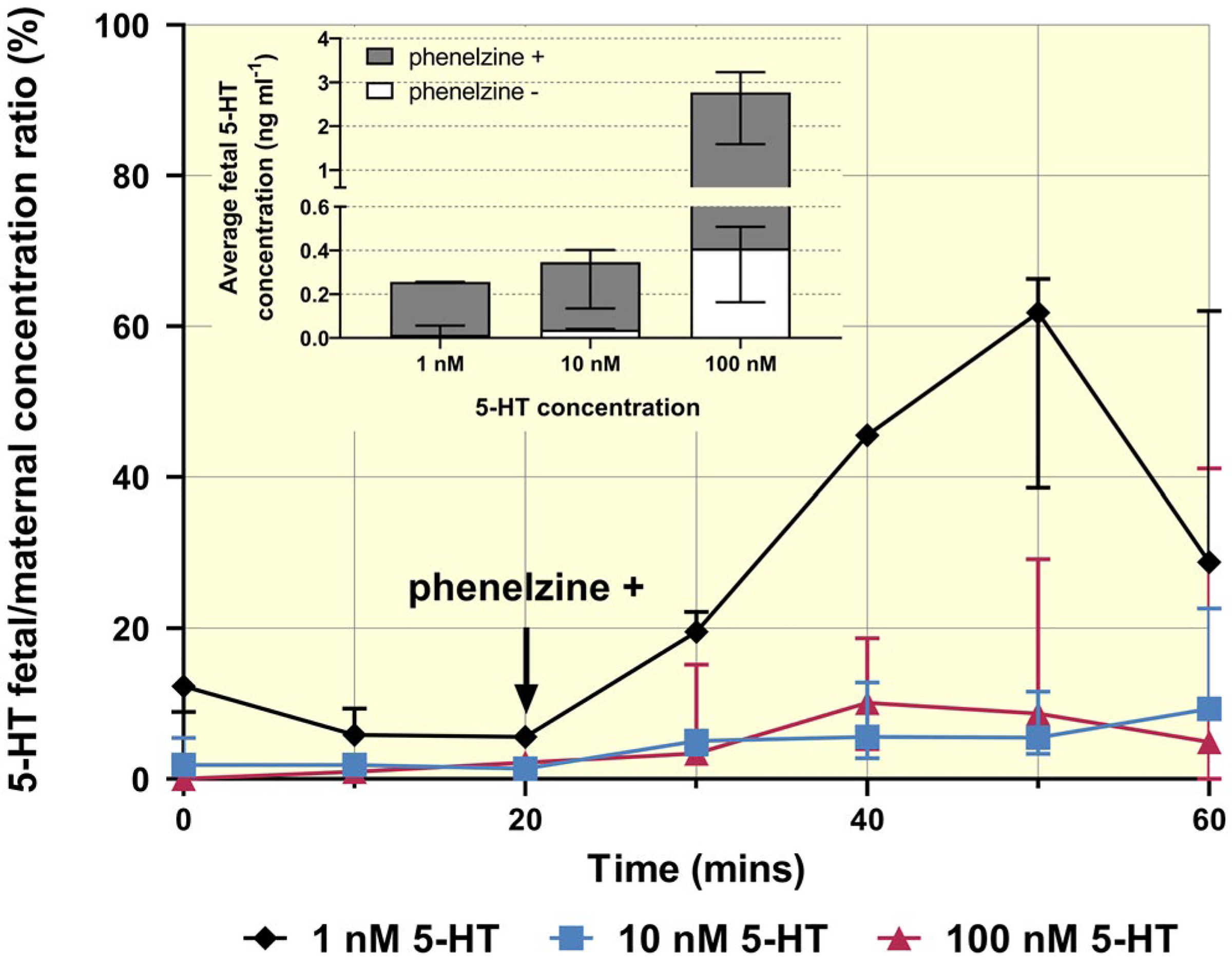
Materno-foetal transport and metabolism of 5-HT in the rat term placenta. Analysis of concentration-dependent 5-HT transport in the materno-foetal direction before and after addition of phenelzine revealed a high degree of metabolism by MAO-A within the placenta and negligible transport across the trophoblast. Data are median + IQR of foetal/maternal concentration ratios with the insert displaying average foetal 5-HT concentrations at steady state just before and after addition of phenelzine (at minutes 20 and 50 respectively)
Co-administration of paroxetine, citalopram, venlafaxine, or reserpine with 5-HT in the mother-to-foetus direction resulted in no significant changes in 5-HT reaching the foetal circulation.
2.5 |. Mother-to-foetus TRP transport and metabolism in rat term placenta
When infusing the maternal side of the placenta with 10 μM TRP, we observed its fast appearance in the foetal circulation, reaching 70%−80% of maternal concentrations within 10 minutes and remaining at steady state thereafter during the perfusion (Figure 5A). At a higher inflow TRP concentration (100 μM) steady-state foetal concentrations stabilized at 25% of maternal levels, indicative of transporter saturation (Figure 5A). No TRP metabolites were detected in the foetal circulation. However, upon addition of phenelzine we observed increasing 5-HT levels in the foetal circulation, likely due to a combination of residual 5-HT synthesizing pathway and inhibition of the 5-HT degrading pathway within the placenta (Figure 5B).
FIGURE 5.
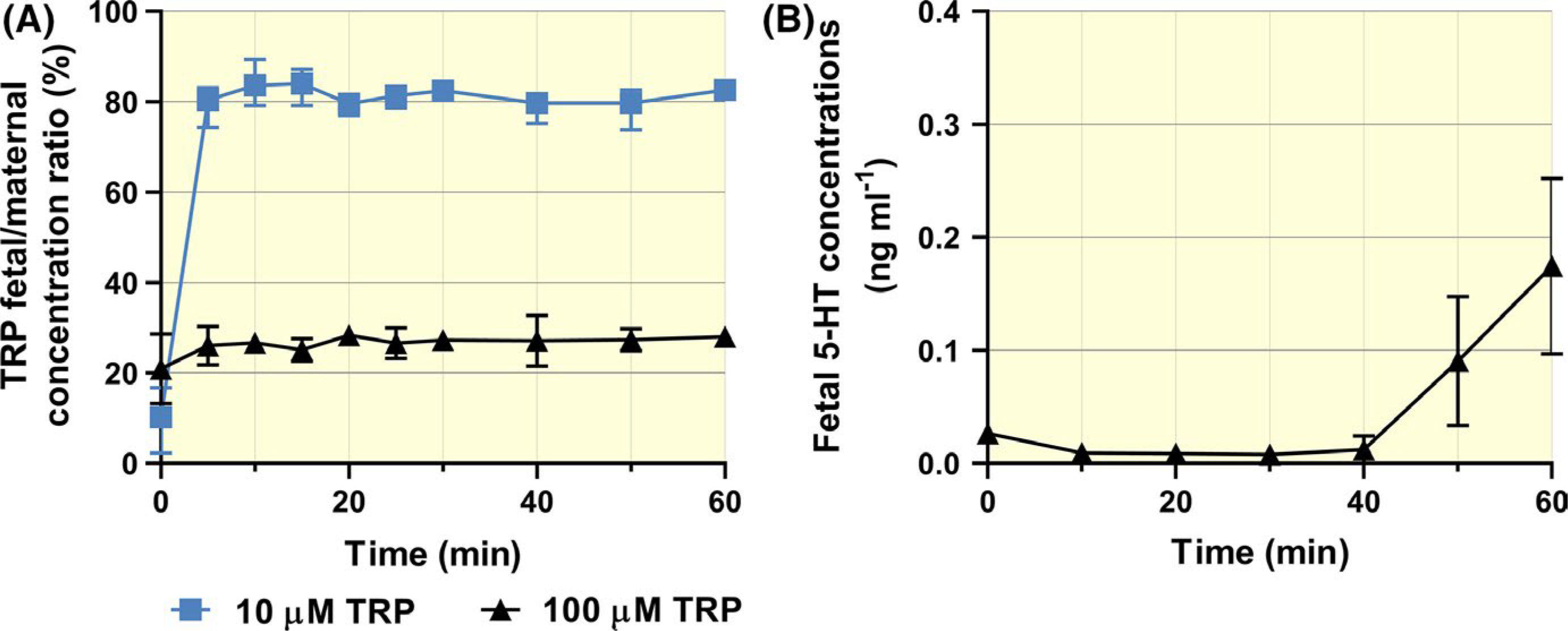
Materno-foetal transport and metabolism of TRP in rat term placenta. (A) Maternal TRP rapidly crossed the placenta and reached the foetal circulation to a high extent, but concentration-dependently. (B) Release of placental 5-HT, synthesized from maternal TRP, into the foetal circulation was evident only after inhibition of MAO-A by phenelzine (100 μM) at 40 min of perfusion. Data are median + IQR (n> 3) for each test condition
2.6 |. Characterization of 5-HT uptake into human placental microvillous membrane (MVM) and basal membrane (BM) vesicles
Membrane vesicles isolated from human term placenta were used to investigate 5-HT uptake by the mother-facing (MVM) and foetus-facing (BM) sides of the syncytiotrophoblast. Analysis of time-dependent uptake into MVM vesicles revealed an “overshoot” phenomenon, with accumulation of 5-HT inside the vesicles being greater within short times than at equilibrium (Figure 6A). This feature has been previously described25 and is typical for systems involving Na+ gradient-dependent transporters such as SERT. In contrast to MVM vesicles, no such “overshoot” was recorded in BM vesicles (Figure 6B), suggesting there are different 5-HT uptake systems on opposite sides of the placenta. Based on these studies and the linearity of 5-HT uptake into MVM and BM vesicles, a 1-minute uptake time was applied in subsequent analysis.
FIGURE 6.
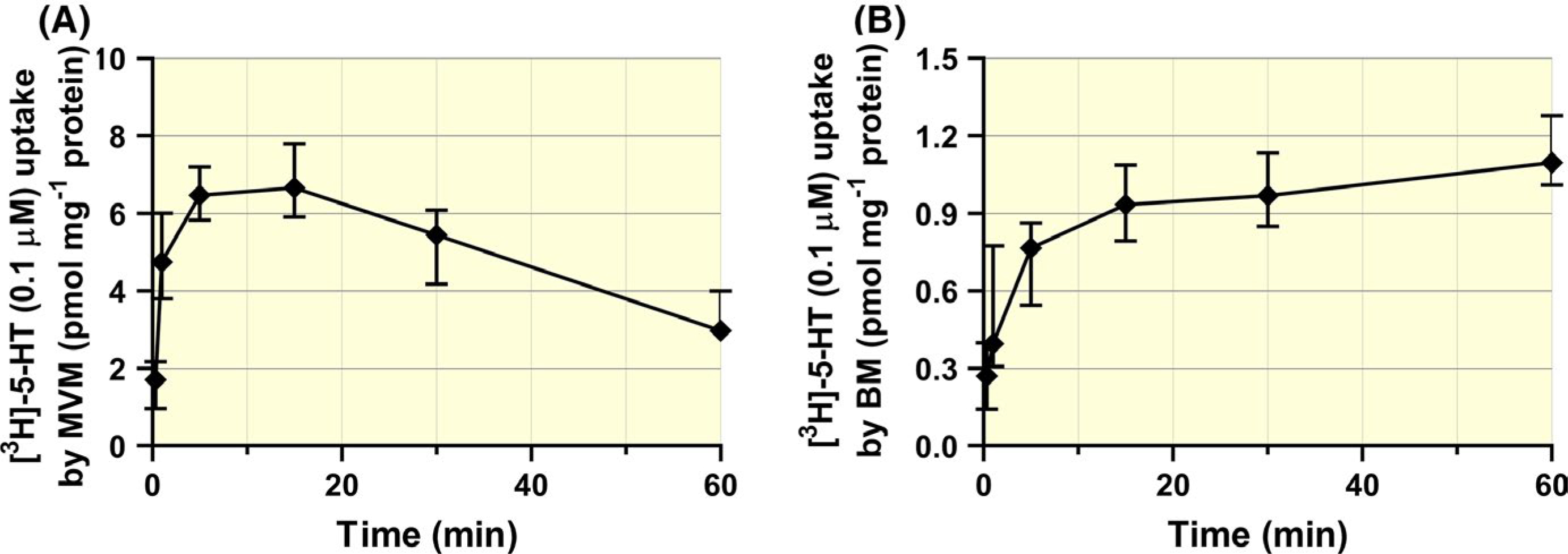
Time-dependent 5-HT uptake into MVM and BM vesicles isolated from human term placentas. 5-HT uptake in the MVM vesicles increased for 15 min, then gradually declined, presumably due to Na+ gradient loss (A). No such effect was detected in the BM vesicles, indicating a Na+-independent mechanism (B). Data are median + IQR (n = 5 donors) for each membrane
[3H]5-HT uptake was measured with concentrations of unlabelled 5-HT ranging from 0.1 to 1000 μM. The resulting isotope displacement kinetics exhibited concentration dependence in both MVM and BM vesicles, but with different patterns (Figure 7). In the MVM vesicles we observed a rapid decrease in [3H]5-HT uptake with increasing 5-HT concentrations (uptake fell below 8% at 100 μM). Uptake by the BM vesicles was affected by increasing 5-HT concentrations less strongly, as uptake remained above 15% even at 1000 μM. These results confirm that OCT3 has higher capacity for taking up 5-HT on the foetus-facing human membrane than SERT on the opposite side, although contribution of non-transporter-mediated transport in the BM, due to higher membrane fluidity,26 cannot be excluded.
FIGURE 7.
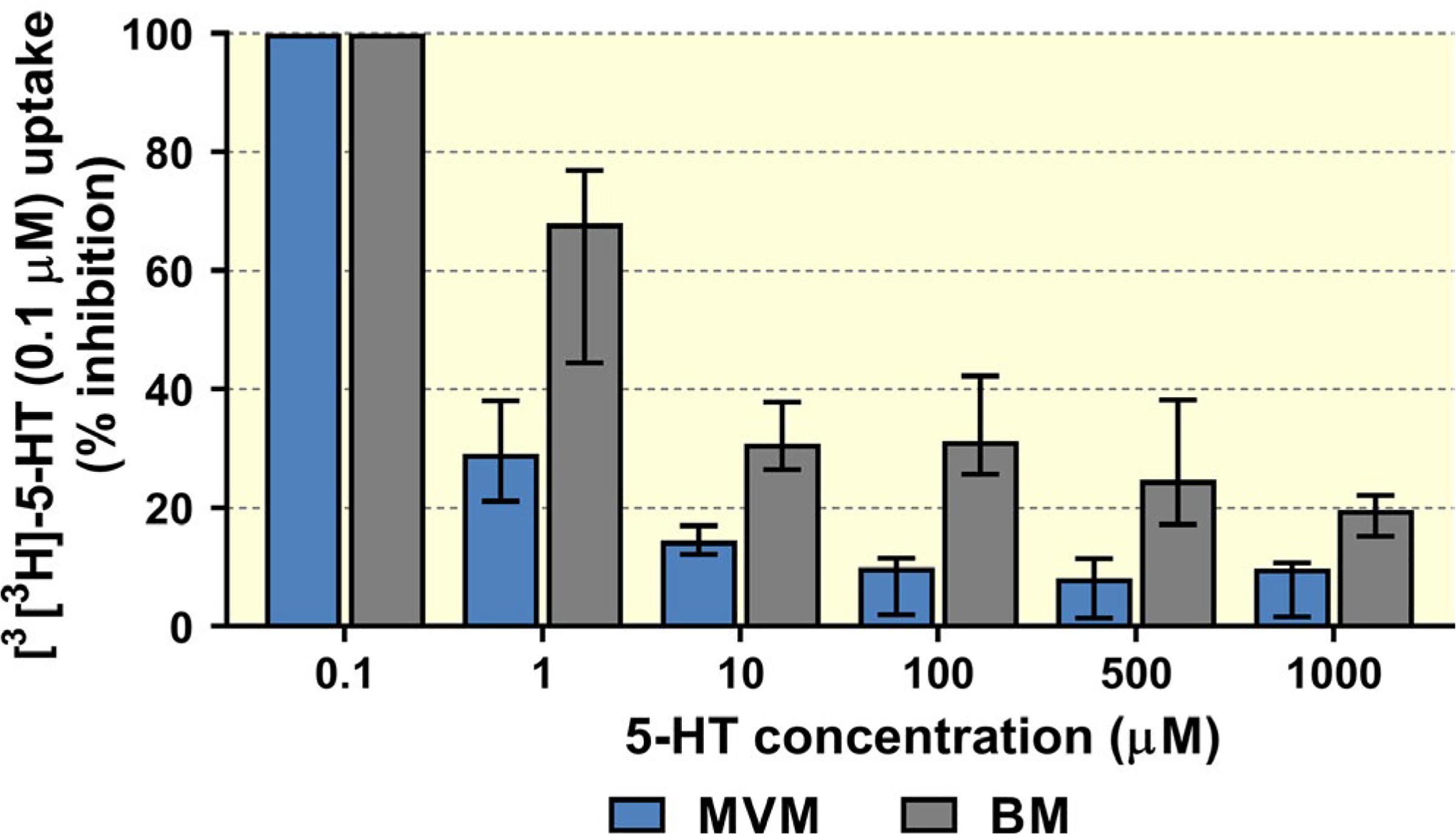
Concentration-dependent 5-HT uptake into MVM and BM vesicles from term human placentas. Increases in substrate concentration affected uptake of [3H]5-HT more strongly in MVM than in BM vesicles. Data are median + IQR (n> 4 donors) for each test condition
2.7 |. 5-HT uptake into human placental MVM and BM vesicles: effects of Na+ and inhibitors
To differentiate the 5-HT transporters in the mother-facing and foetus-facing membranes based on their Na+ dependence, we studied uptake in the presence and absence of Na+. 5-HT uptake into MVM vesicles was significantly lower in the absence of Na+ (Figure 8A), but presence and absence of Na+ had no significant effects on its uptake into the BM vesicles (Figure 8B), confirming that the mechanism responsible for 5-HT transport across the BM is independent of Na+.
FIGURE 8.
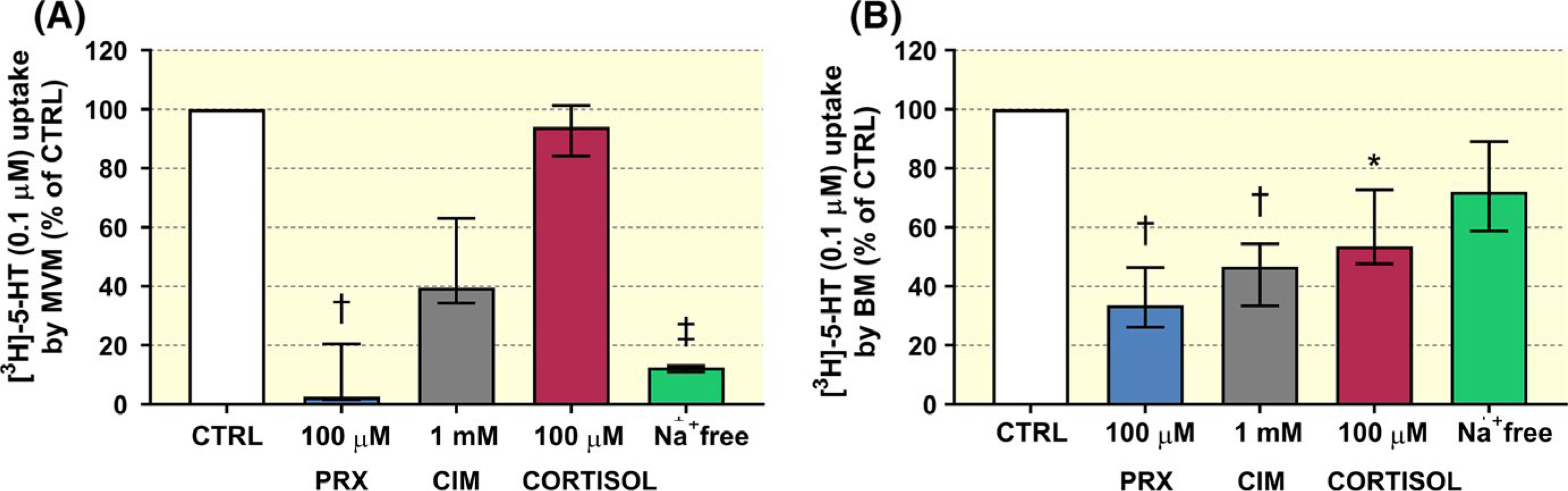
Effects of Na+-gradient and endo/exogenous compounds on 5-HT uptake by the human placental MVM and BM vesicles. (A) Na+ depletion significantly reduced uptake into the MVM vesicles, confirming that the main transporter in the MVM is the Na+-dependent SERT, but not in the BM vesicles (B), suggesting involvement of a different transporter, likely OCT3. In addition, patterns of inhibitory actions of paroxetine (PRX), cimetidine (CIM) and cortisol differed between the membranes. 5-HT uptake by the MVM vesicles was highly inhibited by PRX (A), while its transport across the BM was inhibited by all compounds tested (B). Data are presented as median + IQR (n ≥ 5 donors) for each test condition. The significance of differences between raw values was evaluated by non-parametric Kruskal-Wallis test followed by Dunn’s multiple comparisons test: * (P ≤ .05), †(P ≤ .01), ‡ (P ≤ .001)
Correspondingly, co-administration of SERT or OCT3 inhibitors had different effects on 5-HT uptake into MVM and BM vesicles. Specifically, 5-HT uptake by the MVM vesicles was significantly decreased by 100 μM paroxetine (by 91%) (Figure 8A). In contrast, in the BM we observed inhibitory effect of 100 μM paroxetine (by 64%), 1 mM cimetidine (by 56%) and 100 μM cortisol (by 41%) (Figure 8B).
2.8 |. 5-HT metabolism by MAO-A in rat and human placenta homogenates
5-HT metabolism in term placenta was probed by measuring amounts of 5-HT remaining in placental homogenates after 60 minutes of incubation with 5-HT, with and without MAO inhibitor (Figure 9). After this time, over 70% and 40% of 5-HT had been metabolized by human and rat placentas respectively. 5-HT metabolism was completely inhibited by 100 μM of the MAO inhibitor, phenelzine, indicating that 5-HT degradation was due to activity of this oxidase.
FIGURE 9.
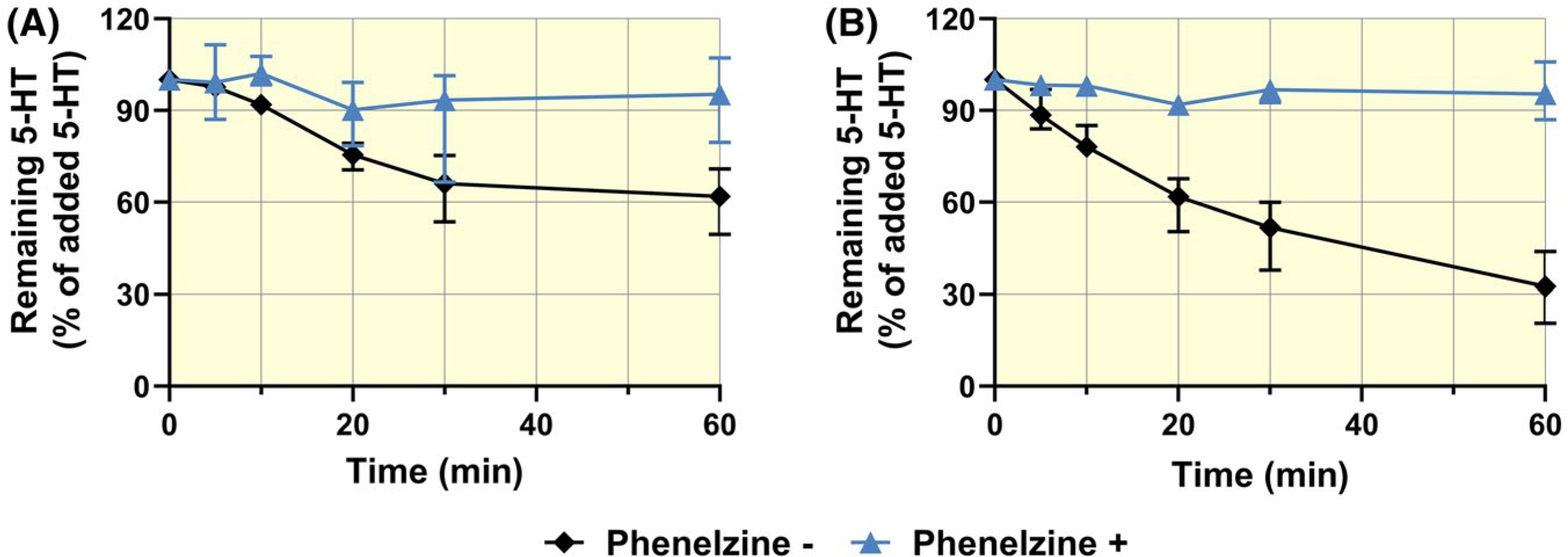
Time courses of 5-HT metabolism in rat (A) and human (B) placental homogenates. Incubation in the presence (+) and absence (−) of phenelzine revealed that activity of placental MAO accounted for most of the loss of 5-HT over time. Values are median + IQR of 5-HT levels at indicated time points relative to levels at time 0
2.9 |. Expression analysis of genes encoding selected enzymes/transporters in human and rat term placentas
Expression of SLC6A4/Slc6a4 (SERT), SLC22A3/Slc22a3 (OCT3), TPH1/Tph1, TPH2/Tph2, MAO-A/Mao-a, MAO-B/Mao-b, SLC7A5/Slc7a5 (LAT1) and SLC7A8/Slc7a8 (LAT2) genes in human and rat term placental tissues was evaluated by quantitative PCR analysis. We found that expression of TPH/Tph enzymes in both human and rat placenta was very low, in several cases below the detection limit, but all other tested genes were expressed in the examined samples (n = 45 and 32 for rat and human placentas respectively). Regarding 5-HT metabolizing enzymes, MAO-A/Mao-a was expressed more strongly than MAO-B/Mao-b in both species. Similarly, expression of both isoforms of LAT was observed, but SLC7A5/Slc7a5 was dominant.
Digital PCR technology was subsequently used to absolutely quantify numbers of SLC6A4/Slc6a4, SLC22A3/Slc22a3, and MAO-A/Mao-a transcripts in both human and rat placentas (Figure 10). The copy number of SLC22A3/Slc22a3 and MAO-A/Mao-a transcripts was also included in the population-based analysis to detect potential influence of genetic variation in placental extraction of 5-HT from the foetal circulation in rats.
FIGURE 10.
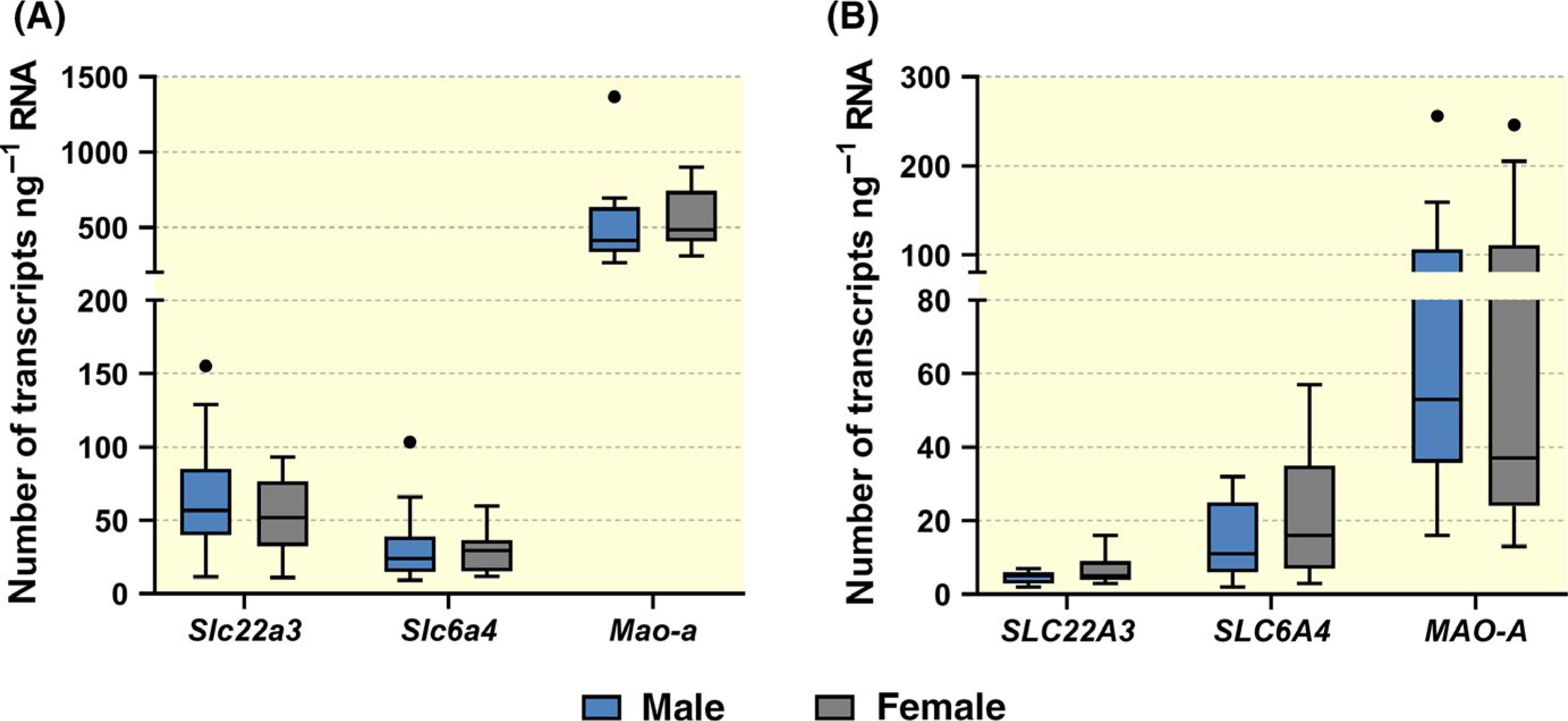
Digital droplet PCR analysis of the expression of MAO-A/Mao-a, SLC6A4/Slc6a4 and SLC22A3/Slc22a3 genes in the rat (A) and human (B) placenta. Large inter-individual variability was observed in the expression of all tested genes, but this phenomenon was independent of foetal sex. The results are reported as Tukey boxplots (1.5-times IQR) of number of transcripts ng−1 RNA; n = 13F/16M and n = 11F/15M for rat and human placentas respectively
3 |. DISCUSSION
Tight regulation of 5-HT levels in the foeto-placental unit is crucial for normal placental function and foetal development. While low 5-HT levels are associated with poor foetal neurodevelopment,2 high 5-HT concentrations promote local vasoconstriction in the placental vasculature that may jeopardize placental circulation and lead to pregnancy complications including pre-eclampsia.27 The current literature suggests that the placenta supplies 5-HT during a limited time window of gestation which is required by the embryo/foetus for proper development and programming.7 However, in later stages of gestation, when the foetus can synthesize its own 5-HT 17 in both brain 16 and enterochromaffin cells15 from maternal TRP,16,17 placental delivery may become unnecessary. This study yielded the first evidence that human and rat term placenta extract 5-HT from foetal circulation via a high-capacity, saturable and inhibitable transport mediated by OCT3. Moreover, we show that rat term placenta no longer transfers maternal or placenta-synthesized 5-HT to the foetus.
Placental uptake of 5-HT from maternal blood across the MVM has been investigated before, and the importance of SERT for materno-placental handling of 5-HT has been clearly established.25 However, there have been no previous published attempts to elucidate 5-HT transport through the foetus-facing basal membrane. Therefore, we perfused the foetal side of the rat placenta with 5-HT at various concentrations and discovered that during a single passage the placenta can extract up to 80% of 5-HT at physiological (nM) levels from the foetal compartment. With increasing concentrations, however, placental extraction of 5-HT decreased (to 24% at 100 μM concentration), suggesting involvement of a saturable transporter-mediated uptake process. We hypothesized that OCT3 is the transporter responsible for 5-HT uptake for three reasons. First, 5-HT is a substrate of OCT3,20 which also provides a buffering mechanism that counters excessive accumulation of 5-HT in the brain.28–30 Second, of all the organic cation transport proteins subfamily 22, only OCT3/SLC22A3 isoform is abundantly expressed in placenta, specifically on the basal side of the syncytiotrophoblast22,24 and both its expression and activity increase during pregnancy, indicating that it has growing importance throughout gestation.24 Third, in a recent evaluation of OCT3 function in the rat placenta we showed that it provides high-capacity for extraction of another substrate, the neurotoxin MPP+, from foetal circulation.23 Thus, placental OCT3 has an important foetal detoxification function.
During our rat placenta perfusion experiments, we noted that the foetal side of the placenta could withstand 5-HT concentrations up to 100 μM without noticeable vasoconstriction. In contrast, perfusing the maternal side of the placenta with a 1000-fold lower 5-HT concentration (100 nM) resulted in increased pressure, decreased flow and oedema formation due to 5-HT-induced vasoconstrictive effects. Since the hemochorial placenta lacks precapillary sphincters, but is responsive to systemic agents acting on vascular bed,31 we believe that the different responses in the utero-placental and the foeto-placental vasculature are due to different trophoblast clearance rates of 5-HT. Indeed SERT, exclusively located in the apical, mother-facing membrane of the placenta, is characterized as a high-affinity, but low-capacity 5-HT transport system.25 In contrast, OCT3 in the basal, foetus-facing, membrane is characterized as a low-affinity but high-capacity 5-HT transporter.32 Evidently, therefore, the rat placenta has higher capacity to clear 5-HT from blood on the foetal side, which reduces the foetal circulation’s vulnerability to 5-HT toxicity.
Since we noticed considerable interindividual variability in extraction of foetal 5-HT by rat placenta, we employed population-based analysis to evaluate the effects of multiple factors (foetal sex, placental weight, number of foetuses in the litter, Slc22a3/Mao-a transcripts) as potential covariates. We identified foetal sex as a factor influencing the transporter-mediated kinetics, obtaining higher Vmax and Km values for placentas of female foetuses than male foetuses (Figure 2B and C). Incorporating foetal sex as a covariate then helped to reduce the unexplained interindividual variability and provided a better description of the base model (Figure S2). These results, if verified in other species and experimental models, might at least partly explain sex-dependent effects observed in behavioural studies of prenatal exposure to OCT3 inhibitors, such as metformin33 or antidepressants.34
To investigate maternal-to-foetal transport and/or synthesis of 5-HT in rat term placenta, we infused the maternal side with either 5-HT or TRP and quantified the appearance of TRP, 5-HT and its metabolites in the foetal circulation. The in situ perfusion of rat term placenta shows that in basal conditions, when placental MAO-A is fully functional, there is negligible dose-dependent maternal-to-foetal transport of 5-HT (from 4% at 1 nM to 2% at 100 nM inflow concentrations). This is consistent with data in mice showing negligible (0.3%) transport in ex vivo perfusions in early pregnancy 7 and with human term placenta perfusion data revealing < 1% transfer rate (S. Gil and A. Bonnin, personal communication). Similarly, we show insignificant placental 5-HT neosynthesis from maternal TRP and release into the foetal circulation. This observation is in a good agreement with studies in mouse term placenta showing very limited 5-HT synthesis.7 Interestingly, when placental MAO-A is inhibited, release of placental 5-HT into the foetal circulation becomes apparent, suggesting that there is residual neosynthetic capacity in term placentas. Significant placental metabolism of 5-HT was subsequently confirmed by analyses of both rat and human placenta homogenates, at rates corresponding to those observed elsewhere.13,27 This suggests that under normal conditions and in contrast to early pregnancy, rat and human term placenta metabolize 5-HT and only minimal amounts of 5-HT reach the foetal bloodstream at term. This is true for 5-HT of both origins, i.e. taken up from maternal circulation by SERT or produced within the trophoblast from maternal TRP via TPH activity.
Based on our findings and previously published research, we hypothesize that placental handling of 5-HT changes during gestation. While the embryo/early foetus requires a placental supply of 5-HT, term placenta takes up 5-HT from both maternal and foetal circulations and deactivates it by MAO, specifically MAO-A as the main isoform present in the placenta 35 (Figure 11C). This hypothesis is supported by the following observations. First, the co-localization and high expression of SERT, OCT3 and MAO-A in trophoblast cells.36,37 Second, the increases in expression and activity of OCT3 throughout gestation in rat,24 murine and human placenta.21 Third, the increases in MAO-A activity throughout gestation observed in rat13,14 and murine7 placentas.
FIGURE 11.
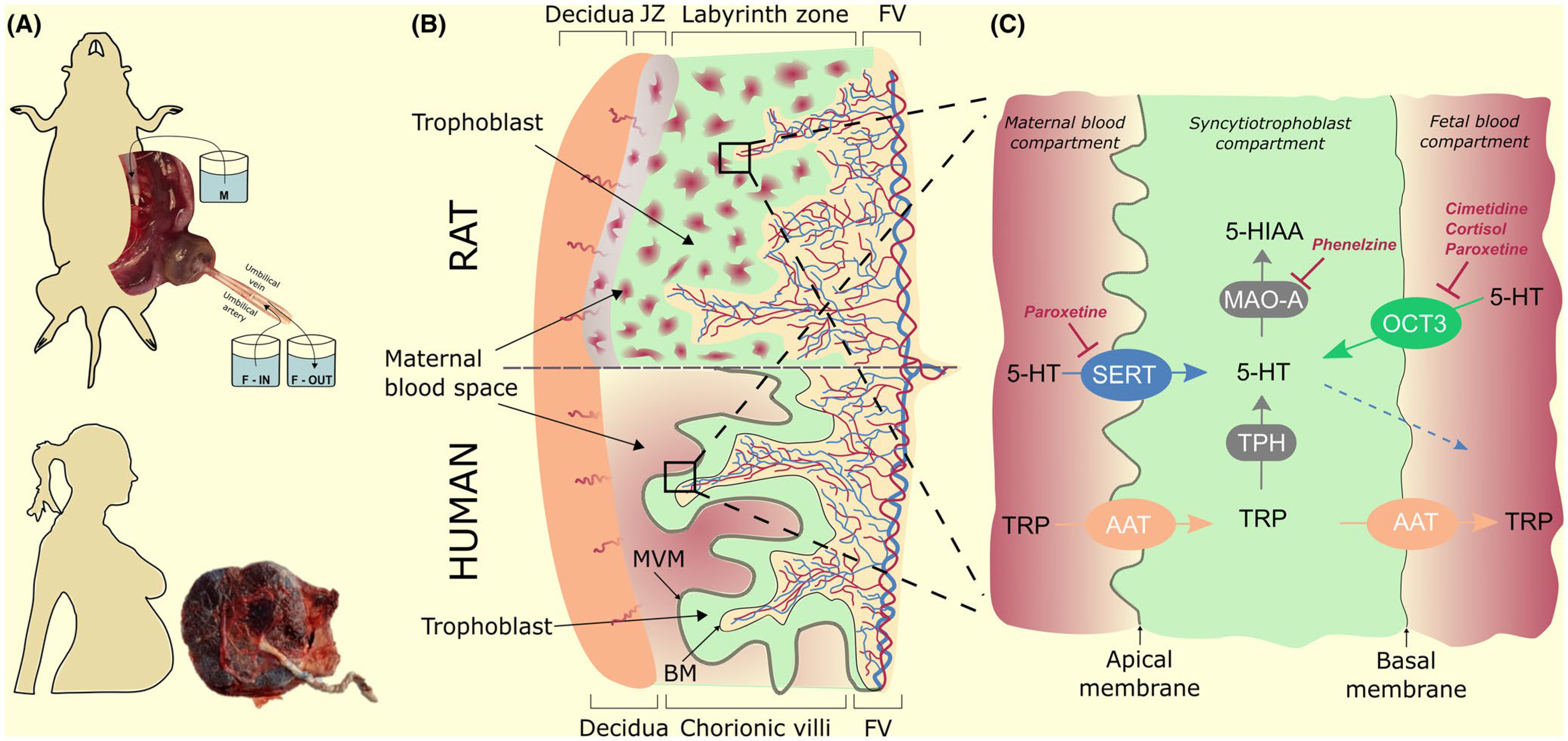
Schematic illustration of 5-HT and TRP homeostasis in human and rat placenta at term. (A) The study was performed in rat term placenta (in situ dual perfusion system) and human term placenta (ex vivo uptake studies by isolated placental membranes). (B) Hemochorial placenta is characterized by a trophoblast layer (in green) providing materno-foetal interdigitation of labyrinthine-type in rats (upper panel) and villous-type in humans (lower panel). The trophoblast layer is composed of cytotrophoblast cells and terminally differentiated syncytiotrophoblasts (cellular layers not shown). The syncytiotrophoblast compartment constitutes the main placental barrier with polarized plasma membranes: apical, maternal-facing and basal, foetal-facing (materno-foetal interface depicted as black squares). (C) Condensed three-compartment depiction of the rat and human materno-foetal interface summarizing the main findings of the study. Mother-to-foetus transport of 5-HT does not occur in the term placenta; only very limited supply of placental-derived 5-HT occurs at term when MAO-A is inhibited (dotted blue arrow). In contrast, 5-HT is taken up from the maternal and foetal circulations by SERT and OCT3 respectively, and subsequently deactivated inside syncytiotrophoblast by MAO-A. This interplay of uptake transporters and degradation enzyme offers a protective mechanism against local vasoconstriction of placental vasculature produced by 5-HT. Importantly, both uptake mechanisms may be inhibited by endogenous and/or pharmacological agents, thereby compromising the placenta’s protective activity against 5-HT. JZ, junctional zone; FV, fetal vasulature
To investigate placental handling of 5-HT in human placenta, we performed uptake studies in mother-facing (MVM) and foetus-facing (BM) membrane vesicles isolated from human term placenta. While 5-HT uptake by MVM has been previously described,25 our study provides the first characterization of 5-HT uptake by BM. Concentration-dependent uptake of 5-HT by both MVM and BM vesicles was detected, confirming that it is a transporter-mediated and saturable process. However, time- and Na+-dependent studies showed that in the BM, 5-HT uptake increased with time regardless of the Na+ gradient, indicating that it hosts a different transport system from MVM. Similarly, isotope displacement kinetics in the presence of increasing concentrations of unlabelled 5-HT confirmed the presence of a high-capacity 5-HT transport system in the BM. Finally, the MVM and BM vesicles’ responses to inhibitors differed. Specifically, cortisol inhibited 5-HT uptake by the BM but not MVM vesicles, supporting the involvement of OCT3 in the former, as OCT3 is known to be a glucocorticoid-sensitive transporter.20 Collectively, the findings from the experiments with human placenta vesicles are consistent with our rat placenta data and confirm that different 5-HT uptake transporters, SERT and OCT3, act on maternal and foetal sides of the placenta respectively.
Our data thus indicate that term placenta does not provide 5-HT to the foetus. In contrast, it actively takes it up from both maternal and foetal circulations, via SERT and OCT3 respectively, and degrades it by MAO-A (Figure 11C). This joint action of uptake transporters and a degradation enzyme provides a protective mechanism against local vasoconstriction effects of 5-HT in the placenta on both maternal and foetal sides in late gestation. We observed sex-dependent differences in placental uptake of 5-HT from the foetal circulation, which were not attributable to differences in transcript-levels of OCT3 or MAO-A expression but could potentially be due to post-transcriptional/translational modifications or other alterations at the functional level of the enzyme or transporter. For example, putative effectors such as 5-HT2A receptor known to control the activity of SERT 38 may affect OCT3 function. This and other possible mechanisms regulating OCT3 activity require further investigation in future studies.
Importantly, OCT3 is known as a polyspecific transporter that can be inhibited by a wide spectrum of compounds.39 Here we report that OCT3-mediated uptake of 5-HT by the placenta may be compromised by both endogenous and exogenous (pharmacological) OCT3 inhibitors. Glucocorticoids were selected in our studies as endogenous molecules with crucial role in foetal growth and programming1 and recognized OCT3 inhibitors.20 Transplacental supply of glucocorticoids from mother to foetus is tightly regulated by placental 11β-hydroxysteroid dehydrogenase which deactivates cortisol to cortisone.40 This enzyme is prone to alteration by various factors such as polymorphisms, stress, hypoxia, diet and inflammation 1; thus, it seems reasonable to speculate that any defect in placental handling of glucocorticoids may result in their suboptimal levels in the foetal circulation, which may subsequently affect placental homeostasis of 5-HT. Various mechanisms of glucocorticoid-5-HT interaction have been described in the CNS41,42 and other organs.43 Our results strongly indicate a mechanism involving close interaction between glucocorticoids and 5-HT in the foeto-placental unit.
In addition, various pharmaceuticals are used during pregnancy despite a lack of safety data. For example, an estimated 13% of pregnant women are prescribed antidepressants during some or all stages of pregnancy – a rate that doubled between 1999 and 2003, and continues to increase.44 Since many antidepressants are potent inhibitors of not only SERT but also OCT3,45 paroxetine, a model antidepressant, was used in our studies to demonstrate that these drugs can affect placental homeostasis of 5-HT through inhibition of OCT3, a phenomenon that has not been reported before. Blocking this protective mechanism could potentially expose the foeto-placental unit to elevated blood concentrations of 5-HT and jeopardize 5-HT-dependent neurogenic and other developmental processes, although it remains to be fully demonstrated. Similarly, pharmacotherapy of pregnant women with other OCT3 inhibitors, such as metformin for gestational diabetes mellitus33 or antiretrovirals for HIV positive pregnant women46 might also dysregulate placental handling of 5-HT and contribute to poor pregnancy outcomes. Taken together, these results indicate that our “physiological” findings have also “pharmacological” relevance; however, additional studies including dose-dependent inhibition experiments and using diverse experimental approaches are necessary to assess clinical importance of our findings.
Finally, apart from 5-HT, other monoamine neurotransmitters such as norepinephrine and dopamine are also substrates of OCT3, although of different affinity when compared to 5-HT.39,47 Therefore, we presume that OCT3 may modulate placental homeostasis of these physiological monoamines, too. This hypothesis, however, needs to be experimentally confirmed in future studies.
In conclusion, our results demonstrate that OCT3 is an important component regulating feto-placental homeostasis of 5-HT. Since this polyspecific transporter can be blocked by a variety of endogenous molecules as well as pharmaceuticals, our findings provide a new mechanistic understanding of unforeseen complications during pregnancy, including (inter alia) prenatal glucocorticoid excess and/or pharmacotherapeutic risks for pregnant women.
4 |. MATERIALS AND METHODS
4.1 |. Chemicals and reagents
[3H]5-hydroxytryptamine ([3H]5-HT), 80 Ci mmol−1 and [3H]dihydroalprenolol, levo-[propyl-1,2,3–3H] hydrochloride, 80 Ci mmol−1 were purchased from American Radiolabeled Chemicals, Inc. Unlabeled serotonin hydrochloride, L-tryptophan, paroxetine hydrochloride, citalopram hydrobromide, venlafaxine hydrochloride, cimetidine hydrochloride, reserpine, corticosterone, cortisol and phenelzine were purchased from Sigma-Aldrich. All other chemicals were of analytical grade. Bicinchoninic acid assay (BCA assay) reagents were purchased from Thermo Fisher Scientific. Tri Reagent solution was obtained from the Molecular Research Centre.
4.2 |. In situ dual perfusion of rat term placenta
Placental transfer of 5-HT or TRP was evaluated using dually perfused rat term placenta in an open setup (Figure 11A), as previously described.48 The experiments were performed in female Wistar rats purchased from Velaz, Ltd. (Czech Republic). All experiments were approved by the Ethical Committee of the Faculty of Pharmacy in Hradec Kralove (approval no. MSMT-4312/2015–8; Charles University, Czech Republic). Descriptive statistics of the rats used in the study are shown in Table S1.
Briefly, the uterine artery proximal to the blood vessel supplying a selected placenta was cannulated with a catheter and the uterine vein, including anastomoses to other foetuses, was ligated behind the perfused placenta and cut so that maternal solution could leave the perfused placenta. The umbilical artery of the selected foetus was catheterized and connected to the foetal reservoir. The umbilical vein was similarly cannulated to collect the foetal effluent. The selected placenta was perfused from the maternal and foetal sides at flow rates of 1 and 0.5 ml min−1 respectively. Foetal effluent was sampled at 5 minutes intervals and steady-state levels of analytes were averaged and used for statistical analysis. To investigate placental extraction of [3H]5-HT from the foetal circulation, only umbilical artery and vein were cannulated and maternal circulation was left intact.
During any perfusion experiment, we monitored the physical condition of the rat (maternal and foetal perfusion pressures, breath, pulse and limb swelling) and selected placenta (swelling, gelling and colouring). If bulk fluid leakage or deviation from normal physical condition was detected the experiment was terminated and obtained data were omitted from analysis. Each experimental perfusion was preceded by a 10–15 minutes wash-out with Krebs buffer containing 1% dextran, to avoid contamination by blood components (proteins, platelets). After experiments with [3H]5-HT, the placenta was perfused with radioactivity-free Krebs buffer for a further 10 minutes and dissolved in Solvable tissue solubilizer (PerkinElmer Life and Analytical Sciences) to detect remaining radioactivity in the placental tissue.
4.3 |. Uptake of 5-HT from foetal circulation: effect of concentration
5-HT with [3H]5-HT as a tracer was added to the foetal reservoir in four concentrations (1 nM, 100 nM, 1 μM or 100 μM) and placental venous effluent was collected for 40 minutes. To quantify placental capacity to take up 5-HT from foetal circulation, the ER was calculated as follows:
| (1) |
where Cfa is the 5-HT concentration in the foetal reservoir entering the perfused placenta via the umbilical artery and Cfv is the drug concentration in the umbilical vein effluent.
Total placenta uptake clearance from the foetal circulation (Cluptake) was calculated as follows:
| (2) |
where Qf is the umbilical flow rate (0.5 ml min−1).
Assuming that Cluptake is a function of passive (Clpassive) and transporter-mediated uptake, the following equation was used to fit our data:
| (3) |
where Vmax is the maximal velocity of the transporter and Km is the concentration at which the velocity is half-maximal. Since we observed high interindividual variability in 5-HT placenta uptake, we used population-based maximum likelihood estimation of mean values of the parameters Clpassive, Vmax and Km for the entire population of subjects, and calculated individual subjects’ deviations from the means. Effects of covariates on the estimated parameters were also assessed.
4.4 |. Uptake of 5-HT from foetal circulation: effects of inhibitors
The potency of effects of three OCT3 inhibitors — paroxetine (100 μM),45 cimetidine (1 mM) 20 and corticosterone (100 μM) 20— on foetal uptake of 5-HT was tested by adding them (separately) to foetal circulation and after a stabilization period of 5–10 minutes adding 1 nM [3H]5-HT to the foetal reservoir and sampling foetal effluent for 40 minutes. The ER was calculated using Equation 1.
4.5 |. Uptake of 5-HT from foetal circulation: effect of foetal sex
One of the aims of the study was to investigate possible effects of foetal sex on 5-HT uptake. Thus, before cannulation of umbilical vessels, the sex of the foetus was assessed by measuring the anogenital distance, and subsequently confirmed by genotyping (see below). Perfusion studies were carried out as described above and the foetal sex was incorporated into the population analysis (see below).
4.6 |. Maternal-to-foetal 5-HT transport and metabolism: effects of concentration and inhibitors
The concentration-dependence of the transport and subsequent metabolism of unlabelled 5-HT were studied by perfusing maternal side of the placenta with 5-HT at final concentrations of 1 nM, 10 nM or 100 nM. After 20 minutes of perfusion, 100 μM phenelzine (a MAO inhibitor) was added to the maternal and foetal compartments. Concentrations of 5-HT and its metabolites, 5-hydroxyindoleacetic acid (5-HIAA) and melatonin (MEL), in foetal circulation were analysed by HPLC, as described below. Transport of 5-HT in the maternal-to-foetal direction was quantified as the ratio between foetal and maternal concentrations at steady-state.
The SERT inhibitors paroxetine (100 μM), venlafaxine (1 mM), citalopram (150 μM) and vesicular monoamine transporter inhibitor reserpine (1 μM) were added, separately, to the maternal and foetal reservoirs to test the potency of their effects on maternal-to-foetal transport of 1 nM 5-HT.
4.7 |. Maternal-to-foetal TRP transport and metabolism
To investigate transport and metabolism of TRP in rat placenta, TRP (100 μM) was added to the maternal reservoir and after five minutes of stabilization foetal effluent samples were collected in pre-weighed vials. After 40 minutes, 100 μM phenelzine was added to both maternal and foetal circulations and sample collection continued for an additional 20 minutes. Concentrations of TRP, 5-HT, 5-HIAA and MEL in the foetal compartment were analysed by HPLC, as described below.
4.8 |. Human placenta sample collection
Human term placentas (n = 26) were obtained from uncomplicated pregnancies at term (38–40 weeks of gestation) immediately after delivery at the University Hospital in Hradec Kralove, after obtaining the women’s written informed consent and approval of the University Hospital Research Ethics Committee (approval no. 201006 S15P). Subjects’ characteristics are shown in Table S2. Only placentas from non-medicated and non-smoking mothers were recruited in the study.
4.9 |. Preparation of microvillous and basal membrane vesicles from human term placenta
Microvillous (MVM) and basal membrane (BM) vesicles were prepared simultaneously, using a previously described differential centrifugation method.49 All procedures were performed at 4 °C. Briefly, the maternal decidua and chorionic plate were removed, placental villous tissue (80–100 g) was cut into small pieces and washed with 0.9% NaCl to remove blood. The tissue was homogenized for 2 minutes in a solution (at 3 ml g−1) containing 250 mM sucrose, 10 mM Tris-Hepes (pH 7.2), 5 mM EGTA, 5 mM EDTA and 1 mM phenylmethylsulfonyl fluoride (PMSF). MVM was separated by precipitation of non-microvillous membranes with Mg2+, and BM was purified on a sucrose gradient.
MVM and BM membranes were resuspended in intravesicular buffer (290 mM sucrose, 5 mM Hepes, 5 mM Tris; pH 7.4), vesiculated by 15 passages through a 25-gauge needle, stored at 4 °C and used for uptake experiments within 3 days of isolation or frozen at −80 °C and equilibrated to room temperature on the day of the experiments. Before initiation of uptake experiments, the comparability of uptake rates of fresh and thawed vesicles was verified. Protein concentrations in the placental homogenate and MVM and BM vesicles were determined using the BCA assay. The purity and enrichment of the membrane fractions were determined by assaying activities of alkaline phosphatase,50 an apical membrane marker, and β-adrenergic receptor (by measuring [3H]dihydroalprenolol, levo-[propyl-1,2,3–3H] hydrochloride binding),51 a basal membrane marker. The right-side out orientation of the vesicles was checked by measuring alkaline phosphatase (for MVM) 50 and Na+/K+-ATPase (for BM) activities 49 before and after vesicles’ lysis by SDS treatment. Purity and orientation results are presented in Table S3.
4.10 |. 5-HT uptake by MVM and BM vesicles
Uptake of [3H]5-HT into MVM or BM vesicles was measured at room temperature using the rapid vacuum filtration technique.50 10 μl suspensions of MVM or BM vesicles (10–20 mg ml−1) were incubated for 1 minute or selected times indicated in the figures (in time dependency studies), in the absence and presence of Na+, and/or selected concentrations of unlabelled 5-HT (in concentration-dependence studies) or inhibitors (paroxetine, cimetidine or cortisol).
Uptake of 125 nM [3H]5-HT was initiated by adding it in extravesicular buffer (145 mM NaCl, 5mM Hepes and 5 mM Tris; pH 7.4) to the pre-incubated vesicles. Uptake was halted after 1 minute or pre-defined time points, by adding 2 ml ice-cold stopping buffer (130 mM NaCl, 10 mM Na2HPO4, 4.2 mM KCl, 1.2 mM MgSO4, 0.75 mM CaCl2; pH 7.4) and filtration through a 0.45 μM mixed cellulose ester filter (MF-Millipore, HAWP00010) under vacuum. Filters were washed with 15 ml stopping solution and the filter-associated radioactivity was determined by liquid scintillation counting, using a Tri-Carb 2910 TR instrument (Perkin Elmer). Nonspecific tracer binding to the filter and/or plasma membranes was determined by measuring protein-free controls and uptake at time zero respectively, which were subtracted from the total vesicle uptake measurements.
4.11 |. 5-HT metabolism by MAO-A in rat and human placenta homogenates
Human and rat term placentas were washed with 0.9% NaCl at 4°C. After weighing and cleaning, the amniochorion and chorionic plate were removed from each sample, then the placentas were cut into small pieces and homogenized at 4°C in a buffer containing 50 mM Tris-Hepes (pH 7.2), 5 mM EGTA, 5 mM EDTA, 1 mM PMSF and 250 mM sucrose. The homogenates were filtered through gauze and centrifuged at 15 000 g (human placenta) or 800 g (rat placenta) for 10 minutes. Supernatants were collected and stored at −80°C until use.
MAO-A activity was determined by a previously published method.27 Briefly, 180 μl of placenta homogenate (1.5–2 mg ml−1) was preincubated for 5 minutes at 37°C, with or without phenelzine (100 μM), and the reaction was initiated by incubation with 20 μl of 5-HT (0.5 mM) for periods indicated in the figures. The reaction was stopped by adding 40 μl of HClO4 (3.4 M) and placed on ice. Samples were centrifuged at 5 000 g for 10 minutes, and 5-HT in the supernatant was measured by HPLC, as described below.
4.12 |. DNA isolation and foetal sex determination by endpoint PCR analysis
Genomic DNA was isolated from weighed rat placental tissues using Tri Reagent solution, according to the manufacturer’s instructions. The purity of the isolated DNA was checked by measuring the A260/A280 ratio and the total DNA concentration was calculated from the A260 measurement.
A single-step PCR method 52 was used to determine foetal sex from the rat placental tissue. Briefly, two unique forward primers for the X chromosome (5’-TTTGTACGACTAGGCCCCAC-3’) and Y chromosome (5’-TTGGTGAGATGGCTGATTCC-3’); and one common reverse primer (3’-GGTTTCTTAAACCGTCGCC-5’) were used for amplifications, yielding amplicons of 692 and 250 bp respectively. Endpoint PCR analysis was carried out in a 25 μl reaction volume using a T100TM Thermal Cycler (Bio-Rad). 10 ng of genomic DNA was amplified with the final concentration of 0.2 μM of forward and reverse primers and 0.625 units of MyTaq™ Red DNA Polymerase (Bioline) according to the manufacturer’s instructions. As positive controls, samples of genomic DNA isolated from male and female rat kidney were used. The PCR amplification conditions were as follows: 95°C for 1 minute followed by 30 cycles at 95°C for 15 s, 52°C for 15 s and 72°C for 30 s. Amplicons were separated on a 1.5% agarose gel with the HyperLadder™ 100 bp length marker (Bioline) and imaged for analysis using a ChemiDoc MP detection system (Bio-Rad).
4.13 |. RNA isolation, reverse transcription and quantitative PCR analysis
Total RNA was isolated from weighed tissue samples using Tri Reagent solution following the manufacturer’s instructions. The purity of the isolated RNA was checked by measuring the A260/A280 ratio, and the A260/230 ratio was measured to evaluate contamination by organic solvents. The integrity of the RNA samples was confirmed by electrophoresis on a 1.5% agarose gel, and total RNA concentrations were calculated from A260 measurements. Reverse transcription (RT) was performed by two-step reactions using a T100™ Thermal Cycler (Bio Rad) with the following settings. In the first step, a mixture containing 1 μl oligo(dT) solution (0.1 mM), 2 μl mRNA solution (500 ng μl−1) and API to 12.5 μl was incubated for 5 minutes at 65 °C. In the second step, 4 μl of ProtoScript II Buffer (5x), 2 μl 10 × DTT, 1 μl dNTP mix (10 mM) and 0.5 μl Protoscript II RT (200 U μl−1) were added to the above mixture and incubated for 50 minutes at 42°C and 20 minutes at 65°C. All chemicals used for RT were purchased from New England Biolabs. The expression of genes encoding six proteins - SERT (SLC6A4/Slc6a4), OCT3 (SLC22A3/Slc22a3), TPH1 (TPH1/Tph1), TPH2 (TPH2/Tph2), MAO-A (MAO-A/Mao-a), MAO-B (MAO-B/Mao-b), LAT1 (L-type amino acid transporter 1; SLC7A5/Slc7a5) and LAT2 (SLC7A8/Slc7a8) - in human and rat term placentas was analyzed by quantitative PCR using a QuantStudio™ 6 instrument (Thermo Fisher Scientific).
Obtained cDNA (25 ng μl−1) was amplified in a 384-well plate, with total reaction volumes of 5 μl per well. PCR was performed using the TaqMan® Universal Master Mix II without UNG (Thermo Fisher Scientific) and predesigned TaqMan® Real Time Expression PCR assays (listed in Table S4). Each sample was amplified in triplicate, using the following PCR cycling profile: 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 60 seconds. Before the beginning of quantitative analysis, stable expression of the reference gene was verified and gene expression was normalized against the pre-selected reference gene: Ywhaz in the rat placenta and B2M in human placenta.
4.14 |. Droplet digital PCR assay
Absolute numbers of SLC6A4/Slc6a4, SLC22A3/Slc22a3, MAO-A/Mao-a transcripts in human and rat placenta were quantified by duplex Droplet Digital PCR (ddPCR) analysis, as previously described.50 Using two different probes (FAM and HEX) we simultaneously analysed the expression of target and housekeeping gene. Briefly, each duplex reaction mixture consisted of 10 μl of ddPCR™ Supermix for Probes, 1 μl of each of predesigned probes (listed in Table S5) and 1 μl of cDNA (50 ng μl−1), in a total volume of 20 μl. After loading the sample mixtures and droplet generation oil into DG8 Cartridges, the cartridges were placed in a QX200 Droplet Generator. The obtained droplets were subsequently amplified to end-point using a T100™ Thermal Cycler and the following amplification conditions: 95°C for 10 minutes, followed by 40 cycles of 94°C for 30 seconds and 60°C for 1 minute, and finally 98°C for 10 minutes. The plate was then placed on a QX200™ Droplet Reader and the concentration of the target gene was calculated from the number of positive droplets using QuantaSoft™ Software. For final data evaluation, only wells in which the number of droplets obtained exceeded 13 000 were used. Expression levels are reported in numbers of transcripts ng−1 of transcribed RNA. The QX200™ Droplet Digital™ PCR System, T100™ Thermal Cycler and all consumables and reagents were obtained from BioRad unless otherwise stated.
4.15 |. HPLC analysis of TRP, 5-HT, 5-HIAA and MEL in placental perfusate and homogenates
The HPLC analyses were performed using a Shimadzu LC20 Performance HPLC chromatograph equipped with two pumps enabling generation of high-pressure gradients and both UV and fluorescence detectors.
For simultaneous chromatographic separation of all tested compounds, a Kinetex EVO C18 100 A 150 × 3 mm, particle size 5 μm column (Phenomenex) with a guard column was used. Analytes were eluted with a biphasic mobile phase consisting of A - 3:97 (v/v) methanol:acetic acid (0.1 M, pH 4.5, adjusted with NaOH) and B - methanol. The proportion of B was 0% for 0–8.4 minutes, then linearly increased to 20% at minute 9.6, held at 20% until minute 22.6, linearly decreased to 0% after minute 23.2 then held at 0% until minute 30.
Excitation and emission wavelengths of the fluorescence detector were set for individual compounds: 280/334 nm for 5-HT and TRP from 0–6.5 minutes, 276/333 nm for 5-HIAA from 6.5–18 minutes, and 307/375 nm for MEL from minute 18. The UV detector was set to 300 nm for TRP if its concentration was higher than 250 ng ml−1. Validation parameters are summarized in Table S6.
4.16 |. Radioisotope analysis
Concentrations of [3H]5-HT (80 Ci mmol−1) and [3H]dihydroalprenolol, levo-[propyl-1,2,3–3H] hydrochloride (80 Ci mmol−1) in experimental samples were measured by liquid scintillation counting using a Tri-Carb 2910 TR instrument.
4.17 |. Statistical analysis
Concentration-dependent uptake rates of 5-HT in the rat placenta were analysed by nonlinear mixed-effect modelling computed using the ADAPT5 software system, Version 5.0.59 (Biomedical Simulations Resource, University of Southern California). Placental uptake clearance was used to construct a base structural model incorporating Michaelis-Menten kinetics and passive diffusion (Equation 3). Model selection was based on goodness-of-fit criteria (Table S7) in combination with likelihood ratio tests, the Akaike information criterion (AIC) and Bayesian information criterion (BIC). For covariate model building, mechanistically plausible covariates — foetal sex, placental weight and expression of both Slc22a3 and Mao-a genes (quantified absolute numbers of transcripts) — were incorporated to explain the interindividual variability of the parameters estimated in the pharmacokinetic model. Descriptive statistics of the population used in the analysis are shown in Table S1.
Effects of tested inhibitors in situ and ex vivo were assessed using non-parametric Kruskal-Wallis followed by Dunn’s multiple comparisons test implemented in GraphPad Prism 8.3.1 software (GraphPad Software, Inc.). Statistical analysis of foetal sex effect on concentration-dependent uptake and parameter estimates (Vmax and Km) was evaluated using non-parametric Mann-Whitney test. Unless otherwise stated, data are presented as median + interquartile range (IQR). Asterisks in the figures indicate significance levels: * (P ≤ .05), † (P ≤ .01), and ‡ (P ≤ .001).
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the Czech Science Foundation (grant no. GACR 20-13017S), by the Grant Agency of Charles University (GAUK 1464119/C/2019, SVV 2020/260414) and the EFSA-CDN project (No. CZ.02.1.01/0.0/0.0/16_01 9/0000841), which is co-funded by the ERDF. AB was supported by a grant from the National Institute of Mental Health (R01MH106806).
Funding information
Grant Agency of Charles University, Grant/Award Number: 1464119/C/2019 and SVV 2020/260414; Czech Science Foundation, Grant/Award Number: 20-13017S; National Institute of Mental Health, Grant/Award Number: R01MH106806
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
REFERENCES
- 1.Staud F, Karahoda R. Trophoblast: The central unit of fetal growth, protection and programming. Int J Biochem Cell Biol. 2018;105:35–40. [DOI] [PubMed] [Google Scholar]
- 2.Bonnin A, Levitt P. Placental source for 5-HT that tunes fetal brain development. Neuropsychopharmacology. 2012;37(1):299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robson JM, Senior JB. The 5-Hydroxytryptamine Content of the Placenta and Foetus during Pregnancy in Mice. Br J Pharmacol Chemother. 1964;22:380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koren Z, Pfeifer Y, Sulman FG. Distribution and placental transfer of C-14-serotonin in pregnant rats. Am J Obstet Gynecol. 1966;95(2):290–295. [DOI] [PubMed] [Google Scholar]
- 5.Cote F, Fligny C, Bayard E, et al. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci USA. 2007;104(1):329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurent L, Deroy K, St-Pierre J, Cote F, Sanderson JT, Vaillancourt C. Human placenta expresses both peripheral and neuronal isoform of tryptophan hydroxylase. Biochimie. 2017;140:159–165. [DOI] [PubMed] [Google Scholar]
- 7.Bonnin A, Goeden N, Chen K, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472(7343):347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kliman HJ, Quaratella SB, Setaro AC, et al. Pathway of maternal serotonin to the human embryo and fetus. Endocrinology. 2018;159(4):1609–1629. [DOI] [PubMed] [Google Scholar]
- 9.Wu HH, Choi S, Levitt P. Differential patterning of genes involved in serotonin metabolism and transport in extra-embryonic tissues of the mouse. Placenta. 2016;42:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganapathy V, Ramamoorthy S, Leibach FH. Transport and metabolism of monoamines in the human placenta: A review. Placenta. 1993;14:35–51. [Google Scholar]
- 11.Prasad PD, Hoffmans BJ, Moe AJ, Smith CH, Leibach FH, Ganapathy V. Functional expression of the plasma membrane serotonin transporter but not the vesicular monoamine transporter in human placental trophoblasts and choriocarcinoma cells. Placenta. 1996;17(4):201–207. [DOI] [PubMed] [Google Scholar]
- 12.Muller CL, Anacker AMJ, Rogers TD, et al. Impact of maternal serotonin transporter genotype on placental serotonin, fetal forebrain serotonin, and neurodevelopment. Neuropsychopharmacol. 2017;42(2):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kono H, Lin YC, Yamaguchi M, et al. Monoamine oxidase activity in rat organs during pregnancy. Tohoku J Exp Med. 1994;172(1):1–8. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Klein DC, Robinson JC. Monoamine oxidase in rat placenta, human placenta, and cultured choriocarcinoma. J Reprod Fertil. 1976;46(2):477–479. [DOI] [PubMed] [Google Scholar]
- 15.Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285(2):262–273. [DOI] [PubMed] [Google Scholar]
- 16.Arevalo R, Afonso D, Castro R, Rodriguez M. Fetal brain serotonin synthesis and catabolism is under control by mother intake of tryptophan. Life Sci. 1991;49(1):53–66. [DOI] [PubMed] [Google Scholar]
- 17.Sano M, Ferchaud-Roucher V, Kaeffer B, Poupeau G, Castellano B, Darmaun D. Maternal and fetal tryptophan metabolism in gestating rats: effects of intrauterine growth restriction. Amino Acids. 2016;48(1):281–290. [DOI] [PubMed] [Google Scholar]
- 18.Bjoro K, Stray-Pedersen S. In vitro perfusion studies on human umbilical arteries. I. Vasoactive effects of serotonin, PGF2 alpha and PGE2. Acta Obstet Gynecol Scand. 1986;65(4):351–355. [DOI] [PubMed] [Google Scholar]
- 19.Marley PB, Robson JM, Sullivan FM. Embryotoxic and teratogenic action of 5-hydroxytryptamine: mechanism of action in the rat. Br J Pharmacol Chemother. 1967;31(3):494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24(7):1227–1251. [DOI] [PubMed] [Google Scholar]
- 21.Lee N, Hebert MF, Prasad B, et al. Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug metabolism and disposition: the biological fate of chemicals. 2013;41(12):2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sata R, Ohtani H, Tsujimoto M, et al. Functional analysis of organic cation transporter 3 expressed in human placenta. The Journal of pharmacology and experimental therapeutics. 2005;315(2):888–895. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadimoghaddam D, Hofman J, Zemankova L, et al. Synchronized activity of organic cation transporter 3 (Oct3/Slc22a3) and multidrug and toxin extrusion 1 (Mate1/Slc47a1) transporter in transplacental passage of MPP+ in rat. Toxicol Sci. 2012;128(2):471–481. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadimoghaddam D, Zemankova L, Nachtigal P, et al. Organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter in the placenta and fetal tissues: expression profile and fetus protective role at different stages of gestation. Biol Reprod. 2013;88(3):55. [DOI] [PubMed] [Google Scholar]
- 25.Balkovetz DF, Tiruppathi C, Leibach FH, Mahesh VB, Ganapathy V. Evidence for an imipramine-sensitive serotonin transporter in human placental brush-border membranes. The Journal of biological chemistry. 1989;264(4):2195–2198. [PubMed] [Google Scholar]
- 26.Jansson T, Illsley NP. Osmotic water permeabilities of human placental microvillous and basal membranes. J Membr Biol. 1993;132(2):147–155. [DOI] [PubMed] [Google Scholar]
- 27.Carrasco G, Cruz MA, Gallardo V, Miguel P, Dominguez A, Gonzalez C. Transport and metabolism of serotonin in the human placenta from normal and severely pre-eclamptic pregnancies. Gynecol Obstet Invest. 2000;49(3):150–155. [DOI] [PubMed] [Google Scholar]
- 28.Feng N, Mo B, Johnson PL, Orchinik M, Lowry CA, Renner KJ. Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus. Brain Res. 2005;1063(1):69–76. [DOI] [PubMed] [Google Scholar]
- 29.Baganz NL, Horton RE, Calderon AS, et al. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105(48):18976–18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt A, Mössner R, Gossmann A, et al. Organic cation transporter capable of transporting serotonin is up-regulated in serotonin transporter-deficient mice. J Neurosci Res. 2003;71(5):701–709. [DOI] [PubMed] [Google Scholar]
- 31.Wooding FBP, Burton G. Comparative Placentation: Structures, Functions, and Evolution. Berlin: Springer-Verlag; 2008. [Google Scholar]
- 32.Fraser-Spears R, Krause-Heuer AM, Basiouny M, et al. Comparative analysis of novel decynium-22 analogs to inhibit transport by the low-affinity, high-capacity monoamine transporters, organic cation transporters 2 and 3, and plasma membrane monoamine transporter. Eur J Pharmacol. 2019;842:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garbarino VR, Santos TA, Nelson AR, et al. Prenatal metformin exposure or organic cation transporter 3 knock-out curbs social interaction preference in male mice. Pharmacol Res. 2019;140:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gobinath AR, Workman JL, Chow C, Lieblich SE, Galea LAM. Sex-dependent effects of maternal corticosterone and SSRI treatment on hippocampal neurogenesis across development. Biol Sex Differ. 2017;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivasubramaniam SD, Finch CC, Billett MA, Baker PN, Billett EE. Monoamine oxidase expression and activity in human placentae from pre-eclamptic and normotensive pregnancies. Placenta. 2002;23(2–3):163–171. [DOI] [PubMed] [Google Scholar]
- 36.Verhaagh S, Barlow DP, Zwart R. The extraneuronal monoamine transporter Slc22a3/Orct3 co-localizes with the Maoa metabolizing enzyme in mouse placenta. Mech Dev. 2001;100(1):127–130. [DOI] [PubMed] [Google Scholar]
- 37.Carrasco G, Cruz MA, Dominguez A, Gallardo V, Miguel P, Gonzalez C. The expression and activity of monoamine oxidase A, but not of the serotonin transporter, is decreased in human placenta from pre-eclamptic pregnancies. Life Sci. 2000;67(24):2961–2969. [DOI] [PubMed] [Google Scholar]
- 38.Viau M, Lafond J, Vaillancourt C. Expression of placental serotonin transporter and 5-HT 2A receptor in normal and gestational diabetes mellitus pregnancies. Reproductive BioMedicine Online. 2009;19(2):207–215. [DOI] [PubMed] [Google Scholar]
- 39.Koepsell H Organic cation transporters in health and disease. Pharmacol Rev. 2020;72(1):253–319. [DOI] [PubMed] [Google Scholar]
- 40.Staud F, Mazancova K, Miksik I, Pavek P, Fendrich Z, Pacha J. Corticosterone transfer and metabolism in the dually perfused rat placenta: effect of 11beta-hydroxysteroid dehydrogenase type 2. Placenta. 2006;27(2–3):171–180. [DOI] [PubMed] [Google Scholar]
- 41.van der Doelen RHA, Calabrese F, Guidotti G, et al. Early life stress and serotonin transporter gene variation interact to affect the transcription of the glucocorticoid and mineralocorticoid receptors, and the co-chaperone FKBP5, in the adult rat brain. Front Behav Neurosci. 2014;8:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32(6):1174–1184. [DOI] [PubMed] [Google Scholar]
- 43.Van de Kar LD. Neuroendocrine pharmacology of serotonergic (5-HT) neurons. Annu Rev Pharmacol Toxicol. 1991;31:289–320. [DOI] [PubMed] [Google Scholar]
- 44.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544 e541–545. [DOI] [PubMed] [Google Scholar]
- 45.Zhu HJ, Appel DI, Grundemann D, Richelson E, Markowitz JS. Evaluation of organic cation transporter 3 (SLC22A3) inhibition as a potential mechanism of antidepressant action. Pharmacol Res. 2012;65(4):491–496. [DOI] [PubMed] [Google Scholar]
- 46.Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS (London, England). 2014;28(7):1049–1057. [DOI] [PubMed] [Google Scholar]
- 47.Amphoux A, Vialou V, Drescher E, et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50(8):941–952. [DOI] [PubMed] [Google Scholar]
- 48.Staud F, Vackova Z, Pospechova K, et al. Expression and transport activity of breast cancer resistance protein (Bcrp/Abcg2) in dually perfused rat placenta and HRP-1 cell line. J Pharmacol Exp Ther. 2006;319(1):53–62. [DOI] [PubMed] [Google Scholar]
- 49.Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM. Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta. 1990;1029(2):218–226. [DOI] [PubMed] [Google Scholar]
- 50.Karahoda R, Ceckova M, Staud F. The inhibitory effect of antiretroviral drugs on the L-carnitine uptake in human placenta. Toxicol Appl Pharmacol. 2019;368:18–25. [DOI] [PubMed] [Google Scholar]
- 51.Farrugia W, de Gooyer T, Rice GE, Moseley JM, Wlodek ME. Parathyroid hormone(1–34) and parathyroid hormone-related protein(1–34) stimulate calcium release from human syncytiotrophoblast basal membranes via a common receptor. The Journal of endocrinology. 2000;166(3):689–695. [DOI] [PubMed] [Google Scholar]
- 52.Dhakal P, Soares MJ. Single-step PCR-based genetic sex determination of rat tissues and cells. Biotechniques. 2017;62(5):232–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


